Abstract
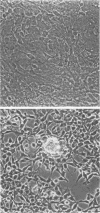
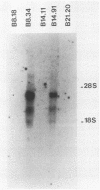
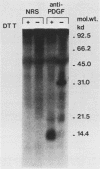
Long-term culturing of brain cells from neonatal BD-IX rats after transplacental treatment with N-ethyl-N-nitrosourea (ENU) results in malignantly transformed cells after a lag period of about 250 days. During culturing, the brain cells undergo a sequence of morphological changes. We examined oncogene expression in cultured cells from ENU-treated animals and found that transformed glioma cells differ from premalignant glial cells by containing high levels of c-sis transcripts. We also report that the transformed cells synthesize functional platelet-derived growth factor. Because glial cells have receptors for platelet-derived growth factor, we propose that an autocrine mechanism plays an important role in ENU-induced brain tumorigenesis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Betsholtz C., Heldin C. H., Nister M., Ek B., Wasteson A., Westermark B. Synthesis of a PDGF-like growth factor in human glioma and sarcoma cells suggests the expression of the cellular homologue to the transforming protein of simian sarcoma virus. Biochem Biophys Res Commun. 1983 Nov 30;117(1):176–182. doi: 10.1016/0006-291x(83)91557-7. [DOI] [PubMed] [Google Scholar]
- Betsholtz C., Westermark B., Ek B., Heldin C. H. Coexpression of a PDGF-like growth factor and PDGF receptors in a human osteosarcoma cell line: implications for autocrine receptor activation. Cell. 1984 Dec;39(3 Pt 2):447–457. doi: 10.1016/0092-8674(84)90452-5. [DOI] [PubMed] [Google Scholar]
- Betsholtz C., Westermark B., Ek B., Heldin C. H. Coexpression of a PDGF-like growth factor and PDGF receptors in a human osteosarcoma cell line: implications for autocrine receptor activation. Cell. 1984 Dec;39(3 Pt 2):447–457. doi: 10.1016/0092-8674(84)90452-5. [DOI] [PubMed] [Google Scholar]
- Bignami A., Eng L. F., Dahl D., Uyeda C. T. Localization of the glial fibrillary acidic protein in astrocytes by immunofluorescence. Brain Res. 1972 Aug 25;43(2):429–435. doi: 10.1016/0006-8993(72)90398-8. [DOI] [PubMed] [Google Scholar]
- Clarke M. F., Westin E., Schmidt D., Josephs S. F., Ratner L., Wong-Staal F., Gallo R. C., Reitz M. S., Jr Transformation of NIH 3T3 cells by a human c-sis cDNA clone. 1984 Mar 29-Apr 4Nature. 308(5958):464–467. doi: 10.1038/308464a0. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F., Hunkapiller M. W., Hood L. E., Devare S. G., Robbins K. C., Aaronson S. A., Antoniades H. N. Simian sarcoma virus onc gene, v-sis, is derived from the gene (or genes) encoding a platelet-derived growth factor. Science. 1983 Jul 15;221(4607):275–277. doi: 10.1126/science.6304883. [DOI] [PubMed] [Google Scholar]
- Eva A., Robbins K. C., Andersen P. R., Srinivasan A., Tronick S. R., Reddy E. P., Ellmore N. W., Galen A. T., Lautenberger J. A., Papas T. S. Cellular genes analogous to retroviral onc genes are transcribed in human tumour cells. Nature. 1982 Jan 14;295(5845):116–119. doi: 10.1038/295116a0. [DOI] [PubMed] [Google Scholar]
- Gazit A., Igarashi H., Chiu I. M., Srinivasan A., Yaniv A., Tronick S. R., Robbins K. C., Aaronson S. A. Expression of the normal human sis/PDGF-2 coding sequence induces cellular transformation. Cell. 1984 Nov;39(1):89–97. doi: 10.1016/0092-8674(84)90194-6. [DOI] [PubMed] [Google Scholar]
- Groffen J., Heisterkamp N., Stephenson J. R., van Kessel A. G., de Klein A., Grosveld G., Bootsma D. c-sis is translocated from chromosome 22 to chromosome 9 in chronic myelocytic leukemia. J Exp Med. 1983 Jul 1;158(1):9–15. doi: 10.1084/jem.158.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin C. H., Westermark B. Growth factors: mechanism of action and relation to oncogenes. Cell. 1984 May;37(1):9–20. doi: 10.1016/0092-8674(84)90296-4. [DOI] [PubMed] [Google Scholar]
