Abstract
Introduction
Collecting duct carcinoma (cdc) is a rare, aggressive form of renal carcinoma that presents at an advanced stage and has a poor prognosis. Little is known concerning the optimal management of cdc. We present the results of a systematic review addressing the management of cdc and the McMaster University cdc series.
Methods
The medline, Cochrane Library, and embase databases and conference proceedings were searched to identify studies relating to the management of cdc. Included studies reported on a minimum of 10 subjects receiving a single intervention. Series in which an evaluation of therapeutic effectiveness was not possible were excluded. The McMaster University (Hamilton, Ontario) series of 6 cases of cdc were retrospectively reviewed.
Results
We identified 3 studies relevant to the management of cdc that included a total of 72 patients. A gemcitabine–cisplatin or –carboplatin regimen resulted in a 26% objective response rate in 23 patients with metastatic cdc. Two additional studies indicated that 49 patients treated with immunotherapy achieved no response. In the McMaster series, cytoreductive nephrectomy was performed in 4 of 6 patients. In 2 patients, mvac therapy (methotrexate–vinblastine–doxorubicin–cisplatin) achieved no response. No significant therapeutic complications occurred, but survival was poor (median: 11 months; range: 10–33 months).
Conclusions
Our review and clinical experience suggest that the current standard of care for metastatic cdc is a gemcitabine–cisplatin regimen.
Keywords: Collecting duct carcinoma, Bellini duct carcinoma, renal cell carcinoma, cytoreductive nephrectomy, gemcitabine, cisplatin, mvac
1. INTRODUCTION
Collecting duct carcinoma (cdc) or Bellini duct carcinoma is a rare type of renal cell carcinoma (rcc) thought to originate from renal collecting duct epithelium. Three multi-institutional retrospective cdc series including 262 patients were recently published from the United States1, Europe2, and Japan3.
The U.S. population–based series by Wright et al. characterized cdc epidemiology in North America (Table i)1. Compared with clear cell rcc, cdc is more common in African American and male patients. The median age at diagnosis of 63 years did not differ from that for clear cell rcc. At diagnosis, collecting duct carcinoma was also more commonly locally advanced, metastatic, and poorly differentiated, resulting in worse 1- and 3-year disease-specific survivals.
TABLE I.
Characteristics of patients with collecting duct carcinoma (cdc) and with clear cell renal cell carcinoma (ccrcc)1
| Characteristica |
Patient group (%)
|
|
|---|---|---|
| cdc (n=160) | ccrcc (n=33,252) | |
| Men | 70 | 62 |
| African American | 23 | 9 |
| Locally advanced diseaseb | 28 | 16 |
| Metastatic diseaseb | 28 | 17 |
| Poorly differentiatedb | 70 | 31 |
| Survival [median (95% ci)]c | ||
| 1-Year | 70 (62–77) | 87 (86–87) |
| 3-Year | 58 (48–67) | 79 (78–80) |
Listed characteristics differ significantly (p < 0.05).
At presentation or staging.
Estimated using the Kaplan–Meier method.
The European2 and Japanese3 series also found that cdc presents at an advanced stage and has a poor prognosis. Additionally, those series indicated that more than two thirds of patients with cdc exhibit locoregional or systemic symptoms on presentation. The most common metastatic sites included regional lymph nodes, lung, bone, and liver3.
Two retrospective series with a total of 35 patients suggest that several computed tomography findings may predict cdc histology4,5. Those findings include medullary location, weak and heterogeneous enhancement, involvement of the renal sinus, infiltrative growth, preserved renal contour, and a cystic component. Nonetheless, the low pre-test probability of cdc and the lack of specificity in the criteria necessitate histopathology for cdc diagnosis.
The major criteria for cdc classification in the World Health Organization’s Classification of Tumors include location in a medullary pyramid; typical histology, with irregular tubular architecture and high nuclear grade; inflammatory desmoplastic stroma with numerous granulocytes; reactivity to antibodies against hmwck, reactivity with uea-i, and absence of urothelial carcinoma6. Contemporary pathology research has focused on excluding urothelial carcinoma and papillary rcc by immunohistochemical staining for pax8, p63, E-cadherin, c-Kit, CD10, and others7,8. Pathology diagnosis of cdc is complex and, at our institution, warrants specialized review.
Recently published series1–3 and conventional secondary sources9 do not provide direction on the appropriate management of cdc. It is for that purpose that we report the results of a systematic review addressing the management of cdc. The McMaster University cdc series is also presented.
