Abstract
Objective
This study examined the effectiveness of prospective stratification to identify and target high-dose glucocorticoid therapy for subjects developing lethal sepsis.
Design
Prospective, randomized, laboratory-controlled experiment.
Setting
University research laboratory.
Subjects
Adult female outbred CD-1 mice.
Interventions
Mice (n = 88) were subjected to sepsis induced by cecal ligation and puncture (CLP). Mice were prospectively divided into two groups, predicted to die (P-DIE) or predicted to live (P-LIVE), based on plasma levels of interleukin (IL)-6 obtained 6 hours after CLP. Following stratification, dexamethasone (DEX, 2.5 mg/kg, two doses) was administered to half the animals in each group whereas the other half received saline.
Measurements and Main Results
Without stratification, DEX conferred no benefit. In the P-DIE group, none of saline-treated mice lived whereas 40% of the DEX-treated mice survived. Of the nonsurvivors, 67% had death delayed by 24 – 48 hours compared with saline-treated mice. Twenty-four hours post-CLP, the lymphocyte count was higher in the P-DIE than in the P-LIVE mice regardless of treatment status, whereas the opposite trend was noted for neutrophils. Plasma cytokine and cytokine inhibitor levels in the saline-treated animals showed that levels in the P-DIE group were higher than those in the P-LIVE group (e.g., 60 vs. 10 ng/mL for IL-6 and 453 vs.129 ng/mL for IL-1 receptor antagonist). Interestingly, DEX therapy did not decrease 24 hours post-CLP circulating cytokines in either the P-DIE or the P-LIVE group.
Conclusions
Following CLP-induced sepsis, early and accurate survival prediction allows targeted immunosuppression that improves survival. Better survival occurred without suppression of the typical proinflammatory mediators, suggesting that the deaths were not mediated by excessive cytokine-driven inflammation. Nonspecific anti-inflammatory/immunosuppressive treatment administered to more rigorously defined cohorts may be more successful than mediator-specific drugs used indiscriminately.
Keywords: stratification, corticosteroids, interleukin-6, sepsis, cytokines, cytokine receptors, inflammation
Standard protocols for sepsis treatment, relying on broad-spectrum antibiotics, fluid resuscitation, and ventilation, have remained essentially unchanged for the last three decades. Although decreased mortality has been observed with early goal-directed therapy and activated protein C (1), aggressive anti-inflammatory trials, either in the form of corticosteroid-based therapy (2–5) or more recent specific anti-cytokine approaches (6), not only failed to improve survival but some were even associated with increased mortality (7). Corticosteroids have a long history in the therapy of sepsis, beginning with the landmark study by Schumer (8), which showed a 30% improvement in survival after early dexamethasone (DEX) and prednisolone treatment in patients with septic shock. However, subsequent clinical trials contradicted the original findings by showing that high-dose glucocorticoids did not improve outcome in septic patients (3, 4, 9, 10). It should be noted that low-dose steroids were reintroduced as an adjunctive therapy in patients with adrenal insufficiency (11).
There is a growing consensus that appropriate therapy for sepsis cannot rely on the “magic bullet” paradigm where specific, targeted therapy directed against a single mediator will be successful. Two major immune conditions proposed to coexist during the course of sepsis, the systemic inflammatory response syndrome and the compensatory anti-inflammatory response syndrome, emphasize that sepsis constantly fluctuates (12). Thus, successful treatment needs to be frequently adjusted based on the patient’s current immunoinflammatory status. Statistical analysis suggested that the failure of the anticytokine trials may have been related to heterogeneous patient populations, because cohorts with the highest mortality risk were more likely to benefit from anti-inflammatory interventions (13). Using inflammatory cytokines as biomarkers, we developed an approach that allows accurate mortality prediction in the cecal ligation and puncture (CLP) model of murine sepsis (14, 15). To verify this approach in a scenario of targeted treatment, we hypothesized that nonspecific corticosteroid (DEX) suppression of inflammation in subjects predicted to die (P-DIE) would improve survival, whereas the identical treatment applied to animals predicted to live (P-LIVE) will produce little benefit.
MATERIALS AND METHODS
Animals
Eighty-eight female CD-1 mice (Harlan-Sprague Dawley, Indianapolis, IN) with an average weight of 22 g were used. Experiments were in accordance with the National Institutes of Health guidelines and the University of Michigan Animal Care and Use Committee.
Sepsis Model
The murine model of CLP used in this study constitutes arguably the best surrogate for abdominal polymicrobial human sepsis to date (16). To generate approximately 50% mortality during the early sepsis phase, the 18-gauge needle size and double cecal puncture were used. We followed the original CLP protocol introduced by the Wichterman and co-workers (17) with modifications including analgesia (buprenorphine) and antibiotic therapy (Imipenem) (18). The experimental reproducibility was assured by performing CLP in small sets of animals (n = 9 –10) at a time and combining the results of eight separate experiments for this study.
Sampling
To collect peripheral blood, the distal tail was clipped (~2 mm) and 20 μL of blood was drawn into a pipette tip rinsed with EDTA (169 mM tripotassium salt). Mice were not killed at the time of sampling. Blood was collected 6 hours after CLP and at 24-hour intervals for the first 5 days or until death. The last individual cytokine measurement represents an animal that died within 24 hours after sampling. Samples were immediately diluted (1:10) in phosphate-buffered saline with a 1:50 dilution of EDTA, centrifuged (5 min/1000g at 4°C), the diluted plasma removed, and stored at −20°C until analysis.
Hematology
Following blood collection, the cell pellet was immediately resuspended in 480 μL of Hemavet solution (CDC Technologies, Oxford, CT). A complete blood count including differential and platelets was performed in a Hemavet 1500 (CDC Technologies).
Prospective Stratification
The classification of relevant subjects was performed by dividing all animals into either P-LIVE or P-DIE mice within 48 hours based on the circulating level of interleukin (IL)-6 at 6 hours after CLP. We elected to simultaneously measure the IL-1 receptor antagonist (ra) percentile method concentration as a safeguard against a failed IL-6 assay. Because all assays were successful, only IL-6 was used as a primary predictor at each of the eight independent experiments. The selected IL-6 cutoff for separation into P-LIVE or P-DIE was established previously (14) at 26 ng/mL. The 6 hours post-CLP time point was selected for blood sampling and subsequent stratification of animals because this precedes the period of highest mortality (14, 15, 19).
