Abstract
Background
Primary prophylaxis with granulocyte colony–stimulating factors (pp-g-csf) is recommended in patients undergoing chemotherapy carrying a febrile neutropenia (fn) risk of 20% or more. In the present study, we examined clinical practice patterns and the impact of pp-g-csf on fn incidence in women with early-stage breast cancer (ebc) treated with modern adjuvant chemotherapy (act).
Methods
This single-centre retrospective cohort study of women with ebc, who were identified from the pharmacy database and who received at least 1 cycle of modern act from January 2009 to December 2011, was conducted at the Cancer Centre of Southeastern Ontario. Data on patient demographics, pathology, stage distribution, chemotherapy, pp-g-csf use, dose reductions, chemotherapy delays, treatment discontinuation, relative dose intensity, and fn events were collected. Chi-square tests, t-tests, univariate and multivariate logistic regression analyses, and nonparametric Mann–Whitney U-tests were used for data analysis.
Results
Of the 239 women eligible for analysis, 145 (61%) received pp-g-csf, and 50 (21%) developed at least 1 episode of fn. Use of pp-g-csf was associated with a significantly lower rate of fn (14% vs. 31%, p = 0.002) and trends to fewer dose delays (17% vs. 27%, p = 0.060) and dose reductions (19% vs. 25%, p = 0.28). Among women receiving pp-g-csf, higher fn rates were associated with an age of 65 years or older, taxane-based chemotherapy, and prophylaxis with filgrastim
Conclusions
Clinical practice patterns at our institution showed that more than 50% of ebc patients treated with modern act received pp-g-csf, which led to fewer fn episodes and increased delivery of planned act. The observed high fn risk despite pp-g-csf was linked to older age, taxane-based chemotherapy, and filgrastim.
Keywords: Adjuvant chemotherapy, early-stage breast cancer, febrile neutropenia, filgrastim, pegfilgrastim, practice patterns, primary prophylaxis with granulocyte colony-stimulating factors
1. INTRODUCTION
Breast cancer is the most common cancer in the female population and the second leading cause of cancer-related death in women in Canada1. Systemic adjuvant chemotherapy (act) with anthracycline- or taxane-containing regimens has substantially improved survival rates for breast cancer patients2,3. However, improvements in outcome are compromised by the considerable toxicities associated with such therapies, most notably an intermediate-to-high risk of febrile neutropenia (fn)4.
Febrile neutropenia is a serious and sometimes life-threatening complication of act5–7. It can lead to delays and dose reductions in chemotherapy treatment5,8, thereby potentially compromising the efficacy of chemotherapy and, subsequently, patient outcome. Deviation from the planned dose intensity of act significantly affects survival rates in women with early-stage breast cancer (ebc), and higher disease-free survival and overall survival were reported in women receiving 85% or more of the planned dose intensity compared with those receiving less9.
Evidence-based guidelines published by the American Society of Clinical Oncology10, the National Comprehensive Cancer Network11, and the European Organisation for Research and Treatment of Cancer12 state that primary prophylaxis with white blood cell growth factors is recommended when the overall fn risk is 20% or greater; however, specific guidance on the type of granulocyte colony–stimulating factor (g-csf) formulation is not provided. The use of g-csfs such as filgrastim (Neupogen: Amgen, Thousand Oaks, CA, U.S.A.)13 and pegfilgrastim (Neulasta: Amgen)14,15 is recommended to reduce the incidence and severity of myelotoxicity, with the aim of maintaining dose intensity. Prophylactic use of g-csf initiated 24 hours after chemotherapy completion was reported to reduce the severity and duration of neutropenia, the incidence of fn, and the associated mortality, morbidity, and cost10,16–18.
Although the risk of fn for several chemotherapy regimens has been widely described within the context of clinical trials, only a few studies have described the occurrence of fn in clinical practice19–23. Interestingly, the reported incidence of fn associated with specific chemotherapy regimens is higher in clinical practice than in clinical trials19,21,23–25. Likewise, although international guidelines are clear on the use of g-csf prophylaxis, data on such use in clinical practice remain scarce.
In the present study, we aimed to evaluate the local practice patterns of primary prophylaxis with g-csf (pp-g-csf) in patients with ebc receiving modern act and the associated rate of fn. We also analyzed the effects of pp-g-csf on chemotherapy delivery and evaluated potential predictors of higher fn incidence in women receiving pp-g-csf.
2. METHODS
2.1. Study Design
This single-centre retrospective cohort study was conducted at the Cancer Centre of Southeastern Ontario after approval by the local Research Ethics Board. Patient informed consent was not required.
2.2. Patients
Women with ebc treated with modern act (taxane-containing regimens with or without anthracyclines) from January 2009 to December 2011, were identified from the pharmacy database at Cancer Centre of Southeastern Ontario. Women who had received neoadjuvant or palliative chemotherapy were excluded. All patients who had received at least 1 cycle of act were included.
2.3. Intervention
In patients with breast cancer positive for the human epidermal growth factor receptor 2 (her2), trastuzumab was given concurrently with docetaxel. The decision for starting prophylaxis with g-csf, and the choice of g-csf agent was made by the treating physician and depended on the funding source. Patients who did not receive pp-g-csf received secondary prophylaxis if they developed fn or required at least a 1-week dose delay because of neutropenia (per Cancer Care Ontario guidelines)26. The g-csf agents used in this cohort were filgrastim (average: once-daily injection for 7 days; range: 5–10 days) and pegfilgrastim (single dose). Filgrastim was administered for a median of 7 days (range: 4–10 days), and pegfilgrastim, at 24 hours after chemotherapy.
