Abstract
Elevated ethanol use during adolescence, a potentially stressful developmental period, is accompanied by insensitivity to many aversive effects of ethanol relative to adults. Given evidence that supports a role for stress and the kappa opioid receptor (KOR) system in mediating aversive properties of ethanol and other drugs, the present study assessed the role of KOR antagonism by norbinaltorphimine (nor-BNI) on ethanol-induced conditioned taste aversion (CTA) in stressed (exposed to repeated restraint) and non-stressed male rats (Experiment 1), with half of the rats pretreated with nor-BNI before stressor exposure. In Experiment 2, CTA induced by the kappa agonist U62,066 was also compared in stressed and non-stressed adolescents and adults. A highly palatable solution (chocolate Boost) was used as the conditioned stimulus (CS), thereby avoiding the need for water deprivation to motivate consumption of the CS during conditioning. No effects of stress on ethanol-induced CTA were found, with all doses eliciting aversions in adolescents and adults in both stress conditions. However, among stressed subjects, adults given nor-BNI before the repeated stressor displayed blunted ethanol aversion relative to adults given saline at that time. This effect of nor-BNI was not seen in adolescents, findings that support a differential role for the KOR involvement in ethanol CTA in stressed adolescents and adults. Results from Experiment 2 revealed that all doses of U62,066 elicited aversions in non-stressed animals of both ages that were attenuated in stressed animals, findings that support a modulatory role for stress in aversive effects of KOR activation. Collectively, these results suggest that although KOR sensitivity appears to be reduced in stressed subjects, this receptor system does not appear to contribute to age differences in ethanol-induced CTA under the present test circumstances.
Keywords: Adolescence, Stress, Rat, Kappa opioid receptor, Ethanol, Conditioned taste aversion
1
Experimentation with alcohol and other drugs is common during adolescence. Results from the 2011 Monitoring the Future study revealed that over 40% of high school seniors have smoked cigarettes or marijuana and 25% report having used other illicit drugs. Ethanol consumption, however, is far more common: among 12th graders, 70% have tried ethanol at least once, and 25% have been drunk within the past 30 days (Johnston et al., 2012). Elevated levels of ethanol use during adolescence may be especially problematic, as early ethanol use is a strong predictor of lifetime alcohol abuse and dependence (Grant and Dawson, 1997).
Adolescence is often considered a stressful and challenging developmental period (Arnett, 1999; Casey et al., 2010). During this time, numerous neural, hormonal, and behavioral alterations occur as individuals make the transition from youth to maturity (Spear, 2000; 2010). Increases in social behavior, impulsivity, risk-taking and novelty seeking are frequently reported during this time (Hartup and Stevens, 1997; Primus & Kellogg, 1989; Varlinskaya & Spear, 2008; Adriani et al., 1998; Laviola et al., 2003; Adriani and Laviola, 2003). Given the ethical constraints that limit such research in humans, animal models are essential for research that aims to illuminate factors contributing to the elevated drug experimentation and abuse that often occurs during adolescence. In rodents, early-mid adolescence is estimated to range from postnatal days 28 to 42, with late adolescence/”emerging adulthood” occurring during postnatal days 42–55 (Vetter-O’Hagen and Spear, 2011). This period encompasses many of the adolescent-typical shifts in physical and behavioral characteristics seen in other species (see Spear, 2000), including altered sleeping habits, increased food consumption and accompanying growth spurt, as well as pubertal maturation. Research conducted in rats and mice suggests that ethanol sensitivity differs drastically across age. For example, adolescents are more sensitive to ethanol-induced social facilitation (Varlinskaya and Spear, 2002) and less sensitive to ethanol’s motor-impairing effects (White et al., 2002), aversive properties (Vetter-O’Hagen et al., 2009; Anderson et al., 2010; Schramm-Sapyta et al., 2010), and withdrawal-induced anxiety (Doremus et al., 2003). This pattern of sensitivity likely promotes elevated ethanol intake during adolescence (for review, see Schramm-Sapyta et al., 2009; Spear, 2011), given that the relative balance of rewarding and aversive effects of drugs of abuse appears to be an important modulator of consumption (Riley, 2011).
Evidence supports a role for the kappa opioid receptor (KOR) system in antagonizing the reinforcing effects of many drugs of abuse, including ethanol (see Shippenberg et al., 2007; Wee and Koob, 2010 for additional references and thorough review). Administration of the KOR agonist U50,488 has been reported to decrease voluntary ethanol consumption in rats (Lindholm et al., 2001) and to block ethanol-induced conditioned place preference (CPP) (Logrip et al., 2009) whereas pretreatment with the KOR antagonist nor-binaltorphimine (nor-BNI) has been reported to enhance ethanol-induced dopamine (DA) release in the nucleus accumbens (Zapata and Shippenberg, 2006), to increase ethanol intake (Mitchell et al., 2005), and to increase state-dependent ethanol CPP (Nguyen et al., 2012). Accordingly, this system has gained attention as a potential target for treating alcohol use disorders (see Walker et al., 2012). Previous research strongly suggests that the KOR system mediates reward and aversion in the brain via regulation of DA release in reward pathway. Typically, effects of KOR activation oppose effects of mu opioid receptor (MOR) activation (see Wee and Koob, 2010). For example, whereas activation of MOR increases DA release in the nucleus accumbens and elicits CPP, activation of KOR decreases DA release in this brain region and precipitates conditioned place aversion (CPA) and conditioned taste aversion (CTA) (Di Chiara and Imperato, 1988; Mucha and Herz, 1985).
KOR sensitivity during adolescence has not been well-characterized. Although KORs are detectable during gestation (Winzer-Serhan et al., 2003), reports on levels of KOR expression across ontogeny and through adolescence are lacking. Prior work in our laboratory examined aversions induced by the kappa agonist U62,066 (spiradoline) in both the CTA and CPA paradigms (Anderson et al., under revision), as well as the impact of KOR antagonism on ethanol consumption (Morales et al., under revision). In both studies, adult males were more sensitive to KOR manipulations than adolescents: whereas adults demonstrated conditioned aversions to the KOR agonist and demonstrated elevated ethanol consumption following KOR antagonism, adolescents were insensitive to these effects. These results have shaped our preliminary hypothesis that attenuated sensitivity to ethanol’s aversive effects may reflect an insensitive KOR system during adolescence.