Corticosteroid Treatment
A water-soluble DEX (cyclodextrin encapsulated; Sigma, St Louis, MO) was given twice intraperitoneally in the supraphysiologic dose of 2.5 mg/kg of body weight 8 and 32 hours after CLP. DEX in a similar dose elicited strong anti-inflammatory properties in another murine CLP model of sepsis when given 15 minutes after surgery (20).
Rapid Enzyme-Linked Immunosorbent Assay
The rapid enzyme-linked immunosorbent assay for IL-6 (and IL-1ra) was based on standard enzyme-linked immunosorbent assay methodology using commercially available matched antibody (Ab) pairs (R&D Systems, Minneapolis, MN) as detailed elsewhere (21). The rapid assay followed a classic format with several modifications to accelerate the detection process. The capture Ab (50 μL/well) was used in a concentration of 3000 ng/mL (in dilution buffer). The next day, the plate(s) was blocked with the custom-made laboratory blocking buffer composed of wash buffer (80% phosphate-buffered saline, 19% distilled deionized water, and 1% Tween 20) with 2% bovine serum albumin and incubated at 37°C for at least 1 hour. Next, the plate was rapidly washed (total three cycles) with the warm wash buffer from a squirt bottle. Standard serial dilutions were prepared in the dilution buffer (as above) with 2% fetal calf serum. Standards and samples were then incubated for 20 minutes with intense (240 rpm) shaking at 37°C. After washing (as above), the detection Ab was used at the concentration of 300 ng/mL (in dilution buffer). The plate was then incubated 20 minutes at 37°C and streptavidin (streptavidine [conjugated]-horseradish peroxidase diluted in the laboratory buffer) was added for 20 minutes at a final concentration of 1:10,000. All consecutive steps followed the regular enzyme-linked immunosorbent assay protocol (21).
Microarray Immunoassay
The microarray immunoassay used has a capacity to simultaneously measure 21 murine cytokines in 20 μL of blood (22). In the microarray immunoassay technique, the primary (capture) Ab against selected cytokines are spotted on nitrocellulose pads affixed to glass slides as detailed elsewhere (22). The 24-hour time point (post-CLP) was selected, because it was characterized by the highest single-day mortality and simultaneously highest expression of circulating cytokines (14).
Statistical Analysis
A receiver operating characteristic analysis was used to evaluate the prognostic accuracy (defined by the area under the curve) of the analyzed cytokines (Table 1) and to determine the sensitivity and specificity for prediction of death, at selected cytokine cutoff values. The 95% confidence intervals for the area under the curve values were estimated using the adjusted bootstrap percentile method to obtain more accurate intervals (23). The accuracy of the receiver operating characteristic area under the curve test is as follows: 0.9–1 = excellent, 0.8–0.9 = good, 0.7–0.8 = fair, and 0.6–0.7 = poor.
Table 1.
Accuracy of IL-6 and IL-1ra for predicting 48-hour survival by receiver operator characteristic test
| Study | Marker | Sensitivity (%) | Specificitya (%) | Area Under the Curveb | SEM | 95% | |
|---|---|---|---|---|---|---|---|
| Confidence | Interval | ||||||
| Current | IL-6 | 89 | 97 | 0.902 | 0.093 | 0.719 | 0.999 |
| Previousc | IL-6 | 61 | 99 | 0.898 | 0.042 | 0.815 | 0.979 |
| Current | IL-1ra | 46 | 98 | 0.866 | 0.048 | 0.774 | 0.959 |
| Previous | IL-1ra | 30 | 100 | 0.862 | 0.042 | 0.780 | 0.944 |
IL, interleukin.
Sensitivity and specificity were chosen based on cutoffs favoring specificity to avoid inclusion of animals with low risk of death into the dexamethasone treatment subgroup;
area under the curve defines value of the given marker as a diagnostic test for outcome;
calculated based on the data from Osuchowski et al (14).
Animals were monitored for 28 days for all-cause mortality. Kaplan-Meier 7-day and 28-day survival curves (Figs. 1 and 2, respectively) were plotted using Prism 4 (GraphPad Software, San Diego, CA). One-tailed p values (by the log-rank test) used to compare groups are depicted along the graphs. One-tailed significance tests were used, given the pre-experimental directional hypotheses that P-DIE DEX-treated mice would have a longer median survival than their P-DIE saline-treated counterparts, and that the P-LIVE DEX-treated mice would have a shorter median survival than the P-LIVE saline-treated subgroup. Given the nonsignificant (p = 0.14) decrease in survival in the 7-day follow-up period in DEX-treated P-LIVE mice (in comparison with P-LIVE saline-treated mice), a power analysis by StatMate 2.0 (GraphPad) estimated the group size at which this effect would reach statistical significance. Approximately 450 mice would have been needed to reach significance with 95% power and an alpha level of 0.05.
Figure 1.
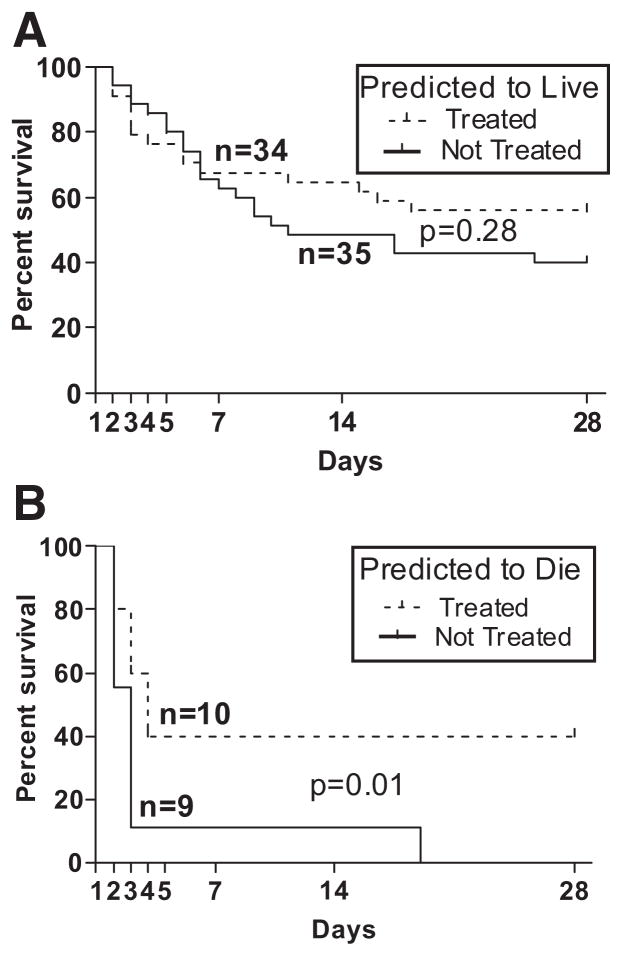
Dexamethasone (DEX) improved short-term survival only in the subpopulation predicted to die. Cecal ligation and puncture was performed with an 18-gauge needle to produce approximately 40% mortality during the early phase of sepsis (first 5 days). All animals were analyzed either based on the treatment alone (A) or risk of death and therapy (B and C). DEX without stratification did not confer any survival benefit (A). When animals predicted to live were treated with DEX (B), their survival was essentially not altered. In mice predicted to die, DEX treatment resulted in a 40% survival. Additionally, death in the nonsurvivors was markedly delayed in comparison with saline-treated counterparts (C); n = 88. All survival comparisons were made by the log-rank test and p values were derived from one-tailed tests.
