Abstract
The antioxidant activity of marine clam, Meretrix casta (Chemnitz) protein hydrolysates prepared from different organs (body, foot and viscera), using the commercial enzymes (pepsin, trypsin and papain) were determined. The protein hydrolysate had a high antioxidant activity where, pepsin hydrolysate of viscera and trypsin hydrolysate of body and foot showed good activity. The viscera pepsin hydrolysate and foot trypsin hydrolysates were purified using FPLC on ion exchange and gel filtration chromatography procedure and activity was determined by DPPH radical scavenging and reducing ability assays. Further the amino acid content of the purified fractions was analyzed using HPLC. Active fractions contained good quantity of both essential and non-essential amino acids.
Keywords: Antioxidant peptide, Clam, Meretrix casta, Protein hydrolysate, FPLC, DPPH radical
Introduction
Aerobic organisms must face free radicals that are generated from sequential reduction of oxygen during their metabolism. Free radicals are molecules with one or two unpaired electron. These reactive radicals are important at physiological level to maintain homeostasis in signal transduction and defense against infections (De Lamirande et al. 1997). In contrast, uncontrolled production of free radicals may cause cellular damage leading to number of diseases including atherosclerosis, arthritis, diabetes and carcinogenesis (Halliwell 1994). Free radicals are also of a great concern in the food industry and among consumers of processed foods. Because, radical mediated oxidation of fats leads for deteriorating the quality of foods during processing and storage. Therefore, synthetic antioxidants such as butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), tert-butylhydroquinone (TBHQ), and propyl gallate are commonly used to solve these problems (Wanita and Lorenz 1996). However, these compounds must use under strict regulation due to their potential health hazards. Therefore, a great interest has been developed to identify new antioxidant compounds from natural sources to overcome these problems.
Antioxidant active peptides derived from different marine animals using various commercial enzymes have been reported effective and safe in recent years (Rajapakse et al. 2005; Shabeena Yousuf Naqash and Nazeer 2010). Biochemical production of protein hydrolysate/peptides may be carried out by employing an autolytic process by endogenous enzyme or an accelerated and controllable method using exogenous enzymes (Shahidi et al. 1995). The use of protein hydrolysates for maintaining the growth of different microorganisms (Gildberg 1993) or food and feed ingredients (Kristinsson and Rasco 2000) were successfully achieved. Many enzymes have been described to be interesting in hydrolysis of animal proteins viz., papain, trypsin, pepsin, alcalase, protamex, flavourzyme, neutrase, etc. (Aspmo et al. 2005; Venugopal and Shahidi 1995; Gildberg 1993).
Therefore, the aim of this study was to identify the antioxidative potentials and amino acid composition of peptides derived from the clam (Meretrix casta) protein hydrolysate. For this purpose, the extracted clam protein from different organs was enzymatically hydrolyzed to obtain antioxidant peptides and assessed for free radical scavenging effects.
Materials and methods
Materials
1,1-Diphenyl-2-picrylhydrazyl (DPPH) were purchased from Sigma Chemical Co. Methanol, ethanol, linoleic acid, ammonium thiocyanate , ferric chloride, tocopherol, 2,2-diphenyl-1 picrylhydrazyl (DPPH), potassium ferricyanide, trichloroacetic acid, DMPO (5,5-dimethyl-1-1-1-pyrroline-N-oxide), trypsin, papain, pepsin, sodium carbonate were analytical grade.
Sample collection
Clams (Meretrix casta) were collected from Kovalam Beach (13o 6′ 15″ N and 30o17′ 30″ E), Chennai, Tamilnadu on 7th January 2010. Clams were washed thoroughly in sea water and brought to laboratory in an ice box containing alternative layers of ice. Further samples were washed with running water followed by double distilled water and the shells were opened by scalpel and then dissected into body, foot and viscera. The dissected samples were stored at −20 oC before further assays.
Enzymatic hydrolysis
To extract antioxidant peptide from different organs of clam, enzymatic hydrolysis was performed using various enzymes at their optimal conditions, papain (0.1 M Na2HPO4-NaH2PO4, pH 6.0 and 37 oC), pepsin (0.1 M Glycine—HCl, pH 2.0 and 37 oC) and trypsin (0.1 M Na2HPO4-NaH2PO4, pH 8.0 and 37 oC). At enzyme/substrate ratio of 1/100 (w/w), 1% substrate and enzyme were mixed. The mixture was incubated for 6 h at each optimal temperature with stirring and then heated in a boiling water bath for 10 min to inactivate the enzyme. Aliquot from each hydrolysate was centrifuged at 12000 rpm for 10 min and supernatant was lyophilized as discussed by Je et al. (2007) and stored under −20 oC until use.
