Abstract
Different antioxidants and salicylic acid were tested to overcome pericarp browning and to maintain the postharvest quality of the litchi fruits at ambient storage. It was found that 0.5% salicylic acid, 1% isoascorbic acid and 1% N-acetyl cysteine performed better over sulphur dioxide (SO2) fumigation for most of the parameters under study. Application of 0.5% salicylic acid found superior to reduce the pericarp browning, relative leakage rate, and decay percentage. It was effective in reduction of polyphenol oxidase activity and improvement of anthocyanin pigments of the fruit pericarp over other treatments. Total soluble solid, titratable acidity and ascorbic acid of the litchi fruits were recorded highest with the application of 1% isoascorbic acid followed by 0.5% salicylic acid treatment. Therefore, 0.5% salicylic acid and 1% isoascorbic could be used as an alternative of SO2 fumigation for quality retention of litchi fruits.
Keywords: Litchi, Isoascorbic acid, N-acetyl cysteine, Salicylic acid, SO2 fumigation
Introduction
Litchi (Litchi chinensis Sonn.) is an evergreen subtropical fruit, known for it’s deliciously flavoured translucent juicy aril, nutritive value and refreshing taste. It is cultivated in China, India, Thailand, Taiwan and South Africa (Ray 1996). The fruits are harvested at ripe stage since it is non-climacteric in nature (Swart 1983). Generally the ripe stage of litchi fruit is adjudged by the development of red colour on the fruit pericarp and flattening of tubercles. Within 1–2 days after harvesting of the fruits, the red colour of the pericarp turn brown, this drastically reduces the commercial value of the fruits. The rapid enzymatic degradation of anthocyanin pigments along with oxidation of phenolic compounds are believed to be the main causes of browning of pericarp (Jaing 2000). Numerous treatments have been tried since the time of commercialization of this fruit but each treatment has it’s certain limitations either relating to tedious and costly method of applications or short duration effect or human health concern.
Recently, antioxidants like N-acetyl cysteine (AC), isoascorbic acid (IAA) have been found very effective to reduce the browning and to prevent the postharvest decay of fruits and vegetables. The polyphenol oxidase activity can be decreased by the use of N-acetyl cysteine (AC), isoascorbic acid (IAA) than commonly used antioxidants like citric acid and glutathione or its combinations (Lei et al. 2004).
On the other hand, salicylic acid (C7H6O3), the active ingredient of aspirin, has been reported to regulate a number of processes in plants. It was also proposed that, salicylic acid has an antagonistic effect on ethylene biosynthesis and/or ethylene action (Raskin 1992). Salicylic acid has been found to delay the senescence of fruits (Hassan et al. 2007).
Though, sulphur dioxide (SO2) fumigation to the fruits has been practiced commercially to overcome pericarp browning related problems. However, SO2-fumigation has certain demerits concerned with residual toxicity and taste of the fruits (Sivakumar et al. 2005). The consumers and packhouse workers have also suffered to health related problems with the consumption and processing of the SO2-fumigant fruits (Koeing et al. 1983). It is also evident that commercial SO2-fumigation intensified micro-cracking of the fruit pericarp (Sivakumar et al. 2005). Therefore, there is an urgent need to find out an alternative postharvest tool that are safe for consumption, ecofriendly and economically viable to over come litchi pericarp browning and maintaining the overall fruit quality.
The main objective of the present investigation is to explore the possibility of these potential promising compounds which may replace the commercial use of SO2-fumigation and also to understand the various effects of these chemicals on fruit quality.
Materials and methods
Freshly harvested mature litchi (cv Rose Scented) fruits were collected from Horticulture Research Centre, G.B. Pant University of Agriculture and Technology, Pantnagar. Fruits were selected for uniformity of shape, colour and size and any blemished or diseased fruits discarded. Destalking of fruits was done by sharp scissor leaving 2 mm pedicel. Prior to application of treatments fruits were precooled followed by air dried. The total 8 treatments comprised of control, isoascorbic acid (0.5% and 1%), N-acetyl cysteine (0.5% and 1%), salicylic acid (0.5% and 1.0%) and SO2 fumigation and replicated thrice. Fruits were dipped for 5 min. in the solutions (except SO2 fumigation) then dried. SO2 fumigation was done by burning pure sulphur powder for 30 min in a closed chamber containing litchi fruits in perforated plastic trays. Fruits dipped in tap water served as control. After application of all the treatments the fruits were placed in polyethylene packet having 2% ventilation. Thereafter, fruits were stored at ambient storage (temperature 25 ± 2 °C, RH 85 ± 5%).
Browning index
Appearance was accessed visually by measuring the extent of the total browned area on each fruit pericarp using 100 fruits during shelf life evaluation, on the following scale: 0 = no browning (excellent quality); 1 = slight browning; 2 =< 1/4 browning; 3 = 1/4–1/2 browning; 4 = 1/2–1/3 browning and 5 = > 1/3 browning (poor quality). The browning index was calculated as ∑ (browning scale x percentage of corresponding fruits within each class).
Decay percentage
On the basis of number of spoiled fruits (unfit for human consumption) observed at every one day interval, the percentage spoilage was worked out and the spoilt fruits were removed.
Relative leakage rate
Membrane permeability expressed as relative electrolyte leakage, was determined by Jiang and Chen (1995). Peel disc were removed with a 5 mm cork borer from the equatorial region of 50 fruits. Fifty discs were rinsed twice in distilled water and then incubated in 30 ml of 0.3 M mannitol solution at 25 °C with shaking for 30 min. Electrolyte leakage was determined with a conductivity meter. Total electrolyte leakage was determined after boiling another batch of 50 discs for 30 min and cooling to 25 °C (total electrolytes).Relative leakage rate was expressed as a proportion of total electrolyte leakage.
Polyphenol oxidase activity
Polyphenol oxidase activity (PPO activity) was assayed with 4-methylcatechol as a substrate according to the method of Zauberman et al. (1991) Peel (6.0 g) from 10 fruits was homogenized in 30 ml of 0.02 M phosphate buffer (pH 6.8) containing 0.6 g of polyvinylpyrrolidone (insoluble). The homogenate was centrifuged for 20 min at 19,000 g and 4 °C and the supernatant was then collected as crude enzyme extract. Assay of PPO activity was performed using 1.0 ml of 0.1 M phosphate buffer (pH 6.8), 0.5 ml of 0.1 M 4-methylcatechol and 0.5 ml enzyme solution. The increase in absorbance at 410 nm at 25 °C was recorded for 3 min. One unit of enzyme activity was defined as the amount which caused a change of 0.01 in absorbance per minute.
Anthocyanin content
Fruit pericarp (10 g) from 15 fruits was finely sliced and extracted with 200 ml of 0.1% HCL-methanol for 2 h, according to the method of Pirie and Mullins (1976). Obtained extract was filtered and diluted; its absorbance was measured at 530 and 600 nm using a sprectophotometer. A unit of anthocyanin was expressed as a difference of 0.1 in the absorbance between 530 and 600 nm and anthocyanin concentrations were presented as unit/g fresh weight.
Total soluble solid, titratable acidity, ascorbic acid content
Total soluble solid (TSS) and titratable acidity concentrations of litchi fruits were analyzed every day starting from first day harvesting date to sixth day of storage. Pulp (20 g) from 15 fruits was homogenized in a grinder and then centrifuged 20 min at 15,000 g. The supernatant was collected for analysis of TSS using a hand refractometer and titratable acidity as percentage citric acid determined by titration with 0.1 M NaOH. For determining the ascorbic acid, aril (10 g) was grind by adding 3% metaphosphoric acid (HPO3) solution and titrated against the dye (2,6-dichlorophenol-indophenol) till the pink colour was appeared as end point. The titre value was recorded and calculated according to the method described by Ranganna (1986).
Physiological loss in weight
Weight of ten fruits in a polythene bag was measured and the rate of weight loss was calculated by the following formula:
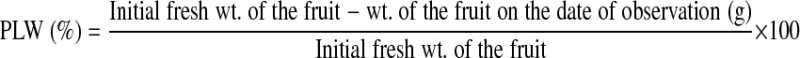 |
Statistical analysis
The experiment was arranged in completely randomized design. Data were tasted by the analysis of variance as given by Cochran and Cox (1959). The over all significance differences among the treatments was tasted, using critical difference (CD) at 5% level of significance.
Results and discussion
Effect on browning index and decay percentage
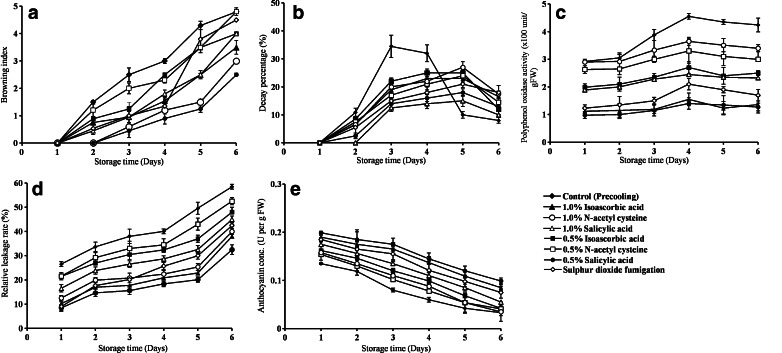
It is evident from the Fig. 1(a) that peel browning index of litchi fruit rapidly increased during storage. The browning indexes of the control fruits were 4.3 and 4.8 after 5 and 6 days of storage at 25 °C, respectively. Treatment with 0.5% salicylic acid was effective in reducing the pericarp browning of the litchi fruits. Treatment 0.5% salicylic acid and 1.0% N-acetyl cysteine were found superior over SO2 fumigation. Pericarp browning was not recorded up to second days in the litchi fruits treated with 0.5% salicylic acid, 1.0% N-acetyl cysteine and SO2 fumigation. However, after second days of storage browning index in SO2 fumigation was significantly higher than 0.5% salicylic acid and 1.0% N-acetyl cysteine treatments. An enhanced browning inhibition with increasing the concentration of salicylic acid from 1 to 4 mM was observed in Chinese water chestnut by Peng and Jiang (2006). This type of findings suggested that salicylic acid delays the browning of pericarp in litchi. However, literature on anti-browning property of salicylic acid in fruits is meagre. In this study the anti-browning effect of salicylic acid was closely related with the 1% N-acetyl cysteine treatment. This sulphur containing amino acid derivative has been reported to be very effective against browning control of fresh-cut pineapple and banana slices (Underhill and Simons 1993; Monsalve-Gonzalez et al. 1995).
Fig. 1.
Effect of antioxidants and salicylic acid on browning index, decay percentage, PPO activity, relative leakage rate and anthocyanin conc. of Rose Scented litchi. [The vertical bars indicates the standard error (n = 3)]
Spoilage pathogens of litchi fruits were significantly inhibited by the use of 1% and 0.5% of salicylic acid (Fig. 1(b)) during storage. Disease development was not observed up to second day of storage in 1% salicylic acid treatment. However, about 34.5% of control fruits began to rot after 5 days of storage. After 6 days of storage, treatment with 1% salicylic acid significantly suppressed disease development. Post harvest decay of fruits is associated with the entry of pathogens in the fruits. Development of microcracking has been reported on litchi fruit pericarp after harvest and during subsequent storage (Underhill and Simons 1993). Application of SO2 fumigation intensified the extent of microcracking in litchi (Sivakumar et al. 2005). This microcracking makes the way for pathogens to take an entry in the fruit. In this present study salicylic acid treatments proved best over other treatments. Salicylic acid has been reported to regulate the expression of pathogenesis related protein genes, which suggest its role as a signal molecule in acquiring the resistance against pathogen attacks (Raskin 1992). Salicylic acid treatment had shown effective in eliciting host defense responses in tobacco leaves (Chen et al. 1993). Application salicylic acid effectively inhibited the disease development in mango and strawberry (Zainuri et al. 2001; Babalar et al. 2007).
Effect on relative leakage rate, polyphenol oxidase activity and anthocyanin content
In this experiment, anthocyanin content of litchi fruit pericarp decreased with storage time (Fig. 1(e)), while polyphenol oxidase activity rapidly increased within 4 days and then remained relatively constant at a high level (Fig. 1(c)). Relative leakage rate was also found in an increasing manner up to 4 days then took a jump in leakage rate in all the treatments (Fig. 1(d)). Treatment with 0.5% salicylic acid and 1% isoascorbic acid recorded more or less similar effect for delayed increase in polyphenol oxidase activity. Treatment with 0.5% salicylic acid and 1% isoascorbic acid were found superior over sulphur dioxide fumigation to reduce the polyphenol oxidase activity and also for maintaining the anthocyanin concentration of the litchi rind. However, anthocayanin concentration decreased progressively after 3 days of storage in all the treatments (Fig. 1(e)). Significant control over relative leakage rate was found with the treatment 0.5% salicylic acid. Sulphur dioxide fumigation performed poorly for reducing the relative leakage rate after 3 days of storage. However, the treatments 0.5% salicylic acid and 1% isoascorbic acid and 1% N-acetyl cysteine had a similar pattern of effect on relative leakage rate. In non-climacteric fruits anthocyanin concentration is increased with the advancement of ripening (Jiang and Joyce 2003). Salicylic acid has a property to delay the ripening process by slowing down the ripening related changes (Hassan et al. 2007). The enzymatic process like poly phenol oxidase activity increased as ripening progressed. The browning of pericarp is positively correlated with the polyphenol oxidase activity (Lin et al. 1988). In this experiment salicylic acid had effectively reduced the browning effect, suggesting that slow rate of poly phenol oxidase activity. Relative leakage rate denotes membrane permeability (Marangoni et al. 1996). Maintenance of membrane integrity is characterized by the activities of three main cell wall degrading enzymes namely cellulose, polygalactouronase (PG) and xylanase (Hurber 1983). In banana decreased level of all these enzymes was reported with the application of salicylic acid in a concentration depending manner during the process of ripening (Srivastava and Dwivedi 2000).
Effect on total soluble solid, titratable acidity, ascorbic acid content, TSS:Acid ratio and Physiological loss in weight
Data on titratable acidity, total soluble solids, TSS:acidity and ascorbic acid content of litchi aril juice and physiological loss in weight of the fruits during sixth days of storage are presented in Table 1. The fruits treated with 1% isoascorbic acid had significantly maintained the highest total soluble solid content of the aril. Second highest total soluble solid content was recorded in the fruits treated with 0.5% salicylic acid. The least soluble solid content was found in control. The titratable acidity content had significantly lower in the fruits treated with 0.5% salicylic acid than control. However, there was no significant difference obtained between 0.5% salicylic acid and 1.0% isoascorbic acid treatments for titratable acidity content of litchi fruits. Fruits which were treated with isoascorbic acid, N-acetyl cysteine and salicylic acid showed a balanced TSS:acidity ratio on sixth days of storage. Application of 1.0% isoascorbic acid significantly maintained the ascorbic acid content over rest of the treatments and also recorded the highest ascorbic acid in the fruit pulp. Minimum physiological loss in weight was obtained with the application of 0.5% salicylic acid. Total soluble solids, titratable acidity, TSS:acidity and ascorbic acid are important factors in assessing flavor and nutritive quality of the litchi. The fruits treated with salicylic acid had higher concentration of total soluble solid, titratable acidity and lower rate of physiological loss in weight. This type of effect found in salicylic acid treatment could be due to the fact that it has the ability to delay the ripening process of the fruits (Leslie and Romani 1988). Salicylic acid delay the process of senescence was also reported in pear (Hassan et al. 2007). However, isoascorbic acid also had a great effect in maintaining the litchi fruit quality in terms of total soluble solid, titratable acidity, TSS:acidity and ascorbic acid content of the pulp (Liu et al. 2006).
Table 1.
Effect of antioxidants and salicylic acid on quality attributes of litchi on sixth day after harvest
| Treatments | TSS (°B) | Titratable acidity (%) | TSS : acidity | Ascorbic acid (mg/100 g pulp) | Physiological loss in weight (%PLW) |
|---|---|---|---|---|---|
| 1.0% isoascorbic acid | 18.5 ± 0.02a | 0.38 ± 0.02a | 48.7 ± 1.66c | 9.0 ± 1.12a | 8.8 ± 0.36b |
| 0.5% isoascorbic acid | 17.9 ± 0.13bc | 0.33 ± 0.03bc | 54.4 ± 3.02b | 5.8 ± 0.83b | 8.8 ± 0.75b |
| 1.0% N-acetyl cysteine | 17.8 ± 0.15cd | 0.31 ± 0.02c | 57.3 ± 1.66ab | 6.0 ± 0.05b | 6.5 ± 0.05d |
| 0.5% N-acetyl cysteine | 17.3 ± 0.10e | 0.34 ± 0.02b | 50.9 ± 0.66bc | 5.3 ± 0.14b | 8.3 ± 0.66c |
| 1.0% salicylic acid | 17.9 ± 0.13c | 0.35 ± 0.01ab | 51.0 ± 1.42bc | 6.0 ± 0.50b | 8.2 ± 0.15c |
| 0.5% salicylic acid | 18.2 ± 0.05b | 0.37 ± 0.03a | 49.1 ± 3.66bc | 6.5 ± 0.48b | 6.0 ± 0.12e |
| SO2 fumigation | 17.6 ±0.11d | 0.33 ± 0.01bc | 54.1 ± 3.98b | 5.1 ± 0.36b | 9.0 ± 0.23b |
| Control (Precooling) | 17.2 ± 0.23ef | 0.28 ± 0.01d | 61.4 ± 2.25a | 5.3 ± 0.61b | 11.0 ± 0.15a |
For each measurement, corresponding means ± SD values followed by the same letter are not significantly different at the 5% level of significance.
Conclusion
The litchi cultivar Rose Scented is having very high export potentiality and popular through out the country. The extent of post harvest browning and decay, limits its profits. We were trying to find out most effective compound which can reduce these problems. Recently, several compounds were used to manage this problem like isoascorbic acid, N-acetyl cystein and salicylic acid in other fruit crops. In this present investigation, the internal processes which cause browning and decay were significantly reduced with the application of 0.5% salicylic acid with a very little change in quality. The quality parameters remained excellent with the application of 1% isoascorbic acid. Therefore, the best way to manage the browning and decay with the maintenance of quality in litchi may be combined application of salicylic acid and isoascorbic acid. But during combined application, the effective concentration of these compounds may vary, which requires further research in this field. However, these findings have given a ray of hope for further substitution of SO2 fumigation with some non-hazardous chemicals in near future.
Acknowledgement
The financial support provided by All India Coordinated Research Project on Sub-Tropical Fruits is greatly appreciated. The authors are also thankful to Dr. Alok Shukla, Associate Professor, College of Basic Science and Humanities, G.B. Pant University of Agriculture and Technology, Pantnagar, Udham Singh Nagar, Uttarakhand, India, for providing laboratory facilities during the work.
Contributor Information
Deepak Kumar, Phone: +91-9473129003.
Daya Shankar Mishra, Phone: +91-9412120708, Email: dsmhort@gmail.com.
Binayak Chakraborty, Phone: +91-9756682434, Email: binayak.hort@gmail.com.
Prabhat Kumar, Phone: +91-9412987323, Email: prabhatflori@gmail.com.
References
- Babalar M, Asghari M, Talaei A, Khosroshahi A. Effect of pre- and postharvest salicylic acid treatment on ethylene production, fungal decay and overall quality of Selva strawberry fruit. Food Chem. 2007;105:449–453. doi: 10.1016/j.foodchem.2007.03.021. [DOI] [Google Scholar]
- Chen Z, Silva H, Klessig DF. Active oxygen species in the induction of plant systemic acquired resistance by salicylic acid. Science. 1993;262:1883–1886. doi: 10.1126/science.8266079. [DOI] [PubMed] [Google Scholar]
- Cochran W, Cox GM. Experimental designs. Bombay: Asia Publication House; 1959. pp. 293–315. [Google Scholar]
- Hassan I, Zhang Y, Guoqiang DU, Wang G, Zhang J. Effect of salicylic acid (SA) on delaying fruit senescence of Huang Kum pear. Front Agric China. 2007;1:456–459. doi: 10.1007/s11703-007-0075-y. [DOI] [Google Scholar]
- Hurber DJ. The role of cell wall hydrolases in fruit softening. Hort Rev. 1983;5:169–219. [Google Scholar]
- Jaing YM. Role of anthocyanins, polyphenol oxidase and phenols in lychee pericarp browning. J Sci Food Agric. 2000;80:305–310. doi: 10.1002/1097-0010(200002)80:3<305::AID-JSFA518>3.0.CO;2-H. [DOI] [Google Scholar]
- Jiang YM, Chen F. A study on polyamine change and browning of fruit during cold storage of litchi fruit. Postharvest Biol Technol. 1995;5:245–250. doi: 10.1016/0925-5214(94)00021-J. [DOI] [Google Scholar]
- Jiang Y, Joyce DC. ABA effect on ethylene production, PAL activity, anthocyanin and phenolic contents of strawberry fruit. Plant Growth Regul. 2003;39:171–174. doi: 10.1023/A:1022539901044. [DOI] [Google Scholar]
- Koeing JK, Pierson WE, Horike M, Frank R. A comparison of the pulmonary effect of 0.5 ppm and 1.0 ppm sulphur dioxide plus sodium chloride droplets in asthmatic adolescents. Toxicol Environ Health. 1983;11:129–132. doi: 10.1080/15287398309530327. [DOI] [PubMed] [Google Scholar]
- Lei DF, Feng F, Jiang DZ. Characterization of polyphenol oxidase from plants. Prog Nat Sci. 2004;14:553–561. doi: 10.1080/10020070412331343941. [DOI] [Google Scholar]
- Leslie CA, Romani RJ. Inhibition of ethylene biosynthesis by salicylic acid. Plant Physiol. 1988;88:833–837. doi: 10.1104/pp.88.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin ZF, Li SS, Chang DL, Lin GZ, Li YB, Liu SX, Chen MD. The changes of pigments, phenolics contents and activities of polyphenol oxidase and phenylalanine ammonia-lyase in pericarp of postharvest litchi fruit. Acta Bot Sin. 1988;30:40–45. [Google Scholar]
- Liu H, Shi J, Song L, You Y, Jiang Y. Browning control and quality maintenance of litchi fruit treated with combination of N-acetyl cysteine and isoascorbic acid. J Food Technol. 2006;4:147–151. [Google Scholar]
- Marangoni AG, Palma T, Sanley DW. Membrane effects in postharvest physiology. Postharvest Biol Technol. 1996;7:193–217. doi: 10.1016/0925-5214(95)00042-9. [DOI] [Google Scholar]
- Monsalve-Gonzalez A, Barbosa-Canovas A, Mcevily A, Iyengar R. Inhibition of enzymatic browning in apple products by 4-heylresorcinol. Food Technol. 1995;49(4):110–118. [Google Scholar]
- Peng L, Jiang Y. Exogenous salicylic acid inhibits browning of fresh-cut Chinese water chestnut. Food Chem. 2006;94:535–540. doi: 10.1016/j.foodchem.2004.11.047. [DOI] [Google Scholar]
- Pirie A, Mullins MG. Changes in anthocyanin and phenolic content of grapevine leaf and fruit tissue treated with sucrose, nitrate and abscisic acid. Plant Physiol. 1976;58:468–472. doi: 10.1104/pp.58.4.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganna S. Handbook of analysis and quality control of fruit and vegetable products. 2. New Delhi: Tata McGrow Hill Publishing Company Ltd; 1986. pp. 9–19. [Google Scholar]
- Raskin I. Role of salicylic acid in plants. Ann Rev Plant Physiol Mol Biol. 1992;43:439–463. doi: 10.1146/annurev.pp.43.060192.002255. [DOI] [Google Scholar]
- Ray PK. Litchi. Breeding of tropical and subtropical fruits. 22 Daryaganj: Narosa Publishing House; 1996. pp. 129–142. [Google Scholar]
- Sivakumar D, Regnier T, Domez B, Korsten L. Effect of different post-harvest treatments on overall quality retention in litchi fruit during low temperature storage. J Hort Sci Biotechnol. 2005;80:32–38. [Google Scholar]
- Srivastava MK, Dwivedi UN. Delayed ripening of banana fruit by salicylic acid. Plant Sci. 2000;158:87–96. doi: 10.1016/S0168-9452(00)00304-6. [DOI] [PubMed] [Google Scholar]
- Swart DH. Post-harvest handling of litchies. Pretoria: Farming in South Africa, Department of Agriculture; 1983. [Google Scholar]
- Underhill SJR, Simons HD. Lychee (Litchi chinensis Sonn.) pericarp desiccation and the importance of postharvest micro-cracking. Sci Hortic. 1993;54:89–97. doi: 10.1016/0304-4238(93)90107-2. [DOI] [Google Scholar]
- Zainuri JDC, Wearing AH, Coates L, Terry L. Effect of phosphonate and salicylic acid treatments on anthracnose disease development and ripening of ‘Kensington Pride’ mango fruit. J Exp Agric. 2001;41:805–813. doi: 10.1071/EA99104. [DOI] [Google Scholar]
- Zauberman G, Ronen R, Akerman M, Weksler A, Rot I, Fuchs Y. Postharvest retention of the red colour of litchi fruit pericarp. Sci Hortic. 1991;47:89–97. doi: 10.1016/0304-4238(91)90030-3. [DOI] [Google Scholar]