2.4. Data Collection
Data were obtained from patient charts and electronic medical records. A data collection form was developed to collect patient demographics, pathology, stage distribution, chemotherapy details, use of pp-g-csf, subsequent dose reductions, chemotherapy delays, treatment discontinuation, relative dose intensity (rdi), and fn events.
2.5. Definitions
Febrile neutropenia was defined as an absolute neutrophil count below 0.5×109/L, together with fever. Fever was defined as a temperature exceeding 38.3°C or a sustained temperature exceeding 38°C for more than 1 hour. Patients experiencing these symptoms were instructed to seek emergency medical assessment either at the host hospital or at a community hospital. Any episode of fn that occurred at least 24 hours after the first dose of g-csf was described as “fn despite pp-g-csf.” Chemotherapy dose delay was defined as a delay in planned chemotherapy of 1 week or more, and chemotherapy dose reduction was defined as a 15% or greater reduction in the planned dose. Relative dose intensity for a combination regimen was defined as the average quantity of drugs delivered over a specific time interval (expressed as a percentage) relative to the standard quantity and was calculated using the formula (dose received / dose planned) / (actual cycle days / planned cycle days).
Because an rdi of less than 85% is associated with compromised outcomes and survival, we compared patients having a rdi of less than 85% with patients having an rdi of 85% or greater.
2.6. Statistical Analysis
Data were initially analyzed descriptively (frequencies and percentages for categorical data, and median and range for age) using IBM SPSS (version 20.0 for Windows: IBM, Armonk, NY, U.S.A.). Characteristics of patients who did and did not receive pp-g-csf were compared using chi-square tests (Pearson or Fisher, as appropriate) and a t-test for age. For subsequent analyses, patients were grouped according to age: less than 65 years of age and 65 years of age or more. The associations of primary g-csf use with fn incidence and with chemotherapy modifications were assessed using chi-square tests (Pearson or Fisher, as appropriate). The rdi was not normally distributed. Therefore, in addition to using chi-square tests to compare patients with a rdi of less than 85% and of 85% or more, values for the groups were also compared using the nonparametric Mann–Whitney U-test.
3. RESULTS
3.1. Patients
Using the pharmacy database, we identified 293 breast cancer patients who received modern act. Of those patients, 239 women met the inclusion criteria and were eligible for analysis. The 54 patients excluded were ineligible for these reasons: neoadjuvant therapy (n = 24), adjuvant therapy with older regimens (alkylating agents, n = 3; anthracycline alone, n = 18), missing charts (n = 7), metastatic cancer (n = 1), and male sex (n = 1). Median age in the eligible cohort was 55 years (range: 32–80 years), and 197 patients (82%) were less than 65 years of age. More than half the women (62%) were postmenopausal (Table i). Baseline characteristics were similar in patients who did and did not receive pp-g-csf.
TABLE I.
Patient, tumour, and treatment characteristics
| Characteristic |
Patient group
|
p Valuea | ||
|---|---|---|---|---|
| Overall | Primary g-csf prophylaxis | |||
| Yes | No | |||
| Patients (n) | 239 | 145 | 94 | |
| Age (years) | ||||
| Median | 55 | 55 | 55 | 0.67 |
| Range | 32–80 | 32–75 | 33–80 | |
| Age ≥65 years [n (%)] | 42 (18) | 26 (18) | 16 (17) | 0.86 |
| Menopausal status [n (%)] | ||||
| Postmenopausal | 148 (62) | 96 (66) | 52 (55) | 0.09 |
| Pathology subtype [n (%)] | ||||
| Ductal | 211 (88) | 130 (90) | 81 (86) | 0.72 |
| Grade iii | 135 (56) | 90 (62) | 45 (48) | 0.10 |
| Receptor status [n (%)] | ||||
| er- or pr-positive | 164 (69) | 106 (73) | 58 (62) | 0.064 |
| her2-positive | 52 (22) | 26 (18) | 26 (28) | 0.06 |
| Triple negative | 52 (22) | 35 (24) | 17 (18) | 0.27 |
| Stage [n (%)] | ||||
| i | 53 (22) | 28 (19) | 25 (27) | 0.11 |
| ii | 127 (53) | 81 (56) | 46 (49) | |
| iii | 53 (22) | 30 (21) | 23 (24) | |
| Unknown | 6 (3) | 6 (4) | 0 (0) | |
| Type of surgery [n (%)] | ||||
| Breast-conservingb | 162 (68) | 100 (69) | 62 (66) | 0.63 |
| Mastectomyc | 77 (32) | 45 (31) | 32 (34) | |
| Chemotherapy agents [n (%)] | ||||
| Anthracycline-taxane regimensd | 212 (89) | 125 (86) | 87 (93) | 0.130 |
| Taxane only regimense | 27 (11) | 20 (14) | 7 (7) | |
| Trastuzumab-based therapy [n (%)] | 51 (21) | 25 (17) | 26 (28) | 0.055 |
Based on the Pearson or Fisher exact chi-square test and the t-test (Age).
