Abstract
AIM: To investigate the survival of individuals with colorectal cancer (CRC) with inflammatory bowel disease (IBD-associated CRC) compared to that of individuals without IBD diagnosed with CRC.
METHODS: Epidemiologic, clinical, and follow-up data were obtained from the Colon Cancer Family Registry (Colon CFR). IBD-associated cases were identified from self-report of physician diagnosis. For a subset of participants, medical records were examined to confirm self-report of IBD. Cox proportional hazards regression was applied to estimate adjusted hazard ratios (aHR) and 95%CI of mortality, comparing IBD-associated to non-IBD-associated CRC, adjusted for age at CRC diagnosis, sex, Colon CFR phase, and number of prior endoscopies. Following imputation to complete CRC stage information, adjustment for CRC stage was examined.
RESULTS: A total of 7202 CRC cases, including 250 cases of IBD-associated CRC, were analyzed. Over a twelve year follow-up period following CRC diagnosis, 2013 and 74 deaths occurred among non-IBD associated CRC and IBD-associated CRC patients, respectively. The difference in survival between IBD-associated and non-IBD CRC cases was not statistically significant (aHR = 1.08; 95%CI: 0.85-1.36). However, the assumption of proportional hazards necessary for valid inference from Cox regression was not met over the entire follow-up period, and we therefore limited analyses to within five years after CRC diagnosis when the assumption of proportional hazards was met. Over this period, there was evidence of worse prognosis for IBD-associated CRC (aHR = 1.36; 95%CI: 1.05-1.76). Results were similar when adjusted for CRC stage, or restricted to IBD confirmed in medical records.
CONCLUSION: These results support the hypothesis that IBD-associated CRC has a worse prognosis than non-IBD-associated CRC.
Keywords: Colorectal cancer, Inflammatory bowel disease, Outcomes research, Cancer survival, Inflammation
Core tip: Inflammatory bowel disease (IBD) - and more generally, inflammation - increases risk of colorectal cancer (CRC). Inflammation may also promote cancer progression and metastasis, and therefore, inflammation might be associated with shorter survival with CRC. This study examined whether CRC that occurs in patients with IBD has a worse prognosis than CRC in patients without IBD.
INTRODUCTION
Inflammatory bowel disease (IBD), comprising ulcerative colitis (UC) and Crohn’s disease (CD), is an inflammatory disease affecting the gastrointestinal tract[1]. IBD is associated with a substantial increase in the risk of colorectal cancer (CRC), especially after 8-10 years of active disease[2-4]. Implicated mechanisms include persistent activation of cyclooxygenase-2 and nuclear factor kappa-B pathways and increased oxidative stress[5,6], leading IBD to be considered a model for understanding the role of chronic inflammation in CRC etiology[2,7].
IBD-associated CRCs have distinct molecular and clinical features compared to sporadic CRCs[6,8]. Specifically, CRC arising in association with IBD has an initiation and progression sequence that differs from the canonical sequence of adenoma-to-cancer characterizing sporadic CRC. Furthermore, IBD-associated CRC typically affects individuals at an earlier age than sporadic CRC, and the distribution of histologic type and site within the colon may differ[6,8]. Despite these differences, the results of many studies support the concept that inflammation at levels more subtle than that seen in IBD is associated with CRC risk[2]; in this model, inflammation acts primarily as a tumor-promoter following environmental mutagenic insult. Non-IBD-associated CRC tumors show infiltration by inflammatory immune cells and elevated inflammatory cytokines[2]. Epidemiologically, circulating C-reactive protein, a marker of inflammation, has been associated with increased risk[9,10], regular use of anti-inflammatory medication such as aspirin reduces risk of CRC[11-15], and genetic variation in inflammatory pathway genes may also modulate CRC risk[16,17]. Inflammation is also hypothesized to enhance metastasis in both IBD-associated and non-IBD-associated CRC[18].
Recent evidence suggests that the use of non-steroidal anti-inflammatory drugs (NSAIDs) improves survival following CRC diagnosis[19,20]. From this it may be inferred that inflammation is involved in disease progression. If this is true, survival of individuals with IBD-associated CRCs would be predicted to be worse than for those with sporadic CRCs. However, the evidence that IBD negatively affects the prognosis of CRC comes primarily from small, hospital-based studies[21-24] and is inconsistent. Among the largest previously published studies, one observed an approximately 20% higher risk of mortality among UC-associated IBD patients compared to other CRC patients[25] and two others found no difference[26,27].
We accessed data from the large, multi-center Colon Cancer Family Registry (Colon CFR)[28] to compare the survival of patients with IBD-associated CRC to those without IBD diagnosed with CRC.
MATERIALS AND METHODS
Study population: The Colon CFR
This study includes incident invasive CRC cases diagnosed between 1997 and 2009 registered with the Colon CFR, an international resource comprising seven sites that has been previously described in detail[28]. Case ascertainment and recruitment methodology differed between Colon CFR centers and between registry phases (1997-2001 and 2002-2009)[28], and included population-based recruitment in addition to targeted recruitment of CRC cases under age 50 or with a family history of CRC. CRC cases associated with familial adenomatous polyposis (n = 89) or Lynch syndrome (n = 1438) were excluded. Invasive CRC cases were eligible if age, sex, and self-reported IBD information was available; for the primary analysis both population-based cases and cases recruited through clinics associated with Colon CFR centers were included. Of 7297 eligible cases, 7202 records had complete information on analytical covariates other than CRC stage, and were included in multivariate adjusted analyses.
