Abstract
In the present study, antioxidant activities of the phenolic extracts from H. isora fruits and C. pentandra seeds were investigated by employing established in vitro systems, which included reducing power, OH●, DPPH●, ABTS●+, linoleic acid emulsion, metal chelation and antihemolytic activity. The extracts of C. pentandra contained relatively higher levels of total phenolics and flavonoids than those of H. isora. All the extracts showed dose dependent reducing power activity and moreover, they were well correlated with the total phenolic substances. A similar dose dependant trend has also been observed for hydroxyl radical scavenging activity and DPPH● radical scavenging activity. Further, addition of 250 μg of extracts to the reaction mixture produced 41.3–54.6% peroxidation inhibiting activity during 60 h of incubation. The potential of multiple antioxidant activity of samples can be further evidenced by inhibition of reactive oxygen mediated erythrocyte cell lysis and metal ion chelating activity.
Keywords: C. pentandra seeds, H. isora fruits, Antioxidant activity, ABTS•+, Phenolics and flavonoids
Introduction
Natural phenolic phytochemicals in plants have been receiving increased interest from consumers and researchers for their beneficial health effects on coronary heart diseases and cancers mainly due their antioxidant activity. Free radicals and other reactive oxygen species generated in living organisms leads to many diseases including cancer, cardiovascular diseases, cataracts, asthma, hepatitis, liver injury and immunodeficiency diseases (Lee et al. 2004). The use of synthetic antioxidants is an old practice and their safety could be questioned by the consumers. The alternative natural compounds with efficient antioxidant activity have been paid increasing attention.
Helicteres isora L. (Sterculiaceae) and Ceiba pentandra L. Gaertn. (Bombacaceae) are two important, traditionally used medicinal plants in India. The former is a shrub or small tree available in forests throughout the central and western India. In tradition, the root juice is claimed to be useful in diabetes, empyema and a favorite cure for snakebite. The root and the bark are expectorant, demulcent, astringent, anti-galactagogue, and useful in constipation, colic, scabies, gastropathy, diabetes, diarrhea and dysentery. The extract from the root and bark possess insulin-sensitizing, hypolipidemic activity and has the potential for use in the treatment of type-2-diabetes (Kumar et al. 2007). Moreover, the root extracts exhibited significant antihyperglycemic activity and the effect was comparable with that of glibenclamide (Venkadesh et al. 2004). Apart from these, petroleum ether, chloroform and aqueous ethanol extracts of root showed significant antinociceptive activity on acetic acid-induced writhing test in mice, at a dose of 250 mg/kg (Kumar et al. 2006). Further, the aqueous extract of the bark showed potential hypoglycaemic and hypolipidaemic effects in streptozotocin induced diabetic rats (Kumar and Murugesan 2008; Kumar et al. 2007). The previous phytochemical analysis showed the presence of phytosterols, saponins, sugars, phlobotannins, lignin, isorin, alkaloids, triterpenoids and their acetates, cucurbitacin B, isocucurbitacin B, flavonoids, flavonoid glucuronides, neolignans, rosmarinic acid derivatives, betulic acid, daucosterol, tannins, anthraquinones, cardiac glycosides, sterols, triterpenes, α- and β-amyrin, lupeol, fridelin, taraxerone, β-sitosterol and volatile oil (Venkadesh et al. 2004; Kumar et al. 2007). The fruits are astringent, refrigerant, stomachic, vermifuge, vulnerary and useful in griping of bowels, flatulence of children and also possess antispasmodic effect. The dried fruit is demulcent and mildly astringent. Among the Bhils of Rajasthan, an extract of the fruit is given orally to children to relieve diarrhea. The powdered fruits mixed with neem oil are used for treatment of paralysis among the tribal inhabitants of eastern Bihar (Satake et al. 1999). From the fruits, three new compounds 49-O- b-D-glucopyranosyl rosmarinic acid (2), 4,49-O-di- b-D-glucopyranosyl rosmarinic acid (3) and 2R-O-(49-O- b-D-glucopyranosyl caffeoyl)-3-(4-hydroxyphenyl) lactic acid named as 49-O- b-D glucopyranosyl isorinic acid (4) were isolated together with rosmarinic acid (1). Among these, compound 3 had greater scavenging activity against superoxide anion produced with xanthine and xanthine oxidase (XOD) than rosamarinic acid (Khare 2007). The fruit extract has also been reported to be a potent inhibitor of human immunodeficiency virus type-1 (HIV-1) (Otake et al. 2006).
C. pentandra is a medium sized, deciduous tropical tree found throughout western and southern India. In Asia, Oceania, Africa and Central America this species is used to treat various disorders, including diarrhea, fever, gonorrhea and parasitic infections and as a diuretic and emollient. The gum and unripe fruits were considered as astringent; root used as antidiabetic and antispasmodic (Noreen et al. 1998). Extract from bark showed anti-inflammatory properties both in in vivo and in vitro, which can be related to several reports of traditional use in the treatment of ailments of an inflammatory nature such as asthma and cough (Ladeji et al. 2003). The bark extract of C. pentandra given orally for a period of 4 weeks, produced a decreased plasma glucose level in diabetic rats, and it does not appear to be toxic, the plant has a hypoglycemic effect and may be safe when taken orally (Ueda et al. 2002). A new naphthoquinone, 2,7-dihydroxy-8-formyl-5-isopropyl-3-methyl-1,4-naphthoquinone (1) together with a known naphthoquinone, 8-formyl-7-hydroxy-5-isopropyl-2-methoxy-3-methyl-1,4-naphthoquinone (2), sesquiterpenoids and isoflavones have been isolated from the heartwood of this plant and particularly the naphthoquinones exhibited antimicrobial activity. Similarly, two new isoflavones, pentandrin and pentandrin glucoside and, vavain and its glucoside were isolated from the stem barks of C. pentandra along with β-sitosterol and its 3-O-β-D-glucopyranoside (Ngounou et al. 2000). In the remote villages, the dry heated/fried seed kernels, after dehulling are being consumed by local peoples as potential protein source. From the above account, it is evident that the plant samples have high level of biologically active components which exhibited most of the medicinal properties. Therefore, the present work aims at further investigating their antioxidant activity through the use of several in vitro assay systems and this will provide insight information with reference to their medicinal value.
