Abstract
Spontaneous regression of metastatic melanoma is an exceedingly rare event, with only 76 well-documented cases in the literature since 1866. Here, we present the case of a patient who developed metastatic melanoma despite interferon therapy and who then achieved spontaneous regression shortly after a reaction to tetanus–diphtheria–pertussis vaccination. A common theme among these cases is the development of febrile illness before remission of the malignant disease. A brief overview of proposed mechanisms for these miraculous recoveries is presented, including a highlight on the potential role of the herv-k-mel viral marker, a nona- or decapeptide that appears in most melanomas, with homologies to peptides in pathogenic microorganisms.
Keywords: Melanoma, regression, fever, tetanus, vaccination, cancer, pertussis, diphtheria
1. CASE DESCRIPTION
A 44-year-old white man with no past medical history developed local irritation of a longstanding nevus on the right anterior tibial area, which progressed to ulceration and bleeding over several months. The lesion was excised by the patient’s family physician, and subsequent analysis showed malignant melanoma characterized by a depth of 2 mm and extension into the reticular dermis, consistent with a Clark level iii/ iv. No other clinical signs of disease or metastases were evident. The patient was referred for more complete excision of the lesion and, because of the depth of the lesion, sentinel node biopsy.
Five months from initial presentation, a sentinel node biopsy and repeat resection of the primary site, with 1 cm margins, was performed. The site of the primary lesion was free of melanoma. However, the sentinel node biopsy showed a 1-mm focus of disease.
After 3 months, a right inguinal lymph node dissection revealed involvement in 1 of 6 lymph nodes. Clinical examination and computed tomography imaging showed no evidence of distant metastasis. The patient was considered to have T2bN1aM0 stage iiib disease.
One month later, the patient started a 4-week induction course of adjuvant high-dose interferon alfa at a daily dose of 36×106 U intravenously 5 days per week, followed by an 11-month course of 18×106 U interferon alfa 3 times weekly subcutaneously for maintenance. He tolerated this regimen well, with some symptoms of muscle pain, diarrhea, fever, and occasional headaches in addition to a slight elevation of liver enzymes.
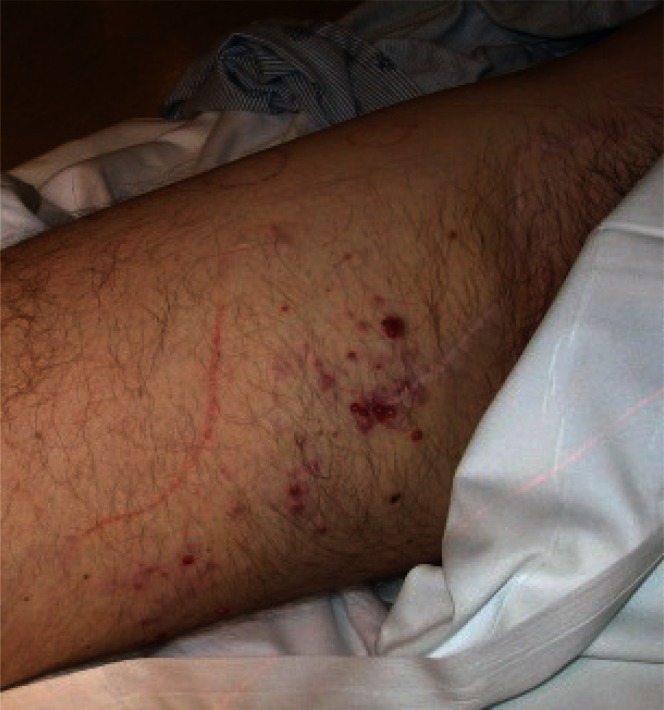
Eleven months into therapy, the patient developed multiple (approximately 15) in-transit metastases in the upper right thigh. These erythematous lesions ranged in size from 1 mm to 10 mm. Two other similar subcutaneous nodules were also seen on the upper lateral aspect of the right thigh (Figure 1). The patient underwent palliative radiotherapy of 40 Gy in 10 fractions to each nodule and 30 Gy in 10 fractions to the intervening skin, with good response. Follow-up imaging showed no other recurrence. The patient completed his 1-year course of interferon.
FIGURE 1.
Numerous in-transit metastases along the patient’s right thigh despite interferon therapy. Local control of the lesions was attempted using radiation therapy.