Scavenging ability on 1, 1-diphenyl-2-picrylhydrazl radicals
DPPH radical scavenging activity was determined according to the method of Yen and Hsieh (1995). An aliquot of sample (1 mg/ml) was mixed with 3 ml of methanol and then added to 1 ml of 0.15 mM DPPH in methanol. The mixture was then mixed vigorously and allowed to stand at room temperature in the dark for 30 min. The absorbance of the mixture was measured at 517 nm using a UV-Vis spectrophotometer. The control was conducted in the same manner but methanol was used instead of sample. DPPH radical scavenging activity was calculated as follows:
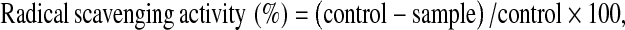 |
Where, sample is the absorbance of sample and control is the absorbance of the control at 517 nm.
Reducing power assay
The ability of the hydrolysate to reduce iron (III) was determined according to the method of Yen and Chen (1995). An aliquot of 1 mg/ml sample of each hydrolysate was mixed with 2.5 ml of 0.2 M phosphate buffer (pH 6.6) and 2.5 ml of 1% potassium ferricyanide. The mixture was incubated at 50 oC for 30 min, followed by addition of 2.5 ml of 10% (w/v) trichloro acetic acid. The mixture was then centrifuged at 1,650×g for 10 min. Finally, 2.5 ml of the supernatant solution were mixed with 2.5 ml of distilled water and 0.5 ml of 0.1% (w/v) ferric chloride. After 10 min reaction the absorbance of the resulting solution was measured at 700 nm. Increased absorbance of the reaction mixture indicated increased reducing power.
Measurement of the antioxidative activity in linoleic acid model system
The antioxidative activity was measured in α-linoleic acid model system according to the methods of Osawa and Namiki (1985). Briefly, a sample (1.3 mg) was dissolved in 10 ml of 50 mM phosphate buffer (pH 7.0), and added to a solution containing 0.13 ml of linoleic acid and 10 ml of 99.5% ethanol. Then the total volume was adjusted to 25 ml with distilled water. The mixture was incubated in a storage bottle with a screw cap at 40 ± 2 oC in a dark room and the degree of oxidation was evaluated by measuring the ferric thiocyanate values. The ferric thiocyanate value was measured according to the method of Mitsuta et al. (1996). The reaction solution (100 μl) incubated in the α-linoleic acid model system was mixed with 4.7 ml of 75% ethanol, 0.1 ml of 30% ammonium thiocyanate, and 0.1 ml of 2 × 10−2 M ferrous chloride solution in 3.5% HCl. After 3 min, the thiocyanate value was measured by reading the absorbance at 500 nm following colour development with FeCl2 and thiocyanate at different intervals during the incubation period.
Purification of the antioxidant peptide
The lyophilized clam body and viscera protein hydrolysate (20 mg/ml) was dissolved in 20 mM sodium acetate buffer (pH 4.0), and loaded onto fast protein liquid chromatography (FPLC) on a XK 26 DEAE anion exchange column equilibrated with 20 mM sodium acetate buffer (pH 4.0), and eluted with a linear gradient of NaCl (0–1.5 M) in the same buffer at a flow rate of 1 ml/min. Each fraction collected at a volume of 6 ml was monitored at 280 nm, pooled fractions were then concentrated using a rotary evaporator; and antioxidant activities were investigated. The fraction having strong antioxidant properties were lyophilized, and loaded onto a Sephadex G-25 gel filtration column (2.5 × 75 cm) which was previously equilibrated with distilled water. The column was then eluted with the distilled water at a flow rate of 1 ml/min. Each fraction collected at a volume of 3 ml was monitored at 280 nm, pooled fractions were then concentrated using a rotary evaporator; and antioxidant activities were investigated. The fraction having strong antioxidant properties were lyophilized, and subjected to HPLC to find the presence of amino acids.