Figure 2.
Dexamethasone (DEX) improved long-term survival only in the subpopulation predicted to die. To examine long-term effects of steroid treatment upon survival in experimental sepsis, all mice were monitored for 28 days. DEX treatment in the subpopulation of mice predicted to live did not significantly alter outcome (A). Administration of DEX to animals predicted to die resulted in a significant improvement in survival compared with saline-treated counterparts (B); n = 88. Survival comparisons were made by the log-rank test and p values were derived from one-tailed tests.
Before analysis, blood differential and cytokine variables were examined for normality. All cytokines, except macrophage inflammatory protein (MIP)-1α, were found to be highly skewed, and thus were analyzed on the natural log scale. The log transformation (rather than nonparametric tests) was selected, because it provided a correction for the unequal variances and the frequent outlying high values. MIP-1α and blood differential values (normally distributed) were analyzed on the original scale. Blood differential variables (Fig. 4) were analyzed using a one-way analysis of variance model fitted at each time point (6, 24, and 48 hours post-CLP) rather than using repeated-measures analysis of variance across all three time points. The one-way analysis of variance model was used given that the differences between groups (not the time trends in separate groups) were the primary end points and given the reduction in sample size across time related to the treatment scenario.
Figure 4.
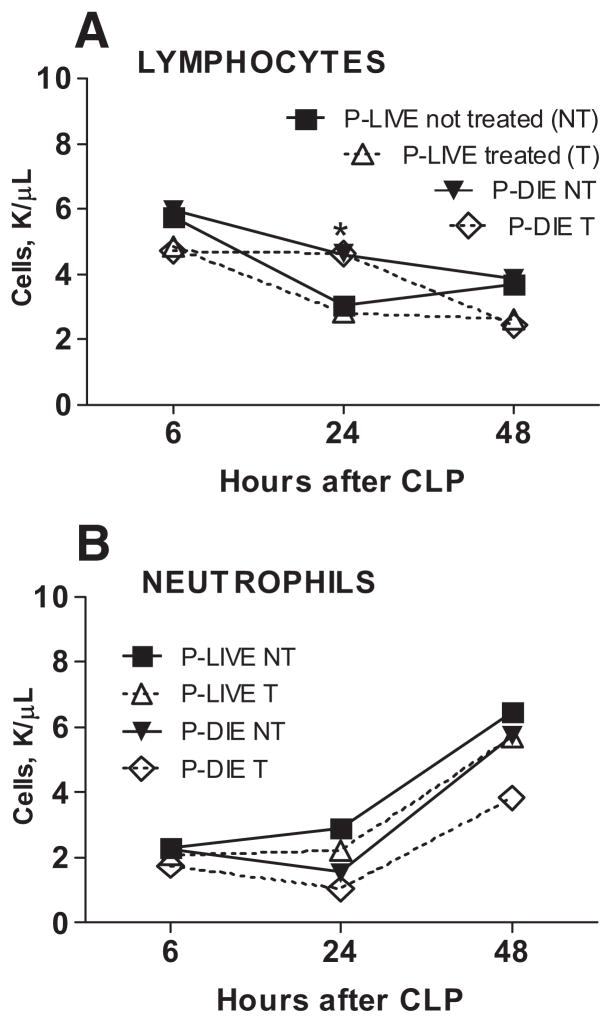
Dexamethasone (DEX) did not alter early circulating leukocyte profiles. Animals designated in the graphs were separated based on the treatment and risk of death as defined in the Methods section. Circulating lymphocytes (A) and neutrophils (B) were measured (counts per μL blood) at 6, 24, and 48 hours postcecal ligation and puncture (CLP). At 6 hours for predicted to die (P-DIE) treated (T), n = 10; for P-DIE not treated (NT), n = 9; for predicted to live (P-LIVE) T, n = 34; and P-LIVE NT, n = 35. At 24 hours for P-DIE T, n = 8; for P-DIE not T, n = 6; for P-LIVE T, n = 31; and P-LIVE not T, n = 33. At 48 hours for P-DIE T, n = 6; for P-DIE not T, n = 3; for P-LIVE T, n = 28; and P-LIVE not T, n = 31. *p < 0.05 for P-DIE compared with P-LIVE (both T and NT, respectively). Data are presented as mean ± SEM.
A one-way analysis of variance model was fitted to compare the mean cytokine levels (Figs. 5 and 6) for the four subgroups of mice (P-LIVE DEX treated, P-LIVE saline treated, P-DIE DEX treated, and P-DIE saline treated) on the appropriate log transformed or original scale. For each analysis, homogeneity of variances was checked using Levene’s test, with α = 0.10. Post hoc tests to compare means for each cytokine were carried out using either the Bonferroni method (equal variances by Levene’s test on natural log: IL-1ra, 2, 5, 6, 12, 10, 18, MIP-2, eotaxin, interferon-γ, monocyte chemoattractant protein-1, tumor necrosis factor [TNF]-α, TNF soluble receptor (SR) I, and II) or the Tamhane method (unequal variances by Levene’s test on natural log: IL-1β, IL-13, keratinocyte-derived chemokine). Data are represented as the mean ± standard error of the means wherever applicable. All tests were carried out using SPSS 15.0 (SPSS, Chicago, IL).
