Abstract
Objective
Maternal infection is a common complication of childbirth, yet little is known about the extent to which infection rates vary among hospitals. We estimated hospital level risk-adjusted maternal infection rates (RAIR) in a large sample of U.S. hospitals and explored associations between RAIR and select hospital features.
Study Design
This retrospective cohort study included hospitals in the Perspective database with more than 100 deliveries over two years. Using a composite measure of infection, we estimated and compared RAIR across hospitals using hierarchical generalized linear models. We then estimated the amount of variation in RAIR attributable to hospital features.
Results
Of the 1,001,189 deliveries at 355 hospitals, 4.1% were complicated by infection. Women ages 15-19 were 50% more likely to experience infection than those ages 25-29. Rupture of membranes >24 hours (OR 3.0, 95% CI 3.24, 3.5), unengaged fetal head (OR 3.11. 95% CI 2.97, 3.27), and blood loss anemia (OR 2.42, 95% CI 2.34, 2.49) had the highest odds ratios among comorbidities commonly found in patients with infection. RAIR ranged from 1.0% to 14.4% (median 4.0%, IQR 2.8%-5.7%). Hospital features such as geographic region, teaching status, urban setting and higher number of obstetric beds were associated with higher infection rates, accounting for 14.8% of the variation observed.
Conclusions
Obstetric RAIR vary among hospitals, suggesting an opportunity to improve obstetric quality of care. Hospital features such as region, number of OB beds and teaching status account for only a small portion of the observed variation in infection rates.
Keywords: Maternal infection rates, variation, hospital characteristics
Introduction
Childbirth is the most common reason for hospital admission in the U.S., with more than 4,000,000 admissions for labor and delivery occurring annually.(1) Although most births are uncomplicated, a small but significant number of women experience complications such as infection, trauma and hemorrhage during childbirth.(2),(3),(4) Reducing obstetric complications has emerged as a national priority, as reflected in goals established by Healthy People 2020(4) and the Centers for Medicare and Medicaid Services’ Partnership for Patients.(5)
Maternal infection is one of the most common perinatal complications, affecting nearly six percent of deliveries,(2) and many of these infections may be preventable. Several small studies and reviews have described clinical practices that can increase the risk of infection, primarily related to cesarean deliveries.(6),(7),(8),(9),(10) Some larger epidemiologic studies have estimated overall regional and national obstetric infection rates(2),(3),(11) and still others have explored the associations between complications and factors such as an obstetrician's residency training site.(12) However, little is known about the extent to which obstetric infection rates vary across hospitals or what impact structural and organizational features of a hospital may have on these rates.
To support the national goal of improving maternal outcomes following childbirth, we used hierarchical generalized linear modeling to estimate risk-adjusted maternal infection rates (RAIR) in a large sample of U.S. hospitals. We then examined whether hospital features, such as the number of hospital beds, teaching status, geographic region, volume of deliveries, and level of implementation of electronic health records (EHR), were associated with higher rates of infection.
Materials and Methods
Study sample and data source
We conducted a cross-sectional study using Perspective, a voluntary, fee-supported database developed by Premier, Inc. (Charlotte NC) that enables participating hospitals to analyze care quality and costs at their institution and to compare their performance to other institutions within the database. The database is comprised of a structurally and geographically diverse set of approximately 450 U.S. hospitals that together account for approximately 20% of all annual hospital admissions in the U.S. In addition to information derived from standard hospital discharge files (i.e., UB-04) Perspective contains a date stamped log of all items (e.g., medications, laboratory, diagnostic tests) and therapeutic services billed to the patient or their insurer.
Women were included in the study if they were discharged between January 1, 2008 and December 31, 2009 and had an International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) principal or secondary diagnosis or procedure code for a vaginal delivery (650, 640.0x through 676.9x (where x=1 or 2) or 73.59) or cesarean delivery (763.4, 669.71, 74.x (x=0-2,4) or 74.99). We excluded discharges for ectopic and molar pregnancies and for pregnancies ending in spontaneous or elective abortion because we were interested in exploring intra/peripartum infections. We also excluded patients who were transferred from or to another institution, because we did not have information about the clinical course or treatments prior to admission or subsequent outcomes and women under age 15 or over age 44 because 15-44 is a common age range for childbearing. In addition we excluded hospitals that recorded fewer than 100 deliveries over the 2 year study period in order to provide stable estimates of infection rates, and because these institutions do not routinely provide obstetric care. Permission to conduct the study was obtained from the Institutional Review Board at Baystate Medical Center in Springfield, MA.
Obstetric infection
A delivery was considered complicated by infection if the patient received one or more diagnoses consistent with infection using a broad set of ICD-9-CM codes that have been used in earlier studies of infections associated with childbirth.(2),(12) (Appendix A) We excluded ICD-9-CM infection codes with a 5th digit of 3, which indicates an antepartum condition, because we were most interested in the association between intra/peripartum infections and hospital features. We organized infection codes into groups of related diagnoses for descriptive purposes. (Table 1) Each infection code was counted towards the overall frequency of each type of infection. When calculating hospital level infection rates, a patient was considered to either have experienced or not experienced an infectious complication regardless of the number of infection codes associated with a single delivery.
Table 1.