2. METHODS
2.1. Systematic Review
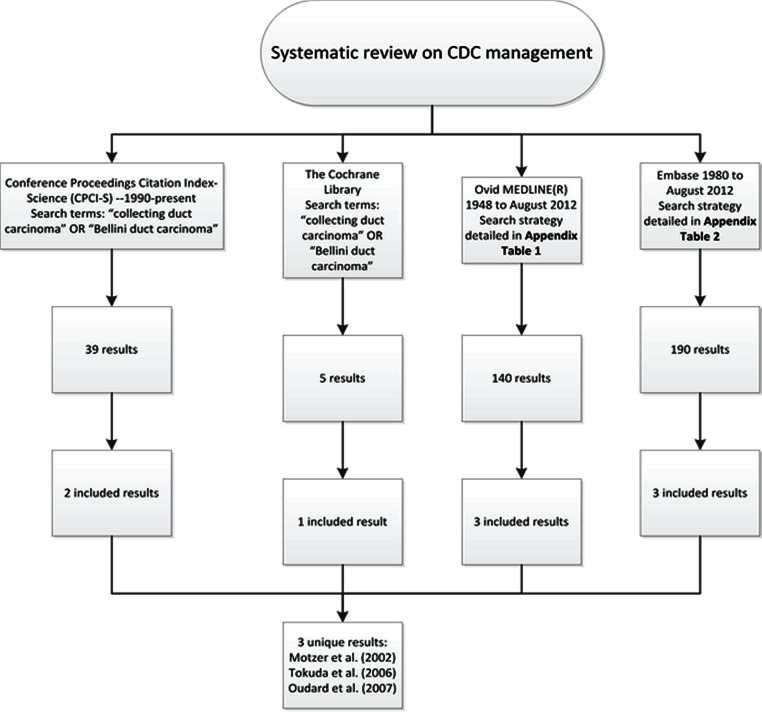
A systematic literature review was performed to evaluate management options for cdc. Databases searched included Ovid medline, the Cochrane Library, embase, and the Thomson Reuters Conference Proceedings Citation Index. Search results were filtered by three researchers.
The literature searches were performed on August 1, 2012, and studies with English abstracts from 1980 to August 2012 were included. Table ii summarizes the search strategies.
TABLE II.
Literature search strategies
| Step | medline search term | Results | embase search term | Results |
|---|---|---|---|---|
| 1 | exp renal neoplasms/ | 50,116 | Collecting duct.mp. | 4,918 |
| 2 | Collecting duct.mp. | 4,854 | Bellini duct.mp. | 89 |
| 3 | Bellini duct.mp. | 65 | (treatment or chemotherapy or radiotherapy or management or mvac or methotrexate or vinblastine or Adriamycin or cisplatin or gemcitabine or carboplatin or Taxol or doxorubicin or ifosfamide or paclitaxel or cisplatin or itp or monoclonal antibody or immunotherapy or tyrosine kinase inhibitor or vegf inhibitor or pazopanib or mtor inhibitor or temsirolimus or everolimus or egfr inhibitor or sunitinib or sorafenib or il2 or interleukin 2 or targeted therapy or molecular therapy or cytoreductive nephrectomy or radical nephrectomy or partial nephrectomy or nephrectomy or metastectomy or surgery or resection).mp. | 5,477,368 |
| 4 | 1 and (2 or 3) | 362 | exp kidney carcinoma/ | 31,931 |
| 5 | (treatment or chemotherapy or radiotherapy or management or mvac or methotrexate or vinblastine or Adriamycin or cisplatin or gemcitabine or carboplatin or Taxol or doxorubicin or ifosfamide or paclitaxel or cisplatin or itp or monoclonal antibody or immunotherapy or tyrosine kinase inhibitor or vegf inhibitor or pazopanib or mtor inhibitor or temsirolimus or everolimus or egfr inhibitor or sunitinib or sorafenib or il2 or interleukin 2 or targeted therapy or molecular therapy or cytoreductive nephrectomy or radical nephrectomy or partial nephrectomy or nephrectomy or metastectomy or surgery or resection).mp. | 3,747,748 | 4 and (1 or 2) | 382 |
| 6 | 4 and 5 | 141 | 3 and 5 | 202 |
| 7 | limit 6 to yr=“1980–Current” | 141 | limit 6 to (human and yr=“1980–Current”) | 190 |
| 8 | limit 7 to humans | 140 |
Included studies reported on a minimum of 10 subjects with a histopathologic diagnosis of cdc receiving a single intervention. Series in which an evaluation of therapeutic effectiveness was not possible were excluded (for example, surgical series with no comparison group). Subgroups within a larger study that fulfilled the inclusion and exclusion criteria were also included.
The primary outcome was response rate (complete or partial, as defined by the study). Secondary outcomes included progression-free survival, overall survival, and toxicity. Also recorded were the study design, number of patients, location of patients, patient and tumour demographics, criteria for diagnosis, and criteria for assessing response. Study quality was evaluated by assigning each study a level of evidence based on the levels of evidence criteria set out by Oxford University’s Centre for Evidence-Based Medicine10. Search terms, inclusion and exclusion criteria, and outcomes were defined a priori.
Figure 1 presents an overview of the systematic review searches.
FIGURE 1.
Overview of the systematic review search methodology. Study selection criteria were a minimum of 10 subjects, a histopathologic diagnosis of collecting duct carcinoma (cdc), and administration of a single intervention. Series in which an evaluation of therapeutic effectiveness was not possible were excluded (surgical series with no comparison group, for instance).
2.2. Case Series
During 2000–2012, 6 patients treated at a single institution (McMaster University, Hamilton, Ontario) received a diagnosis of cdc. Patient information was retrospectively extracted from the office charts for those patients and from regionally-linked electronic medical records. Extracted data included presenting history, staging, treatment, pathology, post-treatment course, and survival. Cytoreductive nephrectomy was defined as nephrectomy performed after a diagnosis of metastatic disease.
3. RESULTS
3.1. Systematic Review
We identified three studies that met our specified inclusion and exclusion criteria. Excluded studies were either isolated case reports, small surgical series, or articles not relevant to the search.