Figure 5.
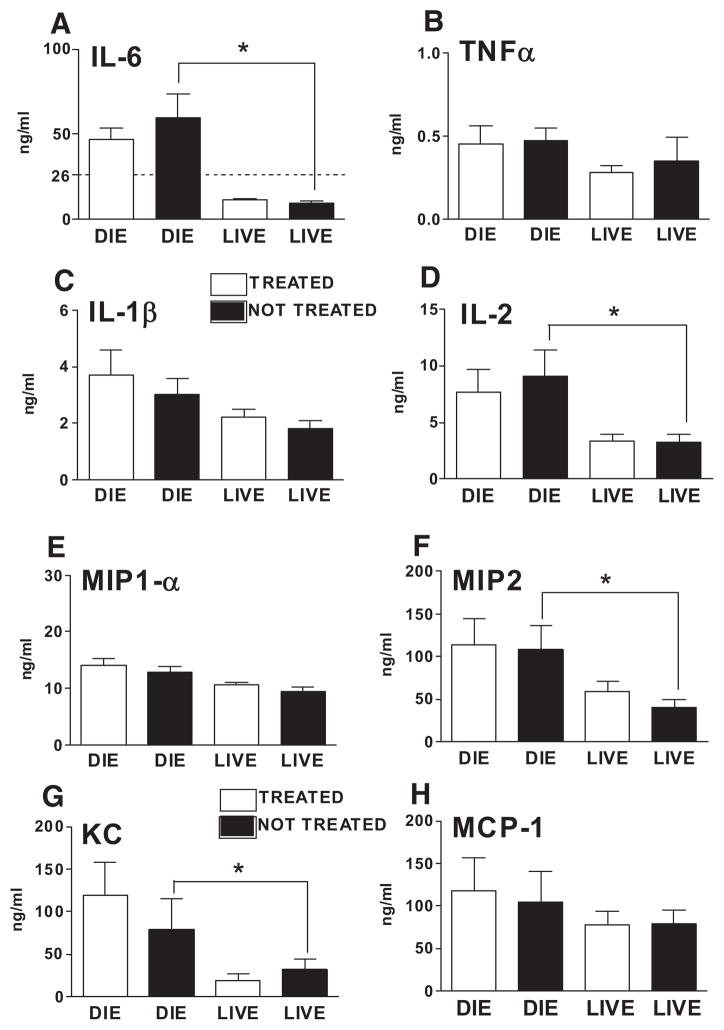
Systemic proinflammatory cytokines measured 24 hours postcecal ligation and puncture were not suppressed by dexamethasone (DEX). To examine the effect of the supraphysiologic dose of DEX (2.5 mg/kg) upon systemic expression of key proinflammatory cytokines (A–D) and chemokines (E–H), plasma concentrations of the indicated cytokines were measured 24 hours postcecal ligation and puncture (15 hours after the first intraperitoneal DEX injection). For treated and nontreated animals predicted to die (depicted as DIE), n = 8 and 6, respectively. For treated and nontreated animals predicted to live (depicted as LIVE), n = 31 and 33, respectively. The dashed line in the y-axis of the interleukin (IL)-6 graph (A) depicts the cutoff used for prediction. *p < 0.01 between the indicated groups. Data are presented as mean ± SEM. TNF-α, tumor necrosis factor-α; MIP, macrophage inflammatory protein; KC, keratinocyte-derived chemokine; MCP, monocyte chemoattractant protein.
Figure 6.
Dexamethasone (DEX) fails to suppress systemic anti-inflammatory cytokines measured 24 hours after cecal ligation and puncture. To examine the effect of the supraphysiologic dose of DEX (2.5 mg/kg) upon systemic expression of key anti-inflammatory cytokines, plasma concentrations of interleukin (IL)-1ra, IL-10, tumor necrosis factor (TNF) soluble receptor (SR) I, and TNF SRII (A–D) were measured 24 hours postcecal ligation and puncture (15 hours after first intraperitoneal DEX injection). For treated and nontreated animals predicted to die (depicted as DIE), n = 8 and 6, respectively. For treated and nontreated animals predicted to live (depicted as LIVE), n = 31 and 33, respectively. *p < 0.01 between the indicated groups. Data are presented as mean ± SEM.
RESULTS
IL-6 and IL-1ra Accurately Predicted Outcome in Acute Sepsis (First 5 days)
Using the murine CLP-induced sepsis model, we have reported that IL-6 as well as anti-inflammatory markers accurately predict outcome in both the acute (14) and chronic (15) phases of sepsis. On the basis of the circulating levels of IL-6 measured at 6 hours after initiation of sepsis and using a previously established cutoff, we separated animals into subpopulations of either high or low risk of death. In this study, both IL-6 and IL-1ra showed high accuracy and reproducibility for predicting mortality (within 48 hours of measurement) as defined by receiver operating characteristic analysis. When data from our previous study (14) were analyzed using the cutoffs and prognostic time frame as in this study, both the previous and current IL-6 and IL-1ra area under the curve scores were comparable (Table 1).
Dexamethasone Improved Survival only in the Subpopulation Predicted to Die
A total of 88 genetically heterogeneous CD-1 outbred mice were subjected to CLP. The acute phase of sepsis (defined as the period from day 1 to 5 after CLP) was selected for intervention, because early deaths are hypothesized to be due to excessive systemic inflammatory response syndrome-type inflammation (12, 24, 25), making inhibition of the exuberant immunoinflammatory response the most rational treatment tactic.
Short-Term Survival (7 days)
Without prospective stratification, i.e., only comparing survival in all DEX-treated mice to saline-treated mice, DEX did not improve survival over the first 7 days (Fig. 1A). Survival curves in both DEX and saline groups were virtually superimposable. Before analysis, mice were divided into two groups: P-LIVE (n = 69) and P-DIE (n = 19) within the next 48 hours. DEX given to half of the P-LIVE subgroup did not significantly increase mortality compared with the other half of saline-treated P-LIVE mice (Fig. 1B). A trend toward increased mortality with corticosteroid therapy was observed between days 2 and 5 (Fig. 1B).
