Abstract
AIM: To investigate gastric antisecretory and gastroprotective activity of bovine hemoglobin (B-Hb) in rats.
METHODS: Adult Albino-Wistar rats were divided into groups of 6 animals each. B-Hb in doses of 100, 300 and 900 mg/kg body weight was tested for gastric acid secretion and antiulcer activity. Gastric secretions were measured 6 h after pylorus ligation in rats pretreated with B-Hb. The acidity was measured by titrating gastric contents against 0.01 mol/L NaOH to pH 7. Indomethacin ulcers were produced by oral administration of 30 mg/kg bw in the rats pretreated with B-Hb one hour before indomethacin. Six hours after indomethacin stomach removed and ulcer index was recorded. Ethanol ulcer were produced by 1 mL of ethanol in the rats pretreated with B-Hb 30 min before the ethanol. One hour after ethanol stomach were cut open to score ulcers. Histological examination and analysis of gastric wall mucus, non-protein sulfhydryl groups (NP-SH), and myeloperoxidase (MPO) were carried in gastric tissue following ethanol administration.
RESULTS: In control rats pylorus ligation for 6 h resulted in the accumulation of 8.1 ± 0.61 mL of gastric secretion. The treatment of the rats with 100, 300 and 900 mg/kg of B-Hb produced a significant decrease in the volume of gastric secretion 5.6 ± 0.63, 5.5 ± 0.75 and 4.7 ± 0.58 mL respectively as compared to the control group [analysis of variance (ANOVA) F = 4.77, P < 0.05]. The lesion area in the control group was found to be 22.4 ± 3.2 mm2 six hours after the administration of indomethacin. Treatment of rats with B-Hb at doses of 100 mg/kg (24.3 ± 3.29 mm2), 300 mg/kg (16.2 ± 1.45 mm2) and 900 mg/kg (12.6 ± 1.85 mm2) produced a dose dependent decreased the lesion scores (ANOVA F = 4.50, P < 0.05). The ulcer index following one hour after 1 mL ethanol was 7.1 ± 0.31. Pretreatment of rats with B-Hb at the doses of 100 mg/kg (2.5 ± 0.42), 300 mg/kg (2.1 ± 0.4) and 900 mg/kg (0.7 ± 0.21) significantly inhibited the formation of gastric lesions (ANOVA F = 63.26, P < 0.0001). Histological examination of gastric mucosa following ethanol showed significant lesions in the form of gastric pits with detachment of the surface epithelium; vacuolation of epithelial cells and elongation of microvessels. The changes were dose-dependently attenuated by B-Hb. The treatment of rats with ethanol significantly decreased the Alcian blue binding capacity of gastric wall mucus (480 ± 25.6 μg Alcian blue/g of tissue) as compared to control rats (667 ± 25.8 μg). Pretreatment of rats with B-Hb at the doses of 100 mg/kg (516 ± 31.6 μg/g), 300 mg/kg (558 ± 28.8 μg/g) and 900 mg/kg (654 ± 33.8 μg/g) significantly attenuated ethanol induced depletion of gastric wall mucus (ANOVA F = 8.05, P < 0.005). A significant and dose dependent increase of gastric mucosal NP-SH (ANOVA F = 19.62, P < 0.001) and decrease in MPO activity (ANOVA F = 3.1, P < 0.05) was observed in B-Hb treated rats.
CONCLUSION: B-Hb possesses significant gastric antisecretory and gastroprotective activity against experimentally induced gastric lesion. The gastroprotective effects of B-Hb are accompanied by inhibition of neutrophils activity, reduction of oxidative stress and maintenance of mucosal integrity.
Keywords: Bovine hemoglobin, Gastric ulcers, Indomethacin, Ethanol, Ischemic injury, Rats
Core tip: In the recent years the treatment strategies for gastric ulcer diseases have significantly changed, mirroring the revolution in the understanding of its pathogenesis. Improved oxygenation in critically ischemic gastric mucosa has emerged as a method of choice to accelerate epithelization of erosive and ulcerative defects. Bovine hemoglobin (B-Hb) has been used in a wide range of applications including restoration of tissue oxygenation in ischemic condition. Our data show that B-Hb has significant anti-gastric acid secretory and gastroprotective activity. Further studies are warranted to determine the role of B-Hb in prophylaxis/treatment of gastric ulcer disease.
INTRODUCTION
A variety of noxious factors and substances like alcohol, drugs, psychological stress, smoking and bacterial infection by Helicobacter pylori, to which the man is exposed in the present day life are known to have deleterious effect on gastric mucosa[1]. Organized at several levels the mucosal defense system comprise the pre-epithelial mucosal layer, the epithelial cell barrier, the mucosal microvasculature, the supply of the mucosa by enteric, extrinsic sensory and extrinsic autonomic neurons and mucosal immune system[2]. Maintenance and repair of gastric mucosa is a dynamic process associated with proliferation and migration of epithelial cells and connective tissue to maintain/regain mucosal architecture[3]. It involves complex host of mechanisms which work in tandem to protect gastric mucosa from damage as well as trigger the mechanism to repair mucosal defects by proliferating and migrating epithelial cells and connective tissue resulting in reconstruction of mucosal architecture[4]. Numerous studies have demonstrated the importance of gastric mucosal haemo-dynamics as a defensive factor of the gastric mucosa against injury[5-7]. Treatment with some prostaglandin derivatives such as prostaglandin E2 have been shown to increase mucosal blood flow and protect gastric mucosa against indomethacin and ethanol induced gastropathy by improving mucosal haemodynamics[8,9]. Oxygen delivery to the gastric mucosa is not only a function of blood flow but also depends on oxygen content in the arterial inflow. A possible strategy would be to supplement oxygen using hyperbaric oxygen (HBO) to overcome ischemic injury[10]. The use of HBO in the treatment of peptic ulcer raised the efficacy of the multimodality therapy and accelerated the epithelization of erosive and ulcerative defects[11,12]. An alternative method to improve oxygenation in critically ischemic tissue has emerged, which consists of using oxygen carriers, such as hemoglobin (Hb) or Hb based products[13]. These biomaterials have been initially developed to avoid the drawbacks of blood transfusions including immunologic reactions, blood-borne transmitted infections, limited availability and restricted storage time[14].
Hb is the iron-containing oxygen-transport metalloprotein in the red blood cells of all vertebrates. In blood Hb carries oxygen from the respiratory organs to the rest of the body where it releases the oxygen to burn nutrients to provide energy to power the functions of the organism, and collects the resultant carbon dioxide to bring it back to the respiratory organs to be dispensed from the organism. In mammals, Hb makes up about 97% of the red blood cells dry content, and around 35% of the total content including water[15]. Hemoglobin has an oxygen binding capacity of 1.34 mL per gram of hemoglobin[16], which increases the total blood oxygen capacity seventy-fold compared to dissolved oxygen in blood. A single mammalian hemoglobin molecule can bind (carry) up to four oxygen molecules[17]. Based on these properties cell free hemoglobin (CF-Hb) products have been successfully used in a variety of ischemic/hypoxic conditions[18,19].