Amino acid composition
The amino acid composition was identified by using the methods of Li et al. (2008) with small modifications. The lyophilized active fractions from clam body and viscera were digested with HCl (6 N) at 110 ºC for 24 h and neutralized with NaoH and loaded onto HPLC. High performance liquid chromatography (HPLC) analysis was carried out in an Agilent 1100 assembly system after precolumn derivatization with o-phthaldialdehyde (OPA). Each sample (1 μl) was injected on a Zorbax 80 A C18 column at 40 ºC with detection at 338 and 262 nm. Mobile phase A was 7.35 mmol/l sodium acetate/ triethylamine (500:0.12, v/v), adjusted to pH 7.2 with acetic acid, while mobile phase B (pH 7.2) was 7.35 mmol/l sodium acetate/ methanol/ acetonitrile (1:2:2, v/v/v). The amino acid composition was expressed as percentage of protein.
Statistical analysis
Experimental results were expressed as mean of triplicate ± standard deviation. Statistical analysis of the result was performed using Statistical Package for the Social Sciences (SPSS) 10.1 for Windows (Chicago, IL, USA).
Results and discussion
Description of the clam
The collected clams (Meretrix casta) were found in moderately large shell (3 ± 0.2 cm) with a brown horny periostracum, extremely smooth, bears three cardinal teeth and another one in front of the cardinals on the left valve and a corresponding depression on the right. The outer surface is pale yellowish brown, fringed with dark gray posteriorly and very faintly rayed with grayish radial line. These features of the specimen were confirmed using bivalves monograph (Shanmugam et al. 1998).
Preparation of clam protein hydrolysates and their antioxidant properties
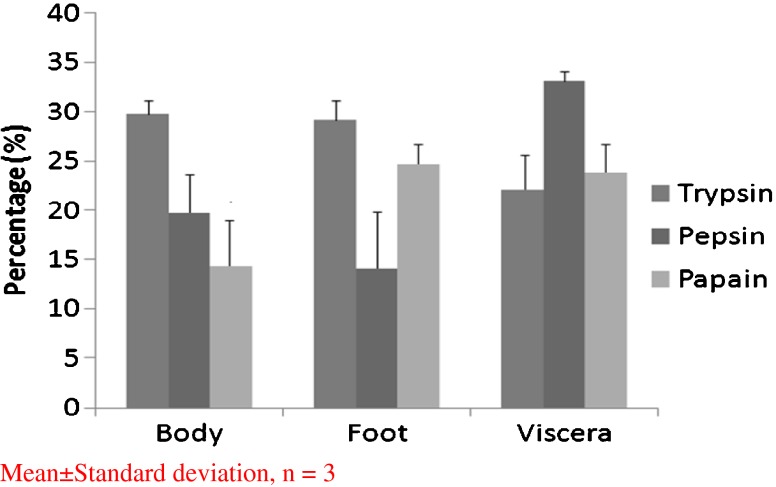
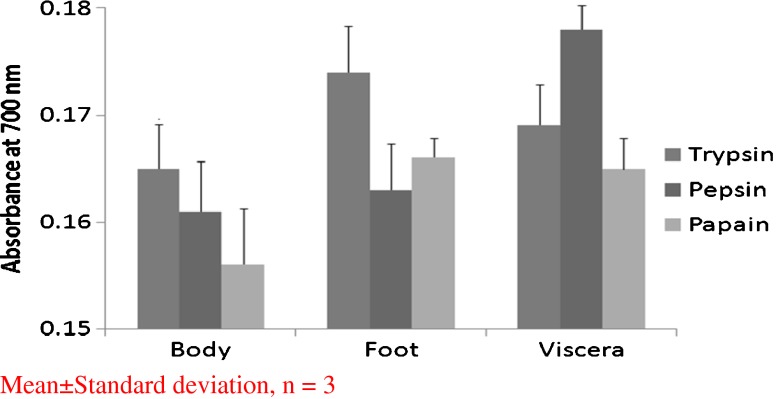