Materials and methods
Chemicals
Potassium ferricyanide, ferric chloride, 2,2-diphenyl-1-picryl-hydrazyl (DPPH●), potassium persulfate, 2,2′azinobis(3-ethylbenzothiozoline-6-sulfonic acid) disodium salt (ABTS), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), linoleic acid, ferrous chloride, ammonium thiocyanate, hydrogen peroxide, ferrous ammonium sulfate, ethylenediamine tetracetic acid (EDTA) disodium salt, 2,2′-bipyridyl and hydroxylamine hydrochloride were obtained from Himedia, Merck or Sigma. All other reagents used were of analytical grade.
Collection of plant material
Fresh dried fruits of H. isora were collected from Vellingiri hills, Coimbatore, Tamil Nadu, India. The dried seeds of C. pentandra were collected from local area in Coimbatore, Tamil Nadu, India. Then, the samples were ground into fine powder and stored in separate screw cap bottles before analysis.
Preparation of extracts
The dried and powdered samples were extracted by stirring with 50 mL of 50% methanol (1:5 w/v) at 25 °C for 24 h and centrifuged at 7,000 rpm for 10 min.Then, the pellet was re-extracted with an additional of 50 mL of 50% methanol, as mentioned above. The residue after air drying, were re-extracted by occasional shaking with 50 mL of 70% acetone, as described above. The solvents of the respective combined extracts were evaporated under reduced pressure, using a rotary vacuum-evaporator at 50 °C (Yamato, RE300) and the remaining water was removed by lyophilization (Vir Tis Benchtop K, Model—#4KBTXL-75). The freeze-dried extracts thus obtained were used directly for assessment of antioxidant capacity through various chemical assays.
Estimation of total phenolics
Twenty μl of each extract (1 mg/mL) was taken in separate test tubes and made up to a volume of 1 mL with distilled water. Then 0.5 mL of Folin ciocalteu’s phenol reagent (1:1 with water) and 2.5 mL of sodium carbonate solution (20%) were added sequentially in each tube. Soon after vortexing the reaction mixture, the test tubes were placed in dark for 40 min and the absorbance was recorded at 725 nm against the reagent blank. The amount of total phenolics was calculated as tannic acid equivalents from the calibration curve (Siddhuraju and Becker 2006).
Estimation of total flavonoid content
The total flavonoid content of sample extracts were determined by the use of a slightly modified colorimetric method described previously (Zhishen et al. 1999). An aliquot of 0.5 mL extract was mixed with 2 mL of distilled water and subsequently with 0.15 mL of a 5% NaNO2 solution. After 6 min, 0.15 mL of 10% AlCl3 solution was added and allowed to stand for 6 min, and then 2 mL of 4% NaOH solution was added to the mixture. Immediately distilled water was added to bring the final volume of 5 mL, and then the mixture was thoroughly mixed and allowed to stand for another 15 min. Absorbance of the mixture was determined at 510 nm versus prepared water blank. Rutin was used as a standard compound for the quantification of total flavonoid. All the values were expressed as gram of rutin equivalent per 100 g of extract.
Reducing power assay
The sample extracts of varying concentration (20–100 μg) were taken in 1 mL of phosphate buffer in a test tube and 5 mL of 0.2 M phosphate buffer, pH 6.6 was added. To this, 5 mL of 1% potassium ferricyanide solution was added. The mixture was incubated at 50 °C for 20 min. After the incubation, 5 mL of 10% TCA was added and the content was centrifuged at 1,000 rpm for 10 min. The upper layer of the supernatant (5 mL) was mixed with 5 mL of distilled water. To this, 1 mL of ferric chloride (0.1%) was added and vortexed. Then, the absorbance of the reaction mixture was read spectrophotometrically at 700 nm against water blank (Oyaizu 1986).
Hydroxyl radical scavenging activity
The scavenging activity of sample extracts on hydroxyl radical was measured according to the method of Klein et al. (1991). Various concentrations (20–100 μg) of extracts were added with 1.0 mL of iron-EDTA solution, 0.5 mL of EDTA solution (0.018%), and 1.0 mL of DMSO (0.85% DMSO (v/v) in 0.1 M phosphate buffer, pH 7.4) sequentially. The reaction was initiated by adding 0.5 mL of ascorbic acid (0.22%) and incubated at 80–90 °C for 15 min in a water bath. After incubation the reaction was terminated by the addition of 1.0 mL of ice-cold TCA (17.5% w/v). Three mL of Nash reagent was added and left at room temperature for 15 min. The reaction mixture without sample was used as control. The intensity of the colour formed was measured spectrophotometrically at 412 nm against reagent blank. The% hydroxyl radical scavenging activity was calculated by the following formula:
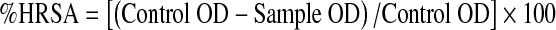 |
Antioxidant activity determination by using linoleic acid emulsion system
A sample extract having the concentration of 1 mg was taken in a screw capped tube and added with 0.5 mL of 2.51% linoleic acid in 99.5% ethanol, 1 mL of 0.05 M phosphate buffer (pH 7), and 0.5 mL of distilled water and then incubated in an oven at 40 °C in dark. A control without sample extract was used. Every 12 h, 0.1 mL aliquots of this solution was taken and 9.7 mL of 75% ethanol and 0.1 mL of 30% ammonium thiocyanate were added. Precisely 3 min after the addition of 0.1 mL of 0.02 M ferrous chloride in 3.5% hydrochloric acid to the reaction mixture, the absorbance was measured at 500 nm until the absorbance of the control reached the maximum (Kikuzaki and Nakatani 1993). The antioxidant activity (AA) was calculated as percentage of inhibition relative to the control using the following equation:
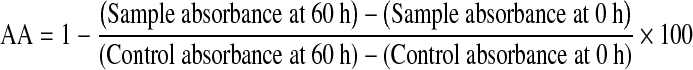 |
Radical scavenging activity using DPPH ● method
Different concentrations of the extracts were taken in separate test tubes. The volume was adjusted to 100 μL with methanol. Five mL of 0.1 mM methanolic solution of DPPH● was added to these tubes and shaken vigorously. The tubes were allowed to stand for 20 min at 27 °C. The control was prepared as above without any extract, and methanol was used for the baseline correction. Changes in the absorbance of the samples were measured at 517 nm (Blois 1958). Radical scavenging activity was expressed as the inhibition concentration (IC50), i.e., The concentration of extract necessary to decrease the initial concentration of DPPH● by 50% (IC50) under the specified experimental condition.