Bovine hemoglobin (B-Hb) has been used for a wide range of applications, including their use to enhance oxygen delivery to tissues during conditions of ischemia or hypoxia[20,21]. Strate et al[13] reported that B-Hb is much more potent than autologous red blood cells in restoring tissue oxygenation in ischemic conditions. In view of the superiority of CF-Hb compared to erythrocyte and whole blood we studied the efficiency of B-Hb against experimentally induced gastric mucosal injury.
MATERIALS AND METHODS
Adult Albino Wistar rats of either sex, weighing 150-200 g and fed on standard chow diet were used. They were divided into groups of 6 animals each. The distribution of animals into groups and the treatment allotted to each group were randomized. All the experiments were started between 8:00 and 10:00 in the morning. The protocol of the study was approved by the Institutional Research and Ethical Committee.
The aqueous solution of ulcerogens and B-Hb were freshly prepared before administration. The concentrations of drug were prepared in such a way that each rat received 0.5 mL of drug solution/100 g body weight.
Gastric secretions
Pylorus ligated (Shay) rats: The animals were fasted for 36 h with access to water ad libitum before the pylorus was ligated under ether anesthesia, care being taken not to cause bleeding or to occlude blood vessels[22]. B-Hb in doses of 100, 300 and 900 mg/kg body weight was given by gavage (ig) half an hour before pylorus ligation by oral route. The animals were sacrificed at 6 h after the pylorus ligation. The stomachs were removed, contents collected, volume measured, centrifuged and subjected to analysis for titratable acidity against 0.01 mol/L NaOH to pH 7 using a pH meter and total acid output was calculated.
Indomethacin-induced gastric lesion: Indomethacin was suspended in 1% carboxymethylcellulose in water and administered by gavage at the dose of 30 mg/kg body weight[23]. B-Hb in the doses of 100, 300 and 900 mg/kg body weight was administered by gavage 1 h before indomethacin. The animals were sacrificed 6 h using ether after indomethacin administration. The stomach were removed and opened along the greater curvature. After washing with saline the gastric lesions were quantified by a person unaware of the treatment protocol. The ulcer were scored according to the method of Valcavi et al[24]. The circular ulcer induced by indomethacin were assessed on the basis of their diameter: deep circular ulcers more than 8 mm diameter = 10; 7-8 mm = 8; 6-7 mm = 7; 5-6 mm = 6; 4-5 mm= 5; 3-4 mm= 4; 2-3 mm= 3; 1-2 mm= 2; and < 1 mm = 1. Deep linear ulcers 10 mm or more in length were scored 6, and linear ucers less than 10 mm in length were scored 3. The scores of each single lesion were then summed up for determination of the ulcer index.
Gastric lesions induced by ethanol (cytoprotection studies): The animals were administered (ig) with 1 mL of absolute ethanol[25]. B-Hb in the doses of 100, 300 and 900 mg/kg body weight was given (ig) 30 min before the administration of ethanol. One hour after the administration of ethanol the animals were sacrificed and examined for the lesions in stomachs. The patcheal lesions of stomach induced by ethanol were scored according to the method described by Schiantarelli et al[26] using the following scale: 0 = normal mucosa; 1 = hyperemic mucosa or up to 3 small patches; 2 = from 4 to 10 small patches; 3 = more than 10 small or up to 3 medium-sized patches; 4= from 4 to 6 medium-sized patches; 5 = more than 6 medium-sized or up to 3 large patches; 6 = from 4 to 6 large patches; 7 = from 7 to 10 large patches; 8 = more than 10 large patches or extensive necrotic zones. “small” was defined as up to 2 mm across (max diameter), “medium-sized” as between 2 and 4 mm across and “large” as more than 4 mm across.
Two separate batches of ethanol treated rats were used for biochemical and histological studies. The assay of gastric wall mucus, non-protein sulfhydryl group (NP-SH), and myeloperoxidase (MPO) in the rats 1 h after ethanol exposure has been described below:
Determination of gastric wall mucus: Gastric wall mucus was determined according to the modified procedure of Corne et al[27]. The glandular segment of the stomach was separated from the lumen of the stomach, weighed, and transferred immediately to 10 mL of 0.1% w/v Alcian blue solution (in 0.16 mol/L sucrose solution buffered with 0.05 mL sodium acetate at pH 5). Tissue was stained for two hours in Alcian blue, excess dye was removed by two successive rinses with 10 mL of 0.25 mol/L sucrose, first for 15 min and then for 45 min. Dye complexed with the gastric wall mucus was extracted with 10 mL of 0.5 mol/L magnesium chloride which was intermittently shaken for 1 min at 30 min intervals for 2 h. Four milliliters of blue extract was then vigorously shaken with an equal volume of diethyl ether. The resulting emulsion was centrifuged at 3600 g for 10 min and the absorbance of aqueous layer was recorded at 580 nm. The quantity of Alcian blue extracted per gram of wet glandular tissue was then calculated.
Estimation of NP-SH: Gastric mucosal NP-SH was measured according to the method of Sedlak and Lindsay[28]. The glandular part of stomach was homogenized in ice-cold 0.02 mmol/L ethylenediaminetetraacetic acid. Aliquots of 5 mL of the homogenates were mixed in 15 mL test tubes with 4 mL of distilled water and 1 mL of 50% trichloroacetic acid. The tubes were shaken intermittently for 10-15 min and centrifuged at 3000 g. Two milliliters of supernatant were mixed with 4 mL of 0.4 mol/L Tris buffer at pH 8.9; 0.1 mL of 5,5’-dithio-bis-(2-nitrobenzoic acid) (DTNB) was added and the sample was shaken. The absorbance was read within 5 min of addition of DTNB at 412 nm against a reagent blank with no homogenates.
Determination of MPO: MPO activity in the gastric mucosa was measured according to the methods described earlier[29]. Preweighed tissue was homogenized (1:10 wt/vol) in 0.5% hexadecyltrimethyl ammonium bromide in 50 mmol potassium phosphate buffer (pH 6.0) before sonication in an ice bath for 20 s. Three freeze/thaw cycles were performed followed by sonication (20 s in ice bath). The samples were centrifuged at 17000 g (5 min, 4 °C) and MPO in the supernatant was assayed by mixing of 0.1 mL of supernatant with 2.9 mL of 50 mmol/potassium phosphate buffer (pH 6.0) containing 0.167 g/L O-dianasidine dihydrochloride and 0.0005% hydrogen peroxide. The change in absorbance at 460 nm was measured for 4 min using ultraviolet (UV)-visible spectrophotometer (UV-160A, Shimadzu, Kyoto, Japan).