Inflammatory bowel disease assessment
IBD prior to CRC diagnosis was assessed through self-report during the Colon CFR baseline epidemiologic and medical history interview. Consecutive questions asked whether a participant had ever been told by doctor that they had CD, UC, irritable bowel syndrome, or diverticulitis. The UC question included a statement that UC is not a stomach ulcer. Each affirmative answer was followed with a question ascertaining the participant’s age or calendar year in which the diagnosis was reported to the participant. Participants reporting either UC or CD diagnosis were counted as having IBD in the primary analysis. Participants reporting irritable bowel syndrome or diverticulitis were not considered to have IBD and included with all other CRC cases.
Clinical records at two Colon CFR centers, Ontario and Seattle, were reviewed for references to UC or CD. Of 169 self-reported IBD diagnoses from these centers, at least some medical or pathology records were available for 150 patients.
Covariate assessment
During the study interview, information was collected on family history of CRC, medical history related to digestive disease including endoscopy and hemoccult testing, height and weight, demographics, and lifestyle (e.g., cigarette smoking, physical activity, and alcohol consumption)[28]. A reference date of 2 years prior to CRC diagnosis was used to categorize participants’ exposures prior to diagnosis, except for endoscopy and hemoccult testing. Hemoccult testing was categorized as never, 1-4, or 5 or more total tests. Similarly the total lifetime number of reported endoscopies (colonoscopies and sigmoidoscopies) was categorized as none or 1, 2, or 3 or more.
Tumor characteristics
Cancer stage, histologic type (i.e., adenocarcinoma, mucinous adenocarcinoma, signet ring cell carcinoma), and anatomic site of the tumor were obtained from registry or pathology reports[28]. For analysis, tumors in the cecum, ascending colon, hepatic flexure, transverse colon, and splenic flexure (ICD-O-3 codes C180, C182-185) were considered proximal; tumors in the descending colon (C186) and sigmoid colon (C187) were grouped as distal colon tumors; and rectosigmoid junction (C199), rectum (C209), and overlapping lesion of the anus and rectum (C218) were considered rectal tumors. Stage information was obtained by Colon CFR centers from the Surveillance Epidemiology and End Results (SEER) registry (Seattle and Hawaii) or medical records and pathology review (all other centers). Records included American Joint Committee on Cancer staging on tumor extent (T-stage), lymph node positivity (N-stage; number of nodes positive for malignant cells), and the presence of distant metastases (M-stage). From these indices, stage was summarized as local, regional, or distant following SEER guidelines. Microsatellite instability (MSI) status was characterized for 4422 cases with available tumor samples in the Colon CFR, combining the results of genetic analysis of a 10-marker panel and immunohistochemicalstaining of DNA mismatch repair proteins as previously described[28-33].
Imputation of stage of CRC
Of 7202 cases eligible for this analysis, 4450 (62%) initially could not be categorized in a SEER summary stage. Of these, 4343 could not be classified in part because of missing M-stage information, primarily because ascertaining distant metastases required medical records not accessible at all Colon CFR centers. However, 2859 of these cases without M-stage information included T- or N-stage information. Missing stage information was imputed in two steps. First, cases without M information but with N information indicating that no lymph nodes tested positive for malignant cells were coded as having no distant metastases. If T-stage information was also available, SEER summary stage was updated to local or regional. In total 1509 of 4450 cases (34%) originally missing SEER summary stage were completed through this direct imputation.
Following direct imputation, multiple imputation with chained equations was used to complete the remaining cases missing stage information[34]. T-stage (T1-3, T4) and N-stage (N0, N > 0) were reduced to dichotomous variables. Fifty datasets were generated with T, N and M imputed using chained logistic regression based on all variables considered in the primary survival analysis (see below), and additional potential predictors of stage: time at risk, vital status, delay between CRC diagnosis and Colon CFR enrollment, histologic type, partial colectomy (ever, never, missing), and tumor site (proximal, distal, rectal, overlapping/NOS). Imputation regression excluded cases with incomplete information in one or more predictors (n = 889). In total, 6313 cases were included in multiple imputation regression; T, N or M were imputed for 1677, 1697 and 2438 cases, respectively. Following imputation, SEER summary stage was derived from imputed T, N and M following SEER coding[35,36]. Together with cases whose records originally included SEER summary stage or were completed with direct imputation, described above, a total of 6809 cases with SEER summary stage were included in analyses involving stage.
Vital status
Vital status was updated at each Colon CFR center through linkage to population-based tumor registries and death indices, in addition to active follow-up 4-5 years after initial enrollment and routine contact with enrollees and family members[28].
Ethical considerations
Each Colon CFR center Institutional Review Board/ethics board approved all study protocols and all participants provided informed consent.
Statistical analysis
Cox proportional hazards regression was used to estimate adjusted hazard ratios (aHRs) of death from any cause with 95%CIs. Time at risk was measured in days from CRC diagnosis, with participants entering observation on the date of Colon CFR baseline interview. Participants alive at the most recent time of confirmed contact were censored at that date. Parallel analyses were completed for the full follow-up time, and with administrative censoring of all participants 5 years after CRC diagnosis. The proportional hazard assumption was examined graphically and by allowing the variable representing IBD status to interact with the logarithm of failure time, and testing the significance of the time-varying factor[37].