Six months after the course of interferon, the patient developed a tender 5-mm nodule above the left nipple, a 5-mm axillary nodule, and a small nodule under the chin and on the central back. The chest lesion was excised and proved to be melanoma. A punch biopsy of the left axillary nodule revealed a malignant non-melanin tumour. Immunohistochemistry for melanoma was positive for S100, mart-1, and tyrosinase, and negative for HMB45, suggesting metastatic disease1. Immunohistochemistry of the primary lesion was not available. In addition, imaging showed new right-middle-lobe lung nodules measuring up to 3.5 mm in diameter, and an enlarged 15×13-mm right external iliac lymph node. Investigations were initiated for enrollment in a trial for what was now stage iv disease.
Three months later, the patient received Adacel vaccine (Sanofi Pasteur, Lyon, France) as a routine preventive measure. The vaccine contains tetanus toxoid, diphtheria toxoid, acellular pertussis, aluminium phosphate, and 2-phenoxyethanol (dtap)2. The patient developed a local and systemic febrile reaction that lasted 2 days. One week later, dramatic improvement in all of the aforementioned nodules was noted on clinical examination. Computed tomography imaging showed a new 20-mm lymph node in the aortopulmonary window and also several new nodules measuring 3–11 mm in the right middle lobe, over the right pectoral muscle, in the left axilla, over the left rectus abdominis, and in the subcutaneous fat overlying the T3 spinous process. A biopsy of a resolved nodule on the patient’s back showed fibrosis, but no disease. The patient underwent human leukocyte antigen (hla) typing in anticipation of being enrolled in a trial for MDX-10 (currently known as ipilimumab), which yielded hla-A2, -A29, -B8, -B44, and -CW7. Repeat imaging before study enrollment after another 3 months showed progressive reduction in the size of the nodules. Two months later, all of the nodules had vanished, except for an enlarged nodule in the left axilla. Because of this remission, the patient was not enrolled.
One month later, the 10-mm lymph node in the left axilla was resected. The surgical specimen showed melanoma, which again stained positive for S100, mart-1, and tyrosinase, and negative for HMB45. Although a second primary tumor cannot be ruled out, it is highly unlikely. This patient has since been regularly followed with clinic visits and imaging that continues to demonstrate a disease-free state. The patient had received 1 year’s worth of adjuvant interferon therapy 9 months after initial presentation (which failed) and superficial local disease control with excision and radiotherapy 20 months after his first biopsy.
2. DISCUSSION
To the best of the authors’ knowledge, this is the only reported case of regression of metastatic melanoma after administration of the dtap vaccine.
Unlike regression of primary melanoma, which has been reported in up to 50% of cases, regression of metastatic melanoma is a rare event3. A review by Kalialis et al. recovered only 76 cases from the literature since 1866, which corresponds to an incidence of 0.23%3. Of particular interest, our patient demonstrated not only subcutaneous and lymphatic regression, but also regression of lung involvement, which has been seen in only 10 cases in the literature4–12.
Currently, no mechanism for spontaneous regressions has been established. However, several concurrent events are often cited in the literature: febrile infection, operative procedures, hormonal influences, immunologic factors, or no objective cause13. Of particular interest in this case is the preceding fever, which we attribute to the patient’s dtap inoculation.
It has been noted for some time that regression of cancers can occur in the context of febrile bacterial infection, particularly those attributable to Streptococcus pyogenes. In fact, Maurer and Koelmel’s review of 68 melanoma regression cases noted that 21 were preceded by a febrile episode, of which 9 were attributed to erysipelas13. Of historical interest is Coley’s toxin, which consisted of heat-treated Streptococcus pyogenes and Serratia marcescens, created by William B. Coley, which was used with variable success a century ago in the treatment of sarcoma14. Later review of his original cases suggested greater effectiveness in the setting of febrile reaction15. Interestingly, it has been shown that tumour cell death occurs to a greater extent in a heated milieu16. Matzinger proposed, through the “danger” model, that although tumour cells are in fact recognized by the immune system, they do not elicit a response because they have means of inducing tolerance, and thus there is an antigen, but no danger signal17. However, in the context of acute bacterial illness or vaccination, there is binding of pathogen-specific molecular patterns such as bacterial components to Toll-like receptors of antigen-presenting cells, which can, in turn, activate tumour-toxic T cells.