Histology of ethanol-induced gastric lesions: The stomach was opened along the greater curvature, washed with saline and fixed in 10% neutral buffered formalin for 24 h. The specimens were then processed overnight for dehydration and clearing steps, using an automatic tissue processor (Shandon Processor MKП; Runcorn. Cheshire, United Kingdom). The specimens were embedded in paraffin blocks and sections of 5 μm thickness were stained with hematoxylin-eosin for light microscopy observations.
Statistical analysis
Data were analyzed by one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test. Differences with P < 0.05 were considered as statistically significant.
RESULTS
Effect of B-Hb on gastric secretion in 6 h pylorus-ligated rats
In control rats pylorus ligation for 6 h resulted in the accumulation of 8.1 ± 0.61 mL of gastric secretion and total acid output of 498.7 ± 31.8 mEq (Table 1). The treatment of the rats with 100, 300 and 900 mg/kg of B-Hb produced a significant decrease in the volume of gastric secretion 5.6 ± 0.63, 5.5 ± 0.75 and 4.7 ± 0.58 mL respectively as compared to the control group. Treatment with B-Hb also dose dependently reduced total acid output in the rats treated with 100 mg/kg (471.1 ± 75 mEq) and 300 mg/kg (438.4 ± 65.6 mEq). However a significant reduction in total acid output was observed in a dose of 900 mg/kg (287.6 ± 24.6 mEq) of B-Hb.
Table 1.
Effect of bovine hemoglobin on the volume of gastric secretion and acidity in 6 h pylorus ligated rats
| Treatment | Dose of B-Hb | Gastric secretion | Total acid output |
| (mg/kg) | (mL) | (mEq) | |
| Control | 0 | 8.1 ± 0.61 | 498.7 ± 31.8 |
| Hemoglobin | 100 | 5.6 ± 0.63a | 471.1 ± 75.0 |
| Hemoglobin | 300 | 5.5 ± 0.75a | 438.4 ± 65.6 |
| Hemoglobin | 900 | 4.7 ± 0.58b | 287.6 ± 24.6a |
Values are mean ± SE.
P < 0.05,
P < 0.01 vs control group using Dunnett’s multiple test. B-Hb: Bovine hemoglobin.
Effect of B-Hb on indomethacin-induced gastric mucosal damage
The administration of indomethacin resulted in production of gastric lesions mainly in the glandular stomach of all the animals. The lesion area in the control group was found to be 22.4 ± 3.2 mm2. Pretreatment of rats with B-Hb at doses of 100 mg/kg (24.3 ± 3.29 mm2), 300 mg/kg (16.2 ± 1.45 mm2) and 900 mg/kg (12.6 ± 1.85 mm2) produced a dose dependent decrease of the lesion area (ANOVA F = 4.50, P < 0.05, Figure 1A).
Figure 1.
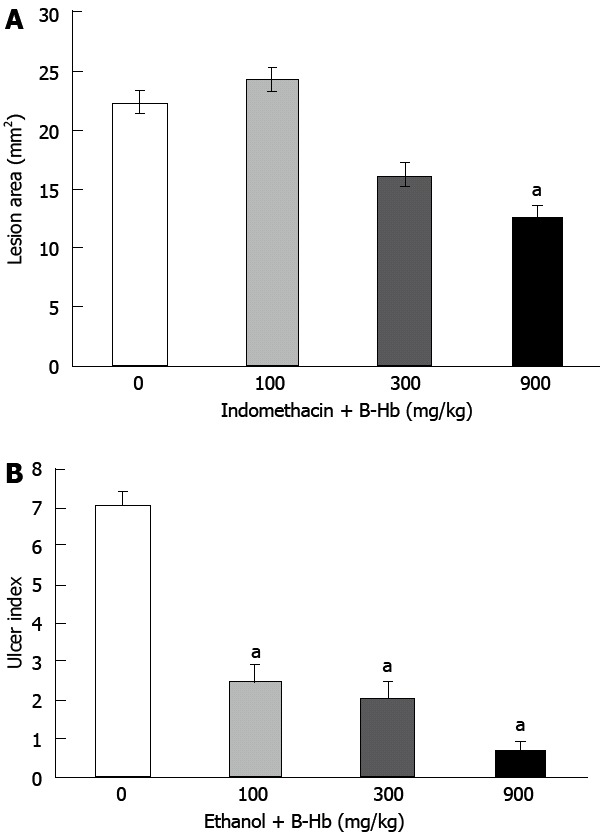
Effect of bovine hemoglobin. A: On indomethacin-induced gastric mucosal damage; B: On ethanol-induced gastric mucosal damage. B-Hb: Bovine hemoglobin. aP < 0.05 vs B-Hb 0 mg/kg.
Effect of B-Hb on ethanol-induced gastric lesions
The treatment of rats with absolute ethanol produced extensive gastric lesion in the glandular mucosa of the stomach. These lesions were characterized by multiple hemorrhagic red bands (patches) of different sizes along the axis of the glandular stomach. The ulcer index in the control group 1 h after ethanol administration was 7.1 ± 0.31. Pretreatment of rats with B-Hb at the doses of 100 mg/kg (ulcer index = 2.5 ± 0.42), 300 mg/kg (2.1 ± 0.4) and 900 mg/kg (0.7 ± 0.21) significantly inhibited the formation of gastric lesions (ANOVA F = 63.26, P < 0.0001, Figure 1B). Histological examination of gastric mucosa showed the appearance of these lesions in the form of gastric pits with detachment of the surface epithelium; vacuolation of epithelial cells and elongation of microvessels. Pretreatment with B-Hb dose-dependently prevented ethanol-induced mucosal damage (Figure 1).
Effect B-Hb on ethanol-induced changes in gastric wall mucus
The treatment of rats with ethanol significantly decreased the Alcian blue binding capacity of gastric wall mucus (480 ± 25.6 μg Alcian blue/g of tissue) as compared to control rats (667 ± 25.8 μg. Pretreatment of rats with B-Hb at the doses of 100 mg/kg (516 ± 31.6 μg/g), 300 mg/kg (558 ± 28.8 μg/g) and 900 mg/kg (654 ± 33.8 μg/g) significantly enhanced the binding capacity of Alcian blue to gastric mucosa (ANOVA F = 8.05, P < 0.005, Table 2).
Table 2.