Conversely, administration of DEX in the group of mice P-DIE resulted in survival improvement (Fig. 1C). After two treatments with DEX, 40% (4 of 10) of mice P-DIE actually survived for the next 7 days, compared with 11% (1 of 9) survival in the saline-treated P-DIE animals. Furthermore, there is a marked difference in the time of death between either of these two subgroups: the deaths in four of six of P-DIE DEX-treated mice were delayed an additional 24 – 48 hours in comparison with saline-treated counterparts (Fig. 1C).
Long-Term Effect (28 days)
Although the deaths are most frequent during the first week of sepsis, mortality continues beyond day 7 in both human (6, 26) and experimental sepsis (14, 15). An increased mortality rate during the late phase of sepsis could easily annul the improved survival achieved in the early phase of the experiment. During the 28-day monitoring, similar to the initial 7-day period, DEX treatment without stratification did not lead to any improvement in survival compared with that in saline-injected mice (Fig. 2A). After stratification, the P-LIVE group did not show any changes in survival between DEX and saline-injected groups. However, in the P-DIE group, DEX therapy provided a substantial improvement in 28-day survival (Fig. 2B). The initial (7 days) beneficial effect of early DEX treatment had a long-lasting effect: none of the rescued mice succumbed to sepsis (or any other complications) in the late phase of sepsis, preserving the 40% survival rate to the end of the study at day 28 (Fig. 2B). These results show that the prospective separation of the entire population into groups based on a lethal inflammatory response (as indicated by IL-6) enabled the DEX treatment to be effective and long lasting.
Equivalent Intensity of Inflammation in DEX and Saline-Treated Groups
It is possible that the beneficial effects of a tested substance can be distorted by an unbalanced distribution of subjects enrolled in the drug and placebo subgroups. Because IL-6 was used to stratify animals, we retrospectively examined the data to ensure that selection bias did not occur. Circulating levels of IL-6 obtained 6 hours after the onset of CLP, and before DEX therapy, showed that the severity of inflammation was similar in those mice assigned to receive DEX therapy or saline (Fig. 3A). This was true for both groups of P-DIE and P-LIVE mice. We also verified the actual IL-6 distribution of individual mice in the P-DIE group, because DEX provided a significant benefit to this cohort. Figure 3B shows that the distribution of data points in both groups (treated or not treated) was virtually identical and that animals rescued by DEX were not necessarily the ones with the lowest IL-6 levels.
Figure 3.
Comparison of the severity of sepsis-induced inflammation in dexamethasone (DEX) and saline-treated subpopulations. Plasma interleukin (IL)-6 measured 6 hours postcecal ligation and puncture served as a marker for the intensity of inflammation in the cecal ligation and puncture sepsis model. The degree of inflammation in the DEX and saline-treated animals was similar in the groups of mice predicted to die and predicted to live (A). To examine the actual distribution of individual subjects in the subgroup where a significant benefit of DEX treatment was observed, all individual IL-6 concentrations from the animals predicted to die were plotted. In both treated and not treated subjects, the IL-6 levels were clustered in a nearly identical manner (B). B also shows that animals saved by DEX were not the ones with the lowest IL-6 levels. C depicts the distribution of all individual IL-6 concentrations taken from mice classified as predicted to live that were either alive or dead at day 28. Note that our prediction model is valid only for the first 5 days of sepsis, not for the entire 28 days of study.
A conservative IL-6 cutoff level was used for this study (26 ng/mL). Retrospective evaluation of the data showed that no untreated animal with a 6 hours IL-6 concentration exceeding 16 ng/mL ever survived (Fig. 3C). The “buffer-zone” between 16 and 26 ng/mL (10 ng/mL) ensured that animals with a potentially nonlethal response were not erroneously included for the immunosuppressive treatment that might have worsened their outcome and vice versa.
Dexamethasone did not Alter Short-Term Systemic Leukocyte Profiles
In both humans and laboratory animals, corticosteroids cause transient (up to 24 hours) lymphopenia and neutrophilia (27). These characteristic effects were absent in this study: approximately 15 hours after the first DEX injection (24 hours post-CLP time point), the lymphocyte (Fig. 4A) and neutrophil (Fig. 4B) counts in both the treated and not treated groups were virtually identical (both the P-DIE and P-LIVE subpopulations). In all animals, there was a CLP-induced gradual decrease in the levels of red blood cells, hemoglobin, and platelets (up to 48 hours), and the rate and degree of this decline was identical for all subpopulations (data not shown). Overall, these results imply that the DEX-induced improvement of survival was not mediated by substantial changes in the levels of circulating leukocytes.
Systemic Proinflammatory Cytokines Unresponsive to Dexamethasone at 24 hours Post-CLP
Detectable levels of TNF-α and IL-1β and other proinflammatory cytokines have been reported in a number of studies investigating both clinical and experimental (14, 19, 28) sepsis. However, the effects of corticosteroid treatment upon the immunoinflammatory profile in CLP septic mice are unknown. Plasma levels of multiple cytokines were measured 24 hours post-CLP, the time point where all treated mice would have been exposed to DEX for approximately 15 hours. Surprisingly, DEX treatment did not significantly decrease any of the plasma concentrations of the proinflammatory cytokines (Fig. 5, A–D) or chemokines (Fig. 5, E–H). This lack of DEX-related cytokine suppression in the systemic circulation was present both in the P-DIE and P-LIVE subpopulations of mice. This failed cytokine suppression was not due to the inadequate immunosuppressive activity of the DEX because its administration (2.5 mg/kg) 2 hours before an endotoxin challenge (Escherichia coli type O111:B4, 10 μg/mouse, intraperitoneal) significantly reduced plasma levels of IL-6, TNF-α, MIP-1α, and MIP-2 at 2 hours post-lipopolysaccharide (data not shown).
Among these circulating biomarkers, there was a clear difference between subpopulations separated based on the predicted outcome and not the treatment criterion (Fig. 5). In all these cases, cytokine levels of P-DIE mice were higher than those recorded in their P-LIVE counterparts. This difference was most prominent in IL-6 (six-fold; compared between saline-treated P-DIE and P-LIVE groups), keratinocyte-derived chemokine (2.5-fold), and MIP-2 (2.5-fold). These results demonstrate the strong correlation of these cytokine mediators with acute sepsis mortality and underscore their previous (18, 22) relevance in predicting short-term outcomes.