3.1.1. Gemcitabine and Cisplatin
Oudard et al. published a prospective multicentre phase ii study evaluating the effect of gemcitabine and either cisplatin or carboplatin (gc) on cdc11 (level of evidence: 2b). This study, conducted at 6 French institutions, reported on patients with metastatic cdc, confirmed by centralized histopathology review, who had received no systemic treatment or radiotherapy in the 4 weeks before inclusion. Median patient age was 65 years (range: 18–74 years), and 87% had previously undergone nephrectomy. In 96% of the patients, Eastern Cooperative Oncology Group performance status was 2 or less.
Participants received 21-day cycles of gemcitabine 1250 mg/m2 on days 1 and 8, plus cisplatin or carboplatin 70 mg/m2 (based on renal function) on day 1 (n = 23 gemcitabine, n = 12 cisplatin, n = 6 carboplatin, and n = 5 cisplatin then carboplatin). Participants underwent a median of 6 cycles of treatment (range: 1–8 cycles).
In the study, participating patients demonstrated a 26% partial or complete response rate [95% confidence interval (ci): 8%–44%], with 1 complete response. Another 10 patients (44%) experienced disease stabilization, and 7 had progressive disease (30%). Responses were assessed using radiologic guidelines from the European Organization for Research and Treatment of Cancer, the U.S. National Cancer Institute, and the National Cancer Institute of Canada12. Progression-free survival was 7.1 months (95% ci: 3.0–11.3 months), and overall survival was 10.5 months (95% ci: 3.8–17.1 months).
In terms of toxicity, no treatment-related deaths occurred. Grades 3 and 4 leukopenia, granulocytopenia, anemia, and thrombocytopenia were experienced by 35%, 52%, 44%, and 26% of patients respectively. Rates of grade 1 or 2 creatinine or liver enzyme rise, neuropathy, and anorexia were less than 10%. One patient experienced grade 3 hypercalcemia.
3.1.2. Immunotherapy
Tokuda et al.3 published a retrospective series based on a multi-institutional survey (66 Japanese institutions—level of evidence: 4). In a subpopulation of that series, immunotherapy was used [interferon (ifn-α, ifn-γ), or interleukin 2 (il-2), n = 34]. The series had central histopathology confirmation by 2 pathologists. In the 34 patients treated with immunotherapy, no responses were observed. Response criteria and baseline characteristics, toxicity, and survival of the subpopulation were not reported. The authors anecdotally mentioned 1 tentative partial response to gc therapy after failure of immunotherapy.
Motzer et al.13 also published a retrospective review for a single U.S. centre (level of evidence: 4). Patients had metastatic disease and a pathology finding of cdc or medullary carcinoma. In a sub-population of these patients, ifn-α or il-2 (n = 15) was used. No responses to immunotherapy were observed. Response criteria and baseline characteristics, toxicity, and survival were not reported for the subpopulation. The authors anecdotally mentioned a 5-month partial response to gc in their series.
3.2. Case Series
In our institutional series, patients ranged in age from 61 to 72 years, and all presented symptomatically with flank pain, hematuria, lower urinary tract symptoms, and weight loss. Table iii summarizes individual patient details. Primary tumours ranged in size from 4 cm to 12 cm. Local invasion, lymphadenopathy, or distant metastases were present in all cases. Metastatic sites included the lumbar vertebrae, lungs, liver, retroperitoneal lymph nodes, and adrenals. Table iv summarizes individual tumour characteristics.
TABLE III.
Patient information
| Pt id | Age | Sex | Presenting history | Management | Survival (months) |
|---|---|---|---|---|---|
| 1 | 64 | Female | Lower urinary tract symptoms, flank pain, 4.5-kg weight loss, 30 pack–year smoking history | Cytoreductive nephrectomy; unfit for chemotherapy | 33 |
| 2 | 61 | Male | Gross hematuria and flank pain, nonsmoker | Initial nephrectomy aborted because of lymphadenopathy; unresponsive to 4 cycles of mvac followed by cytoreductive nephrectomy | 11 |
| 3 | 65 | Female | Gross hematuria, 40 pack–year smoking history | Unresponsive to 3 cycles of mvac | 19 |
| 4 | 70 | Male | Declining performance status, weight loss | Performance status too low for chemotherapy | Lost to follow-up |
| 5 | 69 | Male | Not available | Cytoreductive nephrectomy | 11 |
| 6 | 72 | Male | Lower urinary tract symptoms, no hematuria or flank pain, smoking history | Cytoreductive (partial) nephrectomy (horseshoe kidney) | 10 |
Pt = patient; mvac = methotrexate–vinblastine–doxorubicin–cisplatin chemotherapy.
Cytoreductive nephrectomy was performed in 4 cases (patients 1, 2, 5, and 6). Patients 2 and 3 received mvac chemotherapy, although with no apparent response. Chemotherapy was considered in all other patients, but was not administered because of performance status and comorbidities. No complications requiring hospitalization and no deaths from surgery or chemotherapy occurred. Palliative radiotherapy was administered to patient 1 with no apparent symptomatic response, but in patient 2, palliative radiotherapy was moderately effective for pain control.