- Heldin C. H., Westermark B., Wasteson A. Demonstration of an antibody against platelet-derived growth factor. Exp Cell Res. 1981 Dec;136(2):255–261. doi: 10.1016/0014-4827(81)90003-3. [DOI] [PubMed] [Google Scholar]
- Heldin C. H., Westermark B., Wasteson A. Specific receptors for platelet-derived growth factor on cells derived from connective tissue and glia. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3664–3668. doi: 10.1073/pnas.78.6.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivankovic S., Druckrey H. Transplacentare Erzeugung maligner Tumoren des Nervensystems. I. Athyl-nitroso-harnstoff (ANH) an BD IX-Ratten. Z Krebsforsch. 1968;71(4):320–360. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laerum O. D., Rajewsky M. F. Neoplastic transformation of fetal rat brain cells in culture after exposure to ethylnitrosourea in vivo. J Natl Cancer Inst. 1975 Nov;55(5):1177–1187. doi: 10.1093/jnci/55.5.1177. [DOI] [PubMed] [Google Scholar]
- Libermann T. A., Nusbaum H. R., Razon N., Kris R., Lax I., Soreq H., Whittle N., Waterfield M. D., Ullrich A., Schlessinger J. Amplification, enhanced expression and possible rearrangement of EGF receptor gene in primary human brain tumours of glial origin. Nature. 1985 Jan 10;313(5998):144–147. doi: 10.1038/313144a0. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Mirsky R., Fields K. L., Lisak R. P., Dorfman S. H., Silberberg D. H., Gregson N. A., Leibowitz S., Kennedy M. C. Galactocerebroside is a specific cell-surface antigenic marker for oligodendrocytes in culture. Nature. 1978 Aug 24;274(5673):813–816. [PubMed] [Google Scholar]
- Ross R., Vogel A. The platelet-derived growth factor. Cell. 1978 Jun;14(2):203–210. doi: 10.1016/0092-8674(78)90107-1. [DOI] [PubMed] [Google Scholar]
- Schechter A. L., Stern D. F., Vaidyanathan L., Decker S. J., Drebin J. A., Greene M. I., Weinberg R. A. The neu oncogene: an erb-B-related gene encoding a 185,000-Mr tumour antigen. Nature. 1984 Dec 6;312(5994):513–516. doi: 10.1038/312513a0. [DOI] [PubMed] [Google Scholar]
- Scher C. D., Shepard R. C., Antoniades H. N., Stiles C. D. Platelet-derived growth factor and the regulation of the mammalian fibroblast cell cycle. Biochim Biophys Acta. 1979 Aug 10;560(2):217–241. doi: 10.1016/0304-419x(79)90020-9. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Todaro G. J. Autocrine secretion and malignant transformation of cells. N Engl J Med. 1980 Oct 9;303(15):878–880. doi: 10.1056/NEJM198010093031511. [DOI] [PubMed] [Google Scholar]
- Waterfield M. D., Scrace G. T., Whittle N., Stroobant P., Johnsson A., Wasteson A., Westermark B., Heldin C. H., Huang J. S., Deuel T. F. Platelet-derived growth factor is structurally related to the putative transforming protein p28sis of simian sarcoma virus. Nature. 1983 Jul 7;304(5921):35–39. doi: 10.1038/304035a0. [DOI] [PubMed] [Google Scholar]
- Yen S. H., Fields K. L. Antibodies to neurofilament, glial filament, and fibroblast intermediate filament proteins bind to different cell types of the nervous system. J Cell Biol. 1981 Jan;88(1):115–126. doi: 10.1083/jcb.88.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yssel H., De Vries J. E., Koken M., Van Blitterswijk W., Spits H. Serum-free medium for generation and propagation of functional human cytotoxic and helper T cell clones. J Immunol Methods. 1984 Aug 3;72(1):219–227. doi: 10.1016/0022-1759(84)90450-2. [DOI] [PubMed] [Google Scholar]
- van Zoelen E. J., Twardzik D. R., van Oostwaard T. M., van der Saag P. T., de Laat S. W., Todaro G. J. Neuroblastoma cells produce transforming growth factors during exponential growth in a defined hormone-free medium. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4085–4089. doi: 10.1073/pnas.81.13.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]