Partial mastectomy, lumpectomy, or excisional biopsy with or without lymph node dissection.
Simple and modified radical mastectomy.
fec/d (5-fluorouracil–epirubicin–cyclophosphamide followed by docetaxel), n = 211; ac-t (doxorubicin–cyclophosphamide–paclitaxel), n = 1.
tc (docetaxel–cyclophosphamide), n = 26; tch (docetaxel–platinum and trastuzumab), n = 1. g-csf = granulocyte colony–stimulating factor; er = estrogen receptor; pr = progesterone receptor; her2 = human epidermal growth factor receptor 2.
3.2. Tumour Characteristics
In the study cohort, 162 women (68%) underwent breast-conserving surgery. The most common pathologic subtype of breast cancer was invasive ductal carcinoma (n = 211, 88%), followed by lobular carcinoma (n = 15, 6%). The other breast cancer subtypes included mixed, metaplastic, and mucinous histology (n = 13, 5%). High-grade (grade iii), grades i and ii, and unknown-grade tumours were present in 135 (56%), 102 (43%), and 2 (1%) patients respectively. Tumours 2 cm or greater in size were found in 93 patients (39%). Node-negative disease was noted in 109 patients (46%). In this cohort, 164 tumours (69%) were estrogen or progesterone-receptor positive (or both), 52 (22%) were her2-positive, and 52 (22%) were triple-negative. The stage distribution was as follows: stage i, 22%; stage ii, 53%; stage iii, 22%; and unknown, 3% (Table i).
3.3. Chemotherapy Regimens
We evaluated 1364 chemotherapy cycles in the selected patients. Because of the occurrence of fn, 2 patients (1%) received only 1 cycle of act; 204 patients (85%) received all planned cycles. Of the 239 women, 212 (89%) received an anthracycline–taxane regimen, and 27 (11%) received a taxane-only regimen. The most common chemotherapy was fec/d (5-fluorouracil–epirubicin–cyclophosphamide, followed by docetaxel), which was administered to 211 patients (88%); 51 patients (21%) received trastuzumab therapy (Table i).
3.4. Impact of G-CSF Prophylaxis on FN
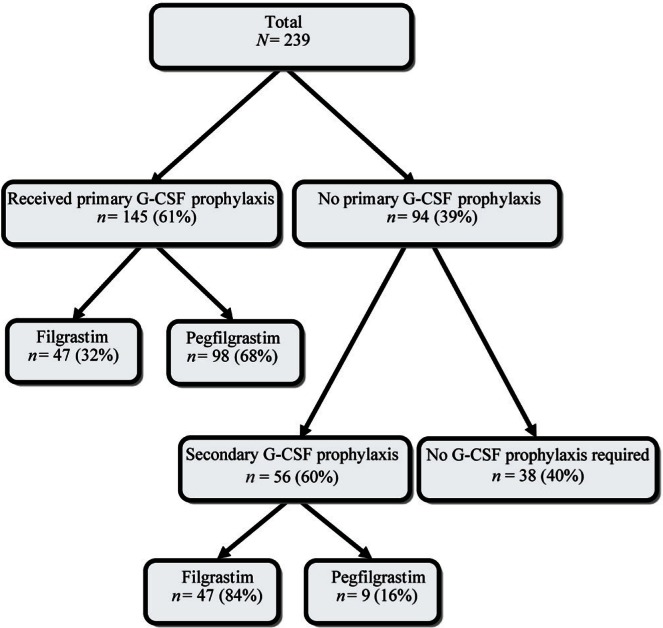
At some point in the course of chemotherapy, 201 patients (84%) received g-csf: 145 (72%) received g-csf as primary prophylaxis, and 56 (28%) received g-csf as secondary prophylaxis (Figure 1). In 107 patients (53%), the g-csf given was pegfilgrastim; in 94 (47%), it was filgrastim. At least 1 episode of fn occurred in 50 patients (21%). A significantly higher incidence of fn was observed among patients who did not receive pp-g-csf (n = 29, 31%) compared with those who did (n = 21, 14%; p = 0.002). In the 29 patients who did not receive pp-g-csf, 13 (45%) and 9 (31%) experienced fn events after the 1st and 4th cycles of chemotherapy respectively. On the other hand, of the 21 patients who experienced fn episodes despite pp-g-csf, 13 (62%) experienced an episode after the 1st cycle of chemotherapy.
FIGURE 1.
Prophylaxis with granulocyte colony–stimulating factor (g-csf) in clinical practice.