We also examined circulating levels of IL-5, 12, 13, 18, eotaxin, IFNγ, and MIP-1α at the 24-hour time point and there were no significant differences between the DEX and saline groups when they were separated based either on the treatment/no treatment or prediction of outcome criterion (data not shown). Overall, these results show that the improvement of survival evident in mice P-DIE and treated with DEX did not occur through inhibition of circulating proinflammatory cytokines and chemokines.
Dexamethasone Fails to Alter Systemic Anti-Inflammatory Cytokines 24 hours Post-CLP
We also evaluated circulating levels of anti-inflammatory cytokines, because IL-1ra and IL-10 have been correlated to severity and outcome of clinical sepsis (29, 30). In the CLP sepsis model, the anti-inflammatory response after injury occurs simultaneously with the early release of proinflammatory mediators (14). As in the case of proinflammatory mediators, DEX treatment did not significantly modulate systemic levels of IL-1ra, IL-10, TNF-SR I, or TNF-SR II anti-inflammatory mediators (Fig. 6, A–D). These results are not surprising, because corticosteroids have limited influence upon anti-inflammatory signaling, although posthydrocortisone reduction of circulating IL-10, TNF SR I, and II levels was reported in septic shock patients (31). Although DEX treatment failed to increase or decrease the anti-inflammatory mediators between either P-LIVE or P-DIE animals in the saline-treated group P-DIE, the circulating concentrations were significantly higher compared with saline-treated mice P-LIVE. Thus, the anti-inflammatory mediators mirrored the changes observed in the proinflammatory mediators with higher levels present in those mice P-DIE.
DISCUSSION
We sought to experimentally verify whether targeting sepsis subgroups offer a better therapeutic strategy compared with indiscriminate treatment of an entire experimental population. In our studies, a more uniform subpopulation was established by clustering animals based on their immediate risk of death using plasma IL-6 as a predictor (14). The potential applicability of circulating IL-6 to direct sepsis therapy has been tried in two randomized, placebo-controlled, clinical trials (26, 32) using an anti-TNF Ab as the therapeutic intervention. These studies used a rapid IL-6 detection test strip with a detection limit of 1000 pg/mL. In both studies, patients who were IL-6 positive had significantly greater mortality than the IL-6 –negative patients, indicating that the IL-6 level prospectively stratified patients appropriately. The first reported study with 994 enrolled patients did not show a significant decrease in mortality with the anti-TNF treatment (32). However, a larger study with 2634 patients did show statistically decreased mortality with the anti-TNF Ab (26). Both these clinical trials validate the use of IL-6 as a biomarker to stratify patients for sepsis interventions, although the appropriate therapeutic intervention still needs to be defined.
Although our protocol using elevated biomarker levels to predict death allows accurate, targeted therapeutic intervention, the underlying mechanism of mortality remains unknown. Thus, though homogeneous by expected outcome, the subpopulation P-DIE may still remain heterogeneous from the mechanistic point of view. To partially correct this shortcoming, the prediction model was applied only in the period of early sepsis associated with systemic inflammatory response syndrome. By predicting death only within the acute phase of the disease characterized by a uniform immunoinflammatory response (featuring elevation of both proinflammatory and anti-inflammatory cytokines), it may be assumed that most of the early deaths are driven by analogous (hyper) inflammatory dynamics. Our treatment approach is clinically relevant given that the first DEX dose was given after mortality was determined and preceded predicted mortality by approximately 40 hours.
The application of high-dose corticosteroids in endotoxin models of acute inflammation consistently showed that inhibition of key proinflammatory mediators such as TNF-α, IL-1β, and IL-6 correlated with improved survival (33, 34). However, when extrapolated to the clinical setting, corticosteroids have not conferred any benefit to septic patients (35, 36).
It has been recognized that the endotoxin model does not adequately recapitulate the multifaceted features of human sepsis (37). Whether the current findings derived from the more appropriate CLP sepsis model bear more weight with regard to the clinical situation remains to be verified. Although adequate in abdominal, polymicrobial sepsis, our treatment strategy may fail in cases/models triggered by a single microorganism and in different organs. In the murine model of E. coli pneumonia, the efficacy of hydrocortisone therapy (no significant benefit) was not modified by the risk of death (38). Interestingly, regardless of the hydrocortisone dose, all blood cytokines and white blood cells remained unchanged at 24 hours post-therapy.
Our study substantiates the concept advocating greater homogeneity among patients enrolled in clinical trials with immunomodulatory treatments and better patient homogeneity triggered the design of Corticosteroid Therapy of Septic Shock trial. The study defined consistent criteria for relative adrenal insufficiency in the critically ill, enabling identification of the ideal target subpopulation for replacement corticosteroid therapy (39). This design was specifically adopted because patients with intact hypothalamic-pituitary-adrenal (axis) function appeared to be adversely affected by such treatment (33). Unfortunately, the treatment with low-dose glucocorticoids lacked benefit when directed toward the subpopulation with adrenal insufficiency (5).
Despite the clear improvement in survival achieved by the new experimental design, the analyzed parameters did not reveal the actual mechanism(s) whereby DEX treatment benefitted the P-DIE sub-population. Because the prognostic accuracy of our markers was not yet validated in our prospective stratification model, we elected not to kill animals for additional end points. However, these findings are no less important because they support a conclusion that inhibition of mortality occurs independent of the suppression of typical proinflammatory mediators such as TNF or IL-6. The lack of a clear connection between systemic cytokines and sepsis mortality has previously been reported in two separate studies where anti-TNF antibodies did not alter mortality (40, 41). Furthermore, IL-6 knockout mice maintained their susceptibility to CLP mortality (42). Similarly, CD14-deficient mice (43) demonstrated a two- to four-fold decrease in both proinflammatory and anti-inflammatory cytokines, but no difference in CLP-induced mortality. Depending on interpretation, clinical evidence could also be viewed as indirectly supportive: 1) none of the phase III trials aimed at reducing sepsis mortality by neutralizing either TNF-α or IL-1 succeeded (44) and 2) a number of studies reported that the majority of septic patients had undetectable levels of TNF and IL-1β in their blood (24).