Total antioxidant activity assay by radical cation (ABTS●+)
ABTS was dissolved in water to a 7 mM concentration. ABTS radical cation (ABTS●+) was produced by reacting ABTS stock solution with 2.45 mM potassium persulfate (final concentration) and allowing the mixture to stand in the dark at room temperature for 12–16 h before use. Prior to assay, the solution was diluted in ethanol (about 1:89 v/v) and equilibrated to 30 °C to give an absorbance at 734 nm of 0.700 ± 0.02 in a 1 cm cuvette. The concentration of sample extract that produced between 20% and 80% inhibition of the blank absorbance was determined and adapted. Triplicate determinations were made at each dilution of the standard, and the percentage inhibition of the blank absorbance at 734 nm was plotted as a function of Trolox concentration (Re et al. 1999). The unit of total antioxidant activity (TAA) was defined as the concentration of Trolox having equivalent antioxidant activity expressed as μmol/g sample extracts on dry matter basis.
Chelating capacity
Metal chelating property of the sample was assessed by bipyridyl assay (Yamaguchi et al. 2000). The reaction mixture contained 1 mg of the extract, 0.25 mL of 1 mM FeSO4 solution, 1 mL of 0.2 M Tris–HCl buffer (pH 7.4), 1 mL of 2, 2′ bipyridyl solution, 0.4 mL of 10% hydroxylamine-HCl and 2.5 mL of ethanol. The final volume was made up to 10 mL with deionised water and the absorbance was determined at 522 nm. The chelating activity of the samples was evaluated using EDTA as standard. The results are expressed as mg EDTA equivalent/g sample extracts.
Antihemolytic activity
The erythrocytes from cow blood were separated by centrifugation and washed with saline phosphate buffer (pH 7.4) until the supernatant was colourless. The erythrocytes were then diluted with saline phosphate buffer to give 4% suspension. One mg of extract/mL saline buffer was added to 2 mL of the suspension of erythrocytes and the volume was made up to 3–5 mL with saline buffer. This mixture was preincubated for 5 min and then 0.5 mL H2O2 solutions of appropriate concentration in saline buffer were added. The concentration of H2O2 in the reaction mixture was adjusted so as to bring about 90% hemolysis of blood cells in control after 240 min. After the incubation time the reaction mixture was centrifuged at 1,500 rpm for10 min and the extent of hemolysis was determined by measurement of the absorbance (at 540 nm) corresponding to haemoglobin liberation (Naim et al. 1976).
Statistical analysis
The data were subjected to a one-way analysis of variance (ANOVA) and the significance of the difference between means was determined by Duncan’s multiple range test (p < 0.05) using statistica (Statsoft Inc., Tulsa, USA). Values expressed are means of three replicate determinations ± standard deviation.
Results and discussion
Yield percentage, total phenolics and total flavonoids
The extract yield percentage, total phenolics and flavonoid contents of the extracts obtained from H. isora and C. pentandra are presented in Table 1. Among the different extracts, the maximum yield was obtained for the methanol extracts of C. pentandra (37.2%) followed by H. Isora (23.2%). However, acetone being a successive solvent, the extract yield percentage of both the samples was found to be much lower (10.0% and 3.8% for C. pentandra and H. isora, respectively). As shown in Table 1, the total phenolic content was found to be higher in methanolic extract of C. pentandra (11.1%) and was considerably decreased in acetone extract (5.4%) of the same sample. Eventhough, the 50% methanol extract of H. Isora registered higher level of extract recovery percentage, the total phenolic content of the respective extract was found to be lower (3.5%).
Table 1.
Extarct yield percentage, total phenolics and total flavonoid content in C. pentandra and H. isora
| Extract yield | Total phenolics | Total flavonoids | |
|---|---|---|---|
| Sample | percent (%) | g/100 g sample | g/100 g sample |
| CPMe | 37.2 | 11.1 ± 1.63 | 9.6 ± 0.30 |
| CPAc | 10.0 | 5.4 ± 1.04 | 2.9 ± 0.10 |
| HIMe | 23.2 | 2.6 ± 0.13 | 1.3 ± 0.10 |
| HIAc | 3.8 | 3.5 ± 0.24 | 1.0 ± 0.10 |
Values are means of triplicate determinations (n = 3) ± standard deviation. CPMe C. pentandra methanol extract; CPAc C. pentandra acetone extract; HIMe H. isora methanol extract; HIAc H. isora acetone extract.
The results obtained in the quantitative analysis flavonoids (Table 1) revealed that higher level of flavonoids was observed in methanol extract of C. pentandra (9.6%) followed by acetone extract (2.9%). However, in H. isora both methanol and acetone extracts registered much lower amounts of flavonoids (HIMth—1.3%; HIAc—1.0%). In previous studies, the presence of phenolic substances including flavonoids and tannins were reported in H. isora (Kumar et al. 2007). Furthermore, Ueda et al. (2002) observed the presence of isoflavones in C. pentandra. The results of the present study strongly suggest that phenolics are important components of these plants and some of their pharmacological effects could be attributed to the presence of these constituents.