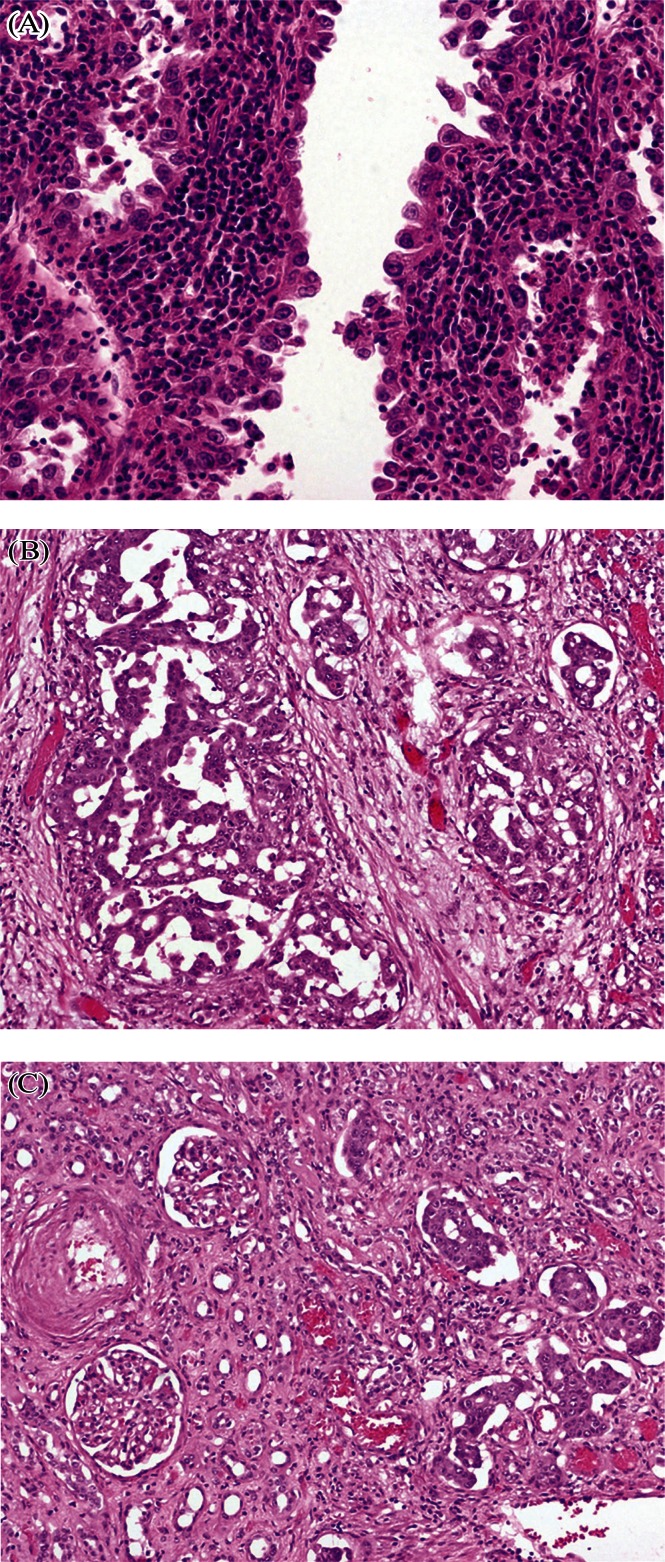
Histology diagnosis based on a combination of hematoxylin and eosin morphology and immunohistochemical profile was obtained in all cases (Table iv). A selection of slides obtained from our patients illustrate the unique histology of cdc (Figure 2). Staging was clinical in patients 3 and 4 because they did not undergo nephrectomy.
TABLE IV.
Tumour information
| Pt id | Primary tumour size (cm) | Distant metastatic sites (clinical) | TNM stagea | Immunohistochemistry |
|---|---|---|---|---|
| 1 | 4 | Lung, vertebral | pT3a (perirenal and hilar fat), N1, M1 | Not available |
| 2 | 7 | Bilateral adrenal | pT4 (splenic invasion), N1, M1 | Positive: CK19, HMW-CK, EMA, vimentin, CK7 Negative: CD10, p63 |
| 3 | 12 | Lung, bilateral | cT3b (ivc extension) possible cT4, N1, M1 | Positive: CK7, CK5/6, p63 Focally positive: MOC31, vimentin, CD10 Negative: CD20 |
| 4 | 11 | Adrenal, liver, lung, vertebral | cT4, N1, M1 | Positive: AE1/AE3, CAM5.2, 34BE12, CK7, CK10 Negative: CK20, PSA, PAP, EMA, vimentin, TTF-1 |
| 5 | 4 | None | pT1, N1, M0 | Not available |
| 6 | 5 | Liver | pT3, N0, M1, R1 mixed Fuhrman 3 ccrcc and cdc | Positive: CK7 |
According to the AJCC Cancer Staging Manual14.
Pt = patient; ivc = inferior vena cava; ccrcc = clear cell renal cell carcinoma; cdc = collecting duct carcinoma.
FIGURE 2.
Collecting duct carcinoma. (A) Hobnail neoplastic cells with high-grade nuclei (centrally) resting on stroma with inflammatory cells. (B) Tumour cells in large nests and some in situ within the collecting ducts. (C) On the left, normal renal parenchyma is visible; on the right in situ neoplastic cells are lining the collecting ducts. All images: hematoxylin and eosin stain, 20× original magnification.