Importantly, the KOR system appears to modulate consequences of stressor exposure. Dynorphins (DYN), the family of endogenous ligands for KOR (Chavkin et al., 1982), are released in response to stressor exposure (Schwarzer, 2009). With the exception of a few studies characterizing chronic mild stress models of anhedonia (e.g., Papp et al., 1991; Valverde et al., 1997), stress is commonly reported to increase rewarding effects (assessed via CPP) of many drugs of abuse. For example, forced swim stress potentiates nicotine and cocaine CPP (Smith et al., 2012; Kreibich et al., 2009). Likewise, inescapable shock stress enhances morphine-induced CPP (Rozeske et al., 2011; Will et al., 1998). Conditioned fear stress has been shown to induce ethanol CPP in rats (Matsuzawa et al., 1998); similarly, stress-induced potentiation of ethanol CPP has also been observed (Sperling et al., 2010). One theoretical model for the reward-potentiating effects of stressor exposure posits that prior stress activates the DYN system, producing a dysphoric state that subsequently increases the relative reward valence of drugs (Bruchas et al., 2010). The inverse interpretation of this model would presumably suggest that the dysphoric state induced by stress would subsequently reduce the relative aversion induced by drugs. Indeed, stress has also been demonstrated to decrease the aversive properties of ethanol: both foot shock and social defeat stress have been reported to attenuate ethanol-induced CPA in adult male rats (Funk et al., 2004). Administration of KOR agonists has been shown to mimic behavioral effects of stressors, including often a potentiation of the rewarding properties of drugs of abuse (e.g., McLaughlin et al., 2006; Sperling et al., 2010). This effect is typically absent in DYN knock-out mice or subjects pretreated with the KOR antagonist nor-binaltorphimine (nor-BNI).
Reward-potentiating effects of KOR agonists appear to be paradoxical, given that these agonists have also been reported to antagonize rewarding effects of many drugs of abuse. These opposing effects on drug reward have been attributed to the timing of agonist administration: suppression of rewarding effects is typically seen shortly following administration, whereas potentiation of rewarding effects is commonly reported after a longer interval. For example, KOR agonists were reported to block ethanol- and cocaine-induced CPP when administered 10–15 minutes prior to drug conditioning (Logrip et al., 2009; McLaughlin et al., 2006). When administered 60–90 minutes prior to drug conditioning sessions, KOR agonists potentiated CPP induced by ethanol and cocaine, thereby mimicking effects of stressor exposure (McLaughlin et al., 2006; Sperling et al., 2010). Similarly, nor-BNI can exert opposing effects on ethanol reward. In stressed subjects, nor-BNI blocked potentiation of ethanol CPP (Sperling et al., 2010) whereas among non-stressed subjects, nor-BNI has been reported to enhance ethanol CPP (Nguyen et al., 2012).
Exposure to stressors has different consequences during adolescence relative to adulthood. Compared to adults, adolescents experience prolonged elevations in the stress hormone corticosterone following acute restraint stress and greater peak corticosterone release in response to repeated stress (Eiland and Romeo, 2012; Doremus-Fitzwater et al., 2009; Romeo and McEwen, 2006). Additionally, adolescents show greater stress-induced reductions in body weight than adults (Eiland and Romeo, 2012; Doremus-Fitzwater et al., 2009). Acute and chronic stressors have also been shown to influence ethanol sensitivity. Following acute restraint, anxiolytic effects of ethanol were reported in adolescents but not adults (Varlinskaya and Spear, 2012). Following repeated restraint, however, age-related differences in the social consequences of ethanol were diminished, with stressor exposure decreasing sensitivity to social inhibitory effects of ethanol and augmenting expression of ethanol’s social facilitatory effects at both ages (Varlinskaya et al., 2010).
The over-arching goal for the present study was to characterize the role of the KOR/DYN system in mediating aversion in stressed and non-stressed adolescent and adult rats. Experiment 1 was designed to examine the effects of KOR blockade on expression of ethanol-induced CTA in animals of both ages. Given interactions between stress and the DYN/KOR system, and evidence that adolescence may be an especially stressful developmental period, both stressed and non-stressed adolescent and adult subjects were included. Finally, to follow up on the results of Experiment 1 and to further explore our hypothesis that KOR sensitivity may be attenuated during adolescence, Experiment 2 was designed to characterize stress effects on the CTA induced by KOR activation in adolescent and adult rats. For both experiments, a highly palatable solution served as the drug-paired conditioned stimulus in order to avoid the potentially stressful consequences of water-deprivation and to more precisely assess the influence of the experimental stressor manipulation.
2 Experimental Procedures
2.1 Subjects
A total of 320 male Sprague-Dawley rats bred in our colony at Binghamton University were used in these experiments (128 in Experiment 1, 192 in Experiment 2). One day following birth, on postnatal day (P)1, litters were culled to 8–10 pups, maintaining a ratio of 6 males to 4 females whenever possible. Male offspring were weaned on P21 and pair-housed with a same-sex littermate in a temperature-controlled vivarium on a 14:10 light/dark cycle (lights on at 0700); females were assigned to other projects. All experimental animals remained pair-housed for the duration of experimentation and were given ad libitum access to food (Purina Rat Chow, Lowell, MA) and water. Subjects were at all times treated in accordance with Binghamton University Institutional Animal Care and Use Committee protocols and within guidelines established by the National Institute of Health (National Research Council, 2011). For both experiments, subjects were assigned to experimental condition via block randomization; in order to avoid confounding litter effects, no more than one animal from a given litter was assigned to the same experimental condition (see Zorilla, 1997; Holson and Pearce, 1992).
2.2 Ethanol-induced CTA
2.2.1 Experiment 1a: KOR involvement in ethanol CTA in adolescents
On experimental day 0 (P28), subjects were injected with either saline or nor-BNI (10 mg/kg, subcutaneous [s.c.]). Nor-BNI is a long-lasting KOR antagonist that achieves peak effects after 24 hours and blocks receptors for up to several weeks (see Metcalf and Coop, 2005). This dose has been shown to produce behavioral effects in a variety of assays (Morales et al., under revision; Sperling et al., 2010). Beginning on P29, animals in the stressed condition were placed in size-adjusted restraint tubes for 90 minutes per day for five days (experimental days 1–5). This restraint procedure has been previously characterized via behavioral and hormonal assays in our laboratory (see Doremus-Fitzwater et al., 2009). Restraint tubes were Plexiglas cylinders measuring 18 × 4.7 cm with a slot across the top and a sliding stopper for size adjustment (Tailveiner, Braintree Scientific, Braintree, MA). Aside from being weighed on experimental days 1, 3, and 5, non-stressed animals were not manipulated during this time. On the conditioning day (experimental day 6: P34), all animals were weighed, and cagemates were separated from each other in the home cage with a wire mesh divider. Subjects were given one hour of access to chocolate Boost® (Nestlé HealthCare Nutrition, Fremont, MI) and their intake was recorded. Immediately after the one-hour access period, subjects were injected intraperitoneally (i.p.) with either saline or ethanol (1.5 or 2.0 g/kg, 18.9% v/v). These ethanol doses have previously been reported to elicit taste aversion in adolescents (see Anderson et al., 2010). Injection volume rather than concentration was adjusted in order to deliver varying ethanol doses (see Linakis and Cunningham, 1979), and saline controls were injected with a volume equivalent to the highest ethanol dose. On the test day (experimental day 7: P35/P75), cagemates were again separated with a wire mesh divider and given two bottles: one containing Boost and one containing water. Individual intakes were recorded after one hour of access.