CONCLUSIONS
This article proposes a new paradigm in the treatment of sepsis. The key feature is an individualized evaluation of the patient’s immunoinflammatory status/risk of death followed by therapy directed only at the appropriate group. Our results suggest that treatment(s) ineffective in large and heterogeneous populations may be beneficial when administered to more rigorously defined cohorts. We can learn from patients with cancer, where patients have their chemotherapy regimen defined by the specific tumor. Appropriate therapy for the septic patient should be tailored toward the inflammatory status of that patient. Given the dynamic nature of sepsis, the mechanism that causes mortality may be heterogeneous. Our data indicate that targeting even nonspecific therapy may be beneficial when the populations demonstrate a similar inflammatory phenotype.
Acknowledgments
Supported, in part, by NIH grant GM 67189.
Footnotes
The authors have not disclosed any potential conflicts of interest.
For information regarding this article: remickd@bu.edu
References
- 1.Dellinger RP, Levy MM, Carlet JM, et al. Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock. Crit Care Med. 2008;36:296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- 2.Klastersky, Cappel JR, Debusscher L, et al. Effectiveness of betamethasone in management of severe infections. A double-blind study. N Engl J Med. 1971;284:1248–1250. doi: 10.1056/NEJM197106032842206. [DOI] [PubMed] [Google Scholar]
- 3.Luce JM, Montgomery AB, Marks JD, et al. Ineffectiveness of high-dose methylpred-nisolone in preventing parenchymal lung injury and improving mortality in patients with septic shock. Am Rev Respir Dis. 1988;138:62–68. doi: 10.1164/ajrccm/138.1.62. [DOI] [PubMed] [Google Scholar]
- 4.Sprung CL, Caralis PV, Marcial EH, et al. The effects of high-dose corticosteroids in patients with septic shock. A prospective, controlled study. N Engl J Med. 1984;311:1137–1343. doi: 10.1056/NEJM198411013111801. [DOI] [PubMed] [Google Scholar]
- 5.Sprung CL, Annane D, Keh D, et al. Hydro-cortisone therapy for patients with septic shock. CORTICUS Study Group. N Engl J Med. 2008;358:111–124. doi: 10.1056/NEJMoa071366. [DOI] [PubMed] [Google Scholar]
- 6.Remick DG. Pathophysiology of sepsis. Am J Pathol. 2007;170:1435–1444. doi: 10.2353/ajpath.2007.060872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fisher CJ, Jr, Agosti JM, Opal SM, et al. Treatment of septic shock with the tumor necrosis factor receptor: Fc fusion protein. The Soluble TNF Receptor Sepsis Study Group. N Engl J Med. 1996;334:1697–1702. doi: 10.1056/NEJM199606273342603. [DOI] [PubMed] [Google Scholar]
- 8.Schumer W. Steroids in treatment of clinical septic shock. Ann Surg. 1976;184:333–341. doi: 10.1097/00000658-197609000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Effect of high-dose glucocorticoid therapy on mortality in patients with clinical signs of systemic sepsis. The Veterans Administration Systemic Sepsis Cooperative Study Group. N Engl J Med. 1987;317:659– 665. doi: 10.1056/NEJM198709103171102. [DOI] [PubMed] [Google Scholar]
- 10.Bone RC, Sibbald WJ, Sprung CL. The ACCP-SCCM consensus conference on sepsis and organ failure. Chest. 1992;101:1481–1483. doi: 10.1378/chest.101.6.1481. [DOI] [PubMed] [Google Scholar]
- 11.Annane D, Sebille V, Charpentier C, et al. Effect of treatment with low doses of hydro-cortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002;288:862–871. doi: 10.1001/jama.288.7.862. [DOI] [PubMed] [Google Scholar]
- 12.Bone RC. Sir Isaac Newton, sepsis, SIRS & CARS. Crit Care Med. 1996;24:1125–1130. doi: 10.1097/00003246-199607000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Deans KJ, Haley M, Natanson C, et al. Novel therapies for sepsis: A review. J Trauma. 2005;58:867–874. doi: 10.1097/01.ta.0000158244.69179.94. [DOI] [PubMed] [Google Scholar]
- 14.Osuchowski MF, Welch K, Siddiqui J, et al. Circulating cytokine/inhibitor profiles reshape the understanding of the SIRS/CARS continuum in sepsis and predict mortality. J Immunol. 2006;177:1967–1974. doi: 10.4049/jimmunol.177.3.1967. [DOI] [PubMed] [Google Scholar]
- 15.Osuchowski MF, Welch K, Yang H, et al. Chronic sepsis mortality characterized by an individualized inflammatory response. J Immunol. 2007;179:623–630. doi: 10.4049/jimmunol.179.1.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hubbard WJ, Choudhry M, Schwacha MG, et al. Cecal ligation and puncture. Shock. 2005;24(Suppl 1):52–57. doi: 10.1097/01.shk.0000191414.94461.7e. [DOI] [PubMed] [Google Scholar]
- 17.Wichterman KA, Baue AE, Chaudry ICH. Sepsis and septic shock—A review of laboratory models and a proposal. J Surg Res. 1980;29:189–201. doi: 10.1016/0022-4804(80)90037-2. [DOI] [PubMed] [Google Scholar]
- 18.Ebong SJ, Call DR, Bolgos G, et al. Immunopathologic responses to non-lethal sepsis. Shock. 1999;12:118–126. doi: 10.1097/00024382-199908000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Remick DG, Bolgos GR, Siddiqui J, et al. Six at six: Interleukin-6 measured 6 h after the initiation of sepsis predicts mortality over 3 days. Shock. 2002;17:463–437. doi: 10.1097/00024382-200206000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Vachharajani V, Vital S, Russell J, et al. Glucocorticoids inhibit the cerebral microvascular dysfunction associated with sepsis in obese mice. Microcirculation. 2006;13:477–487. doi: 10.1080/10739680600777599. [DOI] [PubMed] [Google Scholar]