Effect of bovine hemoglobin on ethanol induced changes in Alcian blue binding capacity, non-protein sulfhydryl groups levels and myeloperoxidase in gastric mucosa of rats
| Treatment | Dose of B-Hb (mg/kg) | Alcian blue binding (mg/g tissue) | Non-protein sulfhydryl (mmol/g tissue) | Myeloperoxidase activity (DA/g tissue) |
| Control | 0 | 667 ± 25.8 | 4.59 ± 0.29 | 15.94 ± 1.6 |
| EtOH alone | 1 mL/animal | 480 ± 25.6b | 1.60 ± 0.12b | 24.54 ± 1.9b |
| EtOH ± B-Hb | 100 | 516 ± 31.6 | 3.11 ± 0.35d | 24.30 ± 4.2 |
| EtOH ± B-Hb | 300 | 558 ± 28.8d | 3.73 ± 0.42d | 14.25 ± 2.6c |
| EtOH ± B-Hb | 900 | 654 ± 33.8d | 5.36 ± 0.34d | 17.21 ± 0.98c |
Values are mean ± SE.
P < 0.01 vs control;
P < 0.05,
P < 0.01 vs EtOH group using Dunnett’s multiple comparison test. B-Hb: Bovine hemoglobin.
Effect of B-Hb on ethanol-induced depletion of gastric mucosal NP-SH
The level of NP-SH in the gastric mucosa of control rats was 4.59 ± 0.29 μmol/g of tissue, which was significantly decreased to 1.60 ± 0.12 μmol/g of tissue following the administration of ethanol. A significant and dose dependent reversal of NP-SH was observed following administration of B-Hb in low (3.11 ± 0.35 mmol/g of tissue), medium (3.73 ± 0.42 mmol/g of tissue) and high dose (5.36 ± 0.34 mmol/g of tissue) (ANOVA F = 19.62, P < 0.001, Table 2).
Effect of B-Hb on ethanol-induced changes in gastric MPO activity
The MPO activity in the normal gastric mucosa was 15.96 ± 1.9 mmol/g of wet tissue which increased significantly to 24.54 ± 1.9 mmol/g following ethanol administration. The MPO activity was slightly reduced to 24.30 ± 4.2 mmol/g in the rats treated with low dose of B-Hb prior to ethanol administration. However medium and high dose of B-Hb significantly reversed ethanol induced increase in MPO the value of MPO in these two groups being 14.25 ± 2.6 mmol/g and 17.21 ± 0.98 mmol/g respectively (ANOVA F = 3.21, P < 0.05, Table 2).
DISCUSSION
The result of this study showed a dose dependent reduction in the volume and acidity of gastric secretions following intra-gastric administration of B-Hb (Table 1). Our findings are supported by several earlier investigators who showed that gastric hemorrhage or intra-gastric administration of blood or blood products exerts inhibitory effect on gastric acid secretions[30-32]. As early as 1953, Chandler et al[33] reported that gastro-duodenal hemorrhage leads to a temporary absence of hydrochloric acid (achlorhydria) causing a significant decrease in gastric acid secretions. Fullarton et al[34] also showed that gastro-duodenal blood infusion significantly inhibits pentagastrin stimulated gastric acid and pepsin secretion. Paradoxically, intra-gastric blood/blood products including hemoglobin which constitute a protein meal instead of stimulating gastric secretions result in gastric antisecretory activity[35,36]. Gastric acid secretion is an elaborate and dynamic process that is regulated by neural (efferent and afferent), hormonal (e.g., gastrin), and paracrine (e.g., histamine, ghrelin, somatostatin) pathways as well as mechanical (e.g., distention) and chemical (e.g., amino acids) stimuli[31]. It has been suggested that intra-gastric blood induced increase in plasma gastric inhibitory polypeptide, secretin or glucogen may modulate gastric acid secretion in animals[32,34,37,38]. These mediators inhibit gastric acid secretions through somatostatin release[39-41]. However this hypothesis has been challenged by some other investigators[42].
Pretreatment of rats with B-Hb dose dependently ameliorated indomethacin-induced gastric mucosal damage (Figure 1). Protective effect of cell free hemoglobin has been reported against a variety of experimentally induced disease models including cardiac ischemic reperfusion injury[18,43,44], pancreatitis[45], renal injury[46] and neuronal injury[18,19,47-49]. The mechanism by which B-Hb exerts its protective effect against indomethacin induced gastropathy is far from clear. Indomethacin causes gastric mucosal damage via several mechanisms, including the impairment of the barrier properties of the mucosa, suppression of gastric prostaglandin synthesis, reduction of gastric mucosal blood flow and interference with the tissue repair mechanism[50]. The ability of non-steroidal anti-inflammatory drugs (NSAIDs) to reduce gastric mucosal blood flow has been recognized for several decades[9]. Prostaglandins (PGs) of the E and I series are potent vasodilators that are continuously produced by the vascular endothelium, inhibition of PG synthesis by NSAIDs lead to a reduced vascular tone and hypoxic injury[51]. Cell free hemoglobin is able to readily diffuse in the microcirculation and transport O2 to hypoxic tissue because of its high O2 affinity, low viscosity and small mean diameter as compared to red blood cells[52]. Once in microcirculation Hb offloads oxygen to the ischemic tissue, which is unlike the vasoconstrictive effect of Hb on large vessels[53-56]. Hence the mitigation of pathogen induced ischemic injury by CF-Hb may be attributed to its ability to transient oxygen delivery[13,57] and preservation of energy metabolism[58]. Moreover, the presence of acid in the lumen of the stomach also contributes to the pathogenesis of indomethacin -induced ulcers and bleeding, by impairing the restitution process, interfering with hemostasis and inactivating several growth factors that are important in mucosal defense and repair[9]. Our experiments using Shay rats model clearly showed an inhibition of gastric acid secretion following B-Hb administration (Table 1). Thus the reduction of acid content in stomach by B-Hb may to some extent contribute to its gastroprotective effect against indomethacin induced ulcers.