Frequency of maternal infections by category of infection
| Infection | N (%) |
|---|---|
| Any of the infections below | 40,605 (4.1) |
| Puerperal infections | 20,519 (2.1) |
| Maternal pyrexia | 16,067 (1.6) |
| Surgical site infection | 3,523 (0.4) |
| Infections of genitourinary tract | 1,964 (0.2) |
| Sepsis | 1,319 (0.1) |
| Other maternal infection | 1,456 (0.2) |
Patient characteristics
We recorded patient demographics (age, gender, race/ethnicity, marital status, and insurance status) and conditions that might confer elevated risk for obstetric infection. We used two complementary methods to identify maternal comorbidities and pregnancy-specific conditions that could influence a patient's risk of infection. The presence of any of 29 comorbidities was computed using the Elixhauser Comorbidity Software, version 3.1, developed by the Agency for Healthcare Research and Quality.(13) In addition, we identified the presence of a set of pregnancy-specific conditions that may confer higher risk for infection.(14) These conditions were originally developed to predict risk for cesarean delivery, but have also been used for risk-adjustment for infection rates in obstetric patients.(12) For conditions that appeared in both sets, such as hypertension and substance abuse, we created combined indicators for patients identified by either method. Gestational diabetes and diabetes existing prior to pregnancy were assessed separately because they confer different risk for infection.(15) A total of 41 maternal comorbidities and pregnancy-specific conditions were evaluated for inclusion in risk-adjustment modeling. (Table 2)
Table 2.
Characteristics of patients included in the study
| Overall (N=1,001,189) | Infection Present (N=40,605) | ||||
|---|---|---|---|---|---|
| N | % | N | (%) | p-Value§ | |
| DEMOGRAPHICS | |||||
| *Age | <0.0001 | ||||
| 15-19 | 94,738 | (9.5) | 6,161 | (15.2) | |
| 20-24 | 236,439 | (23.6) | 10,892 | (26.8) | |
| 25-29 | 280,433 | (28.0) | 10,835 | (26.7) | |
| 30-34 | 232,606 | (23.2) | 8,038 | (19.8) | |
| 35-44 | 156,973 | (15.7) | 4,679 | (11.5) | |
| *Marital Status | <0.0001 | ||||
| Married | 497,959 | (49.7) | 17,283 | (42.6) | |
| Single | 363,647 | (36.3) | 18,557 | (45.7) | |
| Other/Unknown | 139,583 | (13.9) | 4,765 | (11.7) | |
| *Race/Ethnicity | <0.0001 | ||||
| White | 500,170 | (50.0) | 17,434 | (42.9) | |
| Black | 153,258 | (15.3) | 7,963 | (19.6) | |
| Hispanic | 127,105 | (12.7) | 5,323 | (13.1) | |
| Other | 220,656 | (22.0) | 9,885 | (24.3) | |
| *Insurance | <0.0001 | ||||
| Managed care | 419,879 | (41.9) | 16,145 | (39.8) | |
| Medicaid | 417,643 | (41.7) | 17,895 | (44.1) | |
| Medicare | 6,957 | (0.7) | 283 | (0.7) | |
| Commercial - indemnity | 80,777 | (8.1) | 3,002 | (7.4) | |
| Self pay | 26,820 | (2.7) | 979 | (2.4) | |
| Other | 49,113 | (4.9) | 2,301 | (5.7) | |
| ELIXHAUSER | |||||
| Deficiency anemias | 71,578 | (7.2) | 5,528 | (13.6) | <0.0001 |
| *Blood loss anemia | 70,964 | (7.1) | 7,146 | (17.6) | <0.0001 |
| *Valvular disease | 3,941 | (0.4) | 176 | (0.4) | 0.191 |
| *Other neurological disorders | 3,771 | (0.4) | 224 | (0.6) | <0.0001 |
| *Rheumatoid arthritis/CVD | 1,660 | (0.2) | 112 | (0.3) | <0.0001 |
| **Paralysis | 212 | (<0.1) | 16 | (<0.1) | 0.010 |
| *Cancer: lymphoma, metastatic, or solid tumor | 188 | (<0.1) | 17 | (<0.1) | 0.001 |
| **Peripheral vascular disease | 56 | (<0.1) | 5 | (<0.1) | 0.064 |
| PREGNANCY RISK FACTORS | |||||
| *Prior c-section | 182,821 | (18.3) | 4,149 | (10.2) | <0.0001 |
| Advanced maternal age | 156,973 | (15.7) | 4,679 | (11.5) | <0.0001 |
| *Preterm gestation | 75,730 | (7.6) | 5,027 | (12.4) | <0.0001 |
| Fetal malpresentation | 68,696 | (6.9) | 3,175 | (7.8) | <0.0001 |
| *Maternal soft tissue disorder | 36,724 | (3.7) | 2,196 | (5.4) | <0.0001 |
| *Macrosomia | 31,931 | (3.2) | 1,246 | (3.1) | 0.158 |
| *Oligohydramnios | 31,213 | (3.1) | 1,250 | (3.1) | 0.662 |
| *Intrauterine growth restriction | 25,629 | (2.6) | 778 | (1.9) | <0.0001 |
| Isoimmunization | 26,145 | (2.6) | 981 | (2.4) | 0.012 |
| *Herpes | 21,818 | (2.2) | 1,096 | (2.7) | <0.0001 |
| *AP bleed/placental abruption | 18,657 | (1.9) | 1,167 | (2.9) | <0.0001 |
| *Unengaged fetal head | 18,446 | (1.8) | 2,204 | (5.4) | <0.0001 |
| *Multiple gestation | 18,446 | (1.8) | 909 | (2.2) | <0.0001 |
| *Rupture of membranes > 24 hrs | 11,820 | (1.2) | 2.066 | (5.1) | <0.0001 |
| *Polyhydramnios | 9,442 | (0.9) | 374 | (0.9) | 0.639 |
| Uterine scar unrelated to c-section | 2,082 | (0.2) | 72 | (0.2) | 0.166 |
| Congenital fetal anomaly | 1,371 | (0.1) | 65 | (0.2) | 0.198 |
