Abstract
In the present study, artificial sweeteners—aspartame, acesulfame-K and binary sweetener blend of aspartame x acesulfame-K were assessed for stability during storage in whey lemon beverage. A solid phase extraction method using C18 cartridges was standardized for the isolation of aspartame, acesulfame-K and their degradation products in whey lemon beverage. HPLC analytical conditions were standardized over C18 column for simultaneous separation of multiple sweeteners and their degradation products in sample isolates. Storage studies revealed that increase in acidity and viscosity and decrease in pH and ascorbic acid content of artificially sweetened whey lemon beverage samples were similar to the changes occurring in control samples during storage. Analysis using HPLC showed that aspartame (added either singly or in a blend) and acesulfame-K (added in a blend) were stable in whey lemon beverage under refrigerated condition for 15 days.
Keywords: Aspartame, Acesulfame-K, Stability, HPLC, Solid phase extraction, Whey lemon beverage
Introduction
Whey is one of the most important byproducts of the dairy industry obtained mainly during the manufacture of cheese, paneer, chhana, casein and other coagulated milk products. In the production of cheese, paneer and chhana, only 10–20% of milk is recovered as the desired end product and the remaining 80–90% is whey. It is estimated that about 4.84-million tonnes/annum of whey is generated in India (Raju et al. 2005). Whey contains almost all the nutrients of milk except casein and fat, thus making it highly nutritious. Unfortunately, this valuable byproduct is presently being drained-off due to absence of economically viable methods for its utilization, which not only causes losses of precious nutrients but also creates environmental problems due to its high biological oxygen demand (45,000–60,000 ppm). Therefore, there has been a great emphasis all over the world on the utilization of whey solids in different ways.
For orchestrating the growth of dairy industry in the liberalized global economies, product diversification at competitive prices for the domestic and export markets is required. The diversion of whey solids to human food chain employing cost effective technologies appears to be the best alternative to utilize whey. Conversion of whey into a beverage on a commercial scale has an economic advantage, as the whole quantity is being used and there are no problems of left over whey solids. Beverages in general, provide energy, water to digest food, regulate body temperature, prevent dehydration, quench thirst and remove psychological tensions (Shaikh et al. 2001). Use of artificial sweeteners in whey beverage can provide benefit to the diabetic and obese people. Since aspartame and acesulfame-K are high potency sweeteners (approximately 200 times sweeter than sucrose) without major aftertaste problems (Larson-Powers and Pangborn 1978) they may serve as a total sugar replacement in whey beverages, reducing the carbohydrate content significantly. Whey based beverages, in particular the fruit flavoured varieties, have gained popularity in some countries owing to their refreshing taste and healthful image (Beukema and Jelen 1990). The whey from paneer was suited more for preparation of whey lemon beverage than cheese whey as the latter did not have an acidic tang. This may be due to the addition of citric acid for the preparation of paneer, resulting in whey having acidic tinge. The whey flavour particularly that of acid whey, was most compatible with citrus flavours (Holsinger et al. 1974).
Since, the application of artificial sweeteners in dairy products, particularly in whey lemon beverage is new, quantitative information on the degradation/decomposition of sweeteners in dairy products is required. Aspartame is stable to low acidic and alkaline conditions but it is unstable to heat. Prolonged exposure of aspartame to high temperatures and storage leads to its breakdown into aspartylphenylalanine and methanol or in case of cyclisation into diketopiperazine (Jana et al. 1994). The use of aspartame has been of some concern due to the formation of the potentially toxic metabolites, methanol, aspartic acid and phenylalanine (Higton and Thurgood 1994). Acesulfame-K has high degree of stability when exposed to heat; this makes it a versatile sweetener with potential use in a wide range of foods and beverages. Keeping in view the nutritional significance of whey beverages and the importance of use of sweetener/sweetener blend in their production, the present work was conducted to assess the stability of aspartame and acesulfame-K during storage of the product.
Materials and methods
Materials: Lemon essence (International Flavours and Fragrances India Ltd., Andhra Pradesh, India) and lemon juice concentrate (Lemoneez) (Dabur Nepal Pvt. Ltd., Bara, Nepal), aspartame (NutraSweet Company, Augusta, Georgia, USA), aspartame degradation products standards: 2,5-diketopiperazine, L-phenylalanine and L-phenylalanine methyl ester (Sigma-Aldrich Corporation, Lovfs, Missouri, USA), acesulfame-K (Nutrinova, Nutrinova Specialities and Food Ingredients GmbH, Frankfurt, Germany) and acesulfame-K degradation product : acetoacetamide (Sigma-Aldrich Corporation, Lovfs, Missouri, USA) were used in the study. Carrez solution No.1 was prepared by dissolving 3.6 g of potassium ferrocyanide in 100 ml water. Carrez solution No.2 was prepared by dissolving 7.2 g of zinc sulphate in 100 ml water. Mobile phase A: 0.02 M phosphate buffer (pH 5.0): acetonitrile (97:3). Mobile phase B: 0.02 M phosphate buffer (pH 3.5): acetonitrile (80:20) were used for HPLC analysis. Aspartylphenylalanine standard was prepared according to the method of Tsang et al. (1985) by dissolving 50 mg of aspartame in 60 ml of water. The solution was adjusted to pH 8 with diluted sodium hydroxide and then made up to 100 ml with water. Diketopiperazine and aspartylphenylalanine were formed after the solution was stored for a day.
Standard solutions: Ten milligrams each of the sweetener and their degradation products were dissolved separately in 10 ml mixture of mobile phases A and B (1:1), to get standard solutions each of concentration 1 mg/ml. Appropriate dilutions of the stock solutions were made with mixture of mobile phases A and B (1:1) to get standard solutions containing 10 and 12.5 ng/μl. Standard solution of aspartame, acesulfame-K, 2, 5 diketopiperazine, L-phenylalanine and acetoacetamide was also prepared with a mixture of mobile phase A and B (1:1) to get a concentration of each component as 10 ng/μl in the mix.
Equipments: Vacuum filtration assembly (Millipore Corporation, Bedford, MA, USA), solid phase extraction C18 cartridge (Supelco, Bellfonte, PA, USA), ultra-sonifier (Sonics, Vibra Cell, Model VCx750, Newton, CT, USA), solid phase extraction vacuum manifold (VisiprepTM DL, Supelco, Bellfonte, PA, USA), HPLC system with UV dual λ absorbance detector (Model 2487 of Waters, USA), nitrogen analyzer (Gerhardt GmbH & Co. KG, Bonn, Germany) and pH meter (μp Phan, Lab India, New Delhi) were used in the study.
Preparation of whey lemon beverage: Paneer whey (pH 5.47) was collected from Experimental Dairy, NDRI, Karnal. Whey lemon beverage samples with sugar (control) @100 g/kg whey and whey lemon beverage with artificial sweeteners was essentially prepared according to method of Singh et al. (2003) which involved pasteurization (80 °C/5 min) of whey followed by addition of sugar/sweetener (optimized level), addition of lemon juice concentrate (@ 3.5%) and the pH adjustment to 3.8 (using 50% citric acid solution), addition of lemon essence (@ 0.015%) and finally in-bottle pasteurization (85 °C/2-3 min). The most acceptable level of sweeteners when added individually in whey lemon beverage was 0.07% for aspartame, 0.06% for acesulfame-K and when added in blend of aspartame x acesulfame-K, it was 0.05% (i.e. 0.025% each).
Storage and analysis of whey lemon beverage: Control and artificially sweetened samples of whey lemon beverage (with best selected sweetener levels) were stored under refrigerated temperature (6–8 °C). The samples were analyzed on 0, 5th, 10th and 15th day of storage for pH (IS: SP 18 Part XI 1981), ascorbic acid content, titratable acidity of whey lemon beverage samples (Ranganna 2005) and viscosity (Roy and Sen 1994). The control and artificially sweetened samples of whey lemon beverage were also analyzed for fat, lactose and total ash (IS: SP 18 Part XI 1981), total protein (IDF 20–3 2001), total solids and total soluble solids (Ranganna 2005). Stability of the sweeteners was analyzed using HPLC.
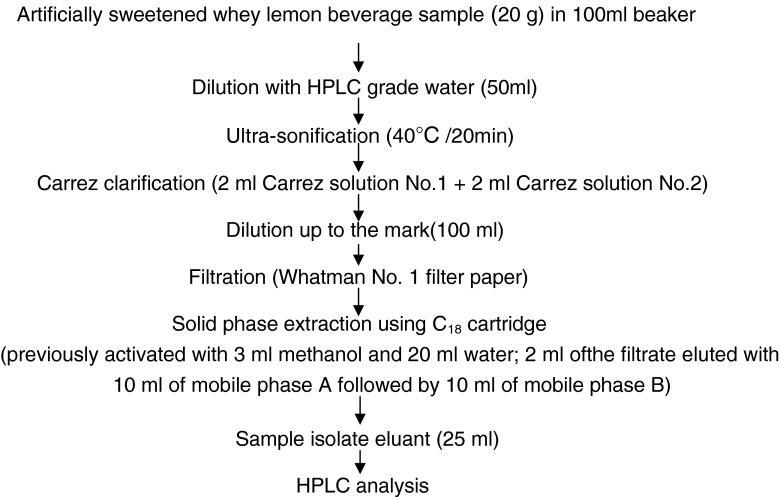
Sample preparation and HPLC analysis: The sample preparation procedure used for isolation of sweeteners (Fig 1) acesulfame-K and aspartame from whey lemon beverage was essentially based upon the method of BSEN 12856 (1999). Elution with 10 ml of mobile phase A followed by 10 ml of mobile phase B gave maximum recovery of aspartame and acesulfame-K. Reverse phase HPLC analysis was performed for the standard samples of aspartame, acesulfame-K, and degradation products (i.e. 2, 5 diketopiperazine, L-phenylalanine and acetoacetamide) and the sample isolates of whey lemon beverage by using the C18 column (Princeton SPHER-100 C18, 100A 5 μm, 250 × 4.6 mm ID column) at V wavelength 200 nm using binary gradient programming with mobile phase A and mobile phase B for 30 min run time. Gradient conditions were 0% mobile phase B for first 8 min, 0–100% B linear gradient from 8–13 min, 100% B from 13—to 25 min, 100—0% B linear gradient from 25–27 min and 0% B from 27–30 min. The actual pressure varied during programming as 80-160-80 Kgmf.
Fig. 1.
Flowchart for isolation of artificial sweeteners
Five-point calibration curves were plotted for acesulfame-K, aspartame and their degradation products representing 50, 100, 150, 200 and 250 ng concentration of the sweeteners and their degradation products. The correlation coefficient of 0.99 for different sweeteners and degradation products showed the linearity of the system. Recovery experiments of acesulfame-K, aspartame and of their respective degradation products were performed at 500, 500, 800 and 625 ppm respectively in whey lemon beverage. The detection limits of the HPLC system were determined by gradually reducing the concentrations of injections till the peaks disappeared. Overlapping of standard spectra and sample spectra was the criteria used for confirmation of presence of degradation product in the sample isolates.
Statistical analysis: In all experiments, one-way/two-way analysis of variance (ANOVA) with a subsequent least significant difference (LSD) test was applied for multiple sample comparison. This was done to test for any significant differences (P < 0.05) in the mean values of all the groups as described by Snedecor and Cochran (1994).
Results and discussion
Composition: Three laboratory scale trials were conducted each for preparation of whey beverages using sucrose/sweetener/sweetener blend. Composition of different whey lemon beverages is presented in Table 1, respectively. Total solids and total soluble solids (%) were higher in control than those in whey lemon beverage manufactured with sweetener/sweetener blend. These differences were due to the presence of sucrose in control whey lemon beverage.
Table 1.
Composition of control, aspartame and aspartame x acesulfame-K sweetened whey lemon beverages
| Parameters | Sucrose sweetened whey lemon beverage (Control) | Aspartame sweetened whey lemon beverage | Aspartame x Acesulfame-K sweetened whey lemon beverage |
|---|---|---|---|
| Fat (%) | 0.04 ± 0.00 | 0.04 ± 0.00 | 0.04 ± 0.00 |
| Total protein (%) | 0.51 ± 0.01 | 0.51 ± 0.01 | 0.51 ± 0.01 |
| Lactose (%) | 4.8 ± 0.14 | 4.8 ± 0.13 | 4.8 ± 0.13 |
| Sucrose (%) | 10.0 ± 0.12 | 0.00 | 0.00 |
| Ash (%) | 0.68 ± 0.02 | 0.67 ± 0.02 | 0.67 ± 0.02 |
| Total solids (%) | 15.2 ± 0.21 | 6.6 ± 0.17 | 6.5 ± 0.18 |
| Total soluble solids (%) | 14.6 ± 0.12 | 6.0 ± 0.10 | 6.0 ± 0.10 |
| Ascorbic acid (mg/100 ml) | 3.3 ± 0.02 | 3.3 ± 0.02 | 3.3 ± 0.02 |
| Moisture (%) | 84.8 ± 0.17 | 93.4 ± 0.15 | 93.4 ± 0.16 |
| Acidity (% citric acid) | 0.55 ± 0.01 | 0.56 ± 0.01 | 0.56 ± 0.01 |
| pH | 3.8 ± 0.01 | 3.8 ± 0.01 | 3.8 ± 0.01 |
Data are presented as means±SEM (n = 3)
Titratable acidity and pH: There was a significant (P < 0.05) increase in acidity of control as well as artificially sweetened whey lemon beverage samples during storage (Table 2). However, non-significant differences (P > 0.05) were observed in titratable acidity between control and whey lemon beverage sweetened with sweetener/sweetener blend throughout the storage period.
Table 2.
Titratable acidity, pH, Ascorbic acid and Viscosity of artificially sweetened whey lemon beverages during storage
| S.No. | Whey lemon beverage sweetened with | Storage period (days) | |||
|---|---|---|---|---|---|
| 0 | 5 | 10 | 15 | ||
| Titratable acidity (% citric acid) | |||||
| 1 | Sucrose (Control) | 0.55 ± 0.01aA | 0.61 ± 0.01bA | 0.67 ± 0.01cA | 0.74 ± 0.01dA |
| 2 | AS (0.07%) | 0.56 ± 0.01aA | 0.62 ± 0.01bA | 0.68 ± 0.01cA | 0.75 ± 0.01dA |
| 3 | AS x AK (50:50, 0.05%*) | 0.56 ± 0.01aA | 0.62 ± 0.01bA | 0.68 ± 0.01cA | 0.75 ± 0.01dA |
| pH | |||||
| 1 | Sucrose (Control) | 3.83 ± 0.01aA | 3.80 ± 0.01bA | 3.77 ± 0.01cA | 3.74 ± 0.01dA |
| 2 | AS (0.07%) | 3.82 ± 0.01aA | 3.79 ± 0.01bA | 3.76 ± 0.01cA | 3.73 ± 0.01dA |
| 3 | AS x AK (50:50, 0.05%*) | 3.82 ± 0.01aA | 3.79 ± 0.01bA | 3.76 ± 0.01cA | 3.73 ± 0.01dA |
| Ascorbic acid content (mg/100 ml) | |||||
| 1 | Sucrose (Control) | 3.33 ± 0.002aA | 3.21 ± 0.002bA | 3.06 ± 0.002cA | 2.58 ± 0.001dA |
| 2 | AS (0.07%) | 3.33 ± 0.002aA | 3.21 ± 0.002bA | 3.06 ± 0.002cA | 2.58 ± 0.001dA |
| 3 | AS x AK (50:50, 0.05%*) | 3.33 ± 0.002aA | 3.21 ± 0.002bA | 3.06 ± 0.002cA | 2.58 ± 0.001dA |
| Viscosity (cp) | |||||
| 1 | Sucrose (Control) | 1.49 ± 0.01aA | 1.53 ± 0.01bA | 1.56 ± 0.01cA | 1.58 ± 0.01dA |
| 2 | AS (0.07%) | 1.18 ± 0.01aB | 1.20 ± 0.01bB | 1.21 ± 0.01cB | 1.24 ± 0.01dB |
| 3 | AS x AK (50:50, 0.05%*) | 1.17 ± 0.01aB | 1.19 ± 0.01bB | 1.21 ± 0.01cB | 1.24 ± 0.01dB |
Means±SEM with different superscripts in each row (a, b, c, d) and in column (A, B) differ significantly (LSD test, P < 0.05) (n = 9) * denotes the actual level of sweeteners added in whey lemon beverage
It is evident from Table 2 that there was a significant (P < 0.05) decrease in pH of artificially sweetened whey lemon beverage samples and control during the entire period of storage. However, pH of control and whey lemon beverage sweetened with sweetener/sweetener blend did not differ significantly (P > 0.05) during storage.
Ascorbic acid: Concentrated lemon juice is rich source of ascorbic acid which may be lost during storage and the degree of loss is greatly influenced by temperature of storage and, oxygen and metal ion contents in the sample (Marcy et al. 1989). There was a significant (P < 0.05) decrease in ascorbic acid of artificially sweetened whey lemon beverage samples and control during the entire period of storage. However, ascorbic acid of control and whey lemon beverage sweetened with sweetener/sweetener blend did not differ significantly (P > 0.05) during storage.
Viscosity: Analysis of variance revealed that control as well as artificially sweetened whey lemon beverage samples showed a significant (P < 0.05) increase in viscosity during storage. Statistical analysis of the data also revealed that viscosity of control whey lemon beverage was significantly higher (P < 0.05) than that of the samples sweetened with sweeteners/sweetener blend during all periods of storage.
The increase in viscosity during storage could result from increased protein-protein interaction, as the precipitation was observed in control as well as in artificially sweetened whey lemon beverage during storage. During the manufacture of whey lemon beverage, denaturation of whey proteins takes place due to thermal treatment. The low pH of the beverage results in a complex phenomenon involving unfolding of globular structure, exposure of buried hydrophilic/hydrophobic groups leading to an increase in size and shape of the molecule, resulting in increase in viscosity. According to Fennema (1977), whey proteins are generally very soluble and thus do not bind large amounts of water in their native conformation. However, heat treatment of proteins results in unfolding and increase in water binding capacity of the protein molecules. Increased citrate content in whey lemon beverage also affects ionic environment, which may lead to changes in structure and functional behaviour of whey proteins. The viscosity of a protein in solution depends primarily on the size and shape of the molecule and its electric charge. It is also influenced by pH and ionic environment (Dewit and Swinkel 1980).
In whey, α-lactalbumin exists as calcium metalloprotein and calcium is released below pH 4.0, making the molecule flexible with large structural changes accompanied by apparent denaturation (Oakenfull et al. 1997). Residual β-lactoglobulin in paneer whey, when adjusted to a low pH (as in whey lemon beverage), is likely to form octomers, resulting in increased viscosity (Pessen et al. 1985). Viscosity of whey proteins decreases near pI (Cayot and Lorient 1997) but there is slight increase towards acidic side of their pI as in the case of whey lemon beverage.
HPLC Analysis of sweeteners and their degradation products: Table 3 depicts the per cent recovery and detection limits of different sweeteners and their degradation products. The detection limits were in the range of 10–40 ng. Lawrence and Charbonneau (1988) also reported similar observations for acesulfame-K and aspartame. However, no information is available in the literature regarding the detection limits of degradation products of sweeteners.
Table 3.
Detection limits of different sweeteners and their degradation products
| Sl. No | Sweetener/Degradation products | Detection limits (ng) at 200 nm | % Recovery |
|---|---|---|---|
| 1 | Aspartame | 30 | 88.3 ± 0.27 |
| 2 | Acesulfame-K | 40, 15 ** | 95.7 ± 0.78 |
| 3 | Diketopiperazine | 10 | 96.1 ± 0.83 |
| 4 | L-Phenylalanine | 20 | 94.9 ± 0.85 |
| 5 | Acetoacetamide | 20 | 96.0 ± 0.68 |
** Detection limit at 220 nm. Data are presented as means ± SEM (n = 3)
Stability of aspartame and acesulfame-K in whey lemon beverage during storage as analyzed using HPLC: Isolates of stored whey lemon beverage samples were analyzed over HPLC after 0, 5th, 10th and 15th day of storage under the standardized analytical conditions. Table 4 depicts the stability of aspartame added singly and in binary blend with acesulfame-K in whey lemon beverage during storage. It is evident that levels of aspartame and acesulfame-K remained unchanged even after 15 days of storage in whey lemon beverage, establishing their stability during storage. HPLC chromatograms obtained on 0, 5th, 10th and 15th day of storage for whey lemon beverage sample isolates sweetened with sweetener/sweetener blend also supported this observation as there was no peak other than aspartame and acesulfame-K in these chromatograms. This clearly established that aspartame and acesulfame-K were not degraded during storage in whey lemon beverage samples under investigation. Among all sweeteners, aspartame is the most heat labile sweetener and its degradation is minimal in the pH range of 4.0–5.0 (Bell and Labuza 1991). As the pH of whey lemon beverage observed in the present study was between 3.7 and 3.85, aspartame remained stable and did not degrade on storage. The findings were also in support of the fact that acesulfame-K had good stability in the pH range common for beverages (Lipinski Von Rymon 1988) and decomposed only under extreme conditions of pH (Arpe 1978) and temperatures well above 200 °C (Lipinski Von Rymon and Hanger 2004).
Table 4.
Stability of aspartame and (aspartame x acesulfame-K) binary sweetener blend in whey lemon beverage during storage as analyzed over HPLC
| Sweetener (level in ppm) | Storage period (days) | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 5 | 10 | 15 | |||||
| Recovery | Recovery | Recovery | Recovery | |||||
| ppm | % | ppm | % | ppm | % | ppm | % | |
| Aspartame (700) | 651.4 | 93.0 | 618.0 | 88.3 | 638.3 | 91.2 | 635.2 | 90.8 |
| Binary sweetener blend (Aspartame x Acesulfame-K) | ||||||||
| Aspartame (250) | 230.4 | 92.2 | 226.7 | 90.7 | 229.3 | 91.7 | 225.3 | 90.1 |
| Acesulfame-K (250) | 245.9 | 98.3 | 244.7 | 97.9 | 246.2 | 98.5 | 243.2 | 97.3 |
The results obtained are in accordance with our earlier findings (Arora et al. 2006, 2007 and 2008) wherein we reported stability of aspartame in burfi and kalakand and acesulfame-K in burfi, kalakand and flavoured milk up to 7 days of storage. However, in case of flavoured milk, aspartame level remained unchanged upto 3rd day of storage but on 5th and 7th day of storage, there was a loss of 25% and 38% in aspartame recovery suggesting that aspartame degraded in flavoured milk after 3 days of storage due to the inherent pH (6.6) of milk. The degradation product of aspartame obtained was aspartylphenylalanine. However, no such peak of aspartylphenylalanine was observed in the HPLC chromatograms of whey lemon beverage, thereby establishing further that aspartame did not degrade in whey lemon beverage samples during storage. Beukema and Jelen (1990) reported a loss of 13.2% of aspartame and no loss of acesulfame-K during heat treatment of 90 °C/30 min used for preparation of whey beverage. The workers however did not report the presence of any degradation product of aspartame in support of sweetener loss.
Conclusion
An isolation procedure involving solid phase extraction (SPE) using C18 cartridges was standardized for the isolation of multiple sweeteners (aspartame and acesulfame-K) and their degradation products (diketopiperazine, L-phenylalanine, and acetoacetamide) in whey lemon beverage followed by HPLC analysis of whey lemon beverage sample isolates. HPLC analytical conditions were standardized over C18 column using UV detector for the simultaneous separation of multiple sweeteners aspartame and acesulfame-K and their degradation products (diketopiperazine, L-phenylalanine and acetoacetamide) in a single run using binary gradient programming in whey lemon beverage sample isolates. Detection limits of sweeteners and their degradation products were in the range of 10–40 ng at 200 nm. The increase in acidity and viscosity and decrease in pH of artificially sweetened whey lemon beverage samples during storage were similar to control and thus were not influenced by the addition of sweeteners/sweetener blend. Analysis of aspartame and acesulfame-K added either singly or in blend in whey lemon beverage, showed their stability throughout the storage period as analyzed over HPLC. Thus, the present investigation established the successful use of two artificial sweeteners aspartame and acesulfame-K in whey lemon beverage providing an alternate variety to the health conscious consumers.
Acknowledgement
The authors would like to express appreciation to NutraSweet Company and Nutrinova Specialities and Food Ingredients for supplying ingredients for this study.
References
- Arora S, Sharma V, Wadhwa BK, Sharma GS, Singh AK (2006) Estimation and stability of low calorie artificial sweeteners in indigenous dairy products. In: IRC proceedings, Annual report of National Dairy Research Institute (2006–2007) Haryana, Karnal
- Arora S, Yarrakula S, Narendra K, Sharma V, Wadhwa BK, Singh AK, Sharma GS. Analysis of saccharin and acesulfame-k and their storage stability in kalakand. Indian J Dairy Sci. 2007;61(3):170–177. [Google Scholar]
- Arora S, Narendra K, Gawande H, Yarrakula S, Sharma V, Wadhwa BK, George V, Sharma GS. Stability of artificial sweeteners saccharin, acesulfame-k and aspartame in flavoured milk. Indian J Dairy Sci. 2008;61(5):335–341. [Google Scholar]
- Arpe HJ (1978) Acesulfame-K, a new noncaloric sweetener. In: Guggenheim, B (Eds.), Health and Sugar Substitutes. Proceeding of the ERGOB Conference, Geneva, 30 October–1 November 1978, Basel: Karger, pp 178–179
- Bell LN, Labuza TP. Aspartame degradation kinetics as affected by pH in intermediate and low moisture food systems. J Food Sci. 1991;56:17–19. doi: 10.1111/j.1365-2621.1991.tb07964.x. [DOI] [Google Scholar]
- Beukema C, Jelen P. High potency sweeteners in formation of whey based beverages. Milchwissenschaft. 1990;45(9):576–579. [Google Scholar]
- BSEN: 12856 Foodstuffs (1999) Determination of acesulfame-K, aspartame and saccharin—High performance liquid chromatographic method. Cited by Wood R, Foster L, Key P (2004) In: Analytical methods for food additives, Woodhead Publishing Ltd., CRC Press, pp 231–252
- Cayot P, Lorient D. Structure–functions relationships of whey proteins. In: Damodaran S, Paraf A, editors. Food protein and their applications. New York: Dekker; 1997. pp. 225–256. [Google Scholar]
- Dewit JN, Swinkel GAM. Structure and functional behaviour of whey proteins. Netherland Milk and Dairy J. 1980;35:47–64. [Google Scholar]
- Fennema O. Water and protein hydration. In: Whitaker JR, Taramenbaum SR, editors. Food proteins. West port: AVI; 1977. pp. 50–90. [Google Scholar]
- Higton FR, Thurgood DM. Aspartame. In: Wade A, Weller PJ, editors. Handbook of pharmaceutical excipients. 2. Washington: American Pharmaceutical Association; 1994. pp. 21–23. [Google Scholar]
- Holsinger VH, Posati LP, De Vilbiss ED. Whey beverages: a review. J Dairy Sci. 1974;57:849–859. doi: 10.3168/jds.S0022-0302(74)84976-3. [DOI] [Google Scholar]
- Milk- determination of nitrogen content-Part 3: Block digestion method (Semi-micro rapid routine method) Brussels: International Dairy Federation; 2001. [Google Scholar]
- ISI handbook for food analysis-dairy products. Manak Bhavan: Bureau of Indian Standards; 1981. [Google Scholar]
- Jana AH, Joshi NSS, Sharma AM. Frozen success—a review. Australian J Dairy Technol. 1994;49:98–108. [Google Scholar]
- Larson-Powers N, Pangborn RM. Descriptive analysis of the sensory properties of beverages and gelatins containing sucrose or synthetic sweeteners. J Food Sci. 1978;43:47–51. doi: 10.1111/j.1365-2621.1978.tb09733.x. [DOI] [Google Scholar]
- Lawrence JF, Charbonneau CF. Determination of seven artificial sweeteners in diet food preparations by reverse phase liquid chromatography with absorbance detection. J Assoc Analytical Chemists. 1988;71:934–937. [PubMed] [Google Scholar]
- Lipinski Von Rymon GW. Einsatz von Sunett in Joghurt und anderen Milcherzeugnissen. Swiss Food. 1988;10:25–29. [Google Scholar]
- Lipinski Von Rymon GW, Hanger LY. Acesulfame-K. In: Nabors LO, editor. Alternative sweeteners. 3. New York: Dekker; 2004. pp. 13–30. [Google Scholar]
- Marcy JE, Hansen AP, Graumilch TR. Effect of storage temperature on the stability of aseptically packaged concentrated orange juice and concentrated orange drink. J Food Sci. 1989;54:227–228. doi: 10.1111/j.1365-2621.1989.tb08610.x. [DOI] [Google Scholar]
- Oakenfull D, Pearce J, Burley RW. Protein gelation. In: Damodaran S, Paraf A, editors. Food proteins and their applications. New York: Dekker; 1997. pp. 111–142. [Google Scholar]
- Pessen H, Purcell JM, Farrell HM., Jr Proton relaxation rates of water in dilute solutions of β-Lactoglobulin determination of cross relaxation and correlation with structural changes by the use of two genetic variants of a self associating globular protein. Biochimica et Biophysica Acta. 1985;828(1):1–12. doi: 10.1016/0167-4838(85)90002-0. [DOI] [Google Scholar]
- Ranganna S (2005) Vitamins, Fruit juices, concentrates and beverages. In: Handbook of analysis and quality control for fruit and vegetable products, (2nd ed) (pp 105–106, 868–882, 891). Tata McGraw-Hill Publishing Company Limited, New Delhi, India
- Raju PN, Rao KH, Devi NL. Whey proteins and their uses in food industry. Indian Food Industry. 2005;24(5):19–27–45. [Google Scholar]
- Roy NK, Sen DC (1994) Chemical Analysis of Fluid Milk. In: Textbook of Practical Dairy Chemistry, Vol. 1, (2nd ed) (pp 22–25) Kalyani Publishers, New Delhi, India
- Shaikh SY, Rathi SD, Pawar VD, Agarkar BS. Studies on development of a process for preparation of fermented carbonated whey beverage. J Food Sci Technol. 2001;38:519–521. [Google Scholar]
- Singh S, Singh AK, Patil GR. Food technology practical manual (Undergraduate level) Karnal: Dairy Technology Division, National Dairy Research Institute; 2003. pp. 25–27. [Google Scholar]
- Snedecor GW, Cochran WG (1994). Statistical methods (8th ed). Affiliated East-West press, Iowa state University Press
- Tsang WS, Clarke MA, Parrish FW. Determination of aspartame and its breakdown products in soft drinks by reverse-phase chromatography. J Agric Food Chem. 1985;33:734–738. doi: 10.1021/jf00064a043. [DOI] [Google Scholar]