The oral administration of absolute ethanol produced significant ulcers in glandular part of gastric mucosa of rats (Figure 2). Our histopathological studies of gastric mucosa showed a significant loss of glandular cells, disruption of epithelium, sub mucosal edema and infiltration of neutrophils following ethanol administration (Figure 2). A significant decrease in Alcian blue binding capacity of gastric mucosa following exposure to ethanol (Table 2) clearly suggests depletion of mucosal gel lining adhering to the gastric surface which is considered the first line of defense in stomach against endogenous and exogenous ulcerogens[59]. Numerous mechanisms have been proposed to explain necrotizing agent induced gastropathy[60]. Besides the direct deleterious effect of ethanol on gastric tissues, disruption of mucosal barrier also results from mucosal capillary necrosis, vascular congestion and thrombosis in the sub-epithelial microvasculature[61]. Oral administration of ethanol results in low or no blood flow to the stomach leading to transient hypoxic mucosal damage[62]. It is well accepted that gastric mucosal protective mechanism largely depend on appropriate microcirculation which help to orchestrate the defense mechanism at various levels of gastric mucosa[63]. Pre-treatment of animal with B-Hb dose dependently attenuated ethanol induced gastric ulcer with almost complete protection in higher dose of 900 mg/kg body weight (Figure 2). The gastroprotective effect of B-Hb against ethanol induced ulcers may to some extent be attributed to its ability to inhibit gastric acid secretions and enhanced oxygen delivery as discussed earlier. Moreover the cytoprotective effect of B-Hb was accompanied by attenuation of ethanol-induced increase in MPO (a marker of neutrophil activity) in glandular tissue of stomach (Table 2). Neutrophils are the major inflammatory cell type infiltrating the injured mucosa following exposure to ethanol[64]. Strategies to counteract the infiltration and/or activation of neutrophils have been shown to protect animals against gastric ulcers[65,66]. Activated neutrophils injure the microvasculature via the release of oxygen derived free radicals (ODFR) and proteases[67,68]. We observed a significant decrease in gastric NP-SH following ethanol administration clearly suggesting a massive generation of ODFR in stomach (Table 2). Our findings are in agreement with earlier reports showing depletion of sulfhydryls in ethanol-induced gastric lesions[69,70]. The treatment of rats with glutathione depletors has been shown to significantly potentiate ulcerogen-induced gastric mucosal injury[71], whereas increase in mucosal NP-SH exerts a gastroprotective effect[72,73]. These findings suggest that gastroprotective effects of B-Hb may to extent be attributed to its antioxidant activity. Dual effect of Hb as prooxidant and antioxidant has been reported earlier[74,75]. In fact, more than 100 years ago Hb was shown to readily react with H2O2[76] and peroxidase activity of Hb has been reported as early as by Wu[77]. Thus the active site of hemeprotein in Hb share peroxide cleavage properties of peroxidase to present antioxidant activity[78]. On the other hand the oxidation of Hb generates potentially cytotoxic products such as ferryl heme intermediate (Fe4+), methemoglobin (Fe3+), hemichromes and free heme or iron[79,80]. Comproportionation of cytotoxic ferryl hemoglobin with oxyhemoglobin has been shown to result in antioxidant function in which ferryl heme intermediate is quenched and resultant methemoglobin is re-reduced by methemoglobin reductase[81]. Moreover there is strong evidence to suggest that Hb exerts its cytoprotective effect through adaptive cytoprotection. The presence of Hb in the tissue has been shown to induce heme oxygenase-1 (HO-1) the enzyme responsible for heme degradation[82]. Studies with HO-1 knock out mice have demonstrated that HO-1 ameliorates NSAIDs induced gastric tissue damage[83,84]. It has been shown that HO-1 exerts its cytoprotective action against necrotizing agents by scavenging the prooxidant heme which is a major hemoglobin degradation product[84,85]. To sum up it may be suggested that cytoprotective effect of B-Hb is complex and involve multiple mechanisms.
Figure 2.
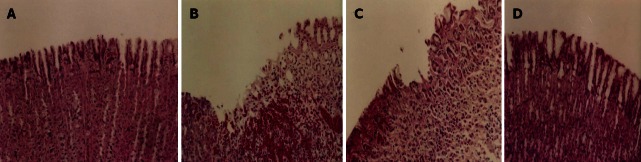
Light micrographs showing the effect of bovine hemoglobin on ethanol-induced gastric lesions in rats (× 200). A: Normal mucosa; B: Ethanol produced lesion; C: Pretreatment of rats with bovine hemoglobin (B-Hb) 100 mg/kg; D: Pretreatment of rats with B-Hb 900 mg/kg.
In conclusion our data show that B-Hb has significant anti-gastric acid secretory and gastroprotective activity. Further studies are warranted to determine the role of B-Hb in prophylaxis/treatment of gastric ulcer disease.
ACKNOWLEDGMENTS
The authors are thankful to the Prince Sultan Military Medical City and the Medical Services Department of Ministry of Defense for their encouragement and support.
COMMENTS
Background
In the recent years, the treatment strategies for gastric ulcer diseases have significantly changed, mirroring the revolution in the understanding of its pathogenesis. Improved oxygenation in critically ischemic gastric mucosa has emerged as a method of choice to accelerate epithelization of erosive and ulcerative defects. Bovine hemoglobin (B-Hb) has been used in a wide range of applications including restoration of tissue oxygenation in ischemic condition.
Research frontiers
Strategies to improve oxygen delivery to ischemic tissues could potentially protect gastric mucosa and other tissues from hypoxic injury. Cell free hemoglobin is able to readily diffuse in the microcirculation and transport O2 to hypoxic tissue because of its high O2 affinity, low viscosity and small diameter.
Innovations and breakthroughs
Recent studies showed protective effect of cell free hemoglobin against disease models of ischemic reperfusion injury including neuronal injury, nephropathy, pancreatitis and myocardial infarction. This study first time demonstrated anti-secretory and gastric antiulcer activity of B-Hb. The gastroprotective effect could be attributed to the ability of B-Hb to improve oxygenation, reduction of oxidative stress and lowering of neutrophil activity.
Applications
The result of this study suggests that bovine hemoglobin besides having significant anti-gastric acid secretory activity protects rats against ethanol and indomethamine induced gastric ulcers. B-Hb being animal protein can be safely explored clinically for the oral treatment of hyperacidity and gastric ulcer diseases.
Terminology
Hb is the iron-containing oxygen-transport metalloprotein in red blood cells of all vertebrates. Hemoglobin is autonomous; it binds oxygen and release it without the need for any cofactor. Cell free Hb has been used for wide range of applications including enhanced oxygen delivery to ischemic tissue. Bovine hemoglobin is superior to autologous red blood cells in restoring tissue oxygen in ischemic conditions.
Peer review
This is the first study showing antisecretory and gastric anti-ulcer activity of bovine hemoglobin. Reduction of oxidative stress and lowering of neutrophil activity might contribute to B-Hb induced gastroprotective effects. The results are valuable to explore pharmacological potential of B-Hb in a variety of ischemic/hypoxic conditions.