The dissected body, foot and viscera were hydrolyzed separately by enzymes (trypsin, pepsin and papain) at optimal conditions. Peptides from all the nine hydrolysates (i.e., three parts hydrolyzed with three different enzymes) were evaluated for their antioxidant activity using DPPH radical scavenging activity and reducing power assay. As shown in Figs. 1 and 2, they were able to induce significant scavenging effects, with peptic hydrolysate exerting the highest in viscera and trypsin hydrolysate in body and foot. However, DPPH radical scavenging activity of trypsin hydrolysates from body and foot was more or less same but, significant variation was observed in reducing power ability assay. In general, antioxidant peptides are obtained by enzymatic modification of various proteins derived from natural bio-resources viz., bullfrog skin (Qian et al. 2008), porcine collagen (Lertittikul et al. 2007), round scad (Thiansilakul et al. 2007), squid muscle (Rajapakse et al. 2005), alaska pollack (Byun and Kim 2001) tuna backbone protein (Je et al. 2007) and fish muscle (Shabeena Yousuf Naqash and Nazeer 2010) using commercial enzymes like alcalase, α-chymotrypsin, neutrase, papain, pepsin and trypsin. But, in the present study only three enzymes (papain, pepsin and trypsin) were used and tested for their antioxidant activity. Just like in tuna backbone (Je et al. 2007) clam viscera also showed good activity with peptic hydrolysate as shown in Fig. 1 than remaining hydrolysates. But, body and foot on hydrolysis with trypsin showed comparatively good activity and the same was observed with the hydrolysate of bullfrog and smooth hound also (Qian et al. 2008; Bougatef et al. 2009).
Fig. 1.
DPPH radical scavenging activity of clam (Meretrix casta) protein hydrolysates
Fig. 2.