Median survival was 11 months (range: 10–33 months). All deaths were attributed to complications of advanced disease. Patient 4 was lost to follow-up.
4. DISCUSSION
4.1. Localized CDC
Localized cdc is an uncommon entity1–3. No studies encountered in our review addressed the management of localized cdc. Long-term survival has been reported in isolated cases with resected localized disease15–17. The role of adjuvant or neoadjuvant therapies is not known.
4.2. Non-localized CDC
4.2.1. Surgery
Almost all reported patients with cdc have undergone surgery1–3,11 and are diagnosed with cdc only after a histopathology examination. To illustrate this point, 87% of patients in the study by Oudard et al. underwent prior cytoreductive nephrectomy. Should cdc be diagnosed on biopsy, evidence for the role of surgery is lacking in the literature. Patients selected for cytoreductive nephrectomy certainly experience improved survival, likely because of selection biases18. Differences in tumour biology suggest that trials demonstrating a benefit for cytoreductive nephrectomy in clear cell rcc are not applicable19–22.
It is important to note that the invasive nature of cdc tumours, combined with the poor preoperative performance status of cdc patients, results in an elevated rate of perioperative morbidity and mortality. For example, Mejean et al.23 reported 3 perioperative deaths in their series of 10 patients undergoing surgery for cdc. Surgical complications and recovery may also delay or prevent a patient from receiving systemic therapy11. Accordingly, surgical therapy for known cdc must be individualized.
4.2.2. Cytotoxic Chemotherapy
Oudard et al. demonstrated the effectiveness of a gc regimen in inducing a 26% (95% ci: 8%–44%) objective response rate in cdc. Interestingly, that rate is similar to the rate in urothelial carcinoma, in which a gc regimen is a standard of care in metastatic or invasive disease24,25. This trial by Oudard et al. is the only one identified in our review that provided evidence of cdc responsiveness to a cytotoxic agent. Given the lack of any other beneficial agent, a gc regimen should be considered the standard of care for first-line systemic treatment of metastatic cdc.
The toxicity of the gc regimen is improved compared with that for traditional mvac therapy25. In their trial, Oudard et al. primarily observed hematologic toxicity: grades 3–4 neutropenia and thrombocytopenia were observed in 52% and 43% of patients respectively. No treatment-related deaths occurred.
This uncontrolled phase ii study by Oudard et al. is limited in several ways. It enrolled 23 patients, falling short of the stated goal of 40 patients. Of the 23 patients, 87% underwent cytoreductive nephrectomy, and thus it is hard to know how the trial findings generalize to patients who have not undergone surgery. Only 52% of the patients had sufficient renal function to complete therapy with cisplatin. Although chemotherapy type (carboplatin, cisplatin, or cisplatin switched to carboplatin) was not a significant predictor of survival on univariate Cox regression, the small number of participants limits that analysis. Furthermore, although the median number of chemotherapy cycles was 6, the range was 1–8 cycles, and how that variation may have affected survival or which factors may have led to the heterogeneity is unknown. As previously mentioned, 87% of the patients in the trial were diagnosed after nephrectomy, and thus it is uncertain how the results apply to patients diagnosed on biopsy. Similarly, 96% of patients had an Eastern Cooperative Oncology Group performance status of 2 or less, which also is a pivotal factor in deciding on cytotoxic therapy.
Despite those limitations, the Oudard et al. trial stands as important hypothesis-generating work, and future phase iii studies might choose to focus on evaluating other cytotoxic urothelial carcinoma regimens in comparison with gc. Chemosensitivity of cdc cell lines to topoisomerase inhibitors such as doxorubicin has been described26. Case reports have also reported responses to mvac27,28, paclitaxel29, and paclitaxel and carboplatin30,31. A phase i study documented a response of metastatic cdc to AQ4N, a novel topoisomerase ii inhibitor32.
4.2.3. Immunotherapy
Although reports of a response of metastatic cdc to immunotherapy have been published33,34, our review indicates that immunotherapy (ifn-α, ifn-γ, and il-2) is not effective in treating metastatic cdc. That conclusion is based on two retrospective series with a total of 49 patients treated with immunotherapy in whom no responses were documented3,13. Although details of the specific treatment regimens used were limited (as were response criteria, survival, toxicity, and baseline patient characteristics), the complete lack of effectiveness in these studies was large and homogeneous.
4.2.4. Targeted Therapy
Outside of small series or case reports, no evidence supports the efficacy of targeted therapy such as sunitinib or sorafenib for cdc. Procopio et al.35 recently reported a series of 7 patients receiving targeted therapies. Two patients experienced periods of disease stabilization and overall survivals of 49 months (sorafenib, then sunitinib) and 19 months (temsirolimus, then sunitinib). Our review also identified three isolated reports. Staehler et al.36 reported a lack of response to sunitinib in 2 patients with metastatic cdc. Miyake et al.37 reported a contribution of sunitinib to a slight decrease in metastatic tumour burden that fell short of a partial response. Finally, Ansari et al.38 reported a response, with minimal side effects, to sorafenib in a patient with metastatic cdc.
These few positive reports and the current dismal prognosis of cdc indicate that targeted therapy is an important area for future investigation. Accordingly, several trials are under way to evaluate the role of targeted therapies in non-clear cell rcc (search for NCT00465179, NCT01185366, and NCT01219751 at http://clinicaltrials.gov/).