Footnotes
P- Reviewer Vorobjova T S- Editor Gou SX L- Editor A E- Editor Li JY
References
- 1.Valle JD. Peptic ulcer disease and related disorders. In: Fauci AS, Kasper DL, Braunwald E, Hauser SL, Longo DL, et al., editors. Harrison’s Principal of Internal Medicine. 17th ed. New York: McGraw Hill; 2008. pp. 1746–1762. [Google Scholar]
- 2.Pai R, Ohta M, Itani RM, Sarfeh IJ, Tarnawski AS. Induction of mitogen-activated protein kinase signal transduction pathway during gastric ulcer healing in rats. Gastroenterology. 1998;114:706–713. doi: 10.1016/s0016-5085(98)70584-0. [DOI] [PubMed] [Google Scholar]
- 3.Johnson LR. Regulation of gastrointestinal mucosal growth. World J Surg. 1979;3:477–486. doi: 10.1007/BF01556110. [DOI] [PubMed] [Google Scholar]
- 4.Holzer P. Gastroduodenal mucosal defense: coordination by a network of messengers and mediators. Curr Opin Gastroenterol. 2001;17:489–496. doi: 10.1097/00001574-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Cheung LY, Chang N. The role of gastric mucosal blood flow and H+ back-diffusion in the pathogenesis of acute gastric erosions. J Surg Res. 1977;22:357–361. doi: 10.1016/0022-4804(77)90157-3. [DOI] [PubMed] [Google Scholar]
- 6.Ritchie WP, Shearburn EW. Influence of isoproterenol and cholestyramine on acute gastric mucosal ulcerogenesis. Gastroenterology. 1977;73:62–65. [PubMed] [Google Scholar]
- 7.Whittle BJ. Mechanisms underlying gastric mucosal damage induced by indomethacin and bile-salts, and the actions of prostaglandins. Br J Pharmacol. 1977;60:455–460. doi: 10.1111/j.1476-5381.1977.tb07522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Konturek SJ, Konturek PC, Brzozowski T. Prostaglandins and ulcer healing. J Physiol Pharmacol. 2005;56 Suppl 5:5–31. [PubMed] [Google Scholar]
- 9.Wallace JL. How do NSAIDs cause ulcer disease? Baillieres Best Pract Res Clin Gastroenterol. 2000;14:147–159. doi: 10.1053/bega.1999.0065. [DOI] [PubMed] [Google Scholar]
- 10.Rachmilewitz D, Karmeli F, Okon E, Rubenstein I, Better OS. Hyperbaric oxygen: a novel modality to ameliorate experimental colitis. Gut. 1998;43:512–518. doi: 10.1136/gut.43.4.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elizavetina GA, Vinnitskiĭ LI, Vorob’eva NT, Chorbinskaia SA, Izmalkova NM. Evaluation of the therapeutic effect of hyperbaric oxygenation and anginin in peptic ulcer. Ter Arkh. 1989;61:46–48. [PubMed] [Google Scholar]
- 12.Guerrin F, Robin H, Gosselin B, Laspeyres M. Effects of oxygen and hyperbaric conditions on various types of experimental ulcers. Lille Med. 1976;21:662–667. [PubMed] [Google Scholar]
- 13.Strate T, Mann O, Kleinhans H, Rusani S, Schneider C, Yekebas E, Freitag M, Standl T, Bloechle C, Izbicki JR. Microcirculatory function and tissue damage is improved after therapeutic injection of bovine hemoglobin in severe acute rodent pancreatitis. Pancreas. 2005;30:254–259. doi: 10.1097/01.mpa.0000157481.22155.2d. [DOI] [PubMed] [Google Scholar]
- 14.Chang TM. Artificial cells for cell and organ replacements. Artif Organs. 2004;28:265–270. doi: 10.1111/j.1525-1594.2004.47343.x. [DOI] [PubMed] [Google Scholar]
- 15.Weed RI, REED CF, BERG G. Is hemoglobin an essential structural component of human erythrocyte membranes? J Clin Invest. 1963;42:581–588. doi: 10.1172/JCI104747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dominguez de Villota ED, Ruiz Carmona MT, Rubio JJ, de Andrés S. Equality of the in vivo and in vitro oxygen-binding capacity of haemoglobin in patients with severe respiratory disease. Br J Anaesth. 1981;53:1325–1328. doi: 10.1093/bja/53.12.1325. [DOI] [PubMed] [Google Scholar]
- 17.Costanzo LS. Physiology. 4th ed. Hagerstown, MD, USA: Lippincott Williams and Wilkins; 2007. [Google Scholar]
- 18.Wei L, Wu RB, Yang CM, Zheng SY, Yu XY. Cardioprotective effect of a hemoglobin-based oxygen carrier on cold ischemia/reperfusion injury. Cardiology. 2011;120:73–83. doi: 10.1159/000333106. [DOI] [PubMed] [Google Scholar]
- 19.Mito T, Nemoto M, Kwansa H, Sampei K, Habeeb M, Murphy SJ, Bucci E, Koehler RC. Decreased damage from transient focal cerebral ischemia by transfusion of zero-link hemoglobin polymers in mouse. Stroke. 2009;40:278–284. doi: 10.1161/STROKEAHA.108.526731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Standl T, Horn P, Wilhelm S, Greim C, Freitag M, Freitag U, Sputtek A, Jacobs E, Schulte am Esch J. Bovine hemoglobin is more potent than autologous red blood cells in restoring muscular tissue oxygenation after profound isovolemic hemodilution in dogs. Can J Anesthesiol. 1996;43:714–723. doi: 10.1007/BF03017957. [DOI] [PubMed] [Google Scholar]
- 21.Harringer W, Hodakowski GT, Svizzero T, Jacobs EE, Vlahakes GJ. Acute effects of massive transfusion of a bovine hemoglobin blood substitute in a canine model of hemorrhagic shock. Eur J Cardiothorac Surg. 1992;6:649–654; discussion 654. doi: 10.1016/1010-7940(92)90189-5. [DOI] [PubMed] [Google Scholar]
- 22.Shay H, Komarov SA, Fels SE, Merze D, Gruenstein H, Siplet H. A simple method for the uniform production of gastric ulceration in the rat. Gastroenterology. 1945;5:43–61. [Google Scholar]
- 23.Bhargava KP, Gupta MB, Tangri KK. Mechanism of ulcerogenic activity of indomethacin and oxyphenbutazone. Eur J Pharmacol. 1973;22:191–195. doi: 10.1016/0014-2999(73)90012-5. [DOI] [PubMed] [Google Scholar]
- 24.Valcavi U, Caponi R, Brambilla A, Palmira M, Minoja F, Bernini F, Musanti R, Fumagalli R. Gastric antisecretory, antiulcer and cytoprotective properties of 9-hydroxy-19,20-bis-nor-prostanoic acid in experimental animals. Arzneimittelforschung. 1982;32:657–663. [PubMed] [Google Scholar]