Reducing power ability of clam (Meretrix casta) protein hydrolysates
Purification of antioxidant peptides
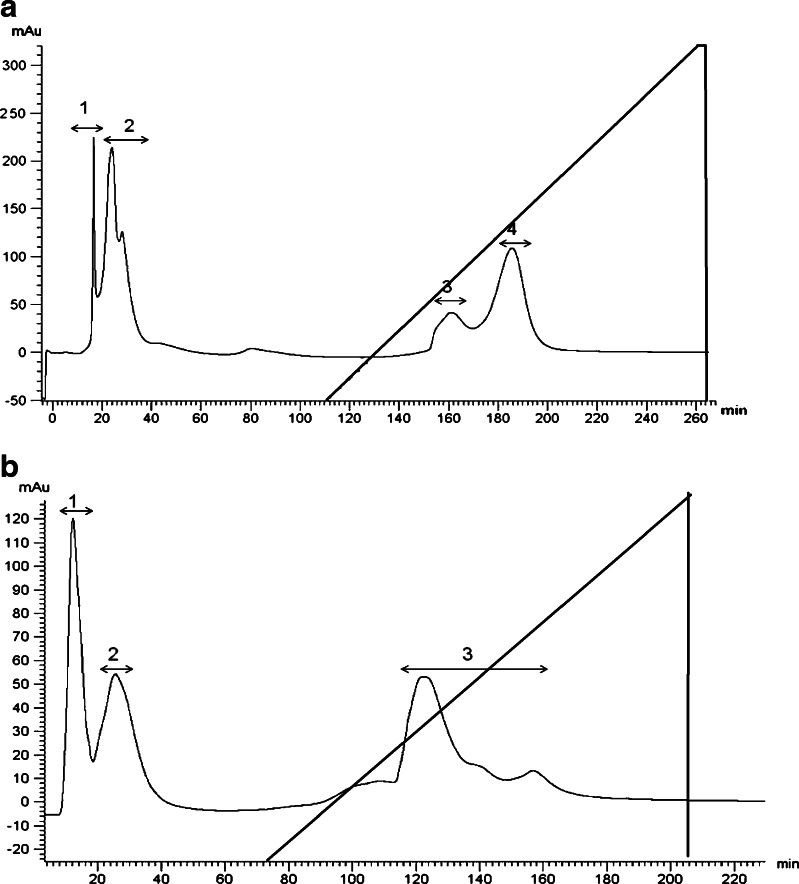
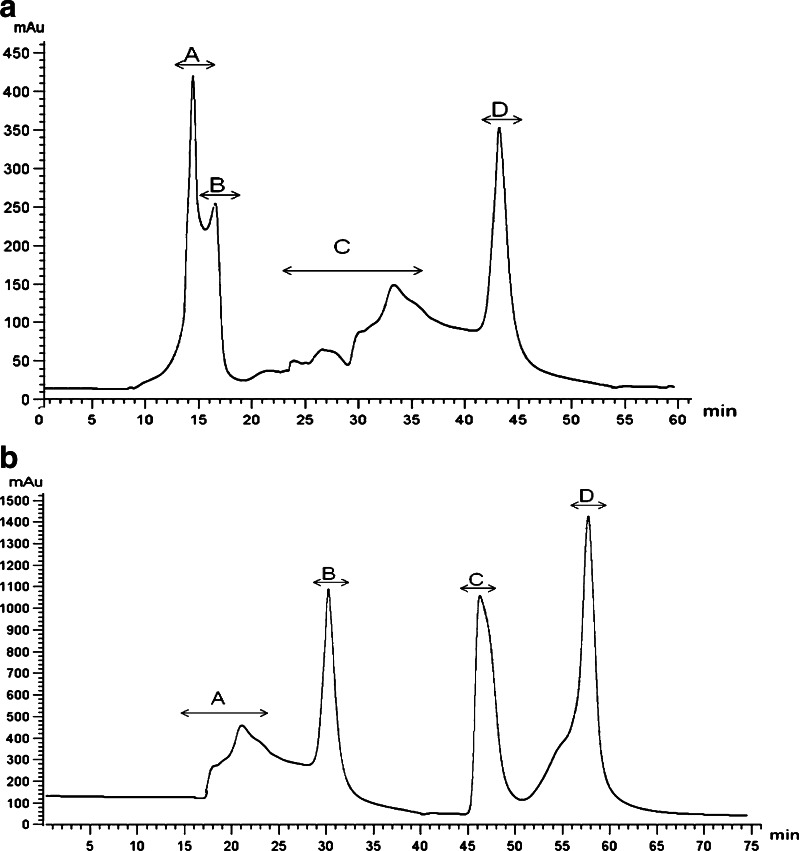
Considering antioxidative effects on DPPH free radical scavenging activity and reducing power ability two protein hydrolysates (were selected out of nine) were employed for the purification and identification of antioxidant peptide. Peptic hydrolysate from viscera and trypsin hydrolysate from foot were dissolved in distillated water at 30 mg/ml separately, and loaded on DEAE anion exchange column. It was fractionated according to variation in charge on elution with buffer B, using the chromatography. As shown in figure, four peptide fractions from peptic viscera hydrolysate (Fig. 3a) and three peptide fractions from trypsin foot hydrolysate (Fig. 3b) was isolated, among which fraction 2 of viscera and fraction 3 of foot hydrolysates was identified to be highly potent. Following activity analysis, the most potent peptides were purified with a Sephadex G-25 column using open column equipment on FPLC. It was fractionated according to molecular size using chromatography. As shown in Fig. 4 a and b, four clear peptides peaks (A–D) were identified and compared for their antioxidant activity. Peptide fractions, showing antioxidant activity from each hydrolysate (Table 1), were finally loaded on an analytical HPLC and their amino acid sequences were determined.
Fig. 3.
Purification of the antioxidant peptide by XK26 DEAE anion exchange chromatography from clam (a) Viscera protein hydrolysate (b) Foot protein hydrolysate
Fig. 4.
Further purification of the antioxidant peptide using Sephadex G-25from clam (a) Viscera protein hydrolysate (fraction 2) (b) Foot protein hydrolysate (fraction 3)
Table 1.
