Abstract
The influence of the packaging films, aerobic and vacuum conditions, and refrigeration storage temperature (0, 4 and 10 °C) on colour and texture of raw meat were studied during a 2-week storage period in order to analyze kinetics of colour and texture changes. The rate of redness decrease was most noticeable at the highest storage temperature and aerobic conditions and these changes were well described by the first-order reaction. Texture parameter reflected a progressive softening during storage for both films. Decrease on shear force of raw beef during storage followed a first-order kinetic model. Temperature dependence of colour and texture change was adequately modelled with the Arrhenius equation.
Keywords: Shelf life, Maximum shear force, Storage temperature, Packaging films
Introduction
During storage, beef muscles undergo several changes that can affect their quality (Ferguson et al. 2001; Mallikarjunan and Mittal 1996). These changes are reflected in many characteristics such as colour, tenderness, flavour, and juiciness.
Colour perception plays a major role in the evaluation of meat quality as consumers use the colour as an indicator of freshness and it strongly influences the consumer’s purchase decision (Feldhusen et al. 1995; Lanari et al. 2002). During storage, distribution and display, the processes of oxygenation and oxidation of myoglobin influence colour (Mancini and Hunt 2005).
On the other hand, texture measurement is associated with the consumer’s perception of food response to mastication and manipulation prior to ingestion (Szczesniak 2002). Meat texture is generally difficult to evaluate using physico-chemical tests, as texture depends on the state and interactions of different muscle components, especially myofibrillar and connective tissues (Campo et al. 2000; Koohmaraie and Geesink 2006).
According to Taoukis and Labuza (1989), it is necessary to know about the quality loss kinetics of a food to predict its shelf life, in this sense, there is much bibliography of mathematical models to quantify and to predict the rate of growth of microorganisms under environmental conditions with the intention of assuring the hygienic quality of food, thus determining its storage life (Koutsoumanis et al. 2006; Limbo et al. 2010; Shimoni and Labuza 2000). However, there has been little information available on modelling kinetics of colour and texture transformations during refrigerated storage in raw meat.
On the other hand, refrigeration and vacuum-packaging are increasingly being used as two techniques for enhancing shelf life of perishable foods such as cuts of fresh meat and meat products, using low-oxygen permeable packing materials (Coll Cárdenas et al. 2008; Kandeepan et al. 2011).
The aims of this work, therefore, were:
-
i)
to analyze the influence of the packaging films and refrigeration storage temperature on colour and texture of raw beef,
-
ii)
to analyze kinetics of colour and texture changes by developing related kinetic models which can well describe the associated changes in these parameters of raw beef during storage.
Materials and methods
Meat samples and storage conditions
Beef samples (n = 24) were obtained from Longissimus dorsi muscle group of natural pH 5.7–5.8, from steers, carcass weighing up to 240 kg, with post-mortem time of 48 h at 4 °C. The samples were divided into sub samples of 50 g (about 2 cm thick) and packaged in two films with different values of oxygen permeability: (a) low density polyethylene (aerobic condition, LPDE film) of 50 μm thick, water vapour permeability WVP = 12 g m−2 day−1 atm−1 at 30 °C and RH = 78 %, oxygen transmission rate OTR = 5,000 cm3 m−2 atm−1 day−1 at 23 °C, and (b) vacuum packaged EVA SARAN EVA (ESE film), being EVA ethyl vinyl acetate and SARAN a polyvinyl and polyvinylidene chloride copolymer (WVP = 7.2 g m−2 day−1 atm−1 at 30 °C and RH = 78 %, OTR = 50 cm3 m−2 atm−1 day−1).
Vacuum packaging was carried out in a Minidual equipment model MW 4980 (Schokolnik SAIC, Bs As, Argentina). Storage experiments with packaged refrigerated beef were performed at 0, 4, and 10 °C. During the experimental study, storage temperatures were recorded using DS1921G Thermochron iButton temperature sensors (USA). Samples were taken out after definite storage periods to determine pH, colour and texture parameters.
Physical determinations
For pH analysis, a pH meter with a puncture electrode (Delta Track Inc., ISFET pH 101, Pleasanton, CA) was used. The pH meter was standardized by a two-point method against standard buffers of pH 4.0 and pH 7.0.
The colour of meat was measured on the surface of meat samples using a hand-held tri-stimulus colorimeter (Minolta Chroma Meter CR-210, Minolta, Osaka, Japan) with an 8 mm diameter measuring area, the equipment was calibrated on the Hunterlab colour space system using a standard white plate (Minolta calibration plate, Y = 92.6, x = 0.3136, y = 0.3196). Colour was described as coordinates: lightness (L: 100 = white, 0 = black), redness (a* ± red–green) and yellowness (b* ± yellow–blue) of the CIELab scale (CIE 1978). Six replicates per storage time and packaging films were recorded. Samples under vacuum were removed from the pouch and allowed to bloom for 40 min before evaluating the colour parameters.
In order to monitor beef colour changes over storage, total colour change (ΔE) was calculated. This parameter can be expressed as:
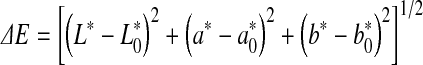 |
1 |
where 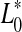 ,
, 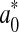 and
and 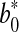 represented the readings at time zero, and L*, a* and b* represented the individual readings after defined storage condition.
represented the readings at time zero, and L*, a* and b* represented the individual readings after defined storage condition.
Texture of beef was analyzed using a texturometer TA-XT2i (Stable Micro Systems Ltd, Godalming, Surrey, UK) operating in the compression mode and using a 25 kg load cell. The equipment was fitted with a Warner-Bratzler knife. Samples of 1 cm2 (square cross-section), with muscle fibers parallel to the longitudinal axis of the sample were placed on the table, under the V blade, and were cut through as the blade moved down with a constant speed (10 mm min−1). Results were expressed in Newton’s (N) as the maximum force applied before breaking (Maximum Shear Force, MSF). For these determinations six replicates per storage time and packaging films were recorded.
Kinetic modelling
Generally, a kinetic model for food quality loss can be expressed as:
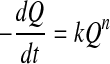 |
2 |
where Q is the measured quality index (e.g. color, texture, sensory attribute), t is time, k is the reaction rate constant, which is temperature dependent, and n is the reaction order. Usually, zero order (n = 0) or first order (n = 1) kinetics models are used (Singh 2000) for overall quality of frozen foods, nonenzymatic browning and microbial death/growth, oxidative color loss, texture loss in heat processing, respectively. Thus integrating Eq. (2) gives:
- Zero order
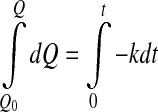
3 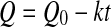
4 - First order
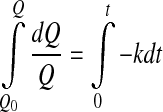
5
or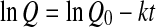
6
where Q0 represent the initial values of the quality factor Q.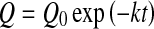
7
The Arrhenius equation is usually applied to evaluate the temperature dependence of reaction rate constant:
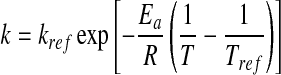 |
8 |
where T is the absolute temperature, Tref the reference absolute temperature, kref is the reaction rate constant at Tref, Ea is the activation energy and R is the universal gas constant (8.31 J/molK).
In the kinetic equations above, (3) and (6) can be fitted by the ordinary methods of linear regression, Q vs time and lnQ vs time, respectively.
In this work, the commercial software OriginPro Version 7.0 (OriginLab Corporation, Northampton, USA) was used to obtain the regression parameters.
Statistical analysis
Analysis of variance and pair wise comparisons were computed using SYSTAT software (SYSTAT, Inc., Evanston, IL). Differences in means and F-tests were considered only when P < 0.05.
Results and discussion
pH analysis
During storage at selected temperatures (0, 4 and 10 °C), the pH value was monitored. The pH of samples did not change significantly and maintained the level of 5.6–5.8 until the end of storage, but for 10 °C and aerobic condition (LDPE film) pH values were increased up to 6.0 at the end of the storage time probably by proteolysis degradation and accumulation of metabolites of bacterial action on meat (Bhat and Pathak 2010; Muchenje et al. 2009).
Colour variation
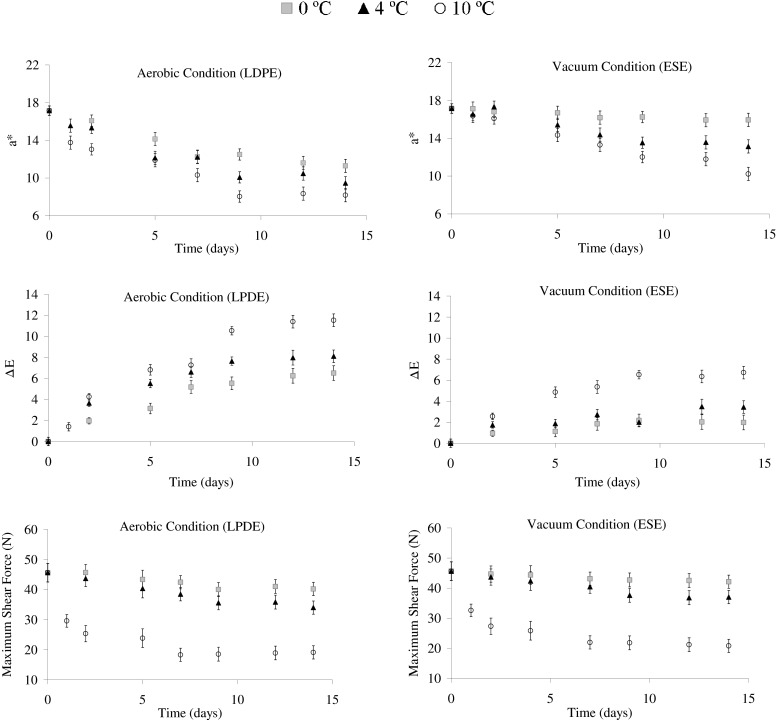
The most important colour parameter for fresh meat is the redness value (a*). Changes of this value during the storage are shown in Fig. 1 for aerobic (LDPE) and vacuum (ESE) conditions. In general, a* values decreased with increasing storage time; however, in the case of storage at 0 °C in vacuum storage, this parameter remained constant along 14 days of storage.
Fig. 1.
Changes in instrumental colour parameters and maximum shear force (MSF) of raw meat during storage under aerobic condition (LDPE) and vacuum condition (ESE) (n = 6). n = 6. a* redness, ΔE total colour change, LDPE low density polyethylene, ESE EVA SARAN EVA (EVA: ethyl vinyl acetate and SARAN: a polyvinyl and polyvinylidene chloride copolymer)
Moreover, our results show that rate of discoloration increases with storage temperature increase (the decrease of redness at 10 °C was 50 %). Gill and McGinnis (1995) informed that muscles are less susceptible to discoloration at temperatures below 0 °C. On the other hand, redness change was most noticeable due to aerobic conditions, probably because vacuum packaged meat when taken out of the vacuum package it will recover its bright red colour (Mancini and Hunt 2005).
In order to study the total colour differences between raw meat in relation to temperature storage and packaging films, the values of ΔE (Eq. 1) were calculated. The reference taken in each case was the colour of the control raw meat. The value of ΔE increased as storage temperature increased, and this increase was higher for LDPE (Fig. 1).
According to Francis and Clydesdale (1975) when ΔE > 3 colour differences are obvious for the human eye, in this sense, for vacuum conditions (Fig. 1) the colour differences were appreciative by the human eye only at 10 °C and after 5 days of storage.
For storage of raw meat, redness and ΔE were adequately modelled by the first-order kinetics (Eqs. 9 and 10, respectively).
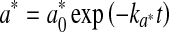 |
9 |
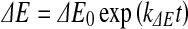 |
10 |
where 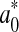 and ∆E0 represented the readings at time zero, a* and ∆E represented the individual readings at any storage condition, and
and ∆E0 represented the readings at time zero, a* and ∆E represented the individual readings at any storage condition, and 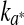 and k∆E are the reaction rate constant for redness and total colour differences, respectively.
and k∆E are the reaction rate constant for redness and total colour differences, respectively.
The corresponding kinetics parameters (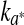 and k∆E) calculated are summarized in Table 1. These parameters increased as temperature storage increased, for both films. Besides, the estimated rates for ESE had lower values, compared to the corresponding k for the LDPE films samples, indicating a lower rate of discoloration for vacuum conditions. Temperature dependence of the rates of colour changes was effectively described by Arrhenius equation across the whole temperature range studied and the values of the activation energies were calculated (Table 1). The lower activation energies for the redness changes of raw meat stored under air atmosphere (aerobic conditions, LPDE film) indicated that the samples were less sensitive to the change in the storage temperature than were the samples stored under vacuum condition (ESE).
and k∆E) calculated are summarized in Table 1. These parameters increased as temperature storage increased, for both films. Besides, the estimated rates for ESE had lower values, compared to the corresponding k for the LDPE films samples, indicating a lower rate of discoloration for vacuum conditions. Temperature dependence of the rates of colour changes was effectively described by Arrhenius equation across the whole temperature range studied and the values of the activation energies were calculated (Table 1). The lower activation energies for the redness changes of raw meat stored under air atmosphere (aerobic conditions, LPDE film) indicated that the samples were less sensitive to the change in the storage temperature than were the samples stored under vacuum condition (ESE).
Table 1.
Reaction rate constant, k, and activation energy, Ea, of colour and texture changes of raw meat during storage
| Storage condition | Storage temperature (°C) | k (day−1) | R2 | Ea (kJ/mol) | R2 | |
|---|---|---|---|---|---|---|
| a* (redness) | Aerobic condition (LDPE) | 0 | 0.0308 | 0.95 | 38.60 | 0.99 |
| 4 | 0.0413 | 0.91 | ||||
| 10 | 0.0565 | 0.95 | ||||
| Vacuum condition (ESE) | 0 | 0.0057 | 0.93 | 112.19 | 0.90 | |
| 4 | 0.0209 | 0.92 | ||||
| 10 | 0.0352 | 0.98 | ||||
| ΔE (total colour change) | Aerobic condition (LDPE) | 0 | 0.0094 | 0.90 | 184.34 | 0.84 |
| 4 | 0.1011 | 0.91 | ||||
| 10 | 0.1918 | 0.95 | ||||
| Vacuum condition (ESE) | 0 | 0.0055 | 0.94 | 207.73 | 0.86 | |
| 4 | 0.0617 | 0.95 | ||||
| 10 | 0.1587 | 0.94 | ||||
| MSF (Maximum shear force) | Aerobic condition (LDPE) | 0 | 0.0102 | 0.97 | 117. 13 | 0.99 |
| 4 | 0.0210 | 0.96 | ||||
| 10 | 0.0630 | 0.90 | ||||
| Vacuum condition (ESE) | 0 | 0.0051 | 0.94 | 148.38 | 0.99 | |
| 4 | 0.0162 | 0.97 | ||||
| 10 | 0.0527 | 0.91 |
n = 6. k Reaction rate constant, Ea activation energy, LDPE low density polyethylene, ESE EVA SARAN EVA (EVA: ethyl vinyl acetate and SARAN: a polyvinyl and polyvinylidene chloride copolymer)
Texture analysis
Variation in the maximum shear force, MSF, for raw meat during storage at 0, 4 and 10 °C was as shown in Fig. 1. There was no significant difference (P > 0.05) between aerobic and vacuum conditions for the MSF value at different storage temperature. Texture parameter reflected a progressive softening in the raw meat during storage at 4 °C and 10 °C, for both films. On the other hand, at 0 °C the MSF showed no variation for period of time studied.
Changes in meat tenderness of raw beef during storage have been reported in several studies. According to Koohmaraie (1996) and Zhang et al. (2005), after 24 h post-mortem, an increase in tenderness is observed as a result of enzymatic degradation of muscle tissue. This degradation is caused by proteolytic enzymes such as calpains and liposomal proteases. Temperature of storage can affect this enzymatic degradation, as well as other factors including: pH, amount and degree of cross-linking of connective tissue, and animal species. Koohmaraie (1996) suggested that ageing can be used to decrease shear force values during post-mortem storage.
Our results agree with Lindahl et al. (2010), who reported that the shear force decreased to the same level when beef cut was stored in high oxygen MA for 10 days as when was stored under vacuum conditions.
The textural changes of raw meat during storage followed a first order kinetic model and fitted well with this model (R2 > 0.95). This behaviour was more noticeable for high temperature of storage showing al lower temperature similar R2 values for both order. Kinetic parameters, k, are summarized in Table 1. These values increased with storage temperature increase. These results clearly indicate that the increase of storage temperature produced an increase of meat tenderness.
Temperature dependence of the rates of maximum shear force for LDPE and ESE was adequately described by Arrhenius equation in the temperature range observed. Ea values (Table 1) were higher, indicating a greater temperature dependence of the texture indices.
Conclusion
The results of this work indicate that the extent of colour and texture change in raw beef depend on the length of storage period, packaging conditions and storage temperature. Higher temperatures of refrigerated storage produced significant changes for both packaging films. These changes were adequately modelled by the first-order reaction.
The Arrhenius equation was effective to describe the dependence of colour and texture change on the storage temperature. The activation energies calculated by the equation could be applied on the prediction of raw meat shelf life because consumer acceptability is based on sensory quality criteria, such as colour, texture, taste and flavour.
Acknowledgments
Authors express their gratitude for the financial support provided by the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Universidad Nacional de La Plata and Frigorífico Calchaqui SA, which provided the meats for this study.
References
- Bhat ZF, Pathak V. Quality evaluation of mutton Harrisa during one week refrigerated storage. J Food Sci Technol. 2010;49:620–625. doi: 10.1007/s13197-010-0199-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campo MM, Santolaria P, Sañudo C, Lepetit JJ, Olleta L, Panea B, Albertí P. Assessment of breed type and ageing time effects on beef meat quality using two different texture devices. Meat Sci. 2000;55:371–378. doi: 10.1016/S0309-1740(99)00162-X. [DOI] [PubMed] [Google Scholar]
- Recommendations on uniform colour spaces—colour difference equations, psychometric colour terms. Supplement No. 2. CIE Publication No. 15(E-1-3.1) 1971/(TC-1-3) Paris: CIE; 1978. [Google Scholar]
- Coll Cárdenas F, Gianuzzi L, Zaritzky N. Mathematical modelling of microbial growth in ground beef from Argentina. Effect of lactic acid addition, temperature and packaging film. Meat Sci. 2008;79:509–520. doi: 10.1016/j.meatsci.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Feldhusen F, Warnatz A, Erdmann R, Wenzel S. Influence of storage time on parameters of colour stability of beef. Meat Sci. 1995;40:235–243. doi: 10.1016/0309-1740(94)00048-C. [DOI] [PubMed] [Google Scholar]
- Ferguson DM, Bruce HL, Thompson JM, Egan AF, Perry D, Shorthose WR. Factors affecting beef palatability- farmgate to chilled carcass. Aust J Exp Agr. 2001;41:879–891. doi: 10.1071/EA00022. [DOI] [Google Scholar]
- Francis F, Clydesdale F. Food colorimetry: theory and applications. Westport: The AVI Publishing Company Inc; 1975. [Google Scholar]
- Gill CO, McGinnis JC. The effects of residual oxygen concentration and temperature on degradation of the color of beef packaged under oxygen depleted atmospheres. Meat Sci. 1995;39:387–394. doi: 10.1016/0309-1740(94)00013-W. [DOI] [PubMed] [Google Scholar]
- Kandeepan G, Anjaneyulu ASR, Kondaiah SK, Mendiratta SK, Rajkumar RS (2011) Evaluation of quality and shelf life of buffalo meat keema at refrigerated storage. J Food Sci Technol. doi:10.1007/s13197-011-0454-5, published online 3 Aug 2011 [DOI] [PMC free article] [PubMed]
- Koohmaraie M. Biochemical factors regulating the toughening and tenderization processes of meat. Meat Sci. 1996;43:S193–S201. doi: 10.1016/0309-1740(96)00065-4. [DOI] [PubMed] [Google Scholar]
- Koohmaraie M, Geesink GH. Contribution of postmortem muscle biochemistry to the delivery of consistent meat quality with particular focus on the calpain system. Meat Sci. 2006;74:34–43. doi: 10.1016/j.meatsci.2006.04.025. [DOI] [PubMed] [Google Scholar]
- Koutsoumanis K, Stamatiou A, Skandamis P, Nychas GJE. Development of a microbial model for the combined effect of temperature and pH on spoilage of ground meat, and validation of the model under dynamic temperature conditions. Appl Environ Microb. 2006;72:124–134. doi: 10.1128/AEM.72.1.124-134.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanari MC, Brewster M, Yang A, Tume RK. Pasture and grain finishing affect the color stability of beef. J Food Sci. 2002;67:2467–2473. doi: 10.1111/j.1365-2621.2002.tb08760.x. [DOI] [Google Scholar]
- Limbo S, Torri L, Sinelli N, Franzetti L, Casiraghi E. Evaluation and predictive modeling of shelf life of minced beef stored in high-oxygen modified atmosphere packaging at different temperatures. Meat Sci. 2010;84:129–136. doi: 10.1016/j.meatsci.2009.08.035. [DOI] [PubMed] [Google Scholar]
- Lindahl G, Lagerstedt A, Ertbjerg P, Sampels S, Lundström K. Ageing of large cuts of beef loin in vacuum or high oxygen modified atmosphere—effect on shear force, calpain activity, desmin degradation and protein oxidation. Meat Sci. 2010;85:160–166. doi: 10.1016/j.meatsci.2009.12.020. [DOI] [PubMed] [Google Scholar]
- Mallikarjunan P, Mittal GS. Selection criteria for beef carcass chilling. Food Res Int. 1996;29:661–666. doi: 10.1016/S0963-9969(96)00064-6. [DOI] [Google Scholar]
- Mancini RA, Hunt MC. Current research in meat color. Meat Sci. 2005;71:100–121. doi: 10.1016/j.meatsci.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Muchenje V, Dzama K, Chimonyo M, Strydom PE, Hugo A, Raats JG. Some biochemical aspects pertaining to beef eating quality and consumer health: a review. Food Chem. 2009;112:279–289. doi: 10.1016/j.foodchem.2008.05.103. [DOI] [Google Scholar]
- Shimoni E, Labuza TP. Modeling pathogen growth in meat products: future challenges. Trends Food Sci Tech. 2000;11:394–402. doi: 10.1016/S0924-2244(01)00023-1. [DOI] [Google Scholar]
- Singh RP. Scientific principles of shelf-life evaluation. In: Man D, Jones A, editors. Shelf-life evaluation of foods. 2. Maryland: Aspen Publishers, Inc; 2000. p. 3. [Google Scholar]
- Szczesniak AS. Texture is a sensory property. Food Qual Prefer. 2002;13:215–225. doi: 10.1016/S0950-3293(01)00039-8. [DOI] [Google Scholar]
- Taoukis PS, Labuza TP. Applicability of time-temperature indicators as shelf life monitors of food products. J Food Sci. 1989;54:783–788. doi: 10.1111/j.1365-2621.1989.tb07882.x. [DOI] [Google Scholar]
- Zhang SX, Farouk MM, Young OA, Wieliczko KJ, Podmore C. Functional stability of frozen normal and high pH beef. Meat Sci. 2005;69:765–772. doi: 10.1016/j.meatsci.2004.11.009. [DOI] [PubMed] [Google Scholar]