3.5. Impact of G-CSF Prophylaxis on Chemotherapy Delivery and RDI
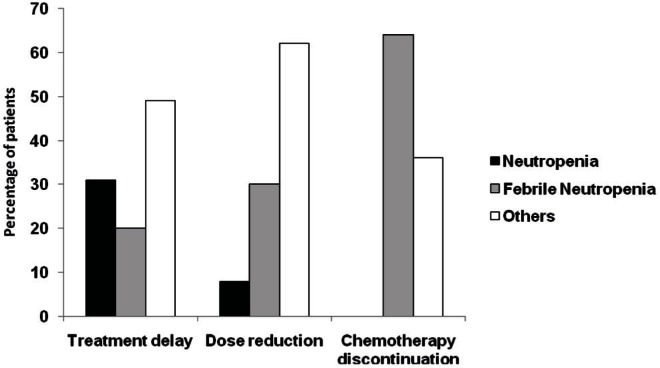
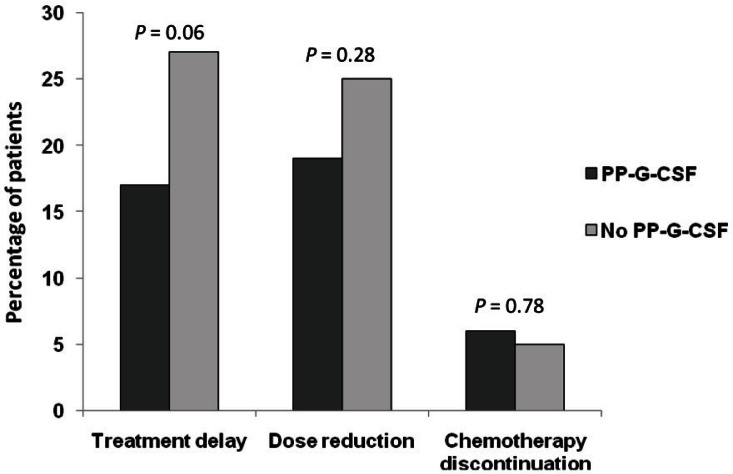
Across all chemotherapy cycles, a median of 6 cycles (range: 1–8 cycles) were delivered. Chemotherapy was delayed in 21% of patients, the dose was reduced in 21%, and chemotherapy was discontinued in 6% of patients. Chemotherapy discontinuation was mostly a consequence of fn (Figure 2). Dose reduction and treatment delay, although also affected by neutropenia or fn, were mostly a result of other toxicities associated with chemotherapy (Figure 2). Patients who received pp-g-csf showed a trend to less dose delay (17% vs. 27%, p = 0.060) and dose reduction (19% vs. 25%, p = 0.28) than was seen in patients who did not receive pp-g-csf; however, those differences were not statistically significant (Figure 3). Discontinuation of chemotherapy was not significantly affected by pp-g-csf (Figure 3).
FIGURE 2.
Reasons for alterations to the planned adjuvant chemotherapy.
FIGURE 3.
Modifications in the planned adjuvant chemotherapy in patients receiving and not receiving primary prophylaxis with granulocyte colony–stimulating factor (pp-g-csf).
The mean rdi was 97% (range: 60%–117%) for the fec/d regimen and 91% (range: 25%–100%) for the taxane–anthracycline regimen. Table ii shows the impact of pp-g-csf on the rdi for patients given fec/d. Patients who received pp-g-csf (98%; range: 75%–117%) received a significantly higher mean rdi of their chemotherapy than did those who were not given pp-g-csf (95%; range: 60%–100%; p = 0.005). Overall, of the 211 patients who received fec/d in the present study, 5% did not achieve 85% of their planned dose intensity, mainly owing to neutropenia or fn. However, of the 120 patients who received pp-g-csf, 97% achieved 85% or more of their planned dose intensity, compared with 92% of the 80 who did not receive pp-g-csf (p = 0.118)—a result that is, however, not statistically significant.
TABLE II.
Impact of primary granulocyte colony–stimulating factor prophylaxis on chemotherapy dose intensity
| Relative dose intensity |
Patient group
|
p Valuea | ||
|---|---|---|---|---|
| Overall | Primary g-csf prophylaxis | |||
| Yes | No | |||
| Received fec/d regimen (n) | 211 | 124 | 87 | |
| Mean (%) | 97 | 98 | 95 | 0.005 |
| Range (%) | 60–117 | 75–117 | 60–100 | |
| ≥85% [n (%)] | 200 (95) | 120 (97) | 80 (92) | 0.118 |
| <85% [n (%)] | 11 (5) | 4 (3) | 7 (8) | |
Based on the Mann–Whitney U-test (continuous data) or the Fisher exact chi-square test.
g-csf = granulocyte colony–stimulating factor; fec/d = 5-fluorouracil–epirubicin–cyclophosphamide followed by docetaxel.
3.6. Predictors of High FN Incidence
To determine the factors that might be associated with higher fn rates in patients who received pp-g-csf, we conducted univariate and multivariate analyses (Table iii). The three variables that showed a significant association with fn in univariate analysis (age, chemotherapy regimen, and primary g-csf type) were assessed using univariate and multivariate logistic regression to calculate odds ratios (ors) and 95% confidence intervals (cis). Higher fn rates were noted in univariate regression in patients 65 years of age and older compared with younger patients (27% vs. 12%; or: 2.8; p = 0.053), but the difference was nonsignificant in multivariate analysis (p = 0.34). Patients receiving taxane-only regimens also had higher fn rates than did those receiving anthracycline–taxane regimens (30% vs. 12%; or: 3.1; p = 0.041), which fell just short of significance in multivariate regression (p = 0.067). In addition, patients receiving pp-g-csf with pegfilgrastim had a fn incidence of 8% compared with 28% in patients receiving filgrastim (or: 4.3; p = 0.003), a difference that remained highly significant in multivariate analysis (p = 0.006).
TABLE III.