2.2.2 Experiment 1b: KOR involvement in ethanol CTA in adults
As with adolescents, adults were administered either saline or nor-BNI (10 mg/kg) on experimental day 0 (P68). For stressor exposure (experimental days 1–5, beginning on P69) animals were placed in restraint tubes measuring 23 × 8 cm for 90 min each day. The test session on day 7 was conducted as described for Experiment 1a. On the conditioning day (experimental day 6; P74), adults received either saline or ethanol (1.0 or 1.5 g/kg) following the one hour access period to the chocolate Boost solution. These ethanol doses have previously been reported to elicit taste aversion in adults (see Anderson et al., 2010). Ethanol was prepared and injected as described for Experiment 1a.
2.2.3 Data Analysis
Intake values from the conditioning and test days were examined for outliers, with data points greater than two standard deviations above or below group means replaced with group means prior to analysis (a total of two adolescent and two adult data points). Conditioning day Boost intake data (mls) were analyzed via a 2 pretreatment (saline; nor- BNI) × 2 stress (restraint and non-manipulated) ANOVA for each age. Test day Boost consumption data (mls) were analyzed via 2 pretreatment × 2 stress condition × 3 ethanol dose ANOVAs. Significant effects and interactions were further explored using Bonferroni-corrected post-hoc tests, focusing on comparisons between animals given each conditioning dose vs. saline control animals to index CTA within each age/stress/pretreatment group.
2.3 Experiment 2: U62,066
2.3.1 Experimental Procedure
Beginning on P29 (adolescents) or P69 (adults), animals in the stressed condition were placed in size-adjusted restraint tubes for 90 minutes per day for five days (experimental days 1–5) as in Experiment 1. On the conditioning day (experimental day 6: P34/P74), all animals were weighed, and cagemates were separated from each other in the home cage with a wire mesh divider. Subjects were given one hour of access to chocolate Boost and their intake was recorded. Immediately after bottles were removed, animals were injected s.c. with saline or U62,066 (0.3, 0.4 or 0.5 mg/kg), with mesh dividers removed approximately 10 min later. Doses of U62,066 were prepared from stock solutions of 0.3, 0.4, and 0.5 mg/ml and administered at a volume of 1 ml/kg. The test session on day 7 was conducted as described for Experiment 1.
2.3.2 Data Analysis
Intake values from the conditioning and test days were examined for outliers, with data points greater than two standard deviations above or below group means replaced with group means prior to analysis (a total of four adult data points). Conditioning day Boost intake data (mls) were analyzed via a 2 age (adolescent, adult) × 2 stress condition (restraint and non-manipulated) ANOVA. Test day Boost intake data (mls) were analyzed via a 2 age × 2 stress × 4 U62,066 dose (0, 0.3, 0.4, 0.5 mg/kg) ANOVA. All significant effects and interactions were further explored using Bonferroni-corrected post-hoc tests, focusing on comparisons between animals given each conditioning dose vs. saline control animals to index CTA within each age/stress group.
3 Results
3.1 Experiment 1a: Adolescents
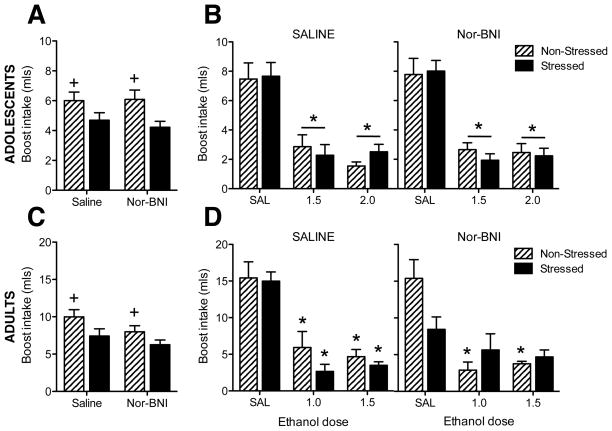
A significant effect of stress emerged in the analysis of conditioning day Boost intake [F(1,85) = 5.1, p < .03], with stressed adolescents consuming less than their non-stressed counterparts (see Figure 1A). Analysis of the test day data revealed a main effect of ethanol dose [F(2,77) = 91.7, p < .0001], with adolescent subjects given 1.5 and 2.0 g/kg ethanol demonstrating CTAs (see Figure 1B). No significant effects of stress or nor-BNI pretreatment emerged.
Fig 1. Ethanol-induced conditioned taste aversion.
(A) Adolescent conditioning day Boost intake. Non-stressed rats consumed more Boost than stressed rats (indicated by +), regardless of pretreatment condition. (B) Adolescent test day Boost intake. Both doses of ethanol elicited aversions (indicated by *), regardless of stress or pretreatment condition. (C) Adult conditioning day Boost intake. Non-stressed rats consumed more Boost than stressed rats (indicated by +), regardless of pretreatment condition. (D) Adult test day Boost intake. Among non-stressed rats, both doses of ethanol elicited conditioned taste aversions regardless of pretreatment condition (indicated by *). Among stressed adults, saline-pretreated rats demonstrated aversions to both ethanol doses (indicated by *) whereas rats pretreated with nor-BNI did not exhibit aversions to any ethanol doses.
3.2 Experiment 1b: Adults
Analysis of conditioning day Boost intake revealed a main effect of stress, with stressed adults consuming less than their non-stressed counterparts [F(1,91) = 7.3, p < .01]. Data are shown in Figure 1C. Analysis of test day data revealed a main effect of ethanol dose [F(2,83) = 45.5, p < .0001] in addition to a 3-way interaction of pretreatment × stress × ethanol dose [F(2,83) = 3.9, p < .03]. Among adults pretreated with saline, both the 1.0 and 1.5 g/kg doses of ethanol produced CTAs, regardless of stress condition. Similarly, among non-stressed adults pretreated with nor-BNI, both ethanol doses elicited aversions. However, neither ethanol dose produced an aversion among stressed adults pretreated with nor-BNI (see Figure 1D).
3.3 Experiment 2
Analysis of conditioning day Boost intake revealed main effects of age [F(1,125) = 16.6, p < .001] and stress [F(1,125) = 10.5, p < 0.01], with adults consuming more Boost than adolescents and non-stressed rats consuming more than stressed rats (see Figure 2A). Analysis of test day intake revealed main effects of dose [F(3,113) = 8.7, p < .001] and a dose × stress interaction [F(3,113) = 3.5, p < .02]. Collapsed across age, all doses of U62,066 elicited CTAs among non-stressed subjects whereas no aversions were seen among stressed subjects (see Figure 2B).