Reducing power
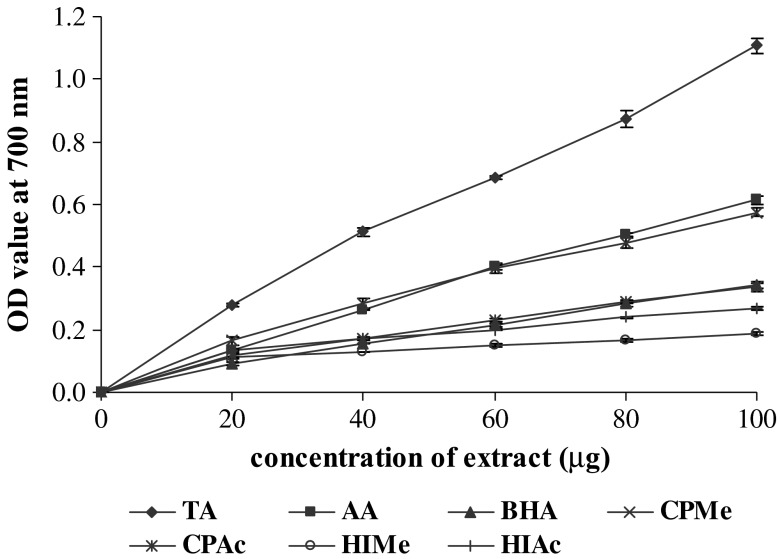
The reducing property is generally associated with the presence of reductants. The antioxidant action of reductants is based on the breaking of free radical chain by donation of a hydrogen atom. Reductants also react with certain precursors of peroxide, thus preventing peroxide formation. The data presented here indicated that the marked reducing activity of the extracts seems to be due to the presence of polyphenols, which may act as reductants by donating the electrons and reacting with free radicals to convert them to more stable products and terminate radical chain reaction. Figure 1 showed the reducing power (as indicated by absorbance at 700 nm) of extracts which increased with increasing concentration to certain extent and then levelled off with further increase in concentrations. A strong reducing power was noted for methanol and acetone extracts of C. pentandra and the values are comparable with the standard antioxidant ascorbic acid. Much lower reducing power was assisted to methanol and acetone extracts of H. isora and BHA. The results showed a significant correlation between total phenolics and reducing power (CPMe, r2 = 0.9465; CPAc, r2 = 0.8841) in C. pentandra. The reducing power of the samples was in the following order: TA>AA>CPMe>CPAc>BHA>HIAc>HIMe. Similar relationship between the polyphenolic constituents and reducing power activity has been reported for several plant extracts (Amarowicz et al. 2004). The results elucidate that polyphenolic contents of the sample extracts appear to function as good electron and hydrogen donors and therefore should be able to terminate radical chain reaction by converting free radicals to more stable products.
Fig. 1.
Reducing power activity of methanol and acetone extract of H. isora and C. pentandra. TA—Tannic acid; AA—Ascorbic acid; BHA- Butylated hydroxyl anisole; CPMe—C. pentandra methanol extract; CPAc—C. pentandra acetone extract; HIMe—H. isora methanol extract; HIAc—H. isora acetone extract. Values are means of triplicate determinations (n = 3) ± standard deviation
Hydroxyl radical scavenging activity
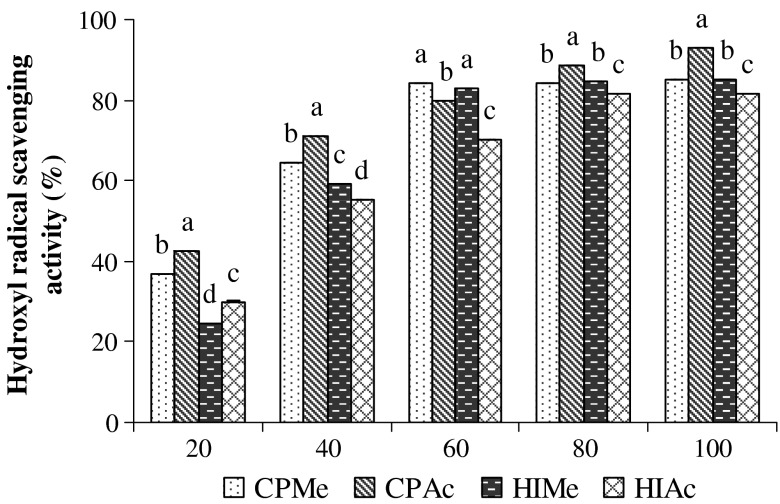
Hydroxyl radical is an extremely reactive free radical formed in biological systems and has been implicated as a highly damaging species in free radical pathology, capable of damaging almost every molecule found in living cells. This radical has the capacity to join nucleotides in DNA and cause strand breakage, which contributes to carcinogenesis, mutagenesis and cytotoxicity. In addition, this species is considered to be one of the quick initiators of lipid peroxidation process, abstracting hydrogen atoms from unsaturated fatty acids. Hydroxyl radical scavenging activity was estimated by generating the hydroxyl radicals using ascorbic acid-iron EDTA. The hydroxyl radical is formed by the oxidation reaction with dimethyl sulfoxide (DMSO) to yield formaldehyde, which provides a convenient method to detect hydroxyl radicals by treatment with Nash reagent (Singh et al. 2002). The scavenging abilities of extracts on hydroxyl radical are shown in Fig. 2. The OH● scavenging ability of extracts were investigated at the concentration range of 20–100 μg/mL and was found to be dose dependent. Among the sample extracts, acetone extract of C. pentandra exhibited highest radical scavenging activity (93.1%) at the concentration of 100 μg/mL. This was closely followed by the methanol extract of C. pentandra and acetone extract of H. isora showing comparable activity (84.1% and 83.1%, respectively) at the concentrations of 60–100 μg/mL. A linear relationship was observed between hydroxyl radical scavenging activity and total phenolic content. These results indicate that the potential scavenging abilities of phenolic substances might be due to the active hydrogen donor ability of hydroxyl substitution. Hagerman et al. (1998) have also reported that high molecular weight and the proximity of many aromatic rings and hydroxyl groups are more important for the free radical scavenging activity by tannins than specific functional groups. In similar lines, methanol extract of pomegranate peel and grape fruit have also been reported to contain sustainable hydroxyl radical scavenging activity and which might be attributed to the presence of phenolic substances (Singh et al. 2002). The investigation reveals that both the sample extracts had good hydroxyl scavenging activity and can be used as a good hydroxyl radical scavenger.
Fig. 2.