Univariate and multivariate logistic regression analyses associated with rate of febrile neutropenia in 145 patients despite primary prophylaxis with granulocyte colony–stimulating factor (g-csf)
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| or | 95% ci | p Value | or | 95% ci | p Value | |
| Age | ||||||
| <65 Years | Reference | |||||
| ≥65 Years | 2.8 | 1.0 to 7.7 | 0.053 | 1.7 | 0.6 to 5.3 | 0.34 |
| Type of chemotherapy | ||||||
| Anthracycline–taxane | Reference | |||||
| Taxane-only | 3.1 | 1.0 to 9.4 | 0.041 | 3.0 | 0.9 to 9.8 | 0.067 |
| Type of primary g-csf prophylaxis | ||||||
| Pegfilgrastim | Reference | |||||
| Filgrastim | 4.3 | 1.6 to 11.3 | 0.003 | 4.1 | 1.5 to 11.1 | 0.006 |
or = odds ratio; ci = confidence interval.
4. DISCUSSION
Existing literature shows that the incidence of fn observed in clinical trials is different from that seen in clinical practice22–25,27. Consistent with such reports, we show that the incidence of fn in “real life” is higher than that reported in clinical trials. In this retrospective review, more than half the ebc patients (61%) treated with modern act received pp-g-csf, most commonly pegfilgrastim. A 50% reduction in the fn rate was noted in women who received pp-gcsf compared with women who did not receive such prophylaxis. Unexpectedly, despite pp-g-csf, the risk of fn in women receiving prophylaxis was still high at 14%. This particular risk was associated with older age, taxane-based chemotherapy, and administration of filgrastim. A trend toward improved rdi for planned chemotherapy was seen with pp-g-csf. Consistently, another retrospective study by Fraser et al. suggested that pp-g-csf alone was not sufficient to achieve a greater-than-85% dose intensity and that prevention and treatment of other toxicities was necessary27.
The overall incidence of fn observed in the present retrospective study was comparable to that in a previous report22, but higher than the incidence reported in clinical trials28,29 and, as might be expected, significantly higher in patients who did not receive pp-g-csf than in those who did. In the present study, patients who developed fn despite primary prophylaxis did so mostly after their 1st cycle of chemotherapy (as did those who did not receive primary prophylaxis), suggesting that this group of patients was at a high risk of developing fn. Assessment of an individual’s inherent risk for developing fn can determine the appropriate use of prophylactic g-csf. The risk of developing fn has been linked to several factors, including tumour type, chemotherapy regimen, and patient-related risk factors such as older age, comorbidities, performance status, and other factors that can lead to increased complications from prolonged neutropenia30,31. In addition, patients experiencing 1 episode of fn are at a significant risk of subsequent episodes, particularly after an episode of neutropenia that is severe and prolonged8, and it is therefore essential that the risk be assessed at each cycle.
Current guidelines indicate the use of g-csf prophylaxis in patients with a 20% or greater fn risk11,12,32; however, for patients with characteristics that increase the overall risk of fn, it may be pertinent to consider primary prophylaxis even though the risk of fn is not above 20%. In the present study, the incidence of fn in the subset of patients receiving pp-g-csf was 14% compared with the 31% seen in those who did not receive primary prophylaxis. That finding suggests that the absence of primary prophylaxis can lead to a high fn risk (>20%) and, therefore, that provision of pp-g-csf should be given serious consideration. Breakthrough fn, or fn despite g-csf prophylaxis, is another factor that should be taken into account. We found that older age, taxane-based therapy, and use of filgrastim were predictors of fn incidence in women receiving pp-g-csf. Although a number of studies have demonstrated the effectiveness of g-csf in preventing fn in patients receiving act, the evidence available to identify patients at risk of developing breakthrough fn is very limited. One study33 reported breakthrough fn in 4.5% of lymphoma patients at the 1st cycle of their chemotherapy regimen and in 13.6% of patients across all cycles. In that study, administration of chemotherapy every 21 days and positive blood cultures in the patients were factors strongly associated with breakthrough fn.
Considering the overall results, further work is necessary to identify patient cohorts vulnerable to breakthrough episodes of fn despite prophylaxis, and to define appropriate treatment guidelines for those patients. In the present study, more than half the patients received pp-g-csf. Consistent with previous studies, we observed that, in addition to a lower incidence of fn, patients receiving g-csf also showed a trend to fewer dose delays or dose reductions of planned chemotherapy and improvement in rdi34,35. That finding is in keeping with results in other studies.
Although current guidelines are clear in their recommendations of g-csf use for women at risk of fn, they lack specific guidance on which g-csf to use. In the present study, the choice of g-csf treatment, although made at the discretion of the treating physician, is reflective mostly of the funding source. Costs for patients receiving primary prophylaxis with a single dose of pegfilgrastim were covered by private third-party health insurance. On the other hand, costs for secondary prophylaxis, which consisted mostly of an average of 7 once-daily injections of filgrastim, were covered through provincial funding by the Ontario Health Insurance Plan (not available for pegfilgrastim).
Previous studies have suggested differences in efficacy between the various g-csf products36. Although some clinical trials have demonstrated equivalent efficacy for pegfilgrastim and filgrastim, those studies had small sample sizes and lacked the statistical power to demonstrate superior outcome for one therapy over the other14,15,37. However, a recent meta-analysis of five studies comparing pegfilgrastim and filgrastim indicated superiority for the former over the latter in reducing the incidence of fn, with a pooled relative risk of 0.64 (95% ci: 0.43 to 0.97)36, although no difference in the incidence or duration of neutropenia or in the incidence of bone pain was observed with either g-csf product. The superiority of pegfilgrastim over filgrastim is further supported by fewer hospitalizations and emergency room visits, less use of antimicrobial agents, improved dose intensity, reduced mortality, and increased cost effectiveness36,38,39. Inherent differences between these g-csf products perhaps account for the difference in their efficacy. Pegfilgrastim has a significantly increased half-life compared with filgrastim, attributed to its lesser renal clearance, with serum clearance directly related to neutrophil numbers. Consequently, although filgrastim requires daily injection, pegfilgrastim persists for approximately 12 days or until neutrophil recovery is achieved40. In the present study, women treated prophylactically with filgrastim experienced a significantly higher incidence of fn than did those treated prophylactically with pegfilgrastim. However, given the observational nature of this study, those results should be interpreted with caution. Given the foregoing reports, it is reasonable to suggest that g-csf formulation should be carefully selected by considering the risk factors mentioned earlier.
Our study has the limitations common to all retrospective studies, such as selection bias because of the absence of randomization, and imbalance in the distribution of, or incomplete measurement of, confounding factors between the treatment groups41.
Detailed clinical information such as duration and severity of fn were missing from the patient records, and rates of hospitalization and emergency room attendance could not be recorded because some patients were managed at outside institutions. Further, the small sample size, bias arising from the single-centre design, and the use of a variety of chemotherapy regimens could limit the generalizability of our results. Moreover, the results and p values have to be interpreted with caution because a sample size calculation was not performed. The proportion of patients reaching a rdi of 85% was not statistically significantly different between the groups that received and did not receive pp-g-csf, and the observed difference therefore represents only a trend. Because filgrastim was provided on compassionate grounds during the time period selected for the study, bias toward the frequent use of filgrastim compared with pegfilgrastim as secondary prophylaxis is a possibility and might have subsequently affected the incidence of fn.
5. CONCLUSIONS
The results of this single-centre study in Ontario are concordant with the existing literature in “reallife” clinical settings and show that the use of g-csf prophylaxis significantly reduces the incidence of fn and results in an improved trend in the delivery of planned chemotherapy dose intensity during adjuvant treatment of breast cancer. Our study identified other risk factors for the incidence of fn, such as older age, taxane-only chemotherapy regimens, and use of the shorter acting g-csf (filgrastim). In addition, it further indicated that the incidence of fn with third-generation act regimens for ebc is higher in the “real-life” clinical setting than in clinical trials. Although our study has a number of limitations, its results support the idea that primary prophylaxis with g-csf should be strongly considered by oncologists treating ebc patients with act. Such prophylaxis will lower the incidence of fn in these patients and allow them to gain the full benefit of chemotherapy by maintaining dose intensity.
7. ACKNOWLEDGMENTS
An abstract version of this paper was presented in part at the annual meeting of the American Society of Clinical Oncology, June 1–5, 2012, Chicago, IL, U.S.A.
6. CONFLICT OF INTEREST DISCLOSURES
The authors have no financial conflicts of interest to declare.
8. REFERENCES
- 1.Canadian Cancer Society’s Steering Committee on Cancer Statistics . Canadian Cancer Statistics 2011. Toronto, ON: Canadian Cancer Society; 2011. [Google Scholar]
- 2.Early Breast Cancer Trialists’ Collaborative Group Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 3.Ginés J, Sabater E, Martorell C, Grau M, Monroy M, Casado MA. Efficacy of taxanes as adjuvant treatment of breast cancer: a review and meta-analysis of randomised clinical trials. Clin Transl Oncol. 2011;13:485–98. doi: 10.1007/s12094-011-0686-x. [DOI] [PubMed] [Google Scholar]