Interestingly, the constituent bacteria of the dtap vaccine have been used in some fashion to combat cancer. The direct cytotoxic effects of the diphtheria toxin have been exploited in a series of conjugates formed with ligands that possess an affinity for tumour antigens18. Our patient received a toxoid rather than a toxin (not designed to have any particular affinity for melanoma cells) and so it is unlikely that direct cellular toxicity from the diphtheria component is a trigger for the observed remission. Pertussis toxin has been shown to possess adjuvant properties that are able to enhance both the humoral response and the Th1 and Th2 responses to antigens19–22.
From a prevention standpoint, research by the Febrile Infections and Melanoma group, a population-based case–control study, showed that smallpox and Bacillus Calmette–Guérin vaccination and prior serious infection can be protective against melanoma23. Krone et al.23 presented a potential mechanism for that protection, which relies on human endogenous retroviruses (hervs). These genetic elements, most of which are functionally degenerate, make up roughly 8% of the human genome. However, some of these elements code for antigenic peptides and retroviral proteins and enzymes. One such peptide is the herv-k-mel viral marker, a nona- or decapeptide that appears in most melanomas and dysplastic nevi24. The herv-k-mel marker is hla-restricted in its presentation to CD8+ T cells. Specifically, it requires hla-A2, which was present in our patient. Interestingly, this peptide possesses homologies to peptides found in many human pathogens, including Clostridium tetani, Bordetella pertussis, and Corynebacterium diphtheriae23. It is thought that previous exposure to pathogens possessing peptides similar to herv-k-mel could induce a cross-reactivity against melanoma by cytotoxic T lymphocytes. Unfortunately, testing for herv-k-mel peptide in our patient’s samples was not available.
3. SUMMARY
Spontaneous regression of metastatic melanoma is a rare event that has been associated with febrile illness. Acute febrile illness may trigger melanoma regression by providing a danger signal that leads to T-cell activation. Available epidemiologic evidence suggests that exposure to or vaccination against certain infectious pathogens may provide protection against the development of melanoma. Lastly, the homologies between the products of the herv-k-mel viral marker prevalent in melanomas and the peptides in human pathogens such as C. tetani, B. pertussis, and C. diphtheriae offer a potential target for an immune-mediated cross-reactivity mechanism in melanoma regression.
4. CONFLICT OF INTEREST DISCLOSURES
The authors have no financial conflicts of interest to report.
5. REFERENCES
- 1.Jungbluth AA. Serological reagents for the immunohistochemical analysis of melanoma metastases in sentinel lymph nodes. Semin Diagn Pathol. 2008;25:120–5. doi: 10.1053/j.semdp.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 2.Sanofi Pasteur Limited . Adacel (Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis Vaccine Adsorbed)[product monograph] Toronto, ON: Sanofi Pasteur Limited; 2009. [Google Scholar]
- 3.Kalialis LV, Drzewiecki KT, Klyver H. Spontaneous regression of metastases from melanoma: review of the literature. Melanoma Res. 2009;19:275–82. doi: 10.1097/CMR.0b013e32832eabd5. [DOI] [PubMed] [Google Scholar]
- 4.Block GE, Hartwell SW. Malignant melanoma: a study of 217 cases: part i: epidemiology. Ann Surg. 1961;154(suppl 6):74–87. doi: 10.1097/00000658-196112000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malleson N. Spontaneous regression of malignant melanoma. Br Med J. 1955;1:668. doi: 10.1136/bmj.1.4914.668-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Everson TC, Cole WH. Spontaneous regression of cancer: preliminary report. Ann Surg. 1956;144:366–83. doi: 10.1097/00000658-195609000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mikhail GR, Gorsulowsky DC. Spontaneous regression of metastatic malignant melanoma. J Dermatol Surg Oncol. 1986;12:497–500. doi: 10.1111/j.1524-4725.1986.tb01939.x. [DOI] [PubMed] [Google Scholar]
- 8.Maurer S, Koelmel KF. Spontaneous Regression of Malignant Melanoma. New York, NY: Cancer Research Institute; 1997. Monograph No. 19. [Google Scholar]
- 9.Wang TS, Lowe L, Smith JW, 2nd, et al. Complete spontaneous regression of pulmonary metastatic melanoma. Dermatol Surg. 1998;24:915–19. doi: 10.1016/S1076-0512(98)00093-4. [DOI] [PubMed] [Google Scholar]
- 10.Cavell B. Transplacental metastasis of malignant melanoma. Report of a case. Acta Paediatr. 1963;(suppl 146):37–40. doi: 10.1111/j.1651-2227.1963.tb05515.x. [DOI] [PubMed] [Google Scholar]
- 11.Levison VB. Spontaneous regression of a malignant melanoma. Br Med J. 1955;1:458–9. doi: 10.1136/bmj.1.4911.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teimourian B, McCune WS. Surgical management of malignant melanoma. Am Surg. 1963;29:515–19. [PubMed] [Google Scholar]
- 13.Maurer S, Kolmel KF. Spontaneous regression of advanced malignant melanoma. Onkologie. 1998;21:14–18. doi: 10.1159/000026785. [DOI] [Google Scholar]
- 14.Coley WB. The treatment of inoperable sarcoma by bacterial toxins (the mixed toxins of the Streptococcus erysipelas and the Bacillus prodigiosus) Proc R Soc Med. 1910;3:1–48. doi: 10.1177/003591571000301601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nauts HC, McLaren JR. Coley toxins—the first century. Adv Exp Med Biol. 1990;267:483–500. doi: 10.1007/978-1-4684-5766-7_52. [DOI] [PubMed] [Google Scholar]
- 16.Trieb K, Sztankay A, Amberger A, Lechner H, Grubeck–Loebenstein B. Hyperthermia inhibits proliferation and stimulates the expression of differentiation markers in cultured thyroid carcinoma cells. Cancer Lett. 1994;87:65–71. doi: 10.1016/0304-3835(94)90410-3. [DOI] [PubMed] [Google Scholar]
- 17.Matzinger P. An innate sense of danger. Semin Immunol. 1998;10:399–415. doi: 10.1006/smim.1998.0143. [DOI] [PubMed] [Google Scholar]
- 18.Frankel AE, Rossi P, Kuzel TM, Foss F. Diphtheria fusion protein therapy of chemoresistant malignancies. Curr Cancer Drug Targets. 2002;2:19–36. doi: 10.2174/1568009023333944. [DOI] [PubMed] [Google Scholar]
- 19.Adkins I, Holubova J, Kosova M, Sadilkova L. Bacteria and their toxins tamed for immunotherapy. Curr Pharm Biotechnol. 2012;13:1446–73. doi: 10.2174/138920112800784835. [DOI] [PubMed] [Google Scholar]
- 20.Samore MH, Siber GR. Pertussis toxin enhanced IgG1 and IgE responses to primary tetanus immunization are mediated by interleukin-4 and persist during secondary responses to tetanus alone. Vaccine. 1996;14:290–7. doi: 10.1016/0264-410X(95)00201-B. [DOI] [PubMed] [Google Scholar]
- 21.Ryan M, McCarthy L, Rappuoli R, Mahon BP, Mills KH. Pertussis toxin potentiates Th1 and Th2 responses to co-injected antigen: adjuvant action is associated with enhanced regulatory cytokine production and expression of the co-stimulatory molecules B7-1, B7-2 and CD28. Int Immunol. 1998;10:651–62. doi: 10.1093/intimm/10.5.651. [DOI] [PubMed] [Google Scholar]
- 22.Roberts M, Bacon A, Rappuoli R, et al. A mutant pertussis toxin molecule that lacks adp–ribosyltransferase activity, PT-9K/129G, is an effective mucosal adjuvant for intranasally delivered proteins. Infect Immun. 1995;63:2100–8. doi: 10.1128/iai.63.6.2100-2108.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krone B, Kolmel KF, Henz BM, Grange JM. Protection against melanoma by vaccination with Bacille Calmette–Guérin (bcg) and/or Vaccinia: an epidemiology-based hypothesis on the nature of a melanoma risk factor and its immunological control. Eur J Cancer. 2005;41:104–17. doi: 10.1016/j.ejca.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 24.Schiavetti F, Thonnard J, Colau D, Boon T, Coulie PG. A human endogenous retroviral sequence encoding an antigen recognized on melanoma by cytolytic T lymphocytes. Cancer Res. 2002;62:5510–16. [PubMed] [Google Scholar]