DPPH radical scavenging activity and reducing power ability of FPLC fractions
| FPLC fractions | Viscera | Foot | ||
|---|---|---|---|---|
| DPPH radical | Reducing power | DPPH radical | Reducing power | |
| Fraction 1 | 19.1 ± 1.74 | 0.2 ± 0.01 | 09.1 ± 1.36 | 0.2 ± 0.06 |
| Fraction 2 | 56.4 ± 2.11 | 0.5 ± 0.09 | 21.2 ± 2.19 | 0.2 ± 0.03 |
| Fraction 3 | 36.2 ± 1.39 | 0.3 ± 0.06 | 43.4 ± 1.18 | 0.3 ± 0.07 |
| Fraction 4 | 29.3 ± 0.89 | 0.2 ± 0.05 | – | – |
| Fraction A | 25.9 ± 2.14 | 0.3 ± 0.01 | 73.5 ± 2.15 | 0.5 ± 0.08 |
| Fraction B | 82.5 ± 1.31 | 0.7 ± 0.05 | 39.8 ± 1.89 | 0.3 ± 0.02 |
| Fraction C | 42.8 ± 1.76 | 0.2 ± 0.04 | 11.1 ± 1.34 | 0.1 ± 0.02 |
| Fraction D | 19.1 ± 1.32 | 0.1 ± 0.04 | 22.1 ± 1.78 | 0.1 ± 0.01 |
Mean ± SD, n = 3; Fraction 1–4: fractions collected from ion exchange chromatography; Fraction A–D: fractions collected from gel filtration chromatography
Amino acid composition
Amino acid composition of both the peptides showed the presence of both essential and non-essential amino acids as tabulated (Table 2). As reported by, Pihlanto Leppala (2001) bioactive peptides usually contain 2–20 amino acid residues per molecule, so to conform this isolated peptides were subjected to HPLC for amino acid composition and clam peptides showed the presence of all the amino acids. The results showed presence of essential amino acid contents (milligrams of amino acid/gram protein) of all samples was higher than the recommended values for human adults (FAO/WHO 1990).These antioxidant active peptides contained glutamic acid, histidine, threonine, arginine in good percentage than remaining amino acids. Presence of amino acids like histidine and methionine might be the reason for good antioxidant activity. But, all the amino acids have shown variation in their presence in the two isolated peptides. Both the peptides contained the lysine requirements for infants (FAO/WHO/UNU 1985). The content of many of the essential amino acids was very close to the requirements (FAO/ WHO/ UNU, 1985). Moreover, amino acids such as, tyrosine, methionine, histidine, lysine and tryptophan, were generally accepted as antioxidants (Chen et al. 1996) and presence of all these amino acids makes the clam peptide a potent antioxidant.
Table 2.
Amino acid composition (% w/w) of the active fractions of purified protein hydrolysates
| Amino acid | Viscera | Body |
|---|---|---|
| Aspartic acid | 5.1 | 3.8 |
| Threoninea | 6.9 | 15.7 |
| Serine | 4.1 | 3.2 |
| Glutamic acid | 10.7 | 9.1 |
| Proline | 2.4 | 2.4 |
| Glycine | 2.9 | 5.0 |
| Alanine | 4.7 | 3.6 |
| Valinea | 4.3 | 2.5 |
| Methioninea | 2.5 | 4.3 |
| Isoleucinea | 2.7 | 2.4 |
| Leucinea | 4.7 | 2.9 |
| Tyrosine | 3.9 | 3.6 |
| Phenylalaninea | 2.6 | 4.2 |
| Lysinea | 3.6 | 3.9 |
| Histidine | 16.4 | 5.2 |
| Arginine | 6.3 | 13.4 |
aEssential amino acids
Measurement of the antioxidative activity in α-linoleic acid model system
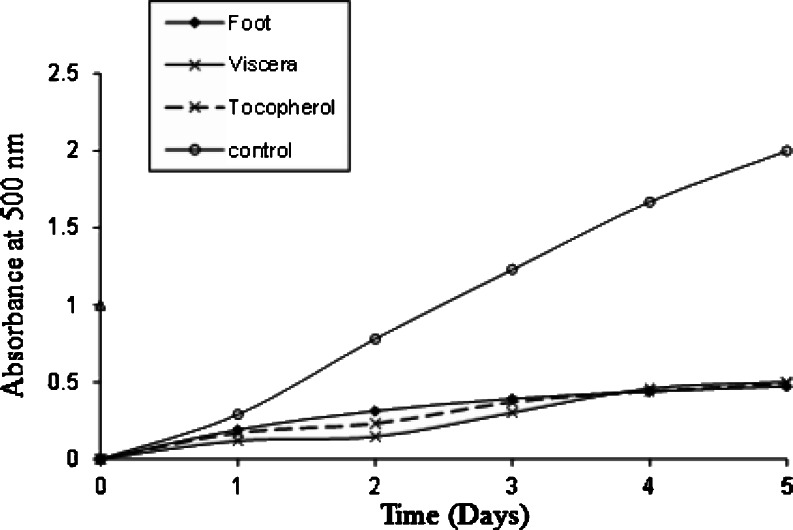
The clam peptides isolated from foot and viscera protein hydrolysates were determined by inhibition of lipid peroxidation in α-linoleic acid model system. As shown Fig. 5, both the peptides were significant retarders of lipid peroxidation and exhibited a nonlinear pattern. For visceral peptide, the effect of inhibiting lipid oxidation decreased in second day and later showed a gradual increase. This activity of clam visceral peptide was higher than natural antioxidant α-tocopherol and followed by clam foot peptide. In the free radical-mediated lipid peroxidation system, antioxidant activity of peptide is mostly dependent on molecular size and chemical properties such as hydrophobicity and electron transferring ability of amino acid residues in the sequence (Qian et al. 2008). Alanine, valine, leucine, proline with non-polar aliphatic groups have high reactivity to hydrophobic PUFAs, and tyrosine, histidine, tryptophan, and phenylalanine with aromatic residues can make reactive oxygen species (ROS) stable through direct electron transfer. May be due to the presence of all positively antioxidant amino acids in the purified peptide made effortless to quench unpaired electrons or radicals by supporting protons in the current study.