- 4.Aapro MS, Cameron DA, Pettengell R, et al. on behalf of the European Organisation for Research and Treatment of Cancer (eortc) Granulocyte Colony-Stimulating Factor (gcsf) Guidelines Working Party eortc guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphomas and solid tumours. Eur J Cancer. 2006;42:2433–53. doi: 10.1016/j.ejca.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Crawford J. Safety and efficacy of pegfilgrastim in patients receiving myelosuppressive chemotherapy. Pharmacotherapy. 2003;23:15S–9S. doi: 10.1592/phco.23.9.15S.32889. [DOI] [PubMed] [Google Scholar]
- 6.Kuderer NM, Dale DC, Crawford J, Cosler LE, Lyman GH. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer. 2006;106:2258–66. doi: 10.1002/cncr.21847. [DOI] [PubMed] [Google Scholar]
- 7.Aapro M, Schwenkglenks M, Lyman GH, et al. Pegfilgrastim primary prophylaxis vs. current practice neutropenia management in elderly breast cancer patients receiving chemotherapy. Crit Rev Oncol Hematol. 2010;74:203–10. doi: 10.1016/j.critrevonc.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Lyman GH, Dale DC, Crawford J. Incidence and predictors of low dose-intensity in adjuvant breast cancer chemotherapy: a nationwide study of community practices. J Clin Oncol. 2003;21:4524–31. doi: 10.1200/JCO.2003.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Bonadonna G, Valagussa P, Moliterni A, Zambetti M, Brambilla C. Adjuvant cyclophosphamide, methotrexate, and fluorouracil in node-positive breast cancer: the results of 20 years of follow-up. N Engl J Med. 1995;332:901–6. doi: 10.1056/NEJM199504063321401. [DOI] [PubMed] [Google Scholar]
- 10.Smith TJ, Khatcheressian J, Lyman GH, et al. 2006 Update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol. 2006;24:3187–205. doi: 10.1200/JCO.2006.06.4451. [DOI] [PubMed] [Google Scholar]
- 11.National Comprehensive Cancer Network (nccn) NCCN Clinical Practice Guidelines in Oncology: Myeloid Growth Factors. Fort Washington, PA: NCCN; 2010. Ver 1.2010. [Google Scholar]
- 12.Aapro MS, Bohlius J, Cameron DA, et al. on behalf of the European Organisation for Research and Treatment of Cancer 2010 Update of eortc guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer. 2011;47:8–32. doi: 10.1016/j.ejca.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 13.Welte K, Gabrilove J, Bronchud MH, Platzer E, Morstyn G. Filgrastim (r-methug-csf): the first 10 years. Blood. 1996;88:1907–29. [PubMed] [Google Scholar]
- 14.Holmes FA, O’Shaughnessy JA, Vukelja S, et al. Blinded, randomized, multicenter study to evaluate single administration pegfilgrastim once per cycle versus daily filgrastim as an adjunct to chemotherapy in patients with high-risk stage ii or stage iii/iv breast cancer. J Clin Oncol. 2002;20:727–31. doi: 10.1200/JCO.20.3.727. [DOI] [PubMed] [Google Scholar]
- 15.Green MD, Koelbl H, Baselga J, et al. on behalf of the International Pegfilgrastim 749 Study Group A randomized double-blind multicenter phase iii study of fixed-dose single-administration pegfilgrastim versus daily filgrastim in patients receiving myelosuppressive chemotherapy. Ann Oncol. 2003;14:29–35. doi: 10.1093/annonc/mdg019. [DOI] [PubMed] [Google Scholar]
- 16.Crawford J, Ozer H, Stoller R, et al. Reduction by granulocyte colony-stimulating factor of fever and neutropenia induced by chemotherapy in patients with small-cell lung cancer. N Engl J Med. 1991;325:164–70. doi: 10.1056/NEJM199107183250305. [DOI] [PubMed] [Google Scholar]
- 17.Trillet–Lenoir V, Green J, Manegold C, et al. Recombinant granulocyte colony stimulating factor reduces the infectious complications of cytotoxic chemotherapy. Eur J Cancer. 1993;29A:319–24. doi: 10.1016/0959-8049(93)90376-Q. [DOI] [PubMed] [Google Scholar]
- 18.Vogel CL, Wojtukiewicz MZ, Carroll RR, et al. First and subsequent cycle use of pegfilgrastim prevents febrile neutropenia in patients with breast cancer: a multicenter, double-blind, placebo-controlled phase iii study. J Clin Oncol. 2005;23:1178–84. doi: 10.1200/JCO.2005.09.102. [DOI] [PubMed] [Google Scholar]
- 19.Soong D, Haj R, Leung MG, et al. High rate of febrile neutropenia in patients with operable breast cancer receiving docetaxel and cyclophosphamide. J Clin Oncol. 2009;27:e101–2. doi: 10.1200/JCO.2009.23.0508. [DOI] [PubMed] [Google Scholar]
- 20.Schwenkglenks M, Jackisch C, Constenla M, et al. Neutropenic event risk and impaired chemotherapy delivery in six European audits of breast cancer treatment. Support Care Cancer. 2006;14:901–9. doi: 10.1007/s00520-006-0034-9. [DOI] [PubMed] [Google Scholar]
- 21.Takabatake D, Taira N, Hara F, et al. Feasibility study of docetaxel with cyclophosphamide as adjuvant chemotherapy for Japanese breast cancer patients. Jpn J Clin Oncol. 2009;39:478–83. doi: 10.1093/jjco/hyp050. [DOI] [PubMed] [Google Scholar]
- 22.Madarnas Y, Dent SF, Husain SF, et al. Real-world experience with adjuvant fec-d chemotherapy in four Ontario regional cancer centres. Curr Oncol. 2011;18:119–25. doi: 10.3747/co.v18i3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerlier L, Lamotte M, Awada A, et al. The use of chemotherapy regimens carrying a moderate or high risk of febrile neutropenia and the corresponding management of febrile neutropenia: an expert survey in breast cancer and non- Hodgkin’s lymphoma. BMC Cancer. 2010;10:642–52. doi: 10.1186/1471-2407-10-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Younis T, Rayson D, Thompson K. Primary g-csf prophylaxis for adjuvant tc or fec-d chemotherapy outside of clinical trial settings: a systematic review and meta-analysis. Support Care Cancer. 2012;20:2523–30. doi: 10.1007/s00520-011-1375-6. [DOI] [PubMed] [Google Scholar]