4.3. Case Series
The patients in our institutional series reflect many characteristics previously described for cdc. All patients were symptomatic at presentation, with classic symptoms of flank pain or gross hematuria3. Presence of flank pain at cdc presentation is ascribed to the infiltrative nature and regional lymphadenopathy of even small cdc tumours, such as the 3.6-cm pT3aN1M1 tumour in patient 1. Gross hematuria may be more likely in cdc because of its central location, allowing for direct access to the collecting system.
Patients were diagnosed in about their 6th decade and had advanced staging at presentation, as in other reported series1. Interestingly, 2 patients in our series had adrenal metastases, a site not commonly reported for cdc metastasis3. The poor prognosis reported in several series also held true for our patients, who had a median survival of 11 months1–3. Histopathology diagnosis of cdc in our series was complex and required specialized review. Discussion of cdc histopathology is beyond the scope of the present paper and is reviewed elsewhere6,7.
Surgical treatment for cdc patients in the present series was individualized. Patients 1, 2, 5, and 6 received cytoreductive nephrectomy. Patient 1 had a small tumour, limited evidence of metastasis, a high performance status, and a diagnosis of cdc that was not known preoperatively. Patient 2 had an invasive tumour, but was thought to be able to tolerate surgery; he had a decent performance status after mvac therapy and a preference for surgery. Patient 5 had a small tumour and, preoperatively, was presumed to have localized non-cdc disease. Patient 6 was thought to have non-cdc organ-confined disease until the final histopathology report confirmed cdc elements, with local invasion and positive margins. Additionally, hepatic nodules in patient 6 may have been cdc in origin. Because of persistent hematuria, palliative nephrectomy was also offered to patient 3, but the procedure was not performed because the hematuria eventually ceased.
In keeping with the recent study by Oudard et al.11, cdc tumours may be treated systemically with cytotoxic therapy, as in urothelial carcinoma. Accordingly, patients 2 and 3 received mvac, a standard of care in metastatic urothelial carcinoma39,40. The remaining patients would also have received mvac, except that patients 1, 5, and 6 were unfit for systemic therapy, and patient 4 was noncompliant and lost to follow-up. No responses were observed, but that result is not unexpected given that 17 of 23 patients in the Oudard et al. trial did not respond to gc11. In our series, mvac was used in preference to gc because treatment occurred before acceptance of equivalent survival and lower toxicity with gc compared with mvac in urothelial carcinoma24,25.
4.4. Limitations of Our Review
The present systematic review is limited by several factors. First, only English-language studies after 1980 were searched, a limitation necessary for practical reasons. Similarly, a minimum sample size of 10 patients was chosen, because that study size was thought to be the minimum needed to appropriately observe responses and toxicities in a consecutive series.
For a study to be included in the review, we also required that an objective outcome be measured. This specification was meant to ensure that studies documenting therapeutic efficacy (or lack thereof) could form the basis of clinical decisions. Those criteria are clearly biased against observational surgical case series because no comparator group is available. However, some of the excluded studies are still relevant in the management of cdc, and so they were included in the discussion.
4.5. Quality of Available Evidence
Because of the rarity of cdc, the overall quality of the available evidence was fairly poor. The study by Oudard et al.11 was the highest quality study, in that it was a prospective phase ii trial with central histopathology review (level of evidence: 2b). Limitations of that study were discussed. Evidence from the studies by Tokuda et al.3 and Motzer et al.13 involved subgroups within a retrospective series that included a cumulative total of 49 patients (level of evidence: 4). Although the quality of this immunotherapy evidence was quite poor, conclusions can be reached from the homogeneity of both studies in demonstrating no effect of immunotherapy. Trying an approach that has worked for other rare diseases, such as a disease registry, may be warranted41.
5. CONCLUSIONS
Collecting duct carcinoma is a rare subtype of rcc. It is aggressive, presents symptomatically at an advanced stage, and has a poor prognosis. A systematic review of the literature on management options for cdc reveals that the only studied treatment is a gc regimen for which a 26% (95% ci: 8%–44%) partial or complete response rate in 23 patients was observed. The role of targeted therapy (tyrosine kinase inhibitors such as sunitinib or sorafenib) in the management of cdc has not been established based on limited data to date.
6. CONFLICT OF INTEREST DISCLOSURES
The authors have no financial conflicts of interest to declare.