Fig. 5.
Antioxidant activity of purified peptide in a linoleic acid emulsion system measured by the ferric thiocyante method
Conclusion
Based on these results, it is suggested that the protein hydrolysates prepared from the body, foot and viscera of clam (Meretrix casta) by enzymatic hydrolysis has potent antioxidant properties. And the purified antioxidant peptide from viscera and foot protein hydrolysates are potent free radical scavenger and effectively inhibited lipid peroxidation, which would be expected to protect against oxidative damage in living systems in relation to atherosclerosis, arthritis, diabetes and carcinogenesis.
Acknowledgment
We gratefully acknowledge Dr. K. Ramasamy, Dean, School of Bioengineering, SRM University, for his support throughout the work. We extend our gratitude to the management, SRM University for providing the facilities.
References
- Bougatef A, Hajji M, Balti R, Lassoued I, Triki-Ellouz Y, Nasri M. Antioxidant and free radical-scavenging activities of smooth hound (Mustelus mustelus) muscle protein hydrolysates obtained by gastrointestinal proteases. Food Chem. 2009;114:1198–1205. doi: 10.1016/j.foodchem.2008.10.075. [DOI] [Google Scholar]
- Aspmo SI, Horn SJ, Eijsink VGH. Enzymatic hydrolysis of Atlantic cod (Gadus morhua L.) viscera. Process Biochem. 2005;40:1957–1966. doi: 10.1016/j.procbio.2004.07.011. [DOI] [Google Scholar]
- Byun HG, Kim SK. Purification and characterization of angiotensin I converting enzyme (ACE) inhibitory peptides from Alaska pollack (Theragra chalcogramma) skin. Process Biochem. 2001;36:1155–1162. doi: 10.1016/S0032-9592(00)00297-1. [DOI] [Google Scholar]
- Chen HM, Muramoto K, Yamauchi F, Nokihara K. Antioxidant activity of designed peptides based on the antioxidative peptide isolated from digests of a soybean protein. J Agric Food Chem. 1996;44:2619–2623. doi: 10.1021/jf950833m. [DOI] [PubMed] [Google Scholar]
- De Lamirande E, Jiang H, Zini A, Kodama H, Gangnon C. Reactive oxygen species and sperm physiology. Rev Reprod. 1997;2:48. doi: 10.1530/ror.0.0020048. [DOI] [PubMed] [Google Scholar]
- Food and Agriculture Org./World Health Org. (1990) Protein quality evaluation. Report of a joint FAO/WHO expert consultation held in Bethesda, Md. 1989 Dec 4–8. Rome: FAO/WHO
- Gildberg A. Enzymatic processing of marine raw materials. Process Biochem. 1993;28:1–15. doi: 10.1016/0032-9592(94)80030-8. [DOI] [Google Scholar]
- Halliwell B. Free radicals, antioxidants, and human disease: curiosity, cause, or consequence. Lancet. 1994;344:721–724. doi: 10.1016/S0140-6736(94)92211-X. [DOI] [PubMed] [Google Scholar]
- Je J-Y, Qian Zhong-Ji, Byun H-G, Kim Se-Kwon. Purification and characterization of an antioxidant peptide obtained from tuna backbone protein by enzymatic hydrolysis. Process Biochem. 2007;42:840–846. doi: 10.1016/j.procbio.2007.02.006. [DOI] [Google Scholar]
- Kristinsson HG, Rasco BA. Biochemical and functional properties of Atlantic salmon (Salmo salar) muscle proteins hydrolysed with various alkaline proteases. J Agri Food Chem. 2000;48:657–666. doi: 10.1021/jf990447v. [DOI] [PubMed] [Google Scholar]
- Lertittikul W, Benjakul S, Tanaka M. Characteristics and antioxidative activity of Maillard reaction products from a porcine plasma protein–glucose model system as influenced by pH. Food Chem. 2007;100:669–677. doi: 10.1016/j.foodchem.2005.09.085. [DOI] [Google Scholar]
- Mitsuta H, Yasumoto K, Iwami K. Antioxidative action of indole compounds during the autoxidation of linoleic acid. Eiyo to Shokuryo. 1996;29:238–244. [Google Scholar]
- Osawa T, Namiki M. Natural antioxidants isolated from eucalyptus leaf waxes. J Agric Food Chem. 1985;33:777–780. doi: 10.1021/jf00065a001. [DOI] [Google Scholar]
- Pihlanto Leppala A. Bioactive peptides derived from whey proteins: opioid and ACE-inhibitory peptides. Trends Food Sci Technol. 2001;11:347–356. doi: 10.1016/S0924-2244(01)00003-6. [DOI] [Google Scholar]
- Qian ZJ, Jung WK, Kim SK. Free radical scavenging activity of a novel antioxidative peptide purified from hydrolysate of bullfrog skin Rana catesbeiana Shaw. Bioresource Technol. 2008;99:1690–1698. doi: 10.1016/j.biortech.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Rajapakse N, Mendis E, Byun HG, Kim SK. Purification and in vitro antioxidative effects of giant squid muscle peptides on free radical-mediated oxidative systems. J Nutr Biochem. 2005;16:562–569. doi: 10.1016/j.jnutbio.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Naqash SY, Nazeer RA. Antioxidant activity of hydrolysates and peptide fractions of_Nemipterus japonicus_and_Exocoetus volitans_muscle. J Aquat Food Prod Technol. 2010;19(3):180–192. doi: 10.1080/10498850.2010.506256. [DOI] [Google Scholar]
- Shahidi F, Han Xiao-Qing H, Synowieck J. Production and characteristics of hydrolysates from capelin (Mallotus villosus) Food Chem. 1995;53:285–293. doi: 10.1016/0308-8146(95)93934-J. [DOI] [Google Scholar]
- Shanmugam A, Rajagopal S, Nazeer RA (1998) Common bivalves of Parangipettai Coast. Centre of Advanced Study in Marine Biology, Annamalai University, Parangipettai, India, 39–40
- Thiansilakul Y, Benjakul S, Shahidi F. Compositions, functional properties and antioxidative activity of protein hydrolysates prepared from round scad (Decapterus maruadsi) Food Chem. 2007;103:1385–1394. doi: 10.1016/j.foodchem.2006.10.055. [DOI] [Google Scholar]
- Venugopal V, Shahidi F. Value-added products from underutilized fish species. Crit Rev Food Sci Nutr. 1995;35:431–453. doi: 10.1080/10408399509527708. [DOI] [PubMed] [Google Scholar]
- Wanita A, Lorenz K. Antioxidant potential of 5-N pentadecylresorcinol. J Food Process Preserv. 1996;20:417–429. doi: 10.1111/j.1745-4549.1996.tb00757.x. [DOI] [Google Scholar]
- Li Y, Zhang T, Wanmeng Mu, Liu J. Antioxidant and free radical-scavenging activities of chickpea protein hydrolysate (CPH) Food Chem. 2008;106:444–450. doi: 10.1016/j.foodchem.2007.04.067. [DOI] [Google Scholar]
- Yen G, Hsieh P. Antioxidant activity and scavenging effects on active oxygen of xylose-lysine Maillard reaction products. J Sci Food Agric. 1995;67:415–420. doi: 10.1002/jsfa.2740670320. [DOI] [Google Scholar]
- Yen GC, Chen HY. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J Agri Food Chem. 1995;431:27–32. doi: 10.1021/jf00049a007. [DOI] [Google Scholar]