- 25.Natale G, Lazzeri G, Blandizzi C, Gherardi G, Lenzi P, Pellegrini A, Del Tacca M. Seriate histomorphometry of whole rat stomach: an accurate and reliable method for quantitative analysis of mucosal damage. Toxicol Appl Pharmacol. 2001;174:17–26. doi: 10.1006/taap.2001.9193. [DOI] [PubMed] [Google Scholar]
- 26.Schiantarelli P, Cadel S, Folco GC. Gastroprotective effects of morniflumate, an esterified anti-inflammatory drug. Arzneimittelforschung. 1984;34:885–890. [PubMed] [Google Scholar]
- 27.Corne SJ, Morrissey SM, Woods RJ. Proceedings: A method for the quantitative estimation of gastric barrier mucus. J Physiol. 1974;242:116P–117P. [PubMed] [Google Scholar]
- 28.Chien CP. Clinical trial of SK& amp; F 14336 concentrate for chronic psychotics. Curr Ther Res Clin Exp. 1969;11:15–21. [PubMed] [Google Scholar]
- 29.Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982;78:206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- 30.Zebrowska T, Low AG, Zebrowska H. Studies on gastric digestion of protein and carbohydrate, gastric secretion and exocrine pancreatic secretion in the growing pig. Br J Nutr. 1983;49:401–410. doi: 10.1079/bjn19830049. [DOI] [PubMed] [Google Scholar]
- 31.Schubert ML. Gastric secretion. Curr Opin Gastroenterol. 2007;23:595–601. doi: 10.1097/MOG.0b013e3282f03462. [DOI] [PubMed] [Google Scholar]
- 32.Fullarton GM, Boyd EJ, Crean GP, Hilditch TE, McColl KE. Effect of simulated intragastric haemorrhage on gastric acid secretion, gastric motility, and serum gastrin. Gut. 1990;31:518–521. doi: 10.1136/gut.31.5.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chandler GN, Watkinson G. Gastric aspiration in haematemesis. Lancet. 1953;265:1170–1175. doi: 10.1016/s0140-6736(53)90726-0. [DOI] [PubMed] [Google Scholar]
- 34.Fullarton GM, Boyd EJ, Crean GP, Buchanan K, McColl KE. Inhibition of gastric secretion and motility by simulated upper gastrointestinal haemorrhage: a response to facilitate haemostasis? Gut. 1989;30:156–160. doi: 10.1136/gut.30.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schubert ML. Gastric secretion. Curr Opin Gastroenterol. 2011;27:536–542. doi: 10.1097/MOG.0b013e32834bd53f. [DOI] [PubMed] [Google Scholar]
- 36.Ramsay PT, Carr A. Gastric acid and digestive physiology. Surg Clin North Am. 2011;91:977–982. doi: 10.1016/j.suc.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 37.Bell FR, Webber DE, Wass JA, Rees LH, Evans J, Morgan LM, Marks V, Lewis J. Correlation of endogenous somatostatin, gastric inhibitory polypeptide, glucagon and insulin with gastric function in the conscious calf. J Endocrinol. 1981;89:451–456. doi: 10.1677/joe.0.0890451. [DOI] [PubMed] [Google Scholar]
- 38.Allison MC, Fullarton GM, Brown IL, Crean GP, McColl KE. Enhanced gastric mucosal haemostasis after upper gastrointestinal haemorrhage. Gut. 1991;32:735–739. doi: 10.1136/gut.32.7.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chiba T, Taminato T, Kadowaki S, Abe H, Chihara K, Seino Y, Matsukura S, Fujita T. Effects of glucagon, secretin, and vasoactive intestinal polypeptide on gastric somatostatin and gastrin release from isolated perfused rat stomach. Gastroenterology. 1980;79:67–71. [PubMed] [Google Scholar]
- 40.McIntosh CH, Pederson RA, Koop H, Brown JC. Gastric inhibitory polypeptide stimulated secretion of somatostatinlike immunoreactivity from the stomach: inhibition by acetylcholine or vagal stimulation. Can J Physiol Pharmacol. 1981;59:468–472. doi: 10.1139/y81-069. [DOI] [PubMed] [Google Scholar]
- 41.Wolfe MM, Reel GM, McGuigan JE. Inhibition of gastrin release by secretin is mediated by somatostatin in cultured rat antral mucosa. J Clin Invest. 1983;72:1586–1593. doi: 10.1172/JCI111117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Konturek SJ, Tasler J, Cieszkowski M, Coy DH, Schally AV. Effect of growth hormone release-inhibiting hormone on gastric secretion, mucosal blood flow, and serum gastrin. Gastroenterology. 1976;70:737–741. [PubMed] [Google Scholar]
- 43.Li T, Li J, Liu J, Zhang P, Wu W, Zhou R, Li G, Zhang W, Yi M, Huang H. Polymerized placenta hemoglobin attenuates ischemia/reperfusion injury and restores the nitroso-redox balance in isolated rat heart. Free Radic Biol Med. 2009;46:397–405. doi: 10.1016/j.freeradbiomed.2008.10.042. [DOI] [PubMed] [Google Scholar]
- 44.Rempf C, Standl T, Schenke K, Chammas K, Gottschalk A, Burmeister MA, Gottschalk A. Administration of bovine polymerized haemoglobin before and during coronary occlusion reduces infarct size in rabbits. Br J Anaesth. 2009;103:496–504. doi: 10.1093/bja/aep233. [DOI] [PubMed] [Google Scholar]
- 45.Strate T, Mann O, Kleinhans H, Schneider C, Knoefel WT, Yekebas E, Standl T, Bloechle C, Izbicki JR. Systemic intravenous infusion of bovine hemoglobin significantly reduces microcirculatory dysfunction in experimentally induced pancreatitis in the rat. Ann Surg. 2003;238:765–771. doi: 10.1097/01.sla.0000094442.12395.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Whitley D, Patterson R, Greenburg AG. Cell-free hemoglobin preserves renal function during normothermic ischemia. J Surg Res. 1998;77:187–191. doi: 10.1006/jsre.1998.5375. [DOI] [PubMed] [Google Scholar]
- 47.Burmeister MA, Rempf C, Standl TG, Rehberg S, Bartsch-Zwemke S, Krause T, Tuszynski S, Gottschalk A, Schulte am Esch J. Effects of prophylactic or therapeutic application of bovine haemoglobin HBOC-200 on ischaemia-reperfusion injury following acute coronary ligature in rats. Br J Anaesth. 2005;95:737–745. doi: 10.1093/bja/aei255. [DOI] [PubMed] [Google Scholar]
- 48.Caswell JE, Strange MB, Rimmer DM, Gibson MF, Cole P, Lefer DJ. A novel hemoglobin-based blood substitute protects against myocardial reperfusion injury. Am J Physiol Heart Circ Physiol. 2005;288:H1796–H1801. doi: 10.1152/ajpheart.00905.2004. [DOI] [PubMed] [Google Scholar]