7. REFERENCES
- 1.Wright JL, Risk MC, Hotaling J, Lin DW. Effect of collecting duct histology on renal cell cancer outcome. J Urol. 2009;182:2595–9. doi: 10.1016/j.juro.2009.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karakiewicz PI, Trinh QD, Rioux–Leclercq N, et al. Collecting duct renal cell carcinoma: a matched analysis of 41 cases. Eur Urol. 2007;52:1140–5. doi: 10.1016/j.eururo.2007.01.070. [DOI] [PubMed] [Google Scholar]
- 3.Tokuda N, Naito S, Matsuzaki O, Nagashima Y, Ozono S, Igarashi T on behalf of the Japanese Society of Renal Cancer. Collecting duct (Bellini duct) renal cell carcinoma: a nationwide survey in Japan. J Urol. 2006;176:40–3. doi: 10.1016/S0022-5347(06)00502-7. [DOI] [PubMed] [Google Scholar]
- 4.Yoon SK, Nam KJ, Rha SH, et al. Collecting duct carcinoma of the kidney: ct and pathologic correlation. Eur J Radiol. 2006;57:453–60. doi: 10.1016/j.ejrad.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 5.Pickhardt PJ, Siegel CL, McLarney JK. Collecting duct carcinoma of the kidney: are imaging findings suggestive of the diagnosis? AJR Am J Roentgenol. 2001;176:627–33. doi: 10.2214/ajr.176.3.1760627. [DOI] [PubMed] [Google Scholar]
- 6.Eble JN, Sauter G, Epstein JI, Sesterhenn IA, editors. Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs. Lyon, France: IARC Press; 2004. [Google Scholar]
- 7.Albadine R, Schultz L, Illei P, et al. pax8 (+)/p63 (–) immunostaining pattern in renal collecting duct carcinoma (cdc): a useful immunoprofile in the differential diagnosis of cdc versus urothelial carcinoma of upper urinary tract. Am J Surg Pathol. 2010;34:965–9. doi: 10.1097/PAS.0b013e3181dc5e8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kobayashi N, Matsuzaki O, Shirai S, Aoki I, Yao M, Nagashima Y. Collecting duct carcinoma of the kidney: an immunohistochemical evaluation of the use of antibodies for differential diagnosis. Hum Pathol. 2008;39:1350–9. doi: 10.1016/j.humpath.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 9.Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA, editors. Campbell–Walsh Urology. 9th ed. Philadelphia, PA: W.B. Saunders; 2007. [Google Scholar]
- 10.Oxford University Centre for Evidence-based Medicine (cebm) CEBM > EBM Tools > Finding the Evidence > Levels of Evidence 2 > Levels of Evidence 1 [Web page] Oxford U.K.: CEBM; 2009. [Available at: http://www.cebm.net/index.aspx?o=1025; cited November 17, 2012] [Google Scholar]
- 11.Oudard S, Banu E, Vieillefond A, et al. Prospective multicenter phase ii study of gemcitabine plus platinum salt for metastatic collecting duct carcinoma: results of a getug (Groupe d’Etudes des Tumeurs Uro-Genitales) study. J Urol. 2007;177:1698–702. doi: 10.1016/j.juro.2007.01.063. [DOI] [PubMed] [Google Scholar]
- 12.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 13.Motzer RJ, Bacik J, Mariani T, Russo P, Mazumdar M, Reuter V. Treatment outcome and survival associated with metastatic renal cell carcinoma of non-clear-cell histology. J Clin Oncol. 2002;20:2376–81. doi: 10.1200/JCO.2002.11.123. [DOI] [PubMed] [Google Scholar]
- 14.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer –Verlag; 2009. [Google Scholar]
- 15.Vázquez–Lavista LG, Uribe–Uribe N, Gabilondo–Navarro F. Collecting duct renal cell carcinoma: two different clinical stages, two different clinical outcomes. Urol Int. 2008;81:116–18. doi: 10.1159/000137652. [DOI] [PubMed] [Google Scholar]
- 16.Yoshida K, Kinoshita H, Taniguti H, et al. Bellini duct carcinoma of the kidney: a case report [Japanese] Hinyokika Kiyo. 2007;53:121–4. [PubMed] [Google Scholar]
- 17.Matsumoto H, Wada T, Aoki A, et al. Collecting duct carcinoma with long survival treated by partial nephrectomy. Int J Urol. 2001;8:401–3. doi: 10.1046/j.1442-2042.2001.00321.x. [DOI] [PubMed] [Google Scholar]
- 18.Abern MR, Tsivian M, Polascik TJ, Coogan CL. Characteristics and outcomes of tumors arising from the distal nephron. Urology. 2012;80:140–6. doi: 10.1016/j.urology.2012.03.034. [DOI] [PubMed] [Google Scholar]
- 19.Flanigan RC, Salmon SE, Blumenstein BA, et al. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. N Engl J Med. 2001;345:1655–9. doi: 10.1056/NEJMoa003013. [DOI] [PubMed] [Google Scholar]
- 20.Mickisch GH, Garin A, van Poppel H, de Prijck L, Sylvester R. Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: a randomised trial. Lancet. 2001;358:966–70. doi: 10.1016/S0140-6736(01)06103-7. [DOI] [PubMed] [Google Scholar]
- 21.Choueiri TK, Xie W, Kollmannsberger C, et al. The impact of cytoreductive nephrectomy on survival of patients with metastatic renal cell carcinoma receiving vascular endothelial growth factor targeted therapy. J Urol. 2011;185:60–6. doi: 10.1016/j.juro.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 22.You D, Jeong IG, Ahn JH, et al. The value of cytoreductive nephrectomy for metastatic renal cell carcinoma in the era of targeted therapy. J Urol. 2011;185:54–9. doi: 10.1016/j.juro.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 23.Mejean A, Roupret M, Larousserie F, Hopirtean V, Thiounn N, Dufour B. Is there a place for radical nephrectomy in the presence of metastatic collecting duct (Bellini) carcinoma? J Urol. 2003;169:1287–90. doi: 10.1097/01.ju.0000050221.51509.f5. [DOI] [PubMed] [Google Scholar]
- 24.von der Maase H, Sengelov L, Roberts JT, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23:4602–8. doi: 10.1200/JCO.2005.07.757. [DOI] [PubMed] [Google Scholar]
- 25.von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase iii study. J Clin Oncol. 2000;18:3068–77. doi: 10.1200/JCO.2000.18.17.3068. [DOI] [PubMed] [Google Scholar]
- 26.Wu ZS, Lee JH, Kwon JA, et al. Genetic alterations and chemosensitivity profile in newly established human renal collecting duct carcinoma cell lines. BJU Int. 2009;103:1721–8. doi: 10.1111/j.1464-410X.2008.08290.x. [DOI] [PubMed] [Google Scholar]
- 27.Furugaki K, Yoshida J, Chijiiwa K, et al. Inferior vena caval thrombus associated with double neoplasms of the retroperitoneum and kidney: report of a case. Surg Today. 1996;26:658–61. doi: 10.1007/BF00311677. [DOI] [PubMed] [Google Scholar]
- 28.Ono K, Nishino E, Nakamine H. Renal collecting duct carcinoma. Report of a case with cytologic findings on fine needle aspiration. Acta Cytol. 2000;44:380–4. doi: 10.1159/000328482. [DOI] [PubMed] [Google Scholar]
- 29.Bagrodia A, Gold R, Handorf C, Liman A, Derweesh IH. Salvage paclitaxel chemotherapy for metastatic collecting duct carcinoma of the kidney. Can J Urol. 2008;15:4425–7. [PubMed] [Google Scholar]
- 30.Gollob JA, Upton MP, DeWolf WC, Atkins MB. Long-term remission in a patient with metastatic collecting duct carcinoma treated with Taxol/carboplatin and surgery. Urology. 2001;58:1058. doi: 10.1016/S0090-4295(01)01411-X. [DOI] [PubMed] [Google Scholar]
- 31.Chao D, Zisman A, Pantuck AJ, et al. Collecting duct renal cell carcinoma: clinical study of a rare tumor. J Urol. 2002;167:71–4. doi: 10.1016/S0022-5347(05)65385-2. [DOI] [PubMed] [Google Scholar]
- 32.Papadopoulos KP, Goel S, Beeram M, et al. A phase 1 open-label, accelerated dose-escalation study of the hypoxia-activated prodrug AQ4N in patients with advanced malignancies. Clin Cancer Res. 2008;14:7110–15. doi: 10.1158/1078-0432.CCR-08-0483. [DOI] [PubMed] [Google Scholar]
- 33.Dimopoulos MA, Logothetis CJ, Markowitz A, Sella A, Amato R, Ro J. Collecting duct carcinoma of the kidney. Br J Urol. 1993;71:388–91. doi: 10.1111/j.1464-410X.1993.tb15978.x. [DOI] [PubMed] [Google Scholar]
- 34.Kirkali Z, Celebi I, Akan G, Yorukoglu K. Bellini duct (collecting duct) carcinoma of the kidney. Urology. 1996;47:921–3. doi: 10.1016/S0090-4295(96)00045-3. [DOI] [PubMed] [Google Scholar]
- 35.Procopio G, Verzoni E, Iacovelli R, Colecchia M, Torelli T, Mariani L. Is there a role for targeted therapies in the collecting ducts of Bellini carcinoma? Efficacy data from a retrospective analysis of 7 cases. Clin Exp Nephrol. 2012;16:464–7. doi: 10.1007/s10157-012-0589-3. [DOI] [PubMed] [Google Scholar]
- 36.Staehler M, Haseke N, Schoppler G, et al. Carcinoma of the collecting ducts of Bellini of the kidney: adjuvant chemotherapy followed by multikinase-inhibition with sunitinib. Eur J Med Res. 2008;13:531–5. [PubMed] [Google Scholar]
- 37.Miyake H, Haraguchi T, Takenaka A, Fujisawa M. Metastatic collecting duct carcinoma of the kidney responded to sunitinib. Int J Clin Oncol. 2011;16:153–5. doi: 10.1007/s10147-010-0116-z. [DOI] [PubMed] [Google Scholar]
- 38.Ansari J, Fatima A, Chaudhri S, Bhatt RI, Wallace M, James ND. Sorafenib induces therapeutic response in a patient with metastatic collecting duct carcinoma of kidney. Onkologie. 2009;32:44–6. doi: 10.1159/000183736. [DOI] [PubMed] [Google Scholar]
- 39.Sternberg CN, Yagoda A, Scher HI, et al. Methotrexate, vinblastine, doxorubicin, and cisplatin for advanced transitional cell carcinoma of the urothelium. Efficacy and patterns of response and relapse. Cancer. 1989;64:2448–58. doi: 10.1002/1097-0142(19891215)64:12<2448::AID-CNCR2820641209>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 40.Loehrer PJ, Sr, Einhorn LH, Elson PJ, et al. A randomized comparison of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group study. J Clin Oncol. 1992;10:1066–73. doi: 10.1200/JCO.1992.10.7.1066. [DOI] [PubMed] [Google Scholar]
- 41.Rubinstein YR, Groft SC, Bartek R, et al. Creating a global rare disease patient registry linked to a rare diseases biorepository database: Rare Disease-hub (rd-hub) Contemp Clin Trials. 2010;31:394–404. doi: 10.1016/j.cct.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]