| Maternal pulmonary embolism | 215 | (<0.1) | 49 | (0.12) | <0.0001 |
| *Maternal hypotension or OB shock | 186 | (<0.1) | 65 | (0.2) | <0.0001 |
| *Cerebral hemorrhage | 60 | (<0.1) | 19 | (0.1) | <0.0001 |
| Gestational diabetes | 56,182 | (5.6) | 2,163 | (5.3) | 0.011 |
| *Premature rupture of membranes | 40,963 | (4.1) | 2,940 | (7.2) | <0.0001 |
| COMBINED RISK FACTORS | |||||
| *Severe hypertension: eclampsia, pre-eclampsia | 14,092 | (1.4) | 900 | (2.2) | <0.0001 |
| *Other types of hypertension | 81,223 | (8.1) | 4,191 | (10.3) | <0.0001 |
| Mental disorder | 39,802 | (4.0) | 1,933 | (4.8) | <0.0001 |
| Obesity | 37,928 | (3.8) | 2,087 | (5.1) | <0.0001 |
| *Chronic pulmonary condition | 32,761 | (3.3) | 1,777 | (4.4) | <0.0001 |
| Thyroid condition | 23,361 | (2.3) | 922 | (2.3) | 0.393 |
| **Abuse of any substance | 12,332 | (1.2) | 618 | (1.5) | <0.0001 |
| **Pre-existing DM | 9,247 | (0.9) | 447 | (1.1) | 0.0002 |
| *CHF and other heart disease | 7,375 | (0.7) | 580 | (1.4) | <0.0001 |
| *Renal condition | 2,210 | (0.2) | 192 | (0.5) | <0.0001 |
| **Liver condition | 1,743 | (0.2) | 101 | (0.2) | 0.0002 |
P-value for chi-square test of association with any infection present vs. not present
Variables retained in model for p<.05
Variables forced into model
Structural and organizational hospital features
Using data from the American Hospital Association (AHA) annual survey and Premier, we noted each hospital's geographic location, number of hospital beds, number of obstetric beds, number of deliveries in the 2 year period, whether the hospital was located in an urban or rural setting, teaching status, and whether a hospital reported full implementation of an electronic health record (EHR). Four questions on the AHA Annual Survey (2008) were used to define a hospital's level of implementation. The questions encompassed EHR use related to patient level health information, results management, order entry management and decision support. A hospital was categorized as having a fully implemented EHR if all four domains were reported as “fully implemented”.
Statistical analysis
We evaluated the association of patient demographics, maternal comorbidities, pregnancy-related conditions and structural and organizational hospital features with the presence of “any infection” using chi-square statistics. We used this composite measure of infection to assess hospital infection rates because it allowed for inclusion of rare diagnoses while reducing the risk that variation in coding practices across hospitals would result in biased rate estimates. Using a model-building strategy that retained factors with p<0.05, or those that were theoretically important to obstetric infections, we employed hierarchical generalized linear modeling (HGLM) to model the log-odds of experiencing infection related to childbirth adjusting for patient demographics, maternal comorbidities and pregnancy-specific conditions that could increase risk of infection, while including a random hospital effect. Conditions, such as diabetes existing prior to pregnancy, that did not meet the significance criterion for inclusion in the model but were clinically important were forced into the model. Selected interaction terms were evaluated. From the final model, we calculated hospital-specific RAIR as the ratio of predicted (using hospital random effect) to expected (using average hospital effect) events multiplied by the overall unadjusted infection rate, a form of indirect standardization that is used in hospital outcomes measurement initiatives sponsored by the Centers for Medicare & Medicaid Services.(16) Our primary model included all deliveries, and we stratified by vaginal or cesarean delivery in a secondary analysis.
We then evaluated the bivariate associations of structural and organizational hospital features with RAIR using ANOVA and t-tests. Lastly, we modeled RAIR across hospitals as a function of structural and organizational hospital features and estimated the proportion of variation in RAIR attributable to hospital features.
Results
Study sample
From the initial sample of 1,038,555 deliveries at 424 hospitals, 3,913 were excluded due to presence of an ICD-9-CM code for ectopic or molar pregnancy or spontaneous or induced abortion, 29,888 due to transfer into or out of the hospital or unknown discharge status, 3,140 for maternal age less than 15 or more than 44 years of age, and 425 because the delivery occurred at a hospital (n=69) with fewer than 100 deliveries during the 2 year study period. Our final sample included 1,001,189 deliveries at 355 hospitals. (Figure 1)
Figure 1.
Flow diagram depicting exclusions and final sample size
The majority of women (75%) were between ages 20-34 years, 50% were married, 25% were black or Hispanic and 42% had a public form of health insurance such as Medicaid. (Table 2) Cesarean deliveries accounted for 39% of the deliveries included in the study. The most commonly identified maternal comorbidities and pregnancy-specific conditions included cesarean delivery during a previous pregnancy (18.3%), advanced maternal age (≥ 35 years) (15.7%), hypertension (8.1%), and preterm delivery (7.6%). (Table 2) Maternal mortality was 0.01% and median length of stay was 2 days (IQR 2-3) for vaginal deliveries and 3 days (IQR 3-4) for cesarean deliveries.
Of the deliveries included in the study, 40,605 (4.1%) were complicated by infection. Puerperal infections were the most common, affecting 2.1% of deliveries, followed by maternal pyrexia (1.6%) and surgical site infections (0.4%). Genitourinary tract infections (0.2%) and sepsis (0.1%) were relatively uncommon. (Table 1) Of the deliveries complicated by infection, maternal mortality was 0.06% and median length of stay was 3 days (IQR 2-3) for vaginal deliveries and 4 days (IQR 3-5) for cesarean deliveries.
Among the hospitals, 28% were teaching hospitals, 77% were in an urban setting, 43% were in the South region and 28% had more than 30 obstetric beds. Relatively few hospitals (19%) reported complete implementation of an EHR.
Hierarchical models
Patient demographics, maternal comorbidities and pregnancy-specific conditions considered in modeling RAIR are shown in Table 2. Adjusted odds ratios from the final main effects model for RAIR are shown in Appendix B. Age was strongly associated with risk for infection; when compared to women ages 25-29, women ages 15-19 had 59% higher risk for infection and women ages 35-44 had 29% lower risk. Maternal comorbidities and pregnancy-specific conditions that were most strongly associated with infection (OR > 2.0) and occurred in more than 5% of deliveries with infection included rupture of membranes > 24 hours (OR 3.0, 95% CI 3.24, 3.5), blood loss anemia (OR 2.54, 95% CI 2.47, 2.62) and unengaged fetal head (OR 3.11, 95% CI 2.97, 3.27). Although other risk factors such as cerebral hemorrhage were strongly associated with infection, they occurred infrequently. Interactions of the patient demographic variables (e.g., age, race, insurance and marital status) with comorbid and pregnancy-specific conditions were included in a final model used to estimate RAIR.
Separate models for vaginal and cesarean deliveries gave results similar in magnitude and direction for most risk factors. (Appendix B)
Hospital risk-adjusted infection rates
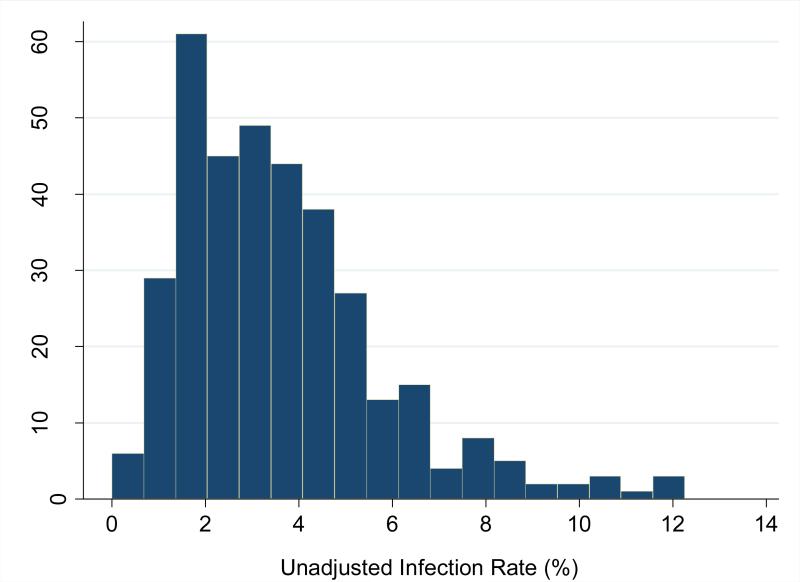
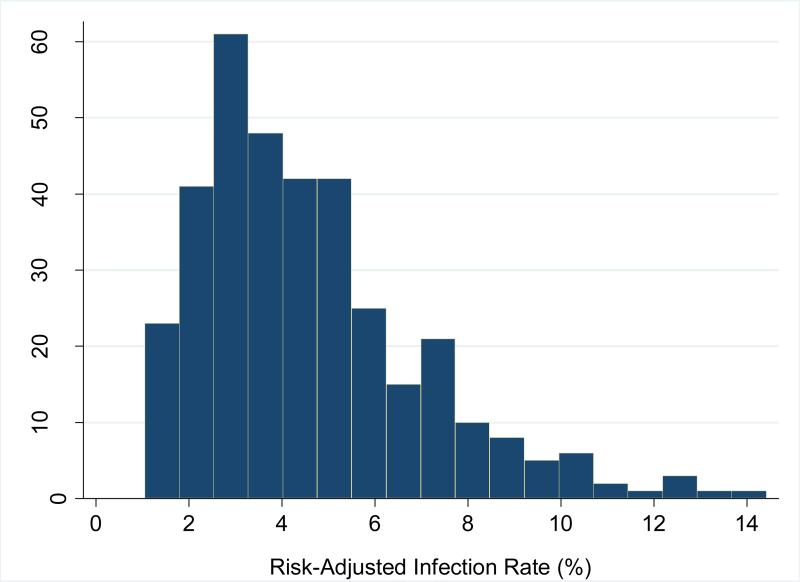
Unadjusted hospital infection rates ranged from 0.0% to 12.3% (median 3.2%, IQR 2.0%-4.6%). However, after adjusting for differences in patient case mix, the RAIR ranged from 1.0% to 14.4% (median 4.0%, IQR 2.8%-5.7%). (Figures 2a and 2b) Women delivering at hospitals at the 75th percentile of infection rates had a twofold risk of experiencing an infection as compared to women delivering at a hospital at the 25th percentile.
Figure 2a.
Distribution of unadjusted hospital-level composite infection rates
Figure 2b.
Distribution of risk-adjusted hospital-level composite infection rates
Secondary analysis of RAIR for cesarean deliveries revealed infection rates ranging from 1.5% to 18.4% (median 5.4%, IQR 3.9%-7.7%). Vaginal delivery infection rates ranged from 0.05% to 13.3 % (median 3.1%, IQR 2.1%-4.6%). (Appendix B) RAIR for vaginal and cesarean deliveries were strongly correlated (Spearman's r=0.69, p<0.0001).
Association between structural and organizational hospital features and risk-adjusted infection rates
RAIR was associated with a number of hospital features. (Table 3) Hospitals in the West region had the highest mean RAIR (5.3, 95% CI 4.7, 5.9) while those in the South had the lowest mean RAIR (4.0, 95% CI 3.7, 4.4). Larger hospitals (400+ beds) had higher rates (5.3, 95% CI 4.8, 5.8) than smaller hospitals (<200 beds) (4.1, 95% CI 3.7, 4.4). Higher RAIR was also observed in hospitals with a greater number of obstetric beds (30+) (5.4, 95% CI 4.9, 5.9) when compared to hospitals with fewer obstetric beds (<15) (3.8, 95% CI 3.5, 4.2). (Table 3) Teaching hospitals had higher RAIR (5.4, 95% CI 4.9, 5.9) compared to nonteaching hospitals (4.3, 95% CI 4.0, 4.6). In a multiple regression model, structural and organizational hospital features explained approximately 14.8% of the observed variation in risk-adjusted infection rates. (Table 4)
Table 3.
Association between hospital features and RAIR
| 95% CI | |||||
|---|---|---|---|---|---|
| Characteristic | N | Mean RAIR | LL | UL | p-Value |
| Region | 0.0003 | ||||
| South | 154 | 4.0 | 3.7 | 4.4 | |
| Midwest | 83 | 4.5 | 4.1 | 5.0 | |
| West | 71 | 5.3 | 4.7 | 5.9 | |
| Northeast | 47 | 5.3 | 4.4 | 6.2 | |
| # of Deliveries in 2 years | 0.0002 | ||||
| 100-999 | 93 | 4.0 | 3.6 | 4.4 | |
| 1000-2149 | 84 | 4.2 | 3.7 | 4.7 | |
| 2150-4099 | 87 | 4.7 | 4.2 | 5.2 | |
| 4100+ | 91 | 5.4 | 4.9 | 5.9 | |
| # of Obstetric Beds | <0.0001 | ||||
| < 15 | 103 | 3.8 | 3.5 | 4.2 | |
| 15-29 | 129 | 4.5 | 4.1 | 4.9 | |
| 30+ | 101 | 5.4 | 4.9 | 5.9 | |
| Unknown | 22 | 4.6 | 3.5 | 5.7 | |
| # of Hospital Beds | 0.0004 | ||||
| < 200 | 116 | 4.1 | 3.7 | 4.4 | |
| 200-399 | 131 | 4.5 | 4.0 | 4.8 | |
| 400+ | 108 | 5.3 | 4.8 | 5.8 | |
| Teaching Status | <0.0001 | ||||
| Nonteaching | 256 | 4.3 | 4.0 | 4.6 | |
| Teaching | 99 | 5.4 | 4.9 | 5.9 | |
| Setting | 0.01 | ||||
| Urban | 275 | 4.8 | 4.5 | 5.0 | |
| Rural | 80 | 4.0 | 3.5 | 4.5 | |
| Electronic Health Record | 0.67 | ||||
| Not complete implementation | 289 | 4.6 | 4.3 | 4.8 | |
| Complete implementation | 66 | 4.7 | 4.1 | 5.3 | |
Table 4.
Risk for higher hospital risk-adjusted infection rate attributable to structural and organizational hospital features (N=355)
| VARIABLE | ESTIMATE* | SE | p-VALUE |
|---|---|---|---|
| Region | |||
| West | 1.51 | 0.32 | <0.0001 |
| Northeast | 0.85 | 0.39 | 0.0280 |
| Midwest | 0.52 | 0.31 | 0.0936 |
| South (reference) | 0 | -- | -- |
| Number of OB beds | |||
| Unknown | -0.76 | 0.54 | 0.1593 |
| < 15 | -1.39 | 0.33 | <0.0001 |
| 15-29 | -0.71 | 0.31 | 0.0206 |
| 30+ (reference) | 0 | -- | -- |
| Teaching status | |||
| Teaching hospital | 0.83 | 0.28 | 0.0036 |
| Nonteaching hospital (reference) | 0 | -- | -- |
Interpretation: adjusting for other factors in the model, hospitals in the West region have RAIR averaging 1.51% higher than those in the South; hospitals with fewer than 15 OB beds have rates averaging 1.39% lower than hospitals with 30 or more OB beds; teaching hospitals have rates averaging 0.83% higher than nonteaching hospitals.
Comment
In this study of more than one million deliveries at 355 hospitals across the U.S., approximately 4.1% of women experienced an infection during hospital admission for childbirth. We observed substantial variation in hospital infection rates that persisted even after adjustment for differences in patient case-mix across institutions, with hospitals at the 75th percentile having an RAIR twice that of hospitals at the 25th percentile. Although several structural and organizational hospital features were associated with higher RAIR, together these features explained only a small fraction of the observed variation in infection rates, consistent with other estimates of the impact such factors have on patient outcomes.(17)
Our study provides important additions to the literature on obstetric infection rates by including a large sample of U.S. hospitals, estimating hospital-specific risk-adjusted maternal infection rates, and examining variation in these rates across hospitals. In a study by Berg et al., obstetric infection rates were compared for two time periods, 1993-1997 and 2001-2005, using the National Hospital Discharge Survey, but this study did not report hospital-specific rates.(2) Srinivas et al. also explored trends in maternal complication rates, but used two state databases for the primary analysis and did not explore variation at the hospital level.(3) Gregory et al. developed a method to measure uncomplicated, or “ideal deliveries”.(18) However, this unique approach does not allow for comparison of rates of undesirable outcomes such as infection.
Our study also contributes to the literature on how structural and organizational hospital characteristics may impact obstetric infection rates. Although this area has been studied for other conditions, such as acute myocardial infarction,(19) there is a paucity of research in this area for obstetrics. A recent study by Kyser et al. demonstrated an association between the volume of deliveries and lower composite complication rates. In that study, hospitals with approximately 100-1600 deliveries annually had the lowest unadjusted infection rates.(20) This is similar to our findings of lower RAIR being associated with lower number of deliveries over 2 years (100-2149). Studies in disciplines other than obstetrics have compared care quality and patient outcomes at teaching hospitals and non-teaching hospitals. These studies have generally shown lower mortality and better overall patient outcomes in the teaching hospitals.(21) In contrast, we found that teaching hospitals had higher infection rates. Although health care associated infections are a common measure of quality and patient safety,(22),(23) infection has not been a commonly selected outcome when comparing teaching and nonteaching hospitals, limiting comparison to other studies. It is possible that differences in the gestational age of patients delivered at teaching vs. non-teaching hospitals might serve as an unmeasured confounder, however gestational age was not available in the data used for this study.
This study had a number of strengths. First, we included a large number of patients drawn from a diverse group of hospitals, enhancing the generalizability of our findings. Second, we used a composite measure of infection rather than individual infection codes to reduce the risk that the variation in the rates we observed could be explained by differences in the coding practices at individual hospitals. Third, while “Present on Admission” codes(24) were not commonly used during the time period studied, we limited diagnoses to those most likely to occur during admission for childbirth by using a subset of 5th digit codes. This made the infections identified more likely to be associated with factors related to hospital practices at the time of the delivery, but may have caused us to miss some pertinent infections. Fourth, we adjusted for a large number of maternal comorbidities and pregnancy-specific conditions found to be associated with the risk of maternal infection, thereby reducing the chance that variation in RAIR across hospitals reflected differences in patient case-mix. Additionally, the use of multi-level regression modeling accounted for the natural clustering of patients within hospitals. Cesarean delivery is a known risk factor for postpartum endometritis and rates of cesarean deliveries vary across hospitals. By including both modes of delivery in the primary analysis, our hospital-level infection risk estimates were not influenced by local preferences for cesarean deliveries and offer a more patient-centric view of the risk of infection associated with the choice of hospital.
The first potential limitation of this study is the accuracy of ICD-9-CM coding and inability to capture outpatient codes. These are inherent limitation of all studies using administrative data. Prior validation studies of obstetric ICD-9-CM codes demonstrate variation in estimates of sensitivity and specificity of codes for a number of conditions, with codes for surgical procedures generally being the most reliable.(25–28) Other studies have found ICD-9-CM coding highly reliable for diagnoses such as diabetes and hospital-based procedures.(29,30) Although we attempted to mitigate some of the potential limitations of coding accuracy by using a composite of multiple diagnosis codes, some of our study's findings may be partly explained by differences in documentation and coding across institutions. For example, our surgical site infection rate was lower than rates found in other studies.(6) This may be due in part to the lack of a specific ICD-9-CM code to identify obstetric surgical site infections or ability to capture only infections identified during the admission. Although the code for maternal pyrexia is associated with infection, this code may also be used for fever from a non-infectious source, such as blood transfusion. A final example of limitations due to coding accuracy relates to obesity codes, which have been found to have high specificity but low sensitivity in obstetrics.(27,30)
The second potential study limitation was our focus on infections that became apparent during the index hospitalization; overall infection rates are undoubtedly higher than we estimated. Third, although we attempted to adjust for a large number of maternal comorbidities and pregnancy-specific factors that could influence the risk of infection, some of the variation in infection rates may be related to limitations on our ability to fully account for the patient-level risks. Fourth, some of the unexpected findings related to the comorbidity rates may be explained by unmeasured factors, such as pre-term births excluded due to transfers of pre-term deliveries. Finally, the over-representation of southern hospitals in the Perspective data set means interpretation of geographic differences in RAIR must be made with care. This geographic over sampling may explain why the rate of cesarean deliveries found in this sample was higher than national averages. (31,32)
In conclusion, we found that risk-adjusted infection rates following childbirth vary considerably across hospitals, and that key structural and organizational hospital features explain only a modest amount of this variation. In order to support large scale efforts to improve the quality of obstetric care, additional research is needed to identify organizational factors and clinical strategies that enable some hospitals to achieve lower infection rates.
Condensation.
Risk-adjusted rates of maternal infection following childbirth vary widely across a large sample of U.S. hospitals, demonstrating a potential opportunity to improve the quality of obstetric care.
Acknowledgments
The project described was supported by the National Center for Research Resources Grant Number KL2 RR025751 and the National Center for Advancing Translational Sciences, National Institutes of Health, Grant Number KL2 TR000074. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: None of the authors report a conflict of interest.
This study was presented in poster format at the 2012 CTSI Annual Meeting in Washington D.C. April 19, 2012.
References
- 1.FASTSTATS - Births and Natality [Internet] [cited 2012 Aug 11]. Available from: http://www.cdc.gov/nchs/fastats/births.htm.
- 2.Berg CJ, Mackay AP, Qin C, Callaghan WM. Overview of maternal morbidity during hospitalization for labor and delivery in the United States: 1993-1997 and 2001-2005. Obstet Gynecol. 2009 May;113(5):1075–81. doi: 10.1097/AOG.0b013e3181a09fc0. [DOI] [PubMed] [Google Scholar]
- 3.Srinivas SK, Epstein AJ, Nicholson S, Herrin J, Asch DA. Improvements in US maternal obstetrical outcomes from 1992 to 2006. Med Care. 2010 May;48(5):487–93. doi: 10.1097/MLR.0b013e3181d68840. [DOI] [PubMed] [Google Scholar]
- 4.Maternal, Infant, and Child Health - Healthy People [Internet] [cited 2012 Aug 11]. Available from: http://www.healthypeople.gov/2020/topicsobjectives2020/objectiveslist.aspx?topicId=26.
- 5.cmmi. Partnership for Patients: A Common Commitment [Internet] [cited 2012 Aug 11]. Available from: http://www.healthcare.gov/compare/partnership-for-patients/about/index.html.
- 6.Conroy K, Koenig AF, Yu Y-H, Courtney A, Lee HJ, Norwitz ER. Infectious morbidity after cesarean delivery: 10 strategies to reduce risk. Rev Obstet Gynecol. 2012;5(2):69–77. [PMC free article] [PubMed] [Google Scholar]
- 7.Olsen MA, Butler AM, Willers DM, Gross GA, Devkota P, Fraser VJ. Risk factors for endometritis after low transverse cesarean delivery. Infect Control Hosp Epidemiol. 2010 Jan;31(1):69–77. doi: 10.1086/649018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dutta S, Reddy R, Sheikh S, Kalra J, Ray P, Narang A. Intrapartum antibiotics and risk factors for early onset sepsis. Arch. Dis. Child. Fetal Neonatal Ed. 2010 Mar;95(2):F99–103. doi: 10.1136/adc.2009.163220. [DOI] [PubMed] [Google Scholar]
- 9.Ghuman M, Rohlandt D, Joshy G, Lawrenson R. Post-caesarean section surgical site infection: rate and risk factors. N. Z. Med. J. 2011 Jul 29;124(1339):32–6. [PubMed] [Google Scholar]
- 10.Leth RA, Møller JK, Thomsen RW, Uldbjerg N, Nørgaard M. Risk of selected postpartum infections after cesarean section compared with vaginal birth: a five-year cohort study of 32,468 women. Acta Obstet Gynecol Scand. 2009 Sep;88(9):976–83. doi: 10.1080/00016340903147405. [DOI] [PubMed] [Google Scholar]
- 11.Lyndon A, Lee HC, Gilbert WM, Gould JB, Lee KA. Maternal morbidity during childbirth hospitalization in California. J. Matern. Fetal. Neonatal. Med. [Internet] 2012 Aug 7; doi: 10.3109/14767058.2012.710280. [cited 2012 Aug 9]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/22779781. [DOI] [PMC free article] [PubMed]
- 12.Asch DA, Nicholson S, Srinivas S, Herrin J, Epstein AJ. Evaluating obstetrical residency programs using patient outcomes. JAMA. 2009 Sep 23;302(12):1277–83. doi: 10.1001/jama.2009.1356. [DOI] [PubMed] [Google Scholar]
- 13.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998 Jan;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Gregory KD, Korst LM, Gornbein JA, Platt LD. Using administrative data to identify indications for elective primary cesarean delivery. Health Serv Res. 2002 Oct;37(5):1387–401. doi: 10.1111/1475-6773.10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piper JM, Georgiou S, Xenakis EM, Langer O. Group B streptococcus infection rate unchanged by gestational diabetes. Obstet Gynecol. 1999 Feb;93(2):292–6. doi: 10.1016/s0029-7844(98)00405-0. [DOI] [PubMed] [Google Scholar]
- 16.Bernheim S, Lin Z, Bhat K, Savage S, Wang Y, Grady J, et al. 2010 Measures Maintenance Technical Report: Acute Myocardial Infarction, Heart Failure, and Pneumonia 30-Day Risk-Standardized Readmission Measures [Internet] Yale New Haven Health Services Corporation / Center for Outcomes Research & Evaluation; Mar. 2010. Available from: http://www.qualitynet.org/dcs/ContentServer?c=Page&pagename=QnetPublic%2FPage%2FQnetTier3&cid=1219069855841. [Google Scholar]
- 17.Brand CA, Barker AL, Morello RT, Vitale MR, Evans SM, Scott IA, et al. A review of hospital characteristics associated with improved performance. Int J Qual Health Care [Internet] 2012 Aug 7; doi: 10.1093/intqhc/mzs044. [cited 2012 Aug 11]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/22871420. [DOI] [PubMed]
- 18.Gregory KD, Fridman M, Shah S, Korst LM. Global measures of quality- and patient safety-related childbirth outcomes: should we monitor adverse or ideal rates? Am. J. Obstet. Gynecol. 2009 Jun;200(6):681, e1–7. doi: 10.1016/j.ajog.2009.02.033. [DOI] [PubMed] [Google Scholar]
- 19.Bradley EH, Curry LA, Spatz ES, Herrin J, Cherlin EJ, Curtis JP, et al. Hospital strategies for reducing risk-standardized mortality rates in acute myocardial infarction. Ann. Intern. Med. 2012 May 1;156(9):618–26. doi: 10.1059/0003-4819-156-9-201205010-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kyser KL, Lu X, Santillan DA, Santillan MK, Hunter SK, Cahill AG, et al. The association between hospital obstetrical volume and maternal postpartum complications. Am. J. Obstet. Gynecol. 2012 Jul;207(1):42.e1–17. doi: 10.1016/j.ajog.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ayanian JZ, Weissman JS. Teaching hospitals and quality of care: a review of the literature. Milbank Q. 2002;80(3):569–593. v. doi: 10.1111/1468-0009.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Welsh CA, Flanagan ME, Hoke SC, Doebbeling BN, Herwaldt L. Reducing health care-associated infections (HAIs): lessons learned from a national collaborative of regional HAI programs. Am J Infect Control. 2012 Feb;40(1):29–34. doi: 10.1016/j.ajic.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 23.Fakih MG, Greene MT, Kennedy EH, Meddings JA, Krein SL, Olmsted RN, et al. Introducing a population-based outcome measure to evaluate the effect of interventions to reduce catheter-associated urinary tract infection. Am J Infect Control. 2012 May;40(4):359–64. doi: 10.1016/j.ajic.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hospital-Acquired Conditions (Present on Admission Indicator) [Internet] Centers for Medicare and Medicaid Services; 2012. [cited 2012 Aug 11]. Available from: http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalAcqCond/index.html?redirect=/HospitalAcqCond. [Google Scholar]
- 25.Yasmeen S, Romano PS, Schembri ME, Keyzer JM, Gilbert WM. Accuracy of obstetric diagnoses and procedures in hospital discharge data. Am. J. Obstet. Gynecol. 2006 Apr;194(4):992–1001. doi: 10.1016/j.ajog.2005.08.058. [DOI] [PubMed] [Google Scholar]
- 26.Romano PS, Yasmeen S, Schembri ME, Keyzer JM, Gilbert WM. Coding of perineal lacerations and other complications of obstetric care in hospital discharge data. Obstet Gynecol. 2005 Oct;106(4):717–25. doi: 10.1097/01.AOG.0000179552.36108.6d. [DOI] [PubMed] [Google Scholar]
- 27.Goff SL, Pekow PS, Markenson G, Knee A, Chasan-Taber L, Lindenauer PK. Validity of using ICD-9-CM codes to identify selected categories of obstetric complications, procedures and co-morbidities. Paediatr Perinat Epidemiol. 2012 Sep;26(5):421–9. doi: 10.1111/j.1365-3016.2012.01303.x. [DOI] [PubMed] [Google Scholar]
- 28.Korst LM, Gregory KD, Gornbein JA. Elective primary caesarean delivery: accuracy of administrative data. Paediatr Perinat Epidemiol. 2004 Mar;18(2):112–9. doi: 10.1111/j.1365-3016.2003.00540.x. [DOI] [PubMed] [Google Scholar]
- 29.Chen G, Khan N, Walker R, Quan H. Validating ICD coding algorithms for diabetes mellitus from administrative data. Diabetes Res. Clin. Pract. 2010 Aug;89(2):189–95. doi: 10.1016/j.diabres.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 30.Andrade SE, Moore Simas TA, Boudreau D, Raebel MA, Toh S, Syat B, et al. Validation of algorithms to ascertain clinical conditions and medical procedures used during pregnancy. Pharmacoepidemiol Drug Saf. 2011 Nov;20(11):1168–76. doi: 10.1002/pds.2217. [DOI] [PubMed] [Google Scholar]
- 31.Kaiser State Health Facts [Internet] [cited 2012 Dec 19]. Available from: http://www.statehealthfacts.org/
- 32.Products - Data Briefs - Number 35 - March 2010 [Internet] [cited 2012 Dec 19]. Available from: http://www.cdc.gov/nchs/data/databriefs/db35.htm.