- 49.Nemoto M, Mito T, Brinigar WS, Fronticelli C, Koehler RC. Salvage of focal cerebral ischemic damage by transfusion of high O2-affinity recombinant hemoglobin polymers in mouse. J Appl Physiol. 2006;100:1688–1691. doi: 10.1152/japplphysiol.01374.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fromm D. How do non-steroidal anti-inflammatory drugs affect gastric mucosal defenses? Clin Invest Med. 1987;10:251–258. [PubMed] [Google Scholar]
- 51.Rainsford KD. Microvascular injury during gastric mucosal damage by anti-inflammatory drugs in pigs and rats. Agents Actions. 1983;13:457–460. doi: 10.1007/BF02176417. [DOI] [PubMed] [Google Scholar]
- 52.Creteur J, Vincent JL. Potential uses of hemoglobin-based oxygen carriers in critical care medicine. Crit Care Clin. 2009;25:311–324, Table of Contents. doi: 10.1016/j.ccc.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 53.Gulati A, Sharma AC, Burhop KE. Effect of stroma-free hemoglobin and diaspirin cross-linked hemoglobin on the regional circulation and systemic hemodynamics. Life Sci. 1994;55:827–837. doi: 10.1016/0024-3205(94)00566-4. [DOI] [PubMed] [Google Scholar]
- 54.Federspiel WJ. Pulmonary diffusing capacity: implications of two-phase blood flow in capillaries. Respir Physiol. 1989;77:119–134. doi: 10.1016/0034-5687(89)90035-2. [DOI] [PubMed] [Google Scholar]
- 55.Page TC, Light WR, McKay CB, Hellums JD. Oxygen transport by erythrocyte/hemoglobin solution mixtures in an in vitro capillary as a model of hemoglobin-based oxygen carrier performance. Microvasc Res. 1998;55:54–64. doi: 10.1006/mvre.1997.2055. [DOI] [PubMed] [Google Scholar]
- 56.George I, Yi GH, Schulman AR, Morrow BT, Cheng Y, Gu A, Zhang G, Oz MC, Burkhoff D, Wang J. A polymerized bovine hemoglobin oxygen carrier preserves regional myocardial function and reduces infarct size after acute myocardial ischemia. Am J Physiol Heart Circ Physiol. 2006;291:H1126–H1137. doi: 10.1152/ajpheart.00076.2006. [DOI] [PubMed] [Google Scholar]
- 57.Plock JA, Rafatmehr N, Sinovcic D, Schnider J, Sakai H, Tsuchida E, Banic A, Erni D. Hemoglobin vesicles improve wound healing and tissue survival in critically ischemic skin in mice. Am J Physiol Heart Circ Physiol. 2009;297:H905–H910. doi: 10.1152/ajpheart.00430.2009. [DOI] [PubMed] [Google Scholar]
- 58.Carlucci F, Miraldi F, Barretta A, Marullo AG, Marinello E, Tabucchi A. Preservation of myocardial energy status by bovine hemoglobin solutions during ischemia. Biomed Pharmacother. 2002;56:247–253. doi: 10.1016/s0753-3322(02)00197-x. [DOI] [PubMed] [Google Scholar]
- 59.Slomiany BL, Piasek A, Sarosiek J, Slomiany A. The role of surface and intracellular mucus in gastric mucosal protection against hydrogen ion. Compositional differences. Scand J Gastroenterol. 1985;20:1191–1196. doi: 10.3109/00365528509089275. [DOI] [PubMed] [Google Scholar]
- 60.Amandeep K, Robin S, Ramica S, Sunil K. Peptic ulcer: A review on etiology and pathogenesis. IRJP. 2012;3:34–38. [Google Scholar]
- 61.Konturek SJ, Stachura J, Konturek JW. Gastric cytoprotection and adaptation to ethanol. In: Preedy VR, Watson RR, et al., editors. Alcohol and the gastrointestinal tract. New York: CRC Press; 1996. pp. 123–141. [Google Scholar]
- 62.Saeki T, Takahashi N, Kanamoto R, Iwami K. Characterization of cloned mouse Na+/taurocholate cotransporting polypeptide by transient expression in COS-7 cells. Biosci Biotechnol Biochem. 2002;66:1116–1118. doi: 10.1271/bbb.66.1116. [DOI] [PubMed] [Google Scholar]
- 63.Holzer P, Lippe IT. Stimulation of afferent nerve endings by intragastric capsaicin protects against ethanol-induced damage of gastric mucosa. Neuroscience. 1988;27:981–987. doi: 10.1016/0306-4522(88)90201-1. [DOI] [PubMed] [Google Scholar]
- 64.Laine L, Weinstein WM. Histology of alcoholic hemorrhagic “gastritis”: a prospective evaluation. Gastroenterology. 1988;94:1254–1262. doi: 10.1016/0016-5085(88)90661-0. [DOI] [PubMed] [Google Scholar]
- 65.Shimizu N, Watanabe T, Arakawa T, Fujiwara Y, Higuchi K, Kuroki T. Pentoxifylline accelerates gastric ulcer healing in rats: roles of tumor necrosis factor alpha and neutrophils during the early phase of ulcer healing. Digestion. 2000;61:157–164. doi: 10.1159/000007752. [DOI] [PubMed] [Google Scholar]
- 66.Kvietys PR, Twohig B, Danzell J, Specian RD. Ethanol-induced injury to the rat gastric mucosa. Role of neutrophils and xanthine oxidase-derived radicals. Gastroenterology. 1990;98:909–920. doi: 10.1016/0016-5085(90)90015-s. [DOI] [PubMed] [Google Scholar]
- 67.Elsbach P, Weiss J. Phagocytosis of bacteria and phospholipid degradation. Biochim Biophys Acta. 1988;947:29–52. doi: 10.1016/0304-4157(88)90018-4. [DOI] [PubMed] [Google Scholar]
- 68.Hernandez LA, Grisham MB, Twohig B. Arfors KE, Harlan JM, Granger DN. Role of neutrophils in ischemia-reperfusion induced microvascular injury. Am J Physiol. 1987;253:H699–H703. doi: 10.1152/ajpheart.1987.253.3.H699. [DOI] [PubMed] [Google Scholar]
- 69.Miller TA, Li D, Kuo YJ, Schmidt KL, Shanbour LL. Nonprotein sulfhydryl compounds in canine gastric mucosa: effects of PGE2 and ethanol. Am J Physiol. 1985;249:G137–G144. doi: 10.1152/ajpgi.1985.249.1.G137. [DOI] [PubMed] [Google Scholar]
- 70.La Casa C, Villegas I, Alarcón de la Lastra C, Motilva V, Martín Calero MJ. Evidence for protective and antioxidant properties of rutin, a natural flavone, against ethanol induced gastric lesions. J Ethnopharmacol. 2000;71:45–53. doi: 10.1016/s0378-8741(99)00174-9. [DOI] [PubMed] [Google Scholar]
- 71.Hiraishi H, Terano A, Ota S, Mutoh H, Sugimoto T, Harada T, Razandi M, Ivey KJ. Protection of cultured rat gastric cells against oxidant-induced damage by exogenous glutathione. Gastroenterology. 1994;106:1199–1207. doi: 10.1016/0016-5085(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 72.Sener-Muratoğlu G, Paskaloğlu K, Arbak S, