Abstract
Background
Alcohol consumption is influenced by genetic factors. Previous studies have examined the heritability of alcohol consumption, or related phenotypes, from adolescence into adulthood, frequently finding that total heritability changes over time. However, it remains unclear whether the same genes underlie liability to alcohol consumption across development versus whether novel risk genes become important over time.
Method
A population-based study of adult male twins (n=1790) born in Virginia, USA, retrospectively reported on their average monthly alcohol consumption from early adolescence through adulthood. We used twin modeling methods to explore genetic and environmental influences on alcohol consumption over time.
Results
One latent genetic factor accounted for the majority of the heritability in alcohol consumption during mid-to late adolescence, but its influence declined thereafter ; from young adulthood forward, heritability was largely attributable to a second genetic factor. The total heritability of alcohol consumption increased from 0 at ages 12–14 years to 0.40 by ages 18–21 years. Shared environmental factors declined in influence over time.
Conclusions
The heritability of alcohol consumption over time is dynamic both quantitatively and qualitatively. These results have important implications for gene identification endeavors. Furthermore, these findings could inform efforts to elucidate developmentally dynamic behaviors, such as antisocial behavior.
Keywords: Adolescent-limited genetic factors, alcohol consumption, gene identification, genetic influences, genetic innovation, twin modeling
Introduction
Alcohol use is common in the USA, with over 70% of Americans between the ages of 18 and 24 years reporting alcohol use within the past year (Chen et al. 2004). Alcohol use typically begins in adolescence, with 33% of US 8th graders (~ages 13–14) reporting a history of alcohol use in 2011; by the 10th grade (~ages 15–16) this figure increases to 56%, and by the 12th grade (~ages 17–18) 70% of adolescents have used alcohol (Johnston et al. 2011). Excessive alcohol consumption is related to an increased probability of alcohol-use disorders (Dawson et al. 2005; McCambridge et al. 2011), as well as to a myriad of other negative health-related outcomes, including hypertension, liver disease and some types of cancer (Giacosa et al. 2012). Among adolescents, alcohol use is related to leading causes of death (e.g. motor vehicle accidents, homicides) and risky behaviors such as physical fighting and sexual activities (Boekeloo & Novik, 2011). Furthermore, the economic cost of excessive alcohol use in the USA, taking into consideration alcohol-related crime, medical consequences, lost productivity, etc, was estimated at $224 billion in 2006 (Bouchery et al. 2011). Clearly, alcohol use affects the majority of the population directly or indirectly.
Alcohol consumption is influenced in part by genetic factors: heritability (h2) estimates for alcohol consumption in adults range from about 0.2 to 0.6 (Kendler et al. 2008b, 2010; Grant et al. 2009; Dick et al. 2011; Geels et al. 2012). For adolescents, heritability estimates for alcohol-related phenotypes vary considerably. Some studies have found that the h2 of alcohol use is quite low (0.1 or lower) (Rhee et al. 2003), while others have reported moderate estimates (0.3–0.5) for frequency of use (Viken et al. 1999; Pagan et al. 2006), frequency of intoxication (0.4–0.7) (Edwards et al. 2011a) and quantity of use (0.6) (Fowler et al. 2007). High levels of alcohol consumption can be a key predictor of alcohol problems (Dawson, 1994; Whitfield et al. 2004; Dawson et al. 2005), and these phenotypes are highly genetically correlated (Whitfield et al. 2004; Grant et al. 2009; Kendler et al. 2010; Dick et al. 2011). Thus, though alcohol use per se does not necessarily predict the development of problems, excessive consumption can be an indicator of genetic liability to problems.
While the heritable nature of alcohol use is well established, there is less information available regarding the continuity of genetic factors between adolescence and adulthood. Generally, genetic influences on alcohol use increase from adolescence into young adulthood before stabilizing (Bergen et al. 2007; Hicks et al. 2007; Kendler et al. 2008b ; Sartor et al. 2008; Edwards et al. 2011a; Geels et al. 2012), though some studies suggest the opposite (Malone et al. 2004; Hicks et al. 2007; van Beek et al. 2012). However, the extant literature largely focuses on total heritability, without exploring whether there are qualitative changes underlying total heritability over time. That is, it remains unclear whether the genetic influences on alcohol use during adolescence are the same as those that are relevant during adulthood. One study, examining symptoms of alcohol-use disorder in a longitudinally assessed sample of Dutch twins, found that while the total heritability of these symptoms varied from the age of 15–17 years to the age of 30–32 years, this variation was only quantitative in nature: a single genetic factor accounted for heritable influences (van Beek et al. 2012). Another study, which used the adult twin sample examined in the current analyses, reported that alcohol consumption in adolescence and adulthood were differentially predicted by genetic risk for externalizing versus for alcohol problems, as indexed by parental and co-twin phenotypes: while a familial liability to externalizing predicted alcohol use in adolescence, the alcohol-specific familial liability predicted later levels of alcohol consumption (Kendler et al. 2011a). This raises the possibility that qualitatively different genetic factors are relevant during different developmental periods, though such a hypothesis was not formally tested in the previous report.
Understanding the biological and environmental etiologies underlying alcohol-use phenotypes is critical for the development of effective prevention programming and treatment strategies. In particular, the use of twin and family studies to delineate dynamic genetic influences on these phenotypes across time can inform gene identification efforts: in the event that a single genetic factor influences an alcohol-related outcome differentially over time, it might be possible to ascertain a sample based on the age range within which those genetic influences are most prominent. Alternatively, if twin studies suggest that qualitatively distinct genetic factors influence the same alcohol phenotype during different time-frames, gene identification studies should be designed that limit the age range of the sample so as to minimize genetic heterogeneity underlying the phenotype of interest. The aim of the current analyses was to use a genetically informative, population-based sample of male twins to determine whether genetic influences on alcohol consumption change, quantitatively (i.e. genetic attenuation) and/or qualitatively (i.e. genetic innovation), from early adolescence into mid-adulthood.
Method
Sample
These analyses use data collected in the third wave of assessment of adult Caucasian male twins participating in the Virginia Adult Twin Study of Psychiatric and Substance Use Disorders (Kendler & Prescott, 2006). Subjects (n=1790) were aged 40.27 (S.D.=9.00) years at the interview. Data were available for 466 complete monozygotic (MZ) twin pairs, 283 complete dizygotic (DZ) twin pairs, and 292 members of incomplete pairs. Zygosity was determined using a combination of self-report measures, photographs and DNA polymorphisms. Most subjects were interviewed over the telephone by interviewers with a Master’s degree in a mental health-related field or a Bachelor’s degree and at least 2 years of clinical experience. The Committee for the Conduct of Human Research at Virginia Commonwealth University approved the project.
Measures
In wave 3 of data collection, participants were asked to report their average monthly alcohol consumption using a life history calendar interview, which improves the accuracy of retrospective recall (Freedman et al. 1988). These data were then combined into ages that correspond to meaningful developmental age ranges (as in previous studies of this sample, e.g. Kendler et al. 2008a) and extending into adulthood: ages 12–14, 15–17, 18–21, 22–25, 26–29, and 30–33 years, which will be referred to as epochs 1–6, respectively. Abstainers were assigned values of 0 (see Discussion). To each average consumption score, 1 was added, followed by a natural log-transformation to adjust for non-normality. Thus, the lowest possible log-transformed score was 0, corresponding to abstainers.
Statistical analyses
Descriptive statistics and preliminary analyses were obtained/conducted using SAS 9.2 and JMP 8 (SAS Institute Inc., USA). Twin modeling was conducted in OpenMx (Boker et al. 2011) using the raw continuous data option. All twins were included in these analyses, including those who were members of incomplete pairs. In twin modeling, liability to phenotypes such as depression or alcohol use can be attributed to several latent sources of variance: additive genetic factors (A), shared environment (C) and unique environment (E). The C variance component represents environmental exposures and experiences that are shared by both members of a twin pair and contribute to twins’ increased similarity, irrespective of zygosity, in a given phenotype. Environmental factors that are unique to one twin are accounted for by the E component; these factors reduce twin similarity for a given phenotype. The E component also includes measurement error. Estimates of each of these variance components are calculated by comparing the phenotypic correlation between MZ twins (who share all their genes) with DZ twins (who share half of their genes, on average, identical by descent).
We used a Cholesky decomposition model (six A factors, six C factors, and six E factors) for these analyses, as this structure allows us to impose a temporal structure on the manifest variables (from left to right as time progresses). This enabled us to evaluate whether new latent A (or C) influences ‘ come online’ over time. We compared submodels in which later (epoch 2–6) C and A factor loadings were sequentially removed from the model using the p value of the χ2 and Akaike’s Information Criterion (Akaike, 1987) as an indicator of model fit and parsimony. We did not test the significance of E factor loadings, as such tests were peripheral to our research question and could result in over-fitting. Thus, the structure of E factor loadings remained saturated throughout the model-fitting process.
Results
Descriptive statistics
Mean monthly alcohol consumption was quite low in epoch 1 (age 12–14 years) at 0.67 (S.E.=0.13) drinks per month; it increased rapidly across epoch 2 (mean=7.24, S.E.=0.55), epoch 3 (mean=38.04, S.E.=1.53) and epoch 4 (mean=39.55, S.E.=1.79), after which consumption decreased to a mean of approximately one drink per day in epoch 5 (mean=30.50, S.E.=1.50) and epoch 6 (mean=29.25, S.E.=1.96). All other statistics and analyses employed natural log-transformed versions of the consumption variables. Phenotypic correlations from one epoch to the immediately subsequent epoch were modest to high (r=0.51–0.86, see Appendix Table A1), and increased over time as the pattern of alcohol use stabilized.
Cross-sectional twin correlations were similar for MZ twins and DZ twins for epochs 1 and 2 (Table 1), suggesting that shared environmental (C) variance contributes to manifestation of these phenotypes, with minimal genetic influence (A). However, from epochs 3–6, the correlation between MZ twins was substantially higher than between DZ twins, suggesting increasing importance of genetic factors and decreasing relevance of C factors.
Table 1.
Twin correlations of log-transformed means of alcohol consumption over time
| Twin 1
|
||||||
|---|---|---|---|---|---|---|
| Twin 2 | Epoch 1 (age 12–14 years) | Epoch 2 (age 15–17 years) | Epoch 3 (age 18–21 years) | Epoch 4 (age 22–25 years) | Epoch 5 (age 26–29 years) | Epoch 6 (age 30–33 years) |
| Monozygotic twins | ||||||
| Epoch 1 | 0.24 | |||||
| Epoch 2 | 0.26 | 0.37 | ||||
| Epoch 3 | 0.18 | 0.37 | 0.56 | |||
| Epoch 4 | 0.14 | 0.32 | 0.49 | 0.51 | ||
| Epoch 5 | 0.12 | 0.28 | 0.39 | 0.45 | 0.45 | |
| Epoch 6 | 0.12 | 0.27 | 0.36 | 0.42 | 0.44 | 0.44 |
| Dizygotic twins | ||||||
| Epoch 1 | 0.28 | |||||
| Epoch 2 | 0.24 | 0.33 | ||||
| Epoch 3 | 0.2 | 0.3 | 0.38 | |||
| Epoch 4 | 0.2 | 0.25 | 0.33 | 0.25 | ||
| Epoch 5 | 0.19 | 0.21 | 0.27 | 0.17 | 0.19 | |
| Epoch 6 | 0.15 | 0.16 | 0.21 | 0.19 | 0.19 | 0.23 |
Twin modeling
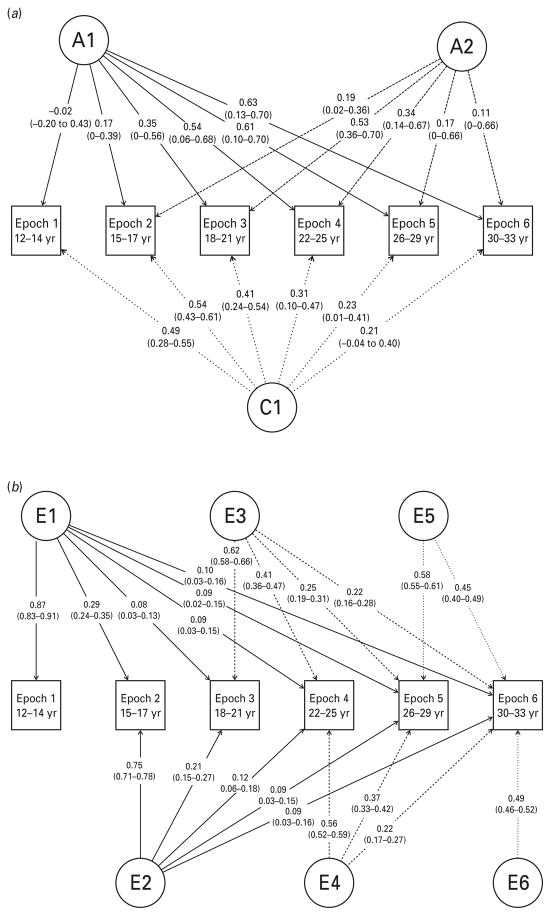
We first tested the significance of C latent factors in the model (Table 2). By setting path estimates to 0, beginning with the C6 factor loadings and working our way back, we found that all of the C factors could be dropped from the model (see Table 2, models 2–7). However, because the phenotypic twin correlations clearly indicated that C influences are relevant in the earlier epochs (see Table 1), we opted to retain a single latent C factor, C1, and all of its factor loadings in the model. We next applied the same approach to A factors, and found that factors A3–A6 could be dropped from the model, but A2 could not. To avoid over-fitting, we did not test the significance of E factor loadings. Therefore, the final model is one in which there are two A factors, one C factor and six E factors. Standardized parameter estimates with confidence intervals (CIs) are provided in Fig. 1. Unstandardized path estimates and CIs are provided in Appendix Table A2.
Table 2.
Model-fitting procedure, with stepwise removal of latent factors and their associated paths
| Model no. | Model description | Model comparison | −2LL | Δχ2 | Δdf | p | ΔAIC |
|---|---|---|---|---|---|---|---|
| 1 | Full | N.A. | 28265.53 | N.A. | (10415) | N.A. | (7435.53) |
| 2 | Drop C6 | 2 v. 1 | 28265.53 | 0 | 1 | 1 | −2.00 |
| 3 | Drop C5 | 3 v. 2 | 28265.53 | 0 | 2 | 1 | −4.00 |
| 4 | Drop C4 | 4 v. 3 | 28265.53 | 0 | 3 | 1 | −6.00 |
| 5 | Drop C3 | 5 v. 4 | 28265.53 | 0 | 4 | 1 | −8.00 |
| 6 | Drop C2 | 6 v. 5 | 28267.37 | 1.84 | 5 | 0.87 | −8.14 |
| 7 | Drop C1 | 7 v. 6 | 28276.07 | 8.7 | 6 | 0.19 | −3.30 |
| 8 | Retain C1, drop A6 | 8 v. 6 | 28267.37 | 0 | 1 | 1 | −2.00 |
| 9 | Drop A5 | 9 v. 8 | 28267.41 | 0.04 | 2 | 0.98 | −3.96 |
| 10 | Drop A4 | 10 v. 9 | 28269.52 | 2.11 | 3 | 0.55 | −3.89 |
| 11a | Drop A3 | 11 v. 10 | 28274.59 | 5.07 | 4 | 0.28 | −2.93 |
| 12 | Drop A2 | 12 v. 11 | 28303.29 | 28.7 | 5 | 0 | 18.7 |
−2LL, −2Log likelihood; df, degrees of freedom; AIC, Akaike’s Information Criterion; N.A., not applicable; C, shared environmental factor; A, additive genetic factor.
Final model.
Fig 1.
Final twin model with standardized path estimates (95% confidence intervals). Genetic (A) and shared environmental (C) influences are presented separately (a) from unique environmental (E) influences (b) for readability.
Table 3 provides details on standardized A, C and E contributions to variance, as a whole and as a function of each latent factor. The total h2 of alcohol use was quite low (<0.01) for ages 12–14 years (epoch 1). The h2 increased to 0.07 for ages 15–17 years (epoch 2), then to 0.40 for ages 18–21 years to 30–33 years (epochs 3–6). Due to the structure of the model, only factor A1 contributes to heritability at epoch 1. During epoch 2, factors A1 and A2 contribute equally to the heritability. The relative contributions of A1 and A2 shift during epoch 3, when factor A1 accounts for 30% of the total heritability in alcohol consumption, and A2 for 70%. This relationship is inverted at epoch 4 (i.e. A1 then accounts for 70% of the heritability). During epochs 5 and 6, A1 accounts for over 90% of the heritability in alcohol consumption. Thus, the effects of A2 are largely limited to a period in mid-adolescence to early adulthood, after which genetic influences from A1 are most relevant (Fig. 2).
Table 3.
Standardized variance attributable to A, C and E overall (with 95% confidence intervals), and as a function of each latent factoa
| Latent Factor | Epoch 1 (age 12–14 years) | Epoch 2 (age 15–17 years) | Epoch 3 (age 18–21 years) | Epoch 4 (age 22–25 years) | Epoch 5 (age 26–29 years) | Epoch 6 (age 30–33 years) |
|---|---|---|---|---|---|---|
| Total A | <0.01 (0–0.04) | 0.07 (0–0.18) | 0.40 (0.27–0.52) | 0.40 (0.26–0.51) | 0.40 (0.26–0.49) | 0.40 (0.26–0.50) |
| Total C | 0.24 (0.08–0.31) | 0.29 (0.18–0.38) | 0.17 (0.06–0.29) | 0.09 (0.01–0.22) | 0.05 (0–0.16) | 0.04 (0–0.16) |
| Total E | 0.76 (0.69–0.83) | 0.64 (0.58–0.71) | 0.43 (0.38–0.49) | 0.50 (0.44–0.57) | 0.55 (0.49–0.62) | 0.55 (0.49–0.63) |
| A1 | <0.01 | 0.03 | 0.12 | 0.28 | 0.37 | 0.40 |
| A2 | N.A. | 0.04 | 0.28 | 0.12 | 0.03 | 0.01 |
| C1 | 0.24 | 0.29 | 0.17 | 0.09 | 0.05 | 0.04 |
| E1 | 0.76 | 0.09 | 0.01 | 0.01 | 0.01 | 0.01 |
| E2 | N.A. | 0.56 | 0.04 | 0.01 | 0.01 | 0.01 |
| E3 | N.A. | N.A. | 0.37 | 0.17 | 0.06 | 0.05 |
| E4 | N.A. | N.A. | N.A. | 0.31 | 0.14 | 0.05 |
| E5 | N.A. | N.A. | N.A. | N.A. | 0.33 | 0.20 |
| E6 | N.A. | N.A. | N.A. | N.A. | N.A. | 0.24 |
A, Additive genetic factor ; C, shared environmental factor; E, unique environmental factor ; N.A., not applicable.
Totals might differ from 1 due to rounding.
Fig 2.
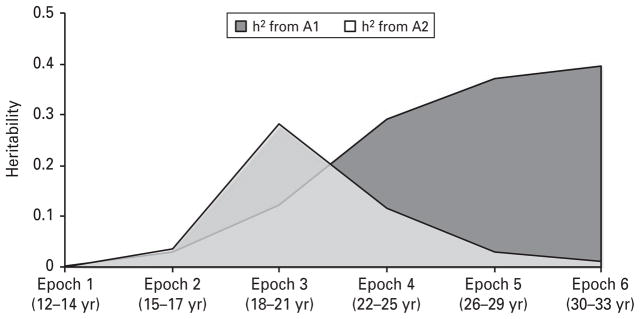
Heritability (h2) accounted for by additive genetic factors A1 and A2 over time.
Shared environmental influences are most prominent during the first two epochs, accounting for 24–29% of the total variance. After this, their influence declines to 4% of the variance by epoch 6. Unique environmental factors also decline in significance over time, though less dramatically: in epoch 1, they account for 76% of the variance; 64% during epoch 2; and 43–55% from epochs 3–6. Factor loadings from E1–E3 are primarily time-specific, with little influence on later epochs; however, even these low cross-time loadings differ significantly from 0 (Fig. 1, Appendix Table A2). Beginning with E4, which loads first onto epoch 4 (ages 22–25 years), unique environmental factors are modestly influential over time rather than being nearly completely occasion-specific.
The total unstandardized phenotypic variance in alcohol consumption is quite low during epoch 1 (VP =0.27). This increases in epochs 2 and 3 (VP =1.59 and VP =2.83, respectively). Afterwards, the variance is relatively stable to epoch 6 (VP =2.85 in epoch 4, VP =2.75 in epoch 5, and VP =2.71 in epoch 6). Thus, while the sources of variance change over time (described above), the actual variance being accounted for changes as well.
Discussion
The goal of this analysis was to describe latent genetic influences on alcohol consumption from early adolescence into adulthood in terms of the number of relevant factors and their relative influence over time. We found that two distinct latent genetic factors account for genetic variance from the ages of 12–14 years through to the ages of 30–33 years. The influence of one of these factors is largely limited to mid-adolescence and early adulthood, declining in significance beyond epoch 3 (ages 18–21 years). Thereafter, the influence of the other latent genetic factor is more pronounced. The total heritability of alcohol consumption – combining that contributed by both latent genetic factors – increases from h2 of about 0 at ages 12–14 years to h2 of about 0.40 from the age of 18–21 years to the age of 30–33 years. These estimates are consistent with those reported previously (Kendler et al. 2008b). Shared environmental influences decline over time, from 24% of the variance to 4%. Likewise, unique environmental influences account for more of the total variance at the age of 12–14 years (76%) than at the age of 30–33 years (55%).
The identification of one genetic factor whose effects decline steadily into young adulthood and another whose effects increase during this time corresponds with some prior evidence of developmentally specific genetic effects. A study using the current sample (Kendler et al. 2011a) examined the genetic risk for externalizing disorders and alcohol-use disorders, which were derived based on co-twin and parental phenotypes, and their respective relationships to alcohol intake. Results indicated that genetic risk for externalizing is more strongly related to intake during adolescence, while genetic risk for alcohol problems is more strongly related to intake beginning in early adulthood. Other studies have found that externalizing disorders and alcohol problems are genetically correlated during adolescence (Button et al. 2007; Hicks et al. 2007; Legrand et al. 2008), though the covariance in phenotypes might also be due to environmental factors (Rose et al. 2004). Such findings raise the possibility that the adolescent-limited genetic factor identified in the current report is actually capturing liability to general externalizing behaviors (which would encompass alcohol use) during that period, rather than liability to alcohol use per se. Additional analyses would be necessary to confirm this, and to examine whether the adult-onset genetic factor is largely specific to alcohol consumption.
Our estimates of the heritability of alcohol consumption in early adolescents are comparable with that of a previous study of adolescent alcohol use (Rhee et al. 2003) and another of adolescent alcohol problems (Rose et al. 2004). However, they are considerably lower than other reports, which range from 0.25 to 0.67 for a variety of alcohol-related phenotypes (Edwards et al. 2011a, b; Geels et al. 2012). Our use of retrospective reports could contribute to discrepancies, though genuine population and cohort differences probably also contribute. Generally, the heritability of alcohol use increases from adolescence into adulthood (Bergen et al. 2007), consistent with the estimates reported here. This increase in heritability is typically accompanied by corresponding decreases in shared environmental influences (Edwards et al. 2011a, b; Geels et al. 2012).
Our findings have implications for gene identification efforts. In recent years, numerous genome-wide association studies have attempted to identify specific genes or variants that are associated with alcohol-related phenotypes (Treutlein et al. 2009; Bierut et al. 2010; Edenberg et al. 2010; Lind et al. 2010; Baik et al. 2011; Heath et al. 2011; Kendler et al. 2011b; Schumann et al. 2011; Zuo et al. 2011, 2012; Agrawal et al. 2012; Frank et al. 2012; Wang et al. 2012), two of which specifically focused on alcohol consumption (Baik et al. 2011; Schumann et al. 2011). The results reported here indicate that the use of a sample population with a wide age range is more likely to result in high levels of genetic heterogeneity underlying liability to alcohol consumption, relative to a sample consisting entirely of mature adults (e.g. age 25 years or older), or a sample consisting entirely of late adolescents. In other words, including both mature adults and late adolescents/young adults in a sample could complicate gene-finding efforts due to the fact that different genetic factors are influential in different subgroups of the sample, a concern previously articulated by Hansell et al. (2009). One potential implication of this possibility is that the use of a phenotype based on a period of highest use – rather than age-specific use – should be carefully considered, as relevant genetic variants could differ depending on whether that period corresponds to adolescence versus adulthood. Furthermore, while the current study is limited to men, researchers should also be cognizant of the possibility that genetic differences in alcohol consumption exist across genders, and make an effort to account for such differences in gene identification studies.
A recent study of Dutch twins (van Beek et al. 2012) is similar to the current report in that it aimed to clarify genetic influences on alcohol problems over time. The sample included individuals assessed from the age of 15 through to 32 years (ages were collapsed into six categories), who were administered the CAGE inventory (Dhalla & Kopec, 2007) to examine alcohol problems. As in the current study, van Beek et al. (2012) found that unique environmental influences were both stable and dynamic; that is, E factors remained relevant over time and were complemented by novel E influences coming ‘online’ at each subsequent age range. They also found that a single C factor, with forward transmission, influenced CAGE scores over time. However, in contrast to our identification of two significant genetic factors, van Beek et al. (2012) found that a single genetic factor, with no innovation over time, accounted for genetic influences on CAGE scores. Previous studies have indicated that alcohol consumption and problems are modestly to strongly genetically correlated in adulthood (Grant et al. 2009; Kendler et al. 2010; Dick et al. 2011); additional analyses would be necessary to determine whether the adolescent-limited or adult-onset genetic factors identified in the current study are more strongly related to alcohol problems. Previous research (described above) suggests that the latter is a more likely candidate. However, other studies have found little to no evidence of genetic influences on alcohol problems in adolescence (Rose et al. 2004; Knopik et al. 2009), raising the possibility that the genetic factors loading onto the CAGE score in late adolescence are atypical.
The presence of an adolescent-limited genetic factor and an adult-onset genetic factor bears some resemblance to previously observed patterns of antisocial behavior across development. Moffitt (1993) characterized adolescent-limited and life-course-persistent patterns of antisocial behavior. As discussed in that work, problem behavior can begin quite early in life and proceed along a persistently high trajectory, or a trajectory that peaks in adolescence and diminishes thereafter. Although those distinctions were originally made in the context of an effort to discriminate between classes of juvenile delinquents and inform theories of antisocial behavior, the current findings could have applications for related research. In the present study, a subset of individuals might carry a high ‘genetic load’ from both the adolescent-limited genetic factor identified in these analyses (A2) and the adult-onset factor (A1); this would increase one’s liability to sustained high alcohol consumption from adolescence into adulthood. Others might harbor multiple risk variants that underlie only the adolescent-limited genetic factor, leading to temporally limited genetic risk to excessive consumption. Still others could carry genetic risk primarily from variants underlying the adult-onset factor, thus leading to a relatively late onset of high consumption. Similar genetic and/or environmental liabilities could account for the phenotypic subtypes of antisocial behavior described by Moffitt (1993). One study of persistent antisocial behavior in a prospectively assessed twin sample reported support for Moffitt’s theory from a genetic perspective: a common latent factor, largely genetic in nature, accounted for continuity of antisocial behavior from mid-childhood through to early adulthood (Tuvblad et al. 2011). This persistent latent liability was supplemented by significant time-specific genetic influences at ages 8–9 years (in both genders) and 13–14 years (females only), indicating that even individuals who were not genetically liable to persistent antisocial behavior might still be genetically liable to such behavior at early ages. Those findings and the current results are complementary to the concept of ‘heterotypic continuity ’: rather than a single latent liability manifesting differently across development, we observe here a consistent phenotype (alcohol consumption) that is influenced by distinct latent liabilities during different developmental periods. The similarities between the current results and those aimed at characterizing risk of antisocial behavior could have a substantive foundation: a broad literature exists examining relationships between antisocial behavior and alcohol problems, including research suggesting that the risk to children of alcoholics developing their own problems could proceed along an antisocial pathway, with executive functioning deficits representing an alternative pathway (Nigg et al. 2004, 2006).
Our results could potentially have differed had abstainers been coded as missing rather than as ‘0’. Addressing this possibility is not entirely straightforward, as participants reporting an average consumption of no drinks for an epoch were not necessarily abstaining entirely: their consumption could simply have been too low to differ substantially from 0 from their perspective. However, we made an effort to explore the possibility by recoding potential abstainers as missing. Model-fitting procedures produced a qualitatively similar final model, with two A factors and one C factor, though model fit statistics were far less satisfactory (results available upon request). The relative contributions of each A factor to total heritability were also similar, with one factor primarily influencing very early drinking and the other influencing consumption in early to mid-adulthood. The most pronounced difference under the recoding scheme is that the total h2 of consumption in epochs 1 and 2 was higher than reported here, at about 0.20 for each time point. These estimates are based on quite small samples of early adolescents who drink, and the CIs are wide and span the estimates reported for the original coding scheme (95% CI 1.64×10−12 to 0.44 for epoch 1; 95% CI 6.38×10−12 to 0.40). Thus, while the true heritability of very early alcohol consumption might be underestimated in the current report, it is difficult to establish a precise estimate for this age range. Importantly, the overall structure of genetic influences is accurately captured by the original coding scheme.
In summary, these analyses explore the dynamic nature of genetic effects on alcohol consumption from adolescence to adulthood in a population-based sample of male twins. We report evidence of two latent genetic factors: one that is influential during mid-adolescence to early adulthood, but whose effects decline thereafter; and a second whose effects are modest during adolescence and increase gradually into adulthood. Consistent with previous reports, shared environmental influences decrease over time. While early unique environmental factors are largely time-specific in their influence, by early adulthood, these factors’ effects are influential across epochs. These findings have important implications in gene identification efforts for alcohol related phenotypes, and could also inform research on the continuity or discontinuity of behavioral phenotypes across development, as they indicate that different genetic factors can underlie the same phenotype from adolescence into adulthood.
Limitations
The results reported here should be considered in the context of several limitations. Data were only available for white men, and the generalizability of these results to women and other ethnicities is not clear. Additionally, alcohol consumption was reported retrospectively, raising the possibility of errors in recall. We were unable to examine changes in A, C and E influences beyond the early 30 s; examination of later age ranges would have resulted in abundant missing data as fewer members of the sample had reached later ages. Finally, we opted to retain shared environmental influences in the model based on theoretical and previous empirical evidence of their relevance to early drinking behaviors; however, this resulted in a final model that was less parsimonious than was possible. These findings warrant replication in a sample of twins that is prospectively assessed and includes both genders and other ethnicities, if possible.
Acknowledgments
This study was supported in part by National Institutes of Health (NIH) grants AA19849 (to A.C.E.) and UL1RR031990 (Virginia Commonwealth University Center for Clinical and Translational Research). S. Aggen assisted with data management.
Appendix Table A1.
Phenotypic correlations of logged means of alcohol consumption over timea
| Epoch 1 (age 12–14 years) | Epoch 2 (age 15–17 years) | Epoch 3 (age 18–21 years) | Epoch 4 (age 22–25 years) | Epoch 5 (age 26–29 years) | Epoch 6 (age 30–33 years) | |
|---|---|---|---|---|---|---|
| Epoch 1 | 1 | |||||
| Epoch 2 | 0.51 | 1 | ||||
| Epoch 3 | 0.26 | 0.56 | 1 | |||
| Epoch 4 | 0.22 | 0.44 | 0.78 | 1 | ||
| Epoch 5 | 0.17 | 0.35 | 0.57 | 0.78 | 1 | |
| Epoch 6 | 0.17 | 0.34 | 0.52 | 0.67 | 0.86 | 1 |
All correlations are significant (p<0.05).
Appendix Table A2.
Unstandardized path estimates
| Path | Estimate (95% CI) |
|---|---|
| a11 | −0.01 (−0.1 to 0.22) |
| a21 | 0.22 (0–0.50) |
| a31 | 0.58 (0–0.95) |
| a41 | 0.91 (0.10–10.16) |
| a51 | 10.01 (0.17–10.17) |
| a61 | 10.03 (0.21–10.18) |
| a22 | 0.24 (0.02–0.45) |
| a32 | 0.89 (0.61–10.19) |
| a42 | 0.57 (0.24–10.15) |
| a52 | 0.28 (0–10.10) |
| a62 | 0.17 (0–10.09) |
| c11 | 0.25 (0.15–0.29) |
| c21 | 0.68 (0.54–0.79) |
| c31 | 0.69 (0.40–0.91) |
| c41 | 0.52 (0.17–0.79) |
| c51 | 0.38 (0.01–0.68) |
| c61 | 0.34 (−0.07 to 0.66) |
| e11 | 0.45 (0.43–0.48) |
| e21 | 0.37 (0.31–0.44) |
| e31 | 0.14 (0.05–0.22) |
| e41 | 0.15 (0.05–0.25) |
| e51 | 0.14 (0.04–0.24) |
| e61 | 0.16 (0.05–0.27) |
| e22 | 0.94 (0.90–0.99) |
| e32 | 0.35 (0.26–0.45) |
| e42 | 0.20 (0.11–0.30) |
| e52 | 0.15 (0.05–0.25) |
| e62 | 0.15 (0.05–0.26) |
| e33 | 10.04 (0.98–10.11) |
| e43 | 0.70 (0.61–0.79) |
| e53 | 0.42 (0.32–0.52) |
| e63 | 0.36 (0.26–0.46) |
| e44 | 0.94 (0.89–0.99) |
| e54 | 0.62 (0.54–0.70) |
| e64 | 0.36 (0.28–0.45) |
| e55 | 0.95 (0.91–10.00) |
| e65 | 0.74 (0.66–0.81) |
| e66 | 0.81 (0.77–0.85) |
CI, Confidence interval; a, additive genetic path; c, shared environmental path; e, unique environmental path.
Footnotes
Declaration of Interest
None.
References
- Agrawal A, Wetherill L, Bucholz KK, Kramer J, Kuperman S, Lynskey MT, Nurnberger JI, Jr, Schuckit M, Tischfield JA, Edenberg HJ, Foroud T, Bierut LJ. Genetic influences on craving for alcohol. Addictive Behavior. 2012 doi: 10.1016/j.addbeh.2012.03.021. Published online 19 March 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike H. Factor analysis and AIC. Psychometrika. 1987;52:317–332. [Google Scholar]
- Baik I, Cho NH, Kim SH, Han BG, Shin C. Genome-wide association studies identify genetic loci related to alcohol consumption in Korean men. American Journal of Clinical Nutrition. 2011;93:809–816. doi: 10.3945/ajcn.110.001776. [DOI] [PubMed] [Google Scholar]
- Bergen SE, Gardner CO, Kendler KS. Age-related changes in heritability of behavioral phenotypes over adolescence and young adulthood : a meta-analysis. Twin Research and Human Genetics. 2007;10:423–433. doi: 10.1375/twin.10.3.423. [DOI] [PubMed] [Google Scholar]
- Bierut LJ, Agrawal A, Bucholz KK, Doheny KF, Laurie C, Pugh E, Fisher S, Fox L, Howells W, Bertelsen S, Hinrichs AL, Almasy L, Breslau N, Culverhouse RC, Dick DM, Edenberg HJ, Foroud T, Grucza RA, Hatsukami D, Hesselbrock V, Johnson EO, Kramer J, Krueger RF, Kuperman S, Lynskey M, Mann K, Neuman RJ, Nothen MM, Nurnberger JI, Jr, Porjesz B, Ridinger M, Saccone NL, Saccone SF, Schuckit MA, Tischfield JA, Wang JC, Rietschel M, Goate AM, Rice JP. A genome-wide association study of alcohol dependence. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:5082–5087. doi: 10.1073/pnas.0911109107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boekeloo BO, Novik MG. Clinical approaches to improving alcohol education and counseling in adolescents and young adults. Adolescent Medicine: State of the Art Reviews. 2011;22:631–648. xiv. [PMC free article] [PubMed] [Google Scholar]
- Boker S, Neale M, Maes H, Wilde M, Spiegel M, Brick T, Spies J, Estabrook R, Kenny S, Bates T, Mehta P, Fox J. OpenMx : an open source extended structural equation modeling framework. Psychometrika. 2011;76:306–317. doi: 10.1007/s11336-010-9200-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchery EE, Harwood HJ, Sacks JJ, Simon CJ, Brewer RD. Economic costs of excessive alcohol consumption in the U.S., 2006. American Journal of Preventative Medicine. 2011;41:516–524. doi: 10.1016/j.amepre.2011.06.045. [DOI] [PubMed] [Google Scholar]
- Button TM, Rhee SH, Hewitt JK, Young SE, Corley RP, Stallings MC. The role of conduct disorder in explaining the comorbidity between alcohol and illicit drug dependence in adolescence. Drug and Alcohol Dependence. 2007;87:46–53. doi: 10.1016/j.drugalcdep.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Chen CM, Dufour MC, Yi H. Alcohol consumption among young adults ages 18–24 in the United States : results from the 2001–2002 NESARC survey. Alcohol Research and Health. 2004;28:269–280. [Google Scholar]
- Dawson DA. Consumption indicators of alcohol dependence. Addiction. 1994;89:345–350. doi: 10.1111/j.1360-0443.1994.tb00901.x. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Grant BF, Li TK. Quantifying the risks associated with exceeding recommended drinking limits. Alcoholism, Clinical and Experimental Research. 2005;29:902–908. doi: 10.1097/01.alc.0000164544.45746.a7. [DOI] [PubMed] [Google Scholar]
- Dhalla S, Kopec JA. The CAGE questionnaire for alcohol misuse: a review of reliability and validity studies. Clinical and Investigative Medicine. 2007;30:33–41. doi: 10.25011/cim.v30i1.447. [DOI] [PubMed] [Google Scholar]
- Dick DM, Meyers JL, Rose RJ, Kaprio J, Kendler KS. Measures of current alcohol consumption and problems: two independent twin studies suggest a complex genetic architecture. Alcoholism, Clinical and Experimental Research. 2011;35:2152–2161. doi: 10.1111/j.1530-0277.2011.01564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edenberg HJ, Koller DL, Xuei X, Wetherill L, McClintick JN, Almasy L, Bierut LJ, Bucholz KK, Goate A, Aliev F, Dick D, Hesselbrock V, Hinrichs A, Kramer J, Kuperman S, Nurnberger JI, Jr, Rice JP, Schuckit MA, Taylor R, Todd Webb B, Tischfield JA, Porjesz B, Foroud T. Genome-wide association study of alcohol dependence implicates a region on chromosome 11. Alcoholism, Clinical and Experimental Research. 2010;34:840–852. doi: 10.1111/j.1530-0277.2010.01156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards AC, Larsson H, Lichtenstein P, Kendler KS. Early environmental influences contribute to covariation between internalizing symptoms and alcohol intoxication frequency across adolescence. Addictive Behaviors. 2011a;36:175–182. doi: 10.1016/j.addbeh.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards AC, Sihvola E, Korhonen T, Pulkkinen L, Moilanen I, Kaprio J, Rose RJ, Dick DM. Depressive symptoms and alcohol use are genetically and environmentally correlated across adolescence. Behavior Genetics. 2011b;41:476–487. doi: 10.1007/s10519-010-9400-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler T, Lifford K, Shelton K, Rice F, Thapar A, Neale MC, McBride A, van den Bree MB. Exploring the relationship between genetic and environmental influences on initiation and progression of substance use. Addiction. 2007;102:413–422. doi: 10.1111/j.1360-0443.2006.01694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank J, Cichon S, Treutlein J, Ridinger M, Mattheisen M, Hoffmann P, Herms S, Wodarz N, Soyka M, Zill P, Maier W, Mossner R, Gaebel W, Dahmen N, Scherbaum N, Schmal C, Steffens M, Lucae S, Ising M, Muller-Myhsok B, Nothen MM, Mann K, Kiefer F, Rietschel M. Genome-wide significant association between alcohol dependence and a variant in the ADH gene cluster. Addiction Biology. 2012;17:171–180. doi: 10.1111/j.1369-1600.2011.00395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman D, Thornton A, Camburn D, Alwin D, Youngdemarco L. The life history calendar : a technique for collecting retrospective data. Sociological Methodology. 1988;18:37–68. [PubMed] [Google Scholar]
- Geels LM, Bartels M, van Beijsterveldt TC, Willemsen G, van der Aa N, Boomsma DI, Vink JM. Trends in adolescent alcohol use: effects of age, sex and cohort on prevalence and heritability. Addiction. 2012;107:518–527. doi: 10.1111/j.1360-0443.2011.03612.x. [DOI] [PubMed] [Google Scholar]
- Giacosa A, Adam-Blondon AF, Baer-Sinnott S, Barale R, Bavaresco L, Di Gaspero G, Dugo L, Ellison RC, Gerbi V, Gifford D, Janssens J, La Vecchia C, Negri E, Pezzotti M, Santi L, Santi L, Rondanelli M. Alcohol and wine in relation to cancer and other diseases. European Journal of Cancer Prevention. 2012;21:103–108. doi: 10.1097/CEJ.0b013e32834761d3. [DOI] [PubMed] [Google Scholar]
- Grant JD, Agrawal A, Bucholz KK, Madden PA, Pergadia ML, Nelson EC, Lynskey MT, Todd RD, Todorov AA, Hansell NK, Whitfield JB, Martin NG, Heath AC. Alcohol consumption indices of genetic risk for alcohol dependence. Biological Psychiatry. 2009;66:795–800. doi: 10.1016/j.biopsych.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansell NK, Agrawal A, Whitfield JB, Morley KI, Gordon SD, Lind PA, Pergadia ML, Montgomery GW, Madden PA, Todd RD, Heath AC, Martin NG. Can we identify genes for alcohol consumption in samples ascertained for heterogeneous purposes? Alcoholism, Clinical and Experimental Research. 2009;33:729–739. doi: 10.1111/j.1530-0277.2008.00890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath AC, Whitfield JB, Martin NG, Pergadia ML, Goate AM, Lind PA, McEvoy BP, Schrage AJ, Grant JD, Chou YL, Zhu R, Henders AK, Medland SE, Gordon SD, Nelson EC, Agrawal A, Nyholt DR, Bucholz KK, Madden PA, Montgomery GW. A quantitative-trait genome-wide association study of alcoholism risk in the community: findings and implications. Biological Psychiatry. 2011;70:513–518. doi: 10.1016/j.biopsych.2011.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks BM, Blonigen DM, Kramer MD, Krueger RF, Patrick CJ, Iacono WG, McGue M. Gender differences and developmental change in externalizing disorders from late adolescence to early adulthood: a longitudinal twin study. Journal of Abnormal Psychology. 2007;116:433–447. doi: 10.1037/0021-843X.116.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Marijuana use continues to rise among US teens, while alcohol use hits historic lows. University of Michigan News Service; Ann Arbor, MI: 2011. [Google Scholar]
- Kendler KS, Gardner C, Dick DM. Predicting alcohol consumption in adolescence from alcohol-specific and general externalizing genetic risk factors, key environmental exposures and their interaction. Psychological Medicine. 2011a;41:1507–1516. doi: 10.1017/S003329171000190X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Jacobson K, Myers JM, Eaves LJ. A genetically informative developmental study of the relationship between conduct disorder and peer deviance in males. Psychological Medicine. 2008a;38:1001–1011. doi: 10.1017/S0033291707001821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Kalsi G, Holmans PA, Sanders AR, Aggen SH, Dick DM, Aliev F, Shi J, Levinson DF, Gejman PV. Genomewide association analysis of symptoms of alcohol dependence in the molecular genetics of schizophrenia (MGS2) control sample. Alcoholism, Clinical and Experimental Research. 2011b;35:963–975. doi: 10.1111/j.1530-0277.2010.01427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Myers J, Dick D, Prescott CA. The relationship between genetic influences on alcohol dependence and on patterns of alcohol consumption. Alcoholism, Clinical and Experimental Research. 2010;34:1058–1065. doi: 10.1111/j.1530-0277.2010.01181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA. Genes, Environment, and Psychopathology. The Guilford Press; Boston, MA: 2006. [Google Scholar]
- Kendler KS, Schmitt E, Aggen SH, Prescott CA. Genetic and environmental influences on alcohol, caffeine, cannabis, and nicotine use from early adolescence to middle adulthood. Archives of General Psychiatry. 2008b;65:674–682. doi: 10.1001/archpsyc.65.6.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopik VS, Heath AC, Bucholz KK, Madden PA, Waldron M. Genetic and environmental influences on externalizing behavior and alcohol problems in adolescence: a female twin study. Pharmacology, Biochemistry and Behavior. 2009;93:313–321. doi: 10.1016/j.pbb.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrand LN, Keyes M, McGue M, Iacono WG, Krueger RF. Rural environments reduce the genetic influence on adolescent substance use and rule-breaking behavior. Psychological Medicine. 2008;38:1341–1350. doi: 10.1017/S0033291707001596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind PA, Macgregor S, Vink JM, Pergadia ML, Hansell NK, de Moor MH, Smit AB, Hottenga JJ, Richter MM, Heath AC, Martin NG, Willemsen G, de Geus EJ, Vogelzangs N, Penninx BW, Whitfield JB, Montgomery GW, Boomsma DI, Madden PA. A genomewide association study of nicotine and alcohol dependence in Australian and Dutch populations. Twin Research and Human Genetics. 2010;13:10–29. doi: 10.1375/twin.13.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone SM, Taylor J, Marmorstein NR, McGue M, Iacono WG. Genetic and environmental influences on antisocial behavior and alcohol dependence from adolescence to early adulthood. Developmental Psychopathology. 2004;16:943–966. doi: 10.1017/s0954579404040088. [DOI] [PubMed] [Google Scholar]
- McCambridge J, McAlaney J, Rowe R. Adult consequences of late adolescent alcohol consumption: a systematic review of cohort studies. PLoS Medicine. 2011;8:e1000413. doi: 10.1371/journal.pmed.1000413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt TE. Adolescence-limited and life-course-persistent antisocial behavior : a developmental taxonomy. Psychological Review. 1993;100:674–701. [PubMed] [Google Scholar]
- Nigg JT, Glass JM, Wong MM, Poon E, Jester JM, Fitzgerald HE, Puttler LI, Adams KM, Zucker RA. Neuropsychological executive functioning in children at elevated risk for alcoholism : findings in early adolescence. Journal of Abnormal Psychology. 2004;113:302–314. doi: 10.1037/0021-843X.113.2.302. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Wong MM, Martel MM, Jester JM, Puttler LI, Glass JM, Adams KM, Fitzgerald HE, Zucker RA. Poor response inhibition as a predictor of problem drinking and illicit drug use in adolescents at risk for alcoholism and other substance use disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45:468–475. doi: 10.1097/01.chi.0000199028.76452.a9. [DOI] [PubMed] [Google Scholar]
- Pagan JL, Rose RJ, Viken RJ, Pulkkinen L, Kaprio J, Dick DM. Genetic and environmental influences on stages of alcohol use across adolescence and into young adulthood. Behavior Genetics. 2006;36:483–497. doi: 10.1007/s10519-006-9062-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee SH, Hewitt JK, Young SE, Corley RP, Crowley TJ, Stallings MC. Genetic and environmental influences on substance initiation, use, and problem use in adolescents. Archives of General Psychiatry. 2003;60:1256–1264. doi: 10.1001/archpsyc.60.12.1256. [DOI] [PubMed] [Google Scholar]
- Rose RJ, Dick DM, Viken RJ, Pulkkinen L, Kaprio J. Genetic and environmental effects on conduct disorder and alcohol dependence symptoms and their covariation at age 14. Alcoholism, Clinical and Experimental Research. 2004;28:1541–1548. doi: 10.1097/01.alc.0000141822.36776.55. [DOI] [PubMed] [Google Scholar]
- Sartor CE, Xian H, Scherrer JF, Lynskey MT, Duncan AE, Haber JR, Grant JD, Bucholz KK, Jacob T. Psychiatric and familial predictors of transition times between smoking stages: results from an offspring-of-twins study. Addictive Behaviors. 2008;33:235–251. doi: 10.1016/j.addbeh.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann G, Coin LJ, Lourdusamy A, Charoen P, Berger KH, Stacey D, Desrivieres S, Aliev FA, Khan AA, Amin N, Aulchenko YS, Bakalkin G, Bakker SJ, Balkau B, Beulens JW, Bilbao A, de Boer RA, Beury D, Bots ML, Breetvelt EJ, Cauchi S, Cavalcanti-Proenca C, Chambers JC, Clarke TK, Dahmen N, de Geus EJ, Dick D, Ducci F, Easton A, Edenberg HJ, Esko T, Fernandez-Medarde A, Foroud T, Freimer NB, Girault JA, Grobbee DE, Guarrera S, Gudbjartsson DF, Hartikainen AL, Heath AC, Hesselbrock V, Hofman A, Hottenga JJ, Isohanni MK, Kaprio J, Khaw KT, Kuehnel B, Laitinen J, Lobbens S, Luan J, Mangino M, Maroteaux M, Matullo G, McCarthy MI, Mueller C, Navis G, Numans ME, Nunez A, Nyholt DR, Onland-Moret CN, Oostra BA, O’Reilly PF, Palkovits M, Penninx BW, Polidoro S, Pouta A, Prokopenko I, Ricceri F, Santos E, Smit JH, Soranzo N, Song K, Sovio U, Stumvoll M, Surakk I, Thorgeirsson TE, Thorsteinsdottir U, Troakes C, Tyrfingsson T, Tonjes A, Uiterwaal CS, Uitterlinden AG, van der Harst P, van der Schouw YT, Staehlin O, Vogelzangs N, Vollenweider P, Waeber G, Wareham NJ, Waterworth DM, Whitfield JB, Wichmann EH, Willemsen G, Witteman JC, Yuan X, Zhai G, Zhao JH, Zhang W, Martin NG, Metspalu A, Doering A, Scott J, Spector TD, Loos RJ, Boomsma DI, Mooser V, Peltonen L, Stefansson K, van Duijn CM, Vineis P, Sommer WH, Kooner JS, Spanagel R, Heberlein UA, Jarvelin MR, Elliott P. Genome-wide association and genetic functional studies identify autism susceptibility candidate 2 gene (AUTS2) in the regulation of alcohol consumption. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:7119–7124. doi: 10.1073/pnas.1017288108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treutlein J, Cichon S, Ridinger M, Wodarz N, Soyka M, Zill P, Maier W, Moessner R, Gaebel W, Dahmen N, Fehr C, Scherbaum N, Steffens M, Ludwig KU, Frank J, Wichmann HE, Schreiber S, Dragano N, Sommer WH, Leonardi-Essmann F, Lourdusamy A, Gebicke-Haerter P, Wienker TF, Sullivan PF, Nothen MM, Kiefer F, Spanagel R, Mann K, Rietschel M. Genome-wide association study of alcohol dependence. Archives of General Psychiatry. 2009;66:773–784. doi: 10.1001/archgenpsychiatry.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuvblad C, Narusyte J, Grann M, Sarnecki J, Lichtenstein P. The genetic and environmental etiology of antisocial behavior from childhood to emerging adulthood. Behavior Genetics. 2011;41:629–640. doi: 10.1007/s10519-011-9463-4. [DOI] [PubMed] [Google Scholar]
- van Beek JH, Kendler KS, de Moor MH, Geels LM, Bartels M, Vink JM, van den Berg SM, Willemsen G, Boomsma DI. Stable genetic effects on symptoms of alcohol abuse and dependence from adolescence into early adulthood. Behavior Genetics. 2012;42:40–56. doi: 10.1007/s10519-011-9488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viken RJ, Kaprio J, Koskenvuo M, Rose RJ. Longitudinal analyses of the determinants of drinking and of drinking to intoxication in adolescent twins. Behavior Genetics. 1999;29:455–461. doi: 10.1023/a:1021631122461. [DOI] [PubMed] [Google Scholar]
- Wang KS, Liu X, Zhang Q, Wu LY, Zeng M. Genome-wide association study identifies 5q21 and 9p24.1 (KDM4C) loci associated with alcohol withdrawal symptoms. Journal of Neural Transmission. 2012;119:425–433. doi: 10.1007/s00702-011-0729-z. [DOI] [PubMed] [Google Scholar]
- Whitfield JB, Zhu G, Madden PA, Neale MC, Heath AC, Martin NG. The genetics of alcohol intake and of alcohol dependence. Alcoholism, Clinical and Experimental Research. 2004;28:1153–1160. doi: 10.1097/01.alc.0000134221.32773.69. [DOI] [PubMed] [Google Scholar]
- Zuo L, Gelernter J, Zhang CK, Zhao H, Lu L, Kranzler HR, Malison RT, Li CS, Wang F, Zhang XY, Deng HW, Krystal JH, Zhang F, Luo X. Genome-wide association study of alcohol dependence implicates KIAA0040 on chromosome 1q. Neuropsychopharmacology. 2012;37:557–566. doi: 10.1038/npp.2011.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo L, Zhang CK, Wang F, Li CS, Zhao H, Lu L, Zhang XY, Lu L, Zhang H, Zhang F, Krystal JH, Luo X. A novel, functional and replicable risk gene region for alcohol dependence identified by genome-wide association study. PLoS One. 2011;6:e26726. doi: 10.1371/journal.pone.0026726. [DOI] [PMC free article] [PubMed] [Google Scholar]