Hydroxyl radical scavengign activity of methanol and acetone extract of H. isora and C. pentandra. CPMe—C. pentandra methanol extract; CPAc—C. pentandra acetone extract; HIMe—H. isora methanol extract; HIAc—H. isora acetone extract. Values are means of triplicate determinations (n = 3) ± standard deviation. Bars having different letters are significantly different (p < 0.05)
Antioxidant activity by linoleic acid emulsion system
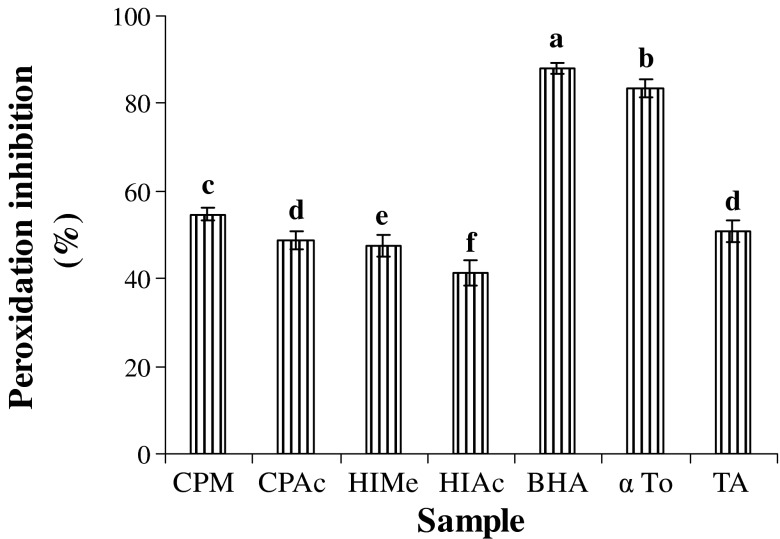
The antioxidant effects of sample extracts, BHA, α tocopherol and tannic acid on the peroxidation of linoleic acid were investigated and the results are presented in Fig. 3. At a concentration of 250 μg in the final reaction mixture, the extracts inhibited 41.3–54.6% peroxidation of linoleic acid after incubation for 60 h. However, these values are significantly lower (p < 0.05) than those of positive controls like BHA (88.0%) and α Tocopherol (83.5%). In summary, the inhibitory potential of extracts obtained was in the following order: BHA>αTo>CPMe>TA>CPAc>HIMe>HIAc. The inhibition of lipid peroxidation might be due to the hydrogen donating ability of phenolic derivatives and subsequent radical stabilization. Many investigations that focused on fruits, vegetables and plant extracts found positive and highly significant relationship between total phenolic content and antioxidant activity (Velioglu et al. 1998). In similar lines, the flavonoid rich extract of Hypericum perforatum obviously decreased the peroxidation of linoleic acid during 84 h incubation (Zou et al. 2004).
Fig. 3.
Peroxidation inhibiting property of methanol and acetone extract of H. isora and C. pentandra. CPMe—C. pentandra methanol extract; CPAc—C. pentandra acetone extract; HIMe—H. isora methanol extract; HIAc—H. isora acetone extract; BHA- Butylated hydroxyl anisole; α To—α Tocopherol; TA—Tannic acid. Values are means of triplicate determinations (n = 3) ± standard deviation. Bars having different letters are significantly different (p < 0.05)
DPPH● radical scavenging activity
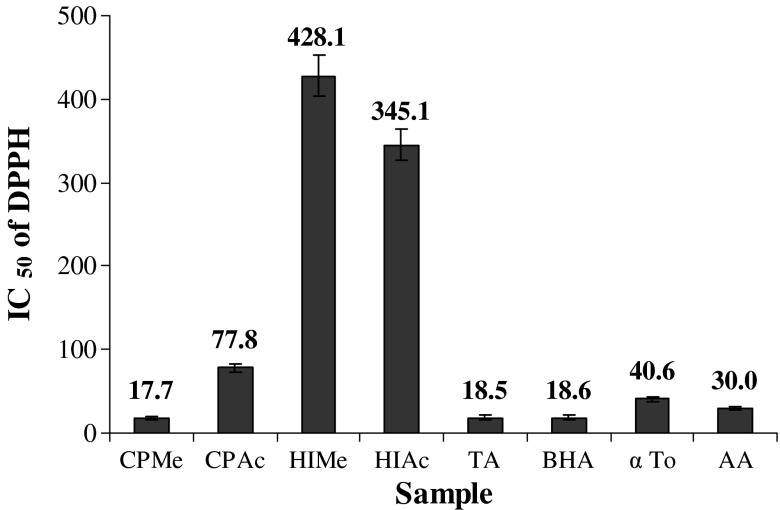
The DPPH● is a stable free radical with a maximum absorbance at 517 nm it can readily undergo scavenging by an antioxidant, and gets converted in to 1,1-diphenyl-2-picrylhydrazine. The degree of discolouration indicates the scavenging potentials of the antioxidant extract. In the present study, the free radical scavenging potentials of the extracts at different concentrations were tested. The concentration of extract necessary to decrease the initial concentration of DPPH● by 50% (IC50) under the specified experimental condition was calculated and the results were shown in Fig. 4. Among the extracts, C. pentandra showed significantly higher DPPH● scavenging activity than H. Isora. In fact, the activity shown by the methanol extract of C. pentandra (CPMe, 17.7 μg) was even higher than those of positive controls like tannic acid (18.5 μg), BHA (18.6 μg), α-tocopherol (40.6 μg) and ascorbic acid (30.0 μg). In the present study, the order of scavenging activity of sample extracts and standards are as follows: CPMe>TA>BHA>AA>α-To>CPAc>HIMe>HIAc. Similar to reducing power activity the DPPH● scavenging activity also appeared to depend on the phenolic concentration. Components such as phenolics, flavonoids, tannins, anthraquinones, isoflavones, and β-sitosterol identified from these plants might be responsible for their powerful antioxidant activity. The DPPH● radical scavenging activity is also known to be related to the nature of phenolics contributing to their electron transfer/hydrogen donating ability (Brand-Williams et al. 1995). Presences of flavonoids generally possess higher antioxidant activity because of double bonds existing in C-ring. Generally the radical scavenging activity of flavonoids depends on their structure and hydroxyl group arrangement. The highest radical scavenging activity is exhibited by compounds that have an ortho 3, 4,-dihydroxy structure at B ring (eg., quercetin), hydroxyl groups in position meta e.g. 5, 7, dihydroxy at ring A (eg., kaemferol), as well as those that have a double bond between the C2 and the C3 hydroxyl group at ring C. It has been further reported that no single compound was able react with all kinds of radicals (Kondo et al. 2000; Rice-Evans et al. 1996).
Fig. 4.