To adjust for age at CRC diagnosis, participants were divided into approximate quartiles of age (cut-points at 47, 56 and 65 years) and a piecewise linear model of hazard of death was constructed. Additional a priori candidate adjustment variables were Colon CFR phase of enrollment (1997-2001 or 2002-2009), Colon CFR center, sex, cigarette smoking history, use of NSAIDs, education, race, body mass index (BMI), first degree family history of CRC, and utilization of hemoccult tests or endoscopy. Colon CFR center, NSAID use, BMI, education, race, smoking, hemoccult tests, and family history were omitted from final statistical models because inclusion did not appreciably change aHRs associated with IBD. CRC stage, when included, was parameterized as SEER summary stage (localized, regional, or distant disease).
RESULTS
Of 7202 eligible CRC patients, 250 (3.5%) reported a physician’s diagnosis of IBD (Table 1). IBD-associated CRC patients were younger at CRC diagnosis than non-IBD cases. In addition, IBD-associated CRC patients were less likely to have ever regularly smoked cigarettes, and were leaner on average than other patients with CRC. IBD-associated CRC patients were much more likely than sporadic CRC patients to be white, report a family history of CRC, or have had multiple endoscopies.
Table 1.
Participant characteristics of colorectal cancer cases at enrollment in the Colon Cancer Family Registry according to self-reported diagnosis of inflammatory bowel disease n (%)
| Characteristics |
Inflammatory bowel disease |
P value | |
| No | Yes | ||
| (n = 6952) | (n = 250) | ||
| Age at CRC diagnosis (yr) | < 0.001 | ||
| < 47 | 1731 (25) | 98 (39) | |
| 47-55 | 1791 (26) | 68 (27) | |
| 56-65 | 1803 (26) | 42 (17) | |
| > 65 | 1627 (23) | 42 (17) | |
| Female | 3515 (51) | 133 (53) | 0.4 |
| Lifetime endoscopies | < 0.001 | ||
| None or one | 2697 (39) | 40 (16) | |
| Two | 2004 (29) | 51 (20) | |
| Three or more | 2251 (32) | 159 (64) | |
| Colon CFR recruitment phase | 0.4 | ||
| I (1997-2001) | 4310 (62) | 161 (64) | |
| II (2002-2009) | 2642 (38) | 89 (36) | |
| Colon CFR center | < 0.01 | ||
| Ontario, Canada | 1775 (26) | 89 (36) | |
| Southern California, United States | 1551 (22) | 39 (16) | |
| Melbourne, Victoria, Australia | 718 (10) | 20 (8) | |
| Hawaii, United States | 348 (5) | 6 (2) | |
| Mayo Clinic, MI, United States | 499 (7) | 16 (6) | |
| Seattle, WA, United States | 2061 (30) | 80 (32) | |
| Education1 | 0.8 | ||
| Secondary or less | 3367 (49) | 123 (50) | |
| More than secondary | 3518 (51) | 124 (50) | |
| Cigarette smoking history12 | 0.02 | ||
| Never | 3022 (44) | 130 (52) | |
| Current | 1259 (18) | 44 (18) | |
| Former | 2650 (38) | 75 (30) | |
| Race1 | < 0.01 | ||
| White | 5033 (74) | 200 (83) | |
| Other | 1769 (26) | 42 (17) | |
| BMI (kg/m2)12 | < 0.01 | ||
| ≤ 23.6 | 1685 (25) | 85 (34) | |
| 23.7-26.5 | 1710 (25) | 59 (24) | |
| 26.6-30.3 | 1726 (25) | 51 (21) | |
| ≥ 30.4 | 1739 (25) | 53 (21) | |
| Regular NSAID user12 | 0.7 | ||
| Yes | 2719 (40) | 95 (39) | |
| No | 4159 (60) | 151 (61) | |
| 1st degree family history of CRC | 1495 (22) | 35 (14) | < 0.01 |
| Tumor site | < 0.01 | ||
| Proximal colon | 2346 (34) | 109 (44) | |
| Distal colon or rectum | 4276 (62) | 136 (54) | |
| Overlapping/not specified | 328 (5) | 5 (2) | |
| Microsatellite instability1 | 0.1 | ||
| None or low | 3743 (88) | 150 (87) | |
| High | 507 (12) | 22 (12) | |
| CRC stage at diagnosis13 | 0.4 | ||
| Local | 3088 (47) | 110 (46) | |
| Regional | 2441 (37) | 98 (40) | |
| Distant | 1080 (16) | 34 (14) | |
Numbers do not sum to total due to missing values;
2 years prior to colorectal cancer (CRC) diagnosis;
Mean of 50 imputed datasets. Colon CFR: Colon Cancer Family Registry; NSAID: Non-steroidal anti-inflammatory drugs; BMI: Body mass index.
Compared to non-IBD cases, a larger proportion of IBD-associated CRC patients were diagnosed with tumors in the proximal colon (Table 1). Among tumors assayed for MSI, no difference in the distribution of MSI between IBD-associated and other CRC was observed.The distribution of SEER summary stage differed only slightly between IBD-associated CRC and other CRC following completion of missing stage information with multiple imputation. However, a more pronounced, but not statistically significant, difference in stage distribution between IBD-associated and sporadic CRC was noted among patients diagnosed before age 50 year; in this age group, 24% of non-IBD associated CRC cases were diagnosed after distant metastases has occurred, compared to 18% of IBD-associated CRC cases (not shown; χ2 test P > 0.15).