- 25.Vandenberg T, Younus J, Al-Khayyat S. Febrile neutropenia rates with adjuvant docetaxel and cyclophosphamide chemotherapy in early breast cancer: discrepancy between published reports and community practice—a retrospective analysis. Curr Oncol. 2010;17:2–3. doi: 10.3747/co.v17i2.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campbell C, Bramwell V, Charette M, Oliver T, on behalf of the Systemic Treatment Disease Site Group . The Role of Colony-Stimulating Factor (CSF) in Patients Receiving Myelosuppressive Chemotherapy for the Treatment of Cancer. Toronto, ON: Cancer Care Ontario; 2003. Practice guideline report 12-2. [Google Scholar]
- 27.Fraser J, Steele N, Al Zaman A, Yule A. Are patients in clinical trials representative of the general population? Dose intensity and toxicities associated with fe100c-d chemotherapy in a nontrial population of node positive breast cancer patients compared with pacs-01 trial group. Eur J Cancer. 2011;47:215–20. doi: 10.1016/j.ejca.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 28.Martin M, Pienkowski T, Mackey J, et al. on behalf of the Breast Cancer International Research Group 001 Investigators Adjuvant docetaxel for node-positive breast cancer. N Engl J Med. 2005;352:2302–13. doi: 10.1056/NEJMoa043681. [DOI] [PubMed] [Google Scholar]
- 29.Roche H, Fumoleau P, Spielmann M, et al. Sequential adjuvant epirubicin-based and docetaxel chemotherapy for nodepositive breast cancer patients: the fnclcc pacs 01 Trial. J Clin Oncol. 2006;4:5664–71. doi: 10.1200/JCO.2006.07.3916. [DOI] [PubMed] [Google Scholar]
- 30.Crawford J, Dale DC, Lyman GH. Chemotherapy-induced neutropenia: risks, consequences, and new directions for its management. Cancer. 2004;100:228–37. doi: 10.1002/cncr.11882. [DOI] [PubMed] [Google Scholar]
- 31.Lyman GH, Kuderer NM, Crawford J, et al. Predicting individual risk of neutropenic complications in patients receiving cancer chemotherapy. Cancer. 2011;117:1917–27. doi: 10.1002/cncr.25691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crawford J, Caserta C, Roila F, on behalf of the esmo Guidelines Working Group Hematopoietic growth factors: esmo clinical practice guidelines for the applications. Ann Oncol. 2010;21(suppl 5):v248–51. doi: 10.1093/annonc/mdq195. [DOI] [PubMed] [Google Scholar]
- 33.Ng JH, Ang XY, Tan SH, Tao M, Lim ST, Chan A. Breakthrough febrile neutropenia and associated complications in non-Hodgkin’s lymphoma patients receiving pegfilgrastim. Acta Haematol. 2011;125:107–14. doi: 10.1159/000321545. [DOI] [PubMed] [Google Scholar]
- 34.Lyman GH, Morrison VA, Dale DC, Crawford J, Delgado DJ, Fridman M, on behalf of the opps Working Group and anc Study Group Risk of febrile neutropenia among patients with intermediate-grade non-Hodgkin’s lymphoma receiving chop chemotherapy. Leuk Lymphoma. 2003;44:2069–76. doi: 10.1080/1042819031000119262. [DOI] [PubMed] [Google Scholar]
- 35.Timmer–Bonte JN, Punt CJ, vd Heijden HF, et al. Prophylactic g-csf and antibiotics enable a significant dose-escalation of triplet-chemotherapy in non-small cell lung cancer. Lung Cancer. 2008;60:222–30. doi: 10.1016/j.lungcan.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 36.Pinto L, Liu Z, Doan Q, Bernal M, Dubois R, Lyman G. Comparison of pegfilgrastim with filgrastim on febrile neutropenia, grade iv neutropenia and bone pain: a metaanalysis of randomized controlled trials. Curr Med Res Opin. 2007;23:2283–95. doi: 10.1185/030079907X219599. [DOI] [PubMed] [Google Scholar]
- 37.Holmes FA, Jones SE, O’Shaughnessy J, et al. Comparable efficacy and safety profiles of once-per-cycle pegfilgrastim and daily injection filgrastim in chemotherapy-induced neutropenia: a multicenter dose-finding study in women with breast cancer. Ann Oncol. 2002;13:903–9. doi: 10.1093/annonc/mdf130. [DOI] [PubMed] [Google Scholar]
- 38.Lyman GH, Lalla A, Barron RL, Dubois RW. Cost-effectiveness of pegfilgrastim versus filgrastim primary prophylaxis in women with early-stage breast cancer receiving chemotherapy in the United States. Clin Ther. 2009;31:1092–104. doi: 10.1016/j.clinthera.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 39.Tan H, Tomic K, Hurley D, Daniel G, Barron R, Malin J. Comparative effectiveness of colony-stimulating factors for febrile neutropenia: a retrospective study. Curr Med Res Opin. 2011;27:79–86. doi: 10.1185/03007995.2010.536527. [DOI] [PubMed] [Google Scholar]
- 40.Yang BB, Kido A, Shibata A. Serum pegfilgrastim concentrations during recovery of absolute neutrophil count in patients with cancer receiving pegfilgrastim after chemotherapy. Pharmacotherapy. 2007;27:1387–93. doi: 10.1592/phco.27.10.1387. [DOI] [PubMed] [Google Scholar]
- 41.Alemayehu D, Cappelleri JC. Revisiting issues, drawbacks and opportunities with observational studies in comparative effectiveness research. J Eval Clin Pract. 2011. [Epub ahead of print]. [DOI] [PubMed]