DPPH● radical scavenging activity of methanol and acetone extract of H. isora and C. pentandra. CPMe—C. pentandra methanol extract; CPAc—C. pentandra acetone extract; HIMe—H. isora methanol extract; HIAc—H. isora acetone extract; BHA- Butylated hydroxyl anisole; α To—α Tocopherol. Values are means of triplicate determinations (n = 3) ± standard deviation
ABTS•+ cation radical scavenging activity
ABTS•+ assay is an excellent tool for determine the antioxidant activity of hydrogen-donating antioxidants (scavenging aqueous phase radicals) and of chain breaking antioxidants (scavenging lipid peroxyl radicals). In ABTS•+ cation radical scavenging method, the activity of tested sample extracts was expressed as micromolar equivalent of Trolox solution, having an antioxidant capacity equivalent to 1 g of the extarct under the experimental investigation. The total antioxidant activities of methanol and acetone extract of the samples are presented in Table 2. The total antioxidant activity of the sample extracts ranges from 4,400 to 8,800 μmol Trolox/g extract and the values are significantly (p < 0.05) different. Among them, the acetone extracts of both the plants exhibited higher and comparable activity (8,800 and 8,100 μmol equivalent of Torolox/g extract, respectively) on a par with the standard antioxidants BHA and α-tocopherol. The antioxidant activity of such samples seems to be sufficient for functioning as potential nutraceuticals when they are ingested along with nutrients. The extensive investigations on antiradical and antioxidant activities of small phenolics including flavonoids and phenolic acids have been reported (Heim et al. 2002). Apart from these, Hagerman et al. (1998) have reported that the high molecular weight phenolics (tannins) have more ability to quench free radicals (ABTS•+) and that effectiveness depends on the molecular weight, the number of aromatic rings and nature of hydroxyl groups substitution than the specific functional groups.
Table 2.
Total antioxidant activity and metal chelating property of H. isora and C. pentandra
| Sample | TAA* (μmol/g extract) | Metal chelating property Mg EDTA/g extract |
|---|---|---|
| CPMe | 4,400 ± 149c | 33.6 ± 2.4b |
| CPAc | 8,100 ± 132a | 31.6 ± 1.3b |
| HIMe | 5,880 ± 804b | 24.6 ± 0.8c |
| HIAc | 8,800 ± 467a | 19.7 ± 3.7c |
| BHA | 8,746 ± 320a | 61.5 ± 1.9a |
| α To | 8,790 ± 194a | 67.6 ±2.1a |
*Total antioxidant activity (μmol equivalent Trolox performed by using ABTS.+ radical cation). Values are means of triplicate determinations (n = 3) ± standard deviation. CPMe C. pentandra methanol extract; CPAc C. pentandra acetone extract; HIMe H. isora methanol extract; HIAc H. isora acetone extract; BHA Butylated hydroxyl anisole; α To α Tocopherol. Mean values followed by different superscript in a column are significantly different (p < 0.05)
Metal chelating activity
Iron is essential for life because it is required for oxygen transport, respiration and activity of many enzymes. However, iron is an extremely reactive metal and will catalyze oxidative changes in lipid, protein, and other cellular components. In addition, liposome peroxidation and oxidative damage of protein model systems ae induced by a Fenton reaction in which ferrous ions catalyze the conversion of hydrogen peroxide to hydroxyl radical with the production of ferric ion. Although metal chelating agents are not antioxidants, they play a vital role in the stabilization of fatty acids against rancidity. Since, Fe2+ has been shown to cause the production of oxyradicals and lipid peroxidation, minimizing Fe2+ concentration in Fenton reactions affords protection against oxidative damage. The metal chelating potential of sample extracts, BHA and α-tocopherol varied significantly (p < 0.05) and the data were presented in Table 2. The iron chelating ability of CPMe and CPAc (33.6 and 31.6 mg EDTA/g extract, respectively) are found to the higher than that of H. Isora extracts, as has been recorded in DPPH● and linoleic acid peroxidation methods. Whereas, in H. isora both the sample extracts exhibited moderate level of activity (HIMe, 24.6; HIAc, 19.7). Nonetheless, the order of metal chelating activity of respective solvent extracts and standards are as follows: α To>BHA>CPMe>CPAc>HIMe>HIAc. The chelating agents, which form σ-bonds with metal, are effective as secondary antioxidants because they reduce the redox potential, thereby stabilizing the oxidized form of the metal ion. However, Gua et al. (1996) reported that polyphenols with dihydroxy groups can conjugate metals, preventing metal catalyzed free radical formation. Heim et al. (2002) reported that both metal chelating properties and radical scavenging ability of polyhydroxylated flavonoids may offer considerable benefit as inhibitors of the Fenton reaction. The data obtained from Table 2 revealed that both sample extracts demonstrate a marked capacity for iron binding, suggesting that their action as a peroxidation protector may be related to their iron binding capacity.
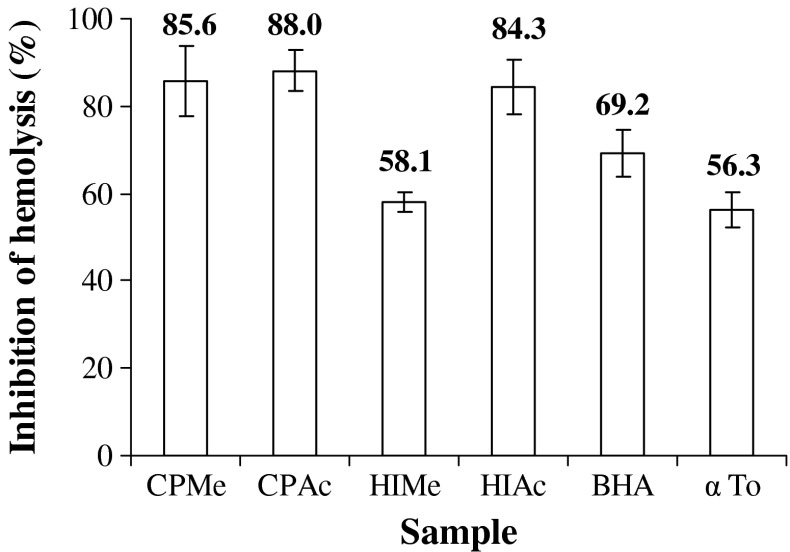
Antihemolytic activity
It is well recognized that the oxidation of polyunsaturated fatty acids in biological membranes can lead to the formation and propagation of lipid radicals, uptake of oxygen, rearrangement of the double bonds in unsaturated lipids, and even destruction of membrane lipids. Many of these biochemical activities can lead to the production of breakdown products that were highly toxic to most cell types. Although hemolysis had a long history of use in measuring free radical mediated damage and its inhibition by antioxidants, only few studies have been performed with erythrocytes in whole blood. RBC hemolysis is a more sensitive system for evaluating the antioxidant properties of the phytoceuticals. In this study, we used a biological test based on free radical-induced erythrocytes lysis in cow blood (Djeridane et al. 2006). Lipid oxidation of cow blood erythrocyte membrane mediated by H2O2 induces membrane damage and subsequently hemolysis. The antihemolytic activity of the various extracts on cow blood erythrocytes are presented in Fig. 5. At the concentration of 1 mg of extract in the final reaction mixture, the methanol and acetone extracts of C. pentandra showed comparable activity (85.6% and 88.0%, respectively). On the other hand in H. isora only acetone extract exhibited higher level of inhibition (84.3%) and the activity was moderate in methanol extract (50.1%). Interestingly, CPAc, CPMe and HIAc were found to have higher level of inhibition of hemolysis than the positive controls BHA (69.2%) and α tocopherol (56.3%). Similar inhibition of radical-induced red blood cell hemolysis has been reported for Oudneyna africanan, Artemisia arboresens and Globularia alpyum, whose activities were comparable with that of caffeic acid (Djeridane et al. 2006). Chaudhuri et al. (2007) observed that binding of flavonoids to the RBC membranes significantly inhibits lipid peroxidation and at the same time enhances their integrity against lysis. The authors suggested that the antihemolytic activity was dependant on the location of the flavonoids in the membrane matrix and they lie in close proximity to the tryptophan residues in the ghost RBC membrane proteins. The above results suggest that the samples having potent antioxidant (electron donating/radical quenching) activity could also protect the cell membrane from lysis.