Over a total follow-up of 12.2 years (median of 4.6 years), 2013 and 74 deaths were reported among non-IBD-associated and IBD-associated CRC cases, respectively. Results of Cox proportional hazards regression using the full follow-up period indicated no association between IBD and risk of mortality from any cause (Figure 1 and Table 2).
Figure 1.
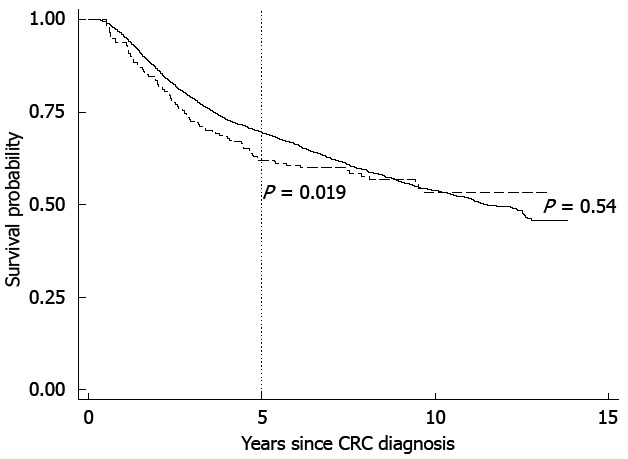
Adjusted survival probability for non-inflammatory bowel disease-associated (solid line) and inflammatory bowel disease-associated colorectal cancer (dashed line). P values are from Wald test of coefficient of inflammatory bowel disease from Cox regression. CRC: Colorectal cancer.
Table 2.
Adjusted hazard ratios and 95%CI for death, either with complete available time-at-risk or with follow-up ended 5 years after colorectal cancer diagnosis
| n | Deaths | aHR1 (95%CI) | n | Deaths | aHR2 (95%CI) | n | Deaths | aHR13 (95%CI) | |
| Complete follow-up | |||||||||
| No IBD | 6952 | 2013 | Ref. | 6567 | 1926 | Ref. | 3836 | 1322 | Ref. |
| IBD | 250 | 74 | 1.08 (0.85-1.36) | 242 | 71 | 1.09 (0.85-1.40) | 81 | 26 | 1.20 (0.81-1.79) |
| P value | 0.54 | 0.48 | 0.36 | ||||||
| 5-yr follow-up | |||||||||
| No IBD | 6903 | 1372 | Ref. | 6526 | 1320 | Ref. | 3801 | 871 | Ref. |
| IBD | 249 | 63 | 1.36 (1.05-1.76) | 241 | 60 | 1.34 (1.02-1.77) | 81 | 24 | 1.52 (1.00-2.30) |
| P value | 0.018 | 0.037 | 0.048 |
Adjusted for age, recruitment phase, sex, and endoscopy;
Additional adjustment for surveillance epidemiology and end results summary stage (local, regional, distant);
Inflammatory bowel disease (IBD) confirmed from medical and pathology records at Seattle and Ontario centers only, non-IBD associated cases restricted to these centers. aHR: Adjusted hazard ratio.
Graphical inspection of the Kaplan-Meier survival curve (Figure 1) and formal testing of time-varying coefficients (not shown), indicated that the proportional hazards assumption was violated by the variable representing IBD status. Ending follow-up at no later than 5 years following diagnosis for all participants resolved the proportional hazards violation based on time-varying models (not shown).
Results from analyses including only the 5 years following diagnosis, but otherwise identical to analysis across the entire follow-up time, showed that IBD-associated CRC patients were at 36% (95%CI: 5%-76%) higher risk of death (Figure 1 and Table 2). Following imputation of CRC stage, additional adjustment for stage left resulting aHR estimates largely unchanged.
Medical and pathology records of 169 eligible cases enrolled at the Seattle and Ontario CFR centers were reviewed; for 14 IBD-associated CRC cases no records were available for review, for 43 only pathology reports or death certificates were available, and for 107 pathology and at least partial medical records were available. During review, 81 of these 169 self-reported IBD diagnoses were confirmed. When proportional hazards regression analysis was repeated including confirmed IBD diagnoses and non-IBD-associated CRC only from the Seattle and Ontario Colon CFR centers, and thus excluding unconfirmed self-reported IBD from analyses, results suggested that IBD was associated with a 52% (95%CI: 0%-130%) higher risk of mortality following CRC diagnosis (Table 2).
In exploratory sub-group analyses of survival through 5 years post-CRC diagnosis, the association between IBD and risk of death did not vary by MSI status of the tumor (not shown). However, IBD was more strongly associated with risk of death among patients with distal or rectal CRC tumors (aHR = 1.62, 95%CI: 1.17-2.26) than among patients with tumors in the proximal colon (aHR = 0.98; 95%CI: 0.64-1.50), although the interaction between IBD and tumor site was not statistically significant (P = 0.06). Survival also did not differ by duration of IBD prior to CRC diagnosis (< 8 years IBD, aHR = 1.38, 95%CI: 0.96-1.99; ≥ 8 years IBD, aHR = 1.38, 95%CI: 0.96-2.00). Patients reporting only CD were apparently at higher risk of mortality than those with UC only, although the difference was not statistically significant (CD, aHR = 1.74, 95%CI: 1.09-2.79; UC, aHR = 1.18, 95%CI: 0.85-1.63, P = 0.16).
Restriction of analysis to 4144 population-based cases enrolled during phase I did not appreciably alter the association between IBD and hazard of mortality within 5 years of CRC diagnosis (aHR = 1.47, 95%CI: 1.08-2.01). Incorporation of probability weights based on the sampling scheme of the Colon CFR[28] also did not change results of death, IBD compared to non-IBD-associated CRC (aHR = 1.62, 95%CI: 1.04-2.54).
DISCUSSION
We observed a higher risk of death from any cause among patients with IBD-associated CRC compared to those with non-IBD-associated CRC when attention was restricted to the first five years following CRC diagnosis. The increase in risk was of borderline statistical significance. However, no increase in risk of death was seen when all available follow-up data were analyzed. The violation of the proportional hazard assumption made the analysis using all available follow-up time difficult to interpret; in contrast, the period within 5 years following CRC diagnosis showed no violations of the proportional hazards assumption.
CRC stage at diagnosis is a strong predictor of survival, and IBD patients may undergo surveillance designed to detect CRC at early stages. Therefore, we compared CRC stage distribution between cases with and without IBD, and conducted stage-adjusted survival analyses. Our results suggest that IBD-associated CRC is only slightly more frequently diagnosed at local or regional stage than non-IBD-associated CRC. This may be due to active endoscopic surveillance for CRC among IBD patients, consistent with earlier reports[38]. We observed that the shift in stage distribution was larger, but not statistically significant, among CRC patients diagnosed prior to age 50 years; this group would generally not receive screening endoscopy in the absence of gastrointestinal symptoms or a strong family history of CRC.
When we repeated survival analysis adjusted for stage, our results still suggested increased risk of mortality among IBD-associated CRC patients. Further exploration revealed that after adjustment for the lifetime number of endoscopic procedures, adjustment for SEER summary stage did not substantially alter the aHR associated with IBD, as would be expected if endoscopy utilization was an important determinant of CRC stage at diagnosis. Examination of our multiple imputation model for CRC stage confirmed that endoscopy was among the strongest predictors of CRC stage. Thus, our results indicate that survival was poorer for IBD-associated CRC patients than for other CRC patients diagnosed at the same SEER stage.
Our results are consistent with reports from several previous studies[24,39] including the largest previous population-based studies of this topic[25,40], which included a similar number of UC- and CD-associated CRC cases. These studies reported about 20% or 50% higher mortality among UC-associated CRC patients[25] and CD-associated CRC patients[40], respectively, compared to other CRC patients, adjusted for CRC stage. A large Japanese study observed worse prognosis for UC-associated CRC but the relationship was restricted to stage III CRC[27]. In contrast with our results, a relatively large (n = 290 IBD-associated CRC cases) clinic-based study did not observe differential risk of death between IBD-associated and other CRC cases, possibly because of the clinical basis of the study[26]. Thus, our overall results, based on a set of relatively unselected cases and confirmed in the population-based portion of the Colon CFR, agree with the population-based results[25,40], and strengthen the evidence that the prognosis for IBD-associated CRC is worse than for non-IBD-associated CRC.
Our study has additional important methodological strengths. Approximately 57% of participants in this study were drawn from Colon CFR centers that utilize population-based case ascertainment strategies. Therefore, our results are likely to provide more general insight into the survival of IBD-associated CRC than clinic-based case series. Although our sample size was limited by the rarity of both IBD and CRC, the large, multi-site Colon CFR included a broad spectrum of CRC. Furthermore, because of the size of the Colon CFR, the cases in this study were recruited over 12 years, in comparison to previous reports that required 20 or more years to recruit a comparable number[25,27].
An important limitation of this study was our reliance on participant report of either UC or CD. We believe that our inability to confirm about 50% of self-reported UC or CD in patients from Ontario and Seattle resulted mainly from lack of access to complete medical records prior to CRC diagnosis. Although we expected IBD to be noted on pathology or medical records related to CRC diagnosis, we cannot be sure why information might be missing; the self-report of UC or CD might be incorrect, or the limited records available for review may be incomplete. Nonetheless, when we restricted analysis to confirmed IBD cases, removing unconfirmed IBD from analysis, the association between IBD-associated CRC and risk of mortality was strengthened. This could be because we successfully reduced misclassification of IBD. Alternatively, IBD confirmed in medical records may have been, on average, more severe than unconfirmed IBD, perhaps because available medical records were those related to CRC diagnosis.
Because we lacked detailed IBD-specific medical records, we were also unable to determine how medications such as 5-aminosalicyclic acid (5-ASA) used to treat IBD may have affected our results. In some studies, 5-ASA use has been associated with a reduced risk of CRC in IBD patients[41,42], and IBD patients who are never diagnosed with CRC are not included in our analysis. Like other anti-inflammatory drugs[19,20], 5-ASA treatment prior to CRC may reduce the risk of CRC mortality, and this may account in part for the relatively small difference in survival we observed between IBD-associated and sporadic CRC.
We also lacked specific cause of death information for deaths recorded outside of the Seattle, Ontario, and Mayo Clinic Colon CFR centers. However, at these centers, CRC was the underlying cause of death for 24 of 31 (77%) IBD-associated CRC patients and 499 of 651 (77%) of non-IBD CRC patients who died within 5 years of CRC diagnosis. Therefore, it is likely that most of the deaths included in the 5-year analysis across all Colon CFR centers were due to CRC, and differences in cause of death between IBD-associated and other CRC is unlikely to have influenced our results.
Finally, our sample size was limited to fully evaluate this association. IBD is associated with a strong increase in CRC risk[2,3,43], and we noted a higher prevalence of IBD, approximately 3.5%, among CRC cases than would be expected in the underlying population in which these CRC cases arose. For example, the prevalence of IBD in the United States is less than 1%[44,45]. However, the number of IBD-associated CRC cases in our study is approximately equal to the largest previous study comparing survival with non-IBD-associated CRC[25]. Nonetheless, sparse numbers certainly hindered our analysis and suggest that cautious interpretation is warranted. Future progress may perhaps be achieved by combining multiple populations through pooled or meta-analyses.
Our observation that IBD-associated CRC appears to have a worse prognosis than non-IBD-associated CRC, even when stage at diagnosis is taken into account, is consistent with evidence that inflammation negatively impacts CRC survival[19,20]. The mechanisms through which this may occur remain to be determined.
COMMENTS
Background
Inflammatory bowel disease (IBD) - more generally, inflammation - increases risk of colorectal cancer (CRC). Inflammation may also be associated with a poorer prognosis in patients with CRC, perhaps because inflammation may promote tumor progression (growth) and metastasis.
Research frontiers
The prevalence of IBD is growing worldwide. It remains unclear whether individuals diagnosed with CRC in the context of IBD are at higher risk of death from CRC, compared to CRC patients without IBD.
Innovations and breakthroughs
The rarity of both IBD and CRC make studying IBD-associated CRC difficult. This study includes a relatively large number of IBD-associated CRC cases, recruited from multiple populations rather than a single clinic case-series. The results suggest that survival with IBD-associated CRC is shorter than survival with CRC arising outside of IBD. This is observed even when comparing CRC cases diagnosed at the same stage, suggesting that IBD-associated CRC progresses more rapidly. However, it may also be due to other factors such as differences in CRC treatment, which could not be measured well in this study.
Applications
This study suggests that more effort is needed to understand why patients with IBD who are later diagnosed with CRC do worse than patients with CRC.
Peer review
The study lacked information on how IBD patients were treated for IBD prior to CRC, and whether they received surveillance for CRC incidence (as is commonly prescribed for this group under certain conditions). Surveillance is intended to catch CRC at early stage in IBD patients and therefore could improve survival with CRC in this group. This is a weakness of the paper. However, the study attempted to account for this by adjusting for stage of CRC at diagnosis (comparing cases diagnosed at the same stage) and noted that there was very little overall stage difference between IBD-associated and other CRC. Also, the difference in survival was noted only for 5 years following CRC diagnosis, but not over a long time (12 years), although most deaths occurred within the first 5 years. The study is interesting and may support the hypothesis that inflammation is worse for CRC survival.
Footnotes
Supported by The American Society of Preventive Oncology/Prevent Cancer Foundation/American Society for Clinical Oncology Cancer Prevention Research Fellowship to SVA; the Australasian Colorectal Cancer Family Registry, No. U01 CA097735; the Familial Colorectal Neoplasia Collaborative Group, No. U01 CA074799; the Mayo Clinic Cooperative Family Registry for Colon Cancer Studies, No. U01 CA074800; the Ontario Registry for Studies of Familial Colorectal Cancer, No. U01 CA074783; the Seattle Colorectal Cancer Family Registry, No. U01 CA074794; the University of Hawaii Colorectal Cancer Family Registry, No. U01 CA074806; and the University of California, Irvine Informatics Center, No. U01 CA078296
P- Reviewers Barreto S, M’Koma A S- Editor Zhai HH L- Editor A E- Editor Li JY
References
- 1.Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066–2078. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Terzić J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138:2101–2114.e5. doi: 10.1053/j.gastro.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 3.Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526–535. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gillen CD, Walmsley RS, Prior P, Andrews HA, Allan RN. Ulcerative colitis and Crohn’s disease: a comparison of the colorectal cancer risk in extensive colitis. Gut. 1994;35:1590–1592. doi: 10.1136/gut.35.11.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Connor PM, Lapointe TK, Beck PL, Buret AG. Mechanisms by which inflammation may increase intestinal cancer risk in inflammatory bowel disease. Inflamm Bowel Dis. 2010;16:1411–1420. doi: 10.1002/ibd.21217. [DOI] [PubMed] [Google Scholar]
- 6.Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol. 2004;287:G7–17. doi: 10.1152/ajpgi.00079.2004. [DOI] [PubMed] [Google Scholar]
- 7.Danese S, Mantovani A. Inflammatory bowel disease and intestinal cancer: a paradigm of the Yin-Yang interplay between inflammation and cancer. Oncogene. 2010;29:3313–3323. doi: 10.1038/onc.2010.109. [DOI] [PubMed] [Google Scholar]
- 8.Jawad N, Direkze N, Leedham SJ. Inflammatory bowel disease and colon cancer. Recent Results Cancer Res. 2011;185:99–115. doi: 10.1007/978-3-642-03503-6_6. [DOI] [PubMed] [Google Scholar]
- 9.Erlinger TP, Platz EA, Rifai N, Helzlsouer KJ. C-reactive protein and the risk of incident colorectal cancer. JAMA. 2004;291:585–590. doi: 10.1001/jama.291.5.585. [DOI] [PubMed] [Google Scholar]
- 10.Tsilidis KK, Branchini C, Guallar E, Helzlsouer KJ, Erlinger TP, Platz EA. C-reactive protein and colorectal cancer risk: a systematic review of prospective studies. Int J Cancer. 2008;123:1133–1140. doi: 10.1002/ijc.23606. [DOI] [PubMed] [Google Scholar]
- 11.Ruder EH, Laiyemo AO, Graubard BI, Hollenbeck AR, Schatzkin A, Cross AJ. Non-steroidal anti-inflammatory drugs and colorectal cancer risk in a large, prospective cohort. Am J Gastroenterol. 2011;106:1340–1350. doi: 10.1038/ajg.2011.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flossmann E, Rothwell PM. Effect of aspirin on long-term risk of colorectal cancer: consistent evidence from randomised and observational studies. Lancet. 2007;369:1603–1613. doi: 10.1016/S0140-6736(07)60747-8. [DOI] [PubMed] [Google Scholar]
- 13.Mahipal A, Anderson KE, Limburg PJ, Folsom AR. Nonsteroidal anti-inflammatory drugs and subsite-specific colorectal cancer incidence in the Iowa women’s health study. Cancer Epidemiol Biomarkers Prev. 2006;15:1785–1790. doi: 10.1158/1055-9965.EPI-05-0674. [DOI] [PubMed] [Google Scholar]
- 14.Chan AT, Giovannucci EL, Meyerhardt JA, Schernhammer ES, Curhan GC, Fuchs CS. Long-term use of aspirin and nonsteroidal anti-inflammatory drugs and risk of colorectal cancer. JAMA. 2005;294:914–923. doi: 10.1001/jama.294.8.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan AT, Ogino S, Giovannucci EL, Fuchs CS. Inflammatory markers are associated with risk of colorectal cancer and chemopreventive response to anti-inflammatory drugs. Gastroenterology. 2011;140:799–808, quiz e11. doi: 10.1053/j.gastro.2010.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Slattery ML, Wolff RK, Herrick JS, Caan BJ, Potter JD. IL6 genotypes and colon and rectal cancer. Cancer Causes Control. 2007;18:1095–1105. doi: 10.1007/s10552-007-9049-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Curtin K, Wolff RK, Herrick JS, Abo R, Slattery ML. Exploring multilocus associations of inflammation genes and colorectal cancer risk using hapConstructor. BMC Med Genet. 2010;11:170. doi: 10.1186/1471-2350-11-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu Y, Zhou BP. Inflammation: a driving force speeds cancer metastasis. Cell Cycle. 2009;8:3267–3273. doi: 10.4161/cc.8.20.9699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan AT, Ogino S, Fuchs CS. Aspirin use and survival after diagnosis of colorectal cancer. JAMA. 2009;302:649–658. doi: 10.1001/jama.2009.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coghill AE, Newcomb PA, Campbell PT, Burnett-Hartman AN, Adams SV, Poole EM, Potter JD, Ulrich CM. Prediagnostic non-steroidal anti-inflammatory drug use and survival after diagnosis of colorectal cancer. Gut. 2011;60:491–498. doi: 10.1136/gut.2010.221143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sugita A, Greenstein AJ, Ribeiro MB, Sachar DB, Bodian C, Panday AK, Szporn A, Pozner J, Heimann T, Palmer M. Survival with colorectal cancer in ulcerative colitis. A study of 102 cases. Ann Surg. 1993;218:189–195. doi: 10.1097/00000658-199308000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gyde SN, Prior P, Thompson H, Waterhouse JA, Allan RN. Survival of patients with colorectal cancer complicating ulcerative colitis. Gut. 1984;25:228–231. doi: 10.1136/gut.25.3.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ritchie JK, Hawley PR, Lennard-Jones JE. Prognosis of carcinoma in ulcerative colitis. Gut. 1981;22:752–755. doi: 10.1136/gut.22.9.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Heerden JA, Beart RW. Carcinoma of the colon and rectum complicating chronic ulcerative colitis. Dis Colon Rectum. 1980;23:155–159. doi: 10.1007/BF02587618. [DOI] [PubMed] [Google Scholar]
- 25.Jensen AB, Larsen M, Gislum M, Skriver MV, Jepsen P, Nørgaard B, Sørensen HT. Survival after colorectal cancer in patients with ulcerative colitis: a nationwide population-based Danish study. Am J Gastroenterol. 2006;101:1283–1287. doi: 10.1111/j.1572-0241.2006.00520.x. [DOI] [PubMed] [Google Scholar]
- 26.Delaunoit T, Limburg PJ, Goldberg RM, Lymp JF, Loftus EV. Colorectal cancer prognosis among patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2006;4:335–342. doi: 10.1016/j.cgh.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 27.Watanabe T, Konishi T, Kishimoto J, Kotake K, Muto T, Sugihara K. Ulcerative colitis-associated colorectal cancer shows a poorer survival than sporadic colorectal cancer: a nationwide Japanese study. Inflamm Bowel Dis. 2011;17:802–808. doi: 10.1002/ibd.21365. [DOI] [PubMed] [Google Scholar]
- 28.Newcomb PA, Baron J, Cotterchio M, Gallinger S, Grove J, Haile R, Hall D, Hopper JL, Jass J, Le Marchand L, et al. Colon Cancer Family Registry: an international resource for studies of the genetic epidemiology of colon cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:2331–2343. doi: 10.1158/1055-9965.EPI-07-0648. [DOI] [PubMed] [Google Scholar]
- 29.Phipps AI, Baron J, Newcomb PA. Prediagnostic smoking history, alcohol consumption, and colorectal cancer survival: the Seattle Colon Cancer Family Registry. Cancer. 2011;117:4948–4957. doi: 10.1002/cncr.26114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW, Meltzer SJ, Rodriguez-Bigas MA, Fodde R, Ranzani GN, et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58:5248–5257. [PubMed] [Google Scholar]
- 31.Lindor NM, Burgart LJ, Leontovich O, Goldberg RM, Cunningham JM, Sargent DJ, Walsh-Vockley C, Petersen GM, Walsh MD, Leggett BA, et al. Immunohistochemistry versus microsatellite instability testing in phenotyping colorectal tumors. J Clin Oncol. 2002;20:1043–1048. doi: 10.1200/JCO.2002.20.4.1043. [DOI] [PubMed] [Google Scholar]
- 32.Shia J, Ellis NA, Paty PB, Nash GM, Qin J, Offit K, Zhang XM, Markowitz AJ, Nafa K, Guillem JG, et al. Value of histopathology in predicting microsatellite instability in hereditary nonpolyposis colorectal cancer and sporadic colorectal cancer. Am J Surg Pathol. 2003;27:1407–1417. doi: 10.1097/00000478-200311000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Poynter JN, Siegmund KD, Weisenberger DJ, Long TI, Thibodeau SN, Lindor N, Young J, Jenkins MA, Hopper JL, Baron JA, et al. Molecular characterization of MSI-H colorectal cancer by MLHI promoter methylation, immunohistochemistry, and mismatch repair germline mutation screening. Cancer Epidemiol Biomarkers Prev. 2008;17:3208–3215. doi: 10.1158/1055-9965.EPI-08-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nur U, Shack LG, Rachet B, Carpenter JR, Coleman MP. Modelling relative survival in the presence of incomplete data: a tutorial. Int J Epidemiol. 2010;39:118–128. doi: 10.1093/ije/dyp309. [DOI] [PubMed] [Google Scholar]
- 35.Young JL Jr, Roffer SD, Ries LAG, Fritz AG, Hurlbut AA. SEER Summary Staging Manual - 2000: Codes and Coding Instructions. NIH Publication number 01-4969 ed. Bethesda: National Cancer Institute; 2001. [Google Scholar]
- 36.Adamo MB, Johnson CH, Ruhl JL, Dickie LA. 2011 SEER Program Coding and Staging Manual. NIH Publication number 11-5581 ed. Bethesda: National Cancer Institute; 2011. [Google Scholar]
- 37.Hosmer DW, Lemeshow S, May S. Applied survival analysis: regression modeling of time-to-event data. 2nd ed. Hoboken: Wiley-Interscience; 2008. [Google Scholar]
- 38.Collins PD, Mpofu C, Watson AJ, Rhodes JM. Strategies for detecting colon cancer and/or dysplasia in patients with inflammatory bowel disease. Cochrane Database Syst Rev. 2006;(2):CD000279. doi: 10.1002/14651858.CD000279.pub3. [DOI] [PubMed] [Google Scholar]
- 39.Aarnio M, Mustonen H, Mecklin JP, Järvinen HJ. Prognosis of colorectal cancer varies in different high-risk conditions. Ann Med. 1998;30:75–80. doi: 10.3109/07853899808999387. [DOI] [PubMed] [Google Scholar]
- 40.Larsen M, Mose H, Gislum M, Skriver MV, Jepsen P, Nørgård B, Sørensen HT. Survival after colorectal cancer in patients with Crohn’s disease: A nationwide population-based Danish follow-up study. Am J Gastroenterol. 2007;102:163–167. doi: 10.1111/j.1572-0241.2006.00857.x. [DOI] [PubMed] [Google Scholar]
- 41.Velayos FS, Terdiman JP, Walsh JM. Effect of 5-aminosalicylate use on colorectal cancer and dysplasia risk: a systematic review and metaanalysis of observational studies. Am J Gastroenterol. 2005;100:1345–1353. doi: 10.1111/j.1572-0241.2005.41442.x. [DOI] [PubMed] [Google Scholar]
- 42.van Staa TP, Card T, Logan RF, Leufkens HG. 5-Aminosalicylate use and colorectal cancer risk in inflammatory bowel disease: a large epidemiological study. Gut. 2005;54:1573–1578. doi: 10.1136/gut.2005.070896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gillen CD, Andrews HA, Prior P, Allan RN. Crohn’s disease and colorectal cancer. Gut. 1994;35:651–655. doi: 10.1136/gut.35.5.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Herrinton LJ, Liu L, Lewis JD, Griffin PM, Allison J. Incidence and prevalence of inflammatory bowel disease in a Northern California managed care organization, 1996-2002. Am J Gastroenterol. 2008;103:1998–2006. doi: 10.1111/j.1572-0241.2008.01960.x. [DOI] [PubMed] [Google Scholar]
- 45.Loftus CG, Loftus EV, Harmsen WS, Zinsmeister AR, Tremaine WJ, Melton LJ, Sandborn WJ. Update on the incidence and prevalence of Crohn’s disease and ulcerative colitis in Olmsted County, Minnesota, 1940-2000. Inflamm Bowel Dis. 2007;13:254–261. doi: 10.1002/ibd.20029. [DOI] [PubMed] [Google Scholar]