- 21.Nemzek JA, Siddiqui J, Remick DG. Development and optimization of cytokine ELISAs using commercial antibody pairs. J Immunol Methods. 2001;255:149–157. doi: 10.1016/s0022-1759(01)00419-7. [DOI] [PubMed] [Google Scholar]
- 22.Knight PR, Sreekumar A, Siddiqui J, et al. Development of a sensitive microarray immunoassay and comparison with standard enzyme-linked immunoassay for cytokine analysis. Shock. 2004;21:26–30. doi: 10.1097/01.shk.0000101668.49265.19. [DOI] [PubMed] [Google Scholar]
- 23.Lasko TA, Bhagwat JG, Zou KH, et al. The use of receiver operating characteristic curves in biomedical informatics. J Biomed Inform. 2005;38:404– 415. doi: 10.1016/j.jbi.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 24.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 25.Oberholzer A, Oberholzer C, Moldawer LL. Sepsis syndromes: Understanding the role of innate and acquired immunity. Shock. 2001;16:83–96. doi: 10.1097/00024382-200116020-00001. [DOI] [PubMed] [Google Scholar]
- 26.Panacek EA, Marshall JC, Albertson TE, et al. Efficacy and safety of the monoclonal anti-tumor necrosis factor antibody F(ab′)2 fragment afelimomab in patients with severe sepsis and elevated interleukin-6 levels. Crit Care Med. 2004;32:2173–2182. doi: 10.1097/01.ccm.0000145229.59014.6c. [DOI] [PubMed] [Google Scholar]
- 27.Prigent H, Maxime V, Annane D. Clinical review: Corticotherapy in sepsis. Crit Care. 2004;8:122–129. doi: 10.1186/cc2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turnbull IR, Javadi P, Buchman TG, et al. Antibiotics improve survival in sepsis independent of injury severity but do not change mortality in mice with markedly elevated interleukin 6 levels. Shock. 2004;21:121–125. doi: 10.1097/01.shk.0000108399.56565.e7. [DOI] [PubMed] [Google Scholar]
- 29.Ashare A, Powers LS, Butler NS, et al. Anti-inflammatory response is associated with mortality and severity of infection in sepsis. Am J Physiol Lung Cell Mol Physiol. 2005;288:L633–L640. doi: 10.1152/ajplung.00231.2004. [DOI] [PubMed] [Google Scholar]
- 30.Neidhardt R, Keel M, Steckholzer U, et al. Relationship of interleukin-10 plasma levels to severity of injury and clinical outcome in injured patients. J Trauma. 1997;42:863–870. doi: 10.1097/00005373-199705000-00017. [DOI] [PubMed] [Google Scholar]
- 31.Keh D, Boehnke T, Weber-Cartens S, et al. Immunologic and hemodynamic effects of “low-dose” hydrocortisone in septic shock: A double-blind, randomized, placebo-controlled, crossover study. Am J Respir Crit Care Med. 2003;167:512–520. doi: 10.1164/rccm.200205-446OC. [DOI] [PubMed] [Google Scholar]
- 32.Reinhart K, Menges T, Gardlund B, et al. Randomized, placebo-controlled trial of the anti-tumor necrosis factor antibody fragment afelimomab in hyperinflammatory response during severe sepsis: The RAMSES Study. Crit Care Med. 2001;29:765–769. doi: 10.1097/00003246-200104000-00015. [DOI] [PubMed] [Google Scholar]
- 33.Sessler CN. Steroids for septic shock? (Con) Chest. 2003;123:482S–489S. doi: 10.1378/chest.123.5_suppl.482s. [DOI] [PubMed] [Google Scholar]
- 34.Balk RA. Steroids for septic shock? (Pro) Chest. 2003;123:490S–499S. doi: 10.1378/chest.123.5_suppl.490s. [DOI] [PubMed] [Google Scholar]
- 35.Lefering R, Neugebauer EA. Steroid controversy in sepsis and septic shock: A meta-analysis. Crit Care Med. 1995;23:1294–1303. doi: 10.1097/00003246-199507000-00021. [DOI] [PubMed] [Google Scholar]
- 36.Cronin L, Cook DJ, Carlet J, et al. Corticosteroid treatment for sepsis: A critical appraisal and meta-analysis of the literature. Crit Care Med. 1995;23:1430–1439. doi: 10.1097/00003246-199508000-00019. [DOI] [PubMed] [Google Scholar]
- 37.Remick DG, Ward PA. Evaluation of endotoxin models for the study of sepsis. Shock. 2005;24(Suppl 1):7–11. doi: 10.1097/01.shk.0000191384.34066.85. [DOI] [PubMed] [Google Scholar]
- 38.Li Y, Xizhong C, Solomon SB, et al. Risk of death does not alter the efficacy of hydrocortisone therapy in a mouse E. coli pneumonia model. Intensive Care Med. 2008;34:568–577. doi: 10.1007/s00134-007-0921-7. [DOI] [PubMed] [Google Scholar]
- 39.Lipiner-Friedman D, Sprung CL, Laterre PF, et al. Adrenal function in sepsis: The retrospective Corticus cohort study. Crit Care Med. 2007;35:1012–1018. doi: 10.1097/01.CCM.0000259465.92018.6E. [DOI] [PubMed] [Google Scholar]
- 40.Remick DG, Manohar P, Bolgos G, et al. Blockade of tumor necrosis factor reduces lipopolysaccharide lethality, but not the lethality of cecal ligation and puncture. Shock. 1995;4:89–94. doi: 10.1097/00024382-199508000-00002. [DOI] [PubMed] [Google Scholar]
- 41.Eskandari MK, Bolgos G, Miller C, et al. Anti-tumor necrosis factor antibody therapy fails to prevent lethality after cecal ligation and puncture or endotoxemia. J Immunol. 1992;148:2724–2730. [PubMed] [Google Scholar]
- 42.Remick DG, Bolgos G, Copeland S, et al. Role of interleukin-6 in mortality from and physiologic response to sepsis. Infect Immun. 2005;73:2751–2757. doi: 10.1128/IAI.73.5.2751-2757.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ebong SJ, Goyert SM, Nemzek JA, et al. Critical role of CD14 for production of proinflammatory cytokines and cytokine inhibitors during sepsis with failure to alter morbidity or mortality. Infect Immun. 2001;69:2099–2104. doi: 10.1128/IAI.69.4.2099-2106.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Remick DG. Cytokine therapeutics for the treatment of sepsis: Why has nothing worked? Curr Pharm Des. 2003;9:75–82. doi: 10.2174/1381612033392567. [DOI] [PubMed] [Google Scholar]