Fig. 5.
Antihemolytic activity of methanol and acetone extract of H. isora and C. pentandra. CPMe—C. pentandra methanol extract; CPAc—C. pentandra acetone extract; HIMe—H. isora methanol extract; HIAc—H. isora acetone extract; BHA- Butylated hydroxyl anisole; α To—α Tocopherol. Values are means of triplicate determinations (n = 3) ± standard deviation
Chu et al. (2000) observed that evaluation of the total antioxidant capacity of fruits, vegetables and other plant products cannot be performed accurately by any single method due to the complex nature of phytochemicals. Because of involvement of multiple reaction characteristics and mechanisms, no single assay could accurately reflect all antioxidants in a mixed or complex system (Du et al. 2009). Therefore, to characterize a substance as an antioxidant, it is necessary to assess its interaction against a wide range of species directly responsible for oxidative damage (Yamaguchi et al. 1999). Interestingly, the extracts from fruits of H. isora and seeds of C. pentandra have the ability to scavenge almost all free radicals and metal ions.
From the foregoing, it may be concluded that the methanol extract of C. pentandra, seeds demonstrated superior antioxidant and free radical scavenging activities over other extracts. This finding substantiates its traditional uses in treating various disorders and increases the interest and potential use of this sample as nutraceutical and pharmacological agent. Further isolation and purification of compounds from this extract and study of their biological effects may provide further information of their medicinal value.
References
- Amarowicz R, Pegg RB, Raim-Mohaddam P, Bral B, Weil JA. Free radical scavenging capacity and antioxidant activity of selected plant species from the Canadian Prairies. Food Chem. 2004;84:551–562. doi: 10.1016/S0308-8146(03)00278-4. [DOI] [Google Scholar]
- Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;26:1199–1200. doi: 10.1038/1811199a0. [DOI] [Google Scholar]
- Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. Lebenson Wiss Technol. 1995;28:25–30. [Google Scholar]
- Chaudhuri S, Banerjee A, Basu K, Sengupta B, Sengupta PK. Interaction of flavonoids with red blood cell membrane lipids and proteins: antioxidant and antihemolytic effects. Int J Biol Macromol. 2007;41:42–48. doi: 10.1016/j.ijbiomac.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Chu YH, Chang CL, Hsu HF. Flavonoid content of several vegetables and their antioxidant activity. J Sci Food Agric. 2000;80:561–565. doi: 10.1002/(SICI)1097-0010(200004)80:5<561::AID-JSFA574>3.0.CO;2-#. [DOI] [Google Scholar]
- Djeridane A, Yousfi M, Nadjemi B, Vidal N, Lesgards JF, Stocker P. Screening of some Algerian medicinal plants for the phenolic compounds and their antioxidant activity. Eur Food Res Technol. 2006;224:801–809. doi: 10.1007/s00217-006-0361-6. [DOI] [Google Scholar]
- Du G, Li M, Ma F, Liang D. Antioxidant capacity and the relationship with polyphenol and vitamin C in Actinidia fruits. Food Chem. 2009;113:557–562. doi: 10.1016/j.foodchem.2008.08.025. [DOI] [Google Scholar]
- Gua Q, Zhao B, Li M, Shen S, Xin W. Studies on protective mechanisms of four components of green tea polyphenols against lipid peroxidation in synaptosomes. Biochem Biophys Acta. 1996;1304:210–222. doi: 10.1016/S0005-2760(96)00122-1. [DOI] [PubMed] [Google Scholar]
- Hagerman AE, Riedl KM, Jones GA, Sovik KN, Ritchard NT, Hartzfeld PW, Riechel TL. High molecular weight plant polyphenolics (Tannins) as biological antioxidants. J Agric Food Chem. 1998;46:1887–1892. doi: 10.1021/jf970975b. [DOI] [PubMed] [Google Scholar]
- Heim KE, Tagliaferro AR, Bobilya DJ. Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. J Nutr Biochem. 2002;13:572–584. doi: 10.1016/S0955-2863(02)00208-5. [DOI] [PubMed] [Google Scholar]
- Khare CP. Indian medicinal plants. New Delhi: Springer private Limited; 2007. [Google Scholar]
- Kikuzaki H, Nakatani N. Antioxidant effects of some ginger constituents. J Food Sci. 1993;58:1407–1410. doi: 10.1111/j.1365-2621.1993.tb06194.x. [DOI] [Google Scholar]
- Klein SM, Cohen G, Cederbaum AI. Production of formaldehyde during metabolism of dimethyl sulphoxide by hydroxyl radical generating system. Biochemistry. 1991;20:6006–6012. doi: 10.1021/bi00524a013. [DOI] [PubMed] [Google Scholar]
- Kondo K, Kurihara M, Fukuhara K, Tanaka T, Suzuki T, Miyata N, Toyoda M. Conversion of procyanidin B-type (catechin dimer) to A-type: evidence for abstraction of C-2 hydrogen in catechin during radical oxidation. Tetrahedron Lett. 2000;41:485–488. doi: 10.1016/S0040-4039(99)02097-3. [DOI] [Google Scholar]
- Kumar G, Murugesan AG. Hypolipidaemic activity of Helicteres isora L. bark extracts in streptozotocin induced diabetic rats. J Ethnopharmacol. 2008;116:161–166. doi: 10.1016/j.jep.2007.11.020. [DOI] [PubMed] [Google Scholar]
- Kumar G, Murugesan AG, Rajasekara-Pandian M. Effect of Helicteres isora bark extract on glucose and hepatic enzymes in experimental diabetes. Pharmazie. 2006;61:353–355. [PubMed] [Google Scholar]
- Kumar G, Banu GS, Murugesan AG, Rajasekara-Pandian M. Preliminary toxicity and phytochemical studies of aqueous bark extract of Helicteres isora L. Int J Pharm. 2007;3:96–100. doi: 10.3923/ijp.2007.96.100. [DOI] [Google Scholar]
- Ladeji O, Omekarah I, Solomon M. Hypoglycemic properties of aqueous bark extract of Ceiba pentandra in streptozotocin-induced diabetic rats. J Ethnopharmacol. 2003;84:139–142. doi: 10.1016/S0378-8741(02)00321-5. [DOI] [PubMed] [Google Scholar]
- Lee J, Koo N, Min DB. Reactive oxygen species, aging, and antioxidative nutraceuticals. Compr Rev Food Sci Food Saf. 2004;3:21–33. doi: 10.1111/j.1541-4337.2004.tb00058.x. [DOI] [PubMed] [Google Scholar]
- Naim M, Gestener B, Bondi A, Birk Y. Antioxidant and antihemolytic activities of soyabean isoflavones. J Agric Food Chem. 1976;24:1174–1177. doi: 10.1021/jf60208a029. [DOI] [PubMed] [Google Scholar]
- Ngounou FN, Meli AL, Lontsi D, Sondengam BL, Rahman AU, Choudhary MI, Malik S, Akhtar F. New isoflavones from Ceiba pentandra. Phytochemistry. 2000;54:107–110. doi: 10.1016/S0031-9422(00)00035-2. [DOI] [PubMed] [Google Scholar]
- Noreen Y, El-Seedi H, Perera P, Bohlin L. Two New isoflavones from Ceiba pentandra and their effect on cyclooxygenase-catalyzed prostaglandin biosynthesis. J Nat Prod. 1998;61:8–12. doi: 10.1021/np970198+. [DOI] [PubMed] [Google Scholar]
- Otake T, Mori H, Morimoto M, Ueba N, Sudardjo S, Kusumoto IT, Hattori M, Namba T. Screening of Indonesian plant extracts for anti-human immunodeficiency virus-type I (HIV-1) activity. Phytother Res. 2006;9:6–10. doi: 10.1002/ptr.2650090103. [DOI] [Google Scholar]
- Oyaizu M. Studies on products of browning reaction: antioxidative activity of products of browning reaction prepared from glucosamine. Jpn J Nutr. 1986;44:307–315. doi: 10.5264/eiyogakuzashi.44.307. [DOI] [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Rice-Evans CA, Miller NJ, Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med. 1996;20:933–956. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- Satake T, Kamiya K, Saiki Y, Hama T, Fujimoto Y, Kitanaka S, Kimura Y, Uzawa J, Endang H, Umar M. Studies on the constituents of fruits of Helicteres isora L. Chem Pharmacol Bull. 1999;47:1444–1447. doi: 10.1248/cpb.47.1444. [DOI] [Google Scholar]
- Siddhuraju P, Becker K. The antioxidant activity and free radical scavenging activities of processed cowpea (Vigna unguiculata (L.) Walp.) seed extracts. Food Chem. 2006;101:10–19. doi: 10.1016/j.foodchem.2006.01.004. [DOI] [Google Scholar]
- Singh RP, Murthy KNC, Jayaprakasha GK. Studies on antioxidant activity of pomegranate (Punica granatum) peel and seed extracts using in vitro models. J Agric Food Chem. 2002;50:81–86. doi: 10.1021/jf010865b. [DOI] [PubMed] [Google Scholar]
- Ueda H, Kaneda N, Kawanishi K, Alves SM, Moriyasu M. A new isoflavone glycoside from Ceiba pentandra (L.) gaertner. Chem Pharm Bull. 2002;50:403–404. doi: 10.1248/cpb.50.403. [DOI] [PubMed] [Google Scholar]
- Velioglu YS, Mazza G, Oomah BD. Antioxidant activity and total phenolics in selected fruits, vegetables and grain products. J Agric Food Chem. 1998;46:4113–4117. doi: 10.1021/jf9801973. [DOI] [Google Scholar]
- Venkadesh S, Reddy GD, Reddy YSR, Sathyavathy D, Reddy BM. Effect of Helicteres isora root extracts on glucose tolerance in glucose-induced hyperglycemic rats. Fitoterapia. 2004;75:364–367. doi: 10.1016/j.fitote.2003.12.025. [DOI] [PubMed] [Google Scholar]
- Yamaguchi F, Yoshihira Y, Nakazawa H, Agria T. Free radical scavenging activity of grape seed extract by Electron Spin Resonance spectrometry in an H2O2/NaOH/DMSO system. J Agric Food Chem. 1999;47:2544–2548. doi: 10.1021/jf9806762. [DOI] [PubMed] [Google Scholar]
- Yamaguchi F, Ariga T, Yoshimura Y, Nakazawa K. Antioxidative and antiglycation activity of garcinol from Garcinia indica fruit rind. J Agric Food Chem. 2000;48:180–185. doi: 10.1021/jf990845y. [DOI] [PubMed] [Google Scholar]
- Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64:555–559. doi: 10.1016/S0308-8146(98)00102-2. [DOI] [Google Scholar]
- Zou Y, Lu Y, Wei D. Antioxidant activity of a flavonoid-rich extract of Hypericum perforatum L. in vitro. J Agric Food Chem. 2004;52:5032–5039. doi: 10.1021/jf049571r. [DOI] [PubMed] [Google Scholar]