Fig 2. U62,066-induced conditioned taste aversion.
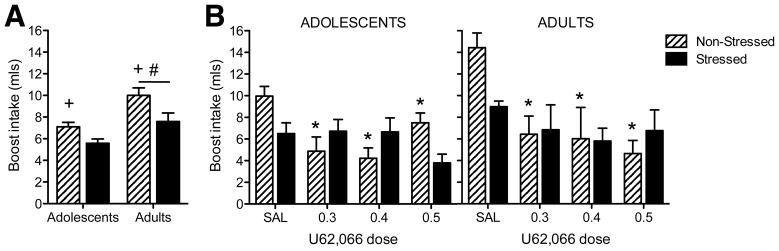
(A) Conditioning day Boost intake. Non-stressed rats consumed more Boost than stressed rats (indicated by +), with adults consuming more Boost than adolescents (indicated by #). (B) Test day Boost intake. Regardless of age, all doses of U62,066 elicited conditioned taste aversions in non-stressed subjects (indicated by *) whereas no aversions were evident in stressed animals.
4. Discussion
The present series of experiments was conducted using a novel model of CTA in non water-deprived subjects to characterize the involvement of the DYN/KOR system in ethanol’s aversive effects in stressed and non-stressed adolescent and adult rats. Results from Experiment 1 revealed no direct effects of stress on ethanol CTA, although nor-BNI pretreatment attenuated aversion to ethanol only in stressed adults. This finding supports a role for the KOR system in mediating ethanol’s aversive effects in these subjects. The KOR system does not appear to modulate the aversive effects of ethanol in adolescents. Experiment 2 was designed to serve as a more general follow-up on KOR sensitivity in stress and non-stressed adolescents and adults. The results demonstrated that restraint stress attenuates U62,066-induced CTA, regardless of age.
4.1 Stress does not modulate ethanol-induced CTA
Results from Experiment 1 provide no evidence of restraint stress effects on CTA to 1.5 or 2.0 g/kg ethanol in adolescent rats or to 1.0 or 1.5 g/kg ethanol in adult rats. Although the present study included only two ethanol doses per age (in addition to other procedural differences), these results are in agreement with our previous study in which we reported no effects of restraint stress on ethanol-induced CTA in water-deprived subjects (Anderson et al., 2010). It is possible that aversive properties of lower ethanol doses may be modulated by stress. Indeed, multiple stressors have been previously reported to attenuate aversion to 1.0 g/kg ethanol in a CPA procedure (Funk et al., 2004).
Stress effects on ethanol’s rewarding properties have also been previously reported in a place conditioning procedure: Matsuzawa and colleagues found that conditioned fear stress induced CPP to 0.15 and 0.3 g/kg ethanol doses (1998) and Sperling and colleagues found that forced swim stress immediately prior to place conditioned potentiated ethanol CPP in mice (2010). Given that stress effects have been reported using both CPP and CPA, it may be possible that place conditioning models are more sensitive to stress modulation than taste conditioning models. Testing a full ethanol dose response in stressed and non-stressed subjects would also provide more conclusive evidence regarding the role of stress in ethanol-induced CTA.
4.2 KOR antagonism attenuates ethanol CTA only in stressed adults
The results from Experiment 1b suggest the KOR system is involved in ethanol’s aversive properties among adult animals when the system is already activated by stress (and thus, potentially more susceptible to KOR antagonism). Among adolescent animals, however, KOR antagonism had no effect on ethanol-induced aversion. These findings suggest age differences in the neural mechanisms involved in ethanol-induced CTA following stress exposure. Given that nor-BNI’s effects are long-lasting, the drug may have affected the outcome of Experiment 1 by either counteracting effects of stressor exposure or by reducing the aversive effects of ethanol. Because nor-BNI pretreatment did not block the stress-induced reduction of Boost intake on the conditioning day in animals of either age, but did attenuate expression of ethanol-induced CTA in stressed adults, the results of the present study support a role for KOR antagonism in reducing the aversive effects of ethanol—but only in stressed adults.
At least one prior study has reported no effect of KOR antagonism on ethanol-induced CTA in non-stressed adult Fischer and Lewis rats (Roma et al., 2008). On the other hand, the DYN/KOR system has been shown to mediate ethanol reward (indexed by place conditioning) in stressed subjects, although not all experimental findings are consistent. For instance, one study revealed that stress-induced activation of the KOR system potentiated CPP to ethanol in mice, an effect successfully blocked by nor-BNI (Sperling et al., 2010), suggesting that stress-induced activation of the DYN/KOR system increases the reward value of ethanol in mice. In contrast, results from another study found activation of the DYN/KOR system to enhance the aversive properties of ethanol in the stressed animals. Specifically, ethanol CPP induced by conditioned fear stress in rats was enhanced by nor-BNI and attenuated by U50,488 (Matsuzawa et al., 1999). Effects of nor-BNI and U50,488 on ethanol place conditioning in non-stressed controls were not assessed in this study. Clearly, more studies are needed for a better understanding of the role this neural system plays in the reinforcing properties of ethanol under basal conditions and following exposure to different stressors. Binding studies and assays of receptor expression under control and stress-exposed conditions may be particularly enlightening.
Whereas taste and place conditioning studies provide more direct assessments of ethanol reward and aversion, data from ethanol self-administration studies can also provide evidence for a role of the DYN/KOR system in ethanol’s motivational properties. Although some studies have reported no effects of KOR manipulation on ethanol intake (e.g., Doyon et al., 2006; Holter et al., 2000), substantial effects of KOR antagonists and agonists have been found as well. Nor-BNI has been shown to increase 24-hr home cage consumption for isolate-housed adult males (Mitchell et al., 2005). These results are similar to those of our recent study that examined effects of nor-BNI on home cage limited access ethanol intake in isolate-housed rats: we found that nor-BNI administration increased ethanol consumption in adult male rats while having no effects in adolescent animals (Morales et al., under revision). Subjects in both studies were isolate-housed, a condition that was likely stressful. The pattern of results seen in these studies suggests that nor-BNI is blocking or reducing the aversive properties of ethanol, thereby promoting enhanced consumption, an effect seen in adults but not adolescents.
This hypothesis is supported by findings of the suppressant effects of the KOR agonist U50,488 on home cage ethanol consumption in adult rats (Lindholm et al., 2001). However, increases in ethanol intake following administration of the same agonist have been reported as well (Holter et al., 2000; Sperling et al., 2010). One study comparing operant self-administration in ethanol-dependent and non-dependent rats found that nor-BNI actually suppressed self-administration among dependent rats following ethanol withdrawal while exerting no effects on control rats (Walker and Koob, 2008), suggesting that the DYN/KOR system is involved in withdrawal-induced dysphoria and, hence, plays a role in the negatively reinforcing, presumably anxiolytic effects of ethanol.
The mixed findings on the effect of KOR manipulations on ethanol intake suggest two possible explanations for a role of the KOR system in ethanol’s motivational effects. KOR activation may increase consumption by inducing a dysphoric state and making ethanol more attractive for its anxiolytic properties (Holter et al., 2000; Sperling et al., 2010, Walker and Koob, 2008). Alternatively, pharmacological blockade of KOR may also increase drinking behavior by blocking ethanol’s aversive properties (Mitchell et al., 2005; Morales et al., under revision). Although these two hypotheses are not mutually exclusive, the current findings are consistent with the idea that activation of the DYN/KOR system mediates aversive responses and dysphoric states. Further research is needed to examine the effect of stress and pharmacological manipulations of KOR on ethanol self-administration in order to better explore the relationship between these two alternative hypotheses.
4.3 Stress modulates KOR-induced CTA
No significant age differences were apparent in our assessment of stress and KOR agonist-induced CTA. All three doses of U62,066 elicited taste aversions in non-stressed adolescents and adults, aversions that were absent in stress-exposed animals of both ages. Attenuation of CTA to the selective KOR agonist following repeated stress in the present study is likely associated with the effects of stress on the DYN/KOR system, in that stress exposure itself elicits a dysphoric state related to DYN release and subsequent activation of KOR (Bruchas et al., 2010; Nabeshima et al., 1992; Shirayama et al., 2004). The KOR system, already activated by stress, may become insensitive to the activating effects of the exogenously administered KOR agonists, thus resulting in attenuated aversions to the selective kappa agonist.
A previous study conducted in our laboratory using water-deprived subjects found that adolescents were less sensitive to U62,066-induced CTA relative to adults (Anderson et al., under revision). However, lower doses of U62,066 (0.1–0.3 mg/kg) were tested in the previous study, results that suggest that age differences might have emerged if lower doses had been assessed in the present study. Alternatively, the findings from Experiment 2 may suggest that adolescents were more stressed by water deprivation than adults in the earlier study, a possibility that offers an explanation for the age differences in sensitivity to the aversive effects of kappa agonists we initially reported.
Results from Experiment 2 showing that stressed subjects were less sensitive to U62,066 are in opposition to a previous study that reported enhanced sensitivity to U50,488-induced CPA in rats subjected to conditioned fear stress relative to non-stressed controls (Matsuzawa et al., 1999). However, the rats in the earlier study were exposed to fear stress (i.e., placement in a context previously paired with foot shocks) immediately prior to each conditioning session, whereas the five days of restraint stress used in our study occurred before induction of CTA. Along with these procedural differences, the varying stressors used may have contributed to the dissimilar results obtained across these studies, given that different stressors produce distinct behavioral changes (Mercier et al., 2003) and neural alterations (Dayas et al., 2001; Bowers et al., 2008).
4.4 Stress effects on ethanol sensitivity across age
The present findings may help explain the relationship between stress and drug-taking behaviors. It has been hypothesized that stress induces a dysphoric state that enhances the reward value of drugs of abuse, thereby contributing to increased drug-taking behaviors (Wee and Koob, 2010). The results of Experiment 2 suggest a direct modulation of the aversive properties of KOR activation by stressor exposure. Since restraint stress attenuates the aversion associated with KOR activation, stress may directly promote drug-taking behavior by reducing the KOR-mediated aversive and/or dysphoric properties of drugs of abuse. However, our findings from Experiment 1 do not support a role for stress in modulation of ethanol’s aversive properties, but instead suggest a differential role for DYN/KOR system in stressed relative to non-stressed adults, with this system becoming involved in ethanol aversion following stress exposure. Aversive properties of ethanol do not appear to be modulated by the KOR system in adolescents, regardless of stress condition. The extent to which stress-induced KOR activation may mediate aversive and rewarding effects of other drugs of abuse differentially across age remains to be tested.
Stressed subjects consumed less Boost than non-stressed on the conditioning day for both experiments (see Figure 1A, 1C, 2A), a result that is consistent with stress-induced anhedonia (Papp, 2012; Willner, 1997) and suggests that the repeated restraint procedure was a significant stressor at both ages. However, an unexpected pattern of test-day Boost consumption emerged in saline-injected control subjects. For Experiment 2, a trend for reduced consumption among stressed subjects (p = .08) was still evident on the test day in saline-injected adolescents and adults. In Experiment 1, this stress reduction was lost, with adolescents and adults in the saline/saline condition consuming similar amounts of Boost regardless of stress condition. Adults in the nor-BNI/saline group, however, appeared to maintain a similar stress pattern on the conditioning day (although this effect did not reach significance). It is possible that saline/saline control subjects in Experiment 1 may have been more stressed by the i.p. injection on the conditioning day than saline control animals from Experiment 2 who received s.c. saline. As a consequence of this acute stress, these animals may have consumed more Boost on the test day for purposes of stress relief (for discussion of the stress-buffering effects of palatable food/drink consumption, see Foster et al., 2009; Ulrich-Lai et al., 2007; 2010). Accordingly, nor-BNI pretreatment may have blocked this effect in adult controls, with adolescents again insensitive to the effects of the KOR antagonist. Clearly, this suggestion is speculative and requires further investigation. Nevertheless, regardless of the factors influencing the persistence or absence of stress-related decreases in conditioned stimulus consumption in animals challenged with saline on the test day, it should be noted that, for all experiments in the present study, attenuated taste aversions were apparent only in groups where the stressed saline controls tended to consume less Boost on the test day.
Given that social interaction has been shown to attenuate ethanol’s aversive effects (e.g., Gauvin et al., 1994), the possible influence of social context should be considered when interpreting our results. Our laboratory has previously shown that ethanol-induced CTA is attenuated in adolescent males given access to a social partner after ethanol administration relative to males that remained isolated (Vetter-O’Hagen et al., 2009). No effects of peer exposure were evident in adults, suggesting a unique social buffering effect on ethanol’s aversive properties among adolescents. Pair housing may have contributed to the lack of stress effects on ethanol’s aversive effects reported in the present study (and those reported in Anderson et al., 2010). Further exploration of social buffering effects on stress in adolescents relative to adults could address this possibility directly.
Dramatic age differences in adolescent and adult sensitivity to numerous drugs of abuse in a CTA paradigm have been consistently reported and are often accompanied by reports of increased sensitivity to rewarding effects as indexed by greater CPP in adolescents relative to adults (see Badanich et al., 2006; Torres et al, 2008; Philpot et al., 2003), results that corroborate the age differences demonstrated in CTA studies. The adolescent-typical enhanced sensitivity to drug reward may be associated with heightened stress sensitivity during adolescence. Indeed, adolescence is a period of development during which many individuals experience stressors that may result in stress-related activation of the DYN/KOR system (Bruchas et al., 2010; Tejeda et al., 2012). This activation, resulting in dysphoria, could enhance the rewarding value of drugs of abuse and foster higher levels of drug consumption. The present study, however, does not support the involvement of the DYN/KOR system in ethanol’s aversive effects.
Highlights.
Stress had no effect on ethanol-induced CTA in adolescents or adults.
Among stressed adults only, kappa antagonism attenuated ethanol-induced CTA.
Stressed adolescents and adults were less sensitive to kappa agonist-induced CTA.
Acknowledgments
The work presented in this manuscript was funded by grants AA012453 to EIV and grants AA017355 and AA017823 to LPS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adriani W, Chiarotti F, Laviola G. Elevated novelty seeking and peculiar d-amphetamine sensitization in periadolescent mice compared with adult mice. Behav Neurosci. 1998;112:1152–66. doi: 10.1037//0735-7044.112.5.1152. [DOI] [PubMed] [Google Scholar]
- Adriani W, Laviola G. Elevated levels of impulsivity and reduced place conditioning with d-amphetamine: two behavioral features of adolescence in mice. Behav Neurosci. 2003;117:695–703. doi: 10.1037/0735-7044.117.4.695. [DOI] [PubMed] [Google Scholar]
- Anderson RI, Morales M, Spear L, Varlinskaya E. Pharmacological activation of kappa opioid receptors: Aversive effects in adolescent and adult male rats. 2012. Under revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RI, Varlinskaya EI, Spear LP. Ethanol-induced conditioned taste aversion in male Sprague-Dawley rats: Impact of age and stress. Alcohol Clin Exp Res. 2010;34:2106–15. doi: 10.1111/j.1530-0277.2010.01307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett JJ. Adolescent storm and stress, reconsidered. Am Psychol. 1999;54:317–26. doi: 10.1037//0003-066x.54.5.317. [DOI] [PubMed] [Google Scholar]
- Badanich KA, Adler KJ, Kirstein CL. Adolescents differ from adults in cocaine conditioned place preference and cocaine-induced dopamine in the nucleus accumbens septi. Eur J Pharmacol. 2006;550:95–106. doi: 10.1016/j.ejphar.2006.08.034. [DOI] [PubMed] [Google Scholar]
- Bowers SL, Bilbo SD, Dhabhar FS, Nelson RJ. Stressor-specific alterations in corticosterone and immune responses in mice. Brain Behav Immun. 2008;22:105–13. doi: 10.1016/j.bbi.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Land BB, Chavkin C. The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res. 2010;1314:44–55. doi: 10.1016/j.brainres.2009.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Jones RM, Levita L, Libby V, Pattwell SS, Ruberry EJ, Soliman F, Somerville LH. The storm and stress of adolescence: insights from human imaging and mouse genetics. Dev Psychobiol. 2010;52:225–35. doi: 10.1002/dev.20447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavkin C, James IF, Goldstein A. Dynorphin is a specific endogenous ligand of the kappa opioid receptor. Science. 1982;215:413–5. doi: 10.1126/science.6120570. [DOI] [PubMed] [Google Scholar]
- Dayas CV, Buller KM, Crane JW, Xu Y, Day TA. Stressor categorization: acute physical and psychological stressors elicit distinctive recruitment patterns in the amygdala and in medullary noradrenergic cell groups. Eur J Neurosci. 2001;14:1143–52. doi: 10.1046/j.0953-816x.2001.01733.x. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Opposite effects of mu and kappa opiate agonists on dopamine release in the nucleus accumbens and in the dorsal caudate of freely moving rats. J Pharmacol Exp Ther. 1988;244:1067–80. [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Varlinskaya EI, Spear LP. Anxiogenic effects during withdrawal from acute ethanol in adolescent and adult rats. Pharmacol Biochem Behav. 2003;75:411–8. doi: 10.1016/s0091-3057(03)00134-5. [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Varlinskaya EI, Spear LP. Social and non-social anxiety in adolescent and adult rats after repeated restraint. Physiology and Behavior. 2009;97:484–94. doi: 10.1016/j.physbeh.2009.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyon WM, Howard EC, Shippenberg TS, Gonzales RA. Kappa-opioid receptor modulation of accumbal dopamine concentration during operant ethanol self-administration. Neuropharmacology. 2006;51:487–96. doi: 10.1016/j.neuropharm.2006.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiland L, Romeo RD. Stress and the developing adolescent brain. Neuroscience. 2012 doi: 10.1016/j.neuroscience.2012.10.048. http://dx.doi.org/10.1016/j.neuroscience.2012.10.048. [DOI] [PMC free article] [PubMed]
- Foster MT, Warne JP, Ginsberg AB, Horneman HF, Pecoraro NC, Akana SF, Dallman MF. Palatable foods, stress, and energy stores sculpt corticotropin-releasing factor, adrenocorticotropin, and corticosterone concentrations after restraint. Endocrinology. 2009;150:2325–33. doi: 10.1210/en.2008-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk D, Vohra S, Le AD. Influence of stressors on the rewarding effects of alcohol in Wistar rats: studies with alcohol deprivation and place conditioning. Psychopharmacology (Berl) 2004;176:82–7. doi: 10.1007/s00213-004-1859-x. [DOI] [PubMed] [Google Scholar]
- Gauvin DV, Briscoe RJ, Goulden KL, Holloway FA. Aversive attributes of ethanol can be attenuated by dyadic social interaction in the rat. Alcohol. 1994;11:247–51. doi: 10.1016/0741-8329(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse. 1997;9:103–10. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Hartup WW, Stevens N. Friendships and adaptation in the life course. Psychological Bulletin. 1997;121:355–370. [Google Scholar]
- Holson RR, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicol Teratol. 1992;14:221–8. doi: 10.1016/0892-0362(92)90020-b. [DOI] [PubMed] [Google Scholar]
- Holter SM, Henniger MS, Lipkowski AW, Spanagel R. Kappa-opioid receptors and relapse-like drinking in long-term ethanol-experienced rats. Psychopharmacology (Berl) 2000;153:93–102. doi: 10.1007/s002130000601. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national results on adolescent drug use: Overview of key findings, 2011. Ann Arbor: Institute for Social Research, The University of Michigan; 2012. [Google Scholar]
- Kreibich AS, Briand L, Cleck JN, Ecke L, Rice KC, Blendy JA. Stress-induced potentiation of cocaine reward: a role for CRF R1 and CREB. Neuropsychopharmacology. 2009;34:2609–17. doi: 10.1038/npp.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviola G, Macri S, Morley-Fletcher S, Adriani W. Risk-taking behavior in adolescent mice: psychobiological determinants and early epigenetic influence. Neurosci Biobehav Rev. 2003;27:19–31. doi: 10.1016/s0149-7634(03)00006-x. [DOI] [PubMed] [Google Scholar]
- Linakis JG, Cunningham CL. Effects of concentration of ethanol injected intraperitoneally on taste aversion, body temperature, and activity. Psychopharmacology. 1979;64:61–65. doi: 10.1007/BF00427346. [DOI] [PubMed] [Google Scholar]
- Lindholm S, Werme M, Brene S, Franck J. The selective kappa-opioid receptor agonist U50,488H attenuates voluntary ethanol intake in the rat. Behav Brain Res. 2001;120:137–46. doi: 10.1016/s0166-4328(00)00368-5. [DOI] [PubMed] [Google Scholar]
- Logrip ML, Janak PH, Ron D. Blockade of ethanol reward by the kappa opioid receptor agonist U50,488H. Alcohol. 2009;43:359–65. doi: 10.1016/j.alcohol.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzawa S, Suzuki T, Misawa M. Conditioned fear stress induces ethanol-associated place preference in rats. Eur J Pharmacol. 1998;341:127–30. doi: 10.1016/s0014-2999(97)01456-8. [DOI] [PubMed] [Google Scholar]
- Matsuzawa S, Suzuki T, Misawa M, Nagase H. Different roles of mu-, delta- and kappa-opioid receptors in ethanol-associated place preference in rats exposed to conditioned fear stress. Eur J Pharmacol. 1999;368:9–16. doi: 10.1016/s0014-2999(99)00008-4. [DOI] [PubMed] [Google Scholar]
- McLaughlin JP, Land BB, Li S, Pintar JE, Chavkin C. Prior activation of kappa opioid receptors by U50,488 mimics repeated forced swim stress to potentiate cocaine place preference conditioning. Neuropsychopharmacology. 2006;31:787–94. doi: 10.1038/sj.npp.1300860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier S, Frederic, Canini, Buguet A, Cespuglio R, Martin S, Bourdon L. Behavioural changes after an acute stress: stressor and test types influences. Behav Brain Res. 2003;139:167–75. doi: 10.1016/s0166-4328(02)00265-6. [DOI] [PubMed] [Google Scholar]
- Metcalf MD, Coop A. Kappa opioid antagonists: past successes and future prospects. AAPS J. 2005;7:E704–22. doi: 10.1208/aapsj070371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JM, Liang MT, Fields HL. A single injection of the kappa opioid antagonist norbinaltorphimine increases ethanol consumption in rats. Psychopharmacology. 2005;182:384–92. doi: 10.1007/s00213-005-0067-7. [DOI] [PubMed] [Google Scholar]
- Morales M, Anderson RI, Spear L, Varlinskaya E. Effects of the kappa opioid receptor antagonist, nor-binaltorphimine, on ethanol intake: impact of age and sex. 2012. Under revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucha RF, Herz A. Motivational properties of kappa and mu opioid receptor agonists studied with place and taste preference conditioning. Psychopharmacology. 1985;86:274–80. doi: 10.1007/BF00432213. [DOI] [PubMed] [Google Scholar]
- Nabeshima T, Katoh A, Wada M, Kameyama T. Stress-induced changes in brain Met-enkephalin, Leu-enkephalin and dynorphin concentrations. Life Sci. 1992;51:211–7. doi: 10.1016/0024-3205(92)90077-3. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals. 8. Washington, DC: The National Academies Press; 2011. [Google Scholar]
- Nguyen K, Tseng A, Marquez P, Hamid A, Lutfy K. The role of endogenous dynorphin in ethanol-induced state-dependent CPP. Behav Brain Res. 2012;227:58–63. doi: 10.1016/j.bbr.2011.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp M. Models of affective illness: chronic mild stress in the rat. Curr Protoc Pharmacol. 2012;Chapter 5(Unit 5):9. doi: 10.1002/0471141755.ph0509s57. [DOI] [PubMed] [Google Scholar]
- Papp M, Willner P, Muscat R. An animal model of anhedonia: attenuation of sucrose consumption and place preference conditioning by chronic unpredictable mild stress. Psychopharmacology (Berl) 1991;104:255–9. doi: 10.1007/BF02244188. [DOI] [PubMed] [Google Scholar]
- Philpot RM, Badanich KA, Kirstein CL. Place conditioning: age-related changes in the rewarding and aversive effects of alcohol. Alcohol Clin Exp Res. 2003;27:593–9. doi: 10.1097/01.ALC.0000060530.71596.D1. [DOI] [PubMed] [Google Scholar]
- Primus RJ, Kellogg CK. Pubertal-related changes influence the development of environment-related social interaction in the male rat. Dev Psychobiol. 1989;22:633–43. doi: 10.1002/dev.420220608. [DOI] [PubMed] [Google Scholar]
- Riley AL. The paradox of drug taking: the role of the aversive effects of drugs. Physiol Behav. 2011;103:69–78. doi: 10.1016/j.physbeh.2010.11.021. [DOI] [PubMed] [Google Scholar]
- Roma PG, Rinker JA, Serafine KM, Chen SA, Barr CS, Cheng K, Rice KC, Riley AL. Genetic and early environmental contributions to alcohol’s aversive and physiological effects. Pharmacol Biochem Behav. 2008;91:134–9. doi: 10.1016/j.pbb.2008.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo RD, McEwen BS. Stress and the adolescent brain. Ann N Y Acad Sci. 2006;1094:202–14. doi: 10.1196/annals.1376.022. [DOI] [PubMed] [Google Scholar]
- Rozeske RR, Greenwood BN, Fleshner M, Watkins LR, Maier SF. Voluntary wheel running produces resistance to inescapable stress-induced potentiation of morphine conditioned place preference. Behav Brain Res. 2011;219:378–81. doi: 10.1016/j.bbr.2011.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, DiFeliceantonio AG, Foscue E, Glowacz S, Haseeb N, Wang N, Zhou C, Kuhn CM. Aversive effects of ethanol in adolescent versus adult rats: potential causes and implication for future drinking. Alcohol Clin Exp Res. 2010;34:2061–9. doi: 10.1111/j.1530-0277.2010.01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Walker QD, Caster JM, Levin ED, Kuhn CM. Are adolescents more vulnerable to drug addiction than adults? Evidence from animal models. Psychopharmacology (Berl) 2009;206:1–21. doi: 10.1007/s00213-009-1585-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzer C. 30 years of dynorphins--new insights on their functions in neuropsychiatric diseases. Pharmacol Ther. 2009;123:353–70. doi: 10.1016/j.pharmthera.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shippenberg TS, Zapata A, Chefer VI. Dynorphin and the pathophysiology of drug addiction. Pharmacol Ther. 2007;116:306–21. doi: 10.1016/j.pharmthera.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirayama Y, Ishida H, Iwata M, Hazama GI, Kawahara R, Duman RS. Stress increases dynorphin immunoreactivity in limbic brain regions and dynorphin antagonism produces antidepressant-like effects. J Neurochem. 2004;90:1258–68. doi: 10.1111/j.1471-4159.2004.02589.x. [DOI] [PubMed] [Google Scholar]
- Smith JS, Schindler AG, Martinelli E, Gustin RM, Bruchas MR, Chavkin C. Stress-induced activation of the dynorphin/kappa-opioid receptor system in the amygdala potentiates nicotine conditioned place preference. J Neurosci. 2012;32:1488–95. doi: 10.1523/JNEUROSCI.2980-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–63. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP. The Behavioral Neuroscience of Adolescence. W. W. Norton, W. W. Norton; 2010. [Google Scholar]
- Spear LP. Rewards, aversions and affect in adolescence: emerging convergences across laboratory animal and human data. Dev Cogn Neurosci. 2011;1:392–400. doi: 10.1016/j.dcn.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RE, Gomes SM, Sypek EI, Carey AN, McLaughlin JP. Endogenous kappa-opioid mediation of stress-induced potentiation of ethanol-conditioned place preference and self-administration. Psychopharmacology (Berl) 2010;210:199–209. doi: 10.1007/s00213-010-1844-5. [DOI] [PubMed] [Google Scholar]
- Tejeda HA, Shippenberg TS, Henriksson R. The dynorphin/kappa-opioid receptor system and its role in psychiatric disorders. Cell Mol Life Sci. 2012;69:857–96. doi: 10.1007/s00018-011-0844-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres OV, Tejeda HA, Natividad LA, O’Dell LE. Enhanced vulnerability to the rewarding effects of nicotine during the adolescent period of development. Pharmacol Biochem Behav. 2008;90:658–63. doi: 10.1016/j.pbb.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Christiansen AM, Ostrander MM, Jones AA, Jones KR, Choi DC, Krause EG, Evanson NK, Furay AR, Davis JF, Solomon MB, de Kloet AD, Tamashiro KL, Sakai RR, Seeley RJ, Woods SC, Herman JP. Pleasurable behaviors reduce stress via brain reward pathways. Proc Natl Acad Sci U S A. 2010;107:20529–34. doi: 10.1073/pnas.1007740107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Ostrander MM, Thomas IM, Packard BA, Furay AR, Dolgas CM, Van Hooren DC, Figueiredo HF, Mueller NK, Choi DC, Herman JP. Daily limited access to sweetened drink attenuates hypothalamic-pituitary-adrenocortical axis stress responses. Endocrinology. 2007;148:1823–34. doi: 10.1210/en.2006-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valverde O, Smadja C, Roques BP, Maldonado R. The attenuation of morphine-conditioned place preference following chronic mild stress is reversed by a CCKB receptor antagonist. Psychopharmacology (Berl) 1997;131:79–85. doi: 10.1007/s002130050268. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Doremus-Fitzwater TL, Spear LP. Repeated restraint stress alters sensitivity to the social consequences of ethanol in adolescent and adult rats. Pharmacol Biochem Behav. 2010;96:228–35. doi: 10.1016/j.pbb.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Acute effects of ethanol on social behavior of adolescent and adult rats: role of familiarity of the test situation. Alcohol Clin Exp Res. 2002;26:1502–11. doi: 10.1097/01.ALC.0000034033.95701.E3. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Social interactions in adolescent and adult Sprague-Dawley rats: impact of social deprivation and test context familiarity. Behav Brain Res. 2008;188:398–405. doi: 10.1016/j.bbr.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Increases in anxiety-like behavior induced by acute stress are reversed by ethanol in adolescent but not adult rats. Pharmacol Biochem Behav. 2012;100:440–50. doi: 10.1016/j.pbb.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter-O’Hagen C, Varlinskaya E, Spear L. Sex differences in ethanol intake and sensitivity to aversive effects during adolescence and adulthood. Alcohol Alcohol. 2009;44:547–54. doi: 10.1093/alcalc/agp048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter-O’Hagen CS, Spear LP. Hormonal and physical markers of puberty and their relationship to adolescent-typical novelty-directed behavior. Dev Psychobiol. 2011 doi: 10.1002/dev.20610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Valdez GR, McLaughlin JP, Bakalkin G. Targeting dynorphin/kappa opioid receptor systems to treat alcohol abuse and dependence. Alcohol. 2012;46:359–70. doi: 10.1016/j.alcohol.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Koob GF. Pharmacological evidence for a motivational role of kappa-opioid systems in ethanol dependence. Neuropsychopharmacology. 2008;33:643–52. doi: 10.1038/sj.npp.1301438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Koob GF. The role of the dynorphin-kappa opioid system in the reinforcing effects of drugs of abuse. Psychopharmacology (Berl) 2010;210:121–35. doi: 10.1007/s00213-010-1825-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AM, Truesdale MC, Bae JG, Ahmad S, Wilson WA, Best PJ, Swartzwelder HS. Differential effects of ethanol on motor coordination in adolescent and adult rats. Pharmacol Biochem Behav. 2002;73:673–7. doi: 10.1016/s0091-3057(02)00860-2. [DOI] [PubMed] [Google Scholar]
- Will MJ, Watkins LR, Maier SF. Uncontrollable stress potentiates morphine’s rewarding properties. Pharmacol Biochem Behav. 1998;60:655–64. doi: 10.1016/s0091-3057(98)00027-6. [DOI] [PubMed] [Google Scholar]
- Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology (Berl) 1997;134:319–29. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]
- Winzer-Serhan UH, Chen Y, Leslie FM. Expression of opioid peptides and receptors in striatum and substantia nigra during rat brain development. J Chem Neuroanat. 2003;26:17–36. doi: 10.1016/s0891-0618(03)00031-0. [DOI] [PubMed] [Google Scholar]
- Zapata A, Shippenberg TS. Endogenous kappa opioid receptor systems modulate the responsiveness of mesoaccumbal dopamine neurons to ethanol. Alcohol Clin Exp Res. 2006;30:592–7. doi: 10.1111/j.1530-0277.2006.00069.x. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP. Multiparous species present problems (and possibilities) to developmentalists. Developmental Psychobiology. 1997;30:141–150. doi: 10.1002/(sici)1098-2302(199703)30:2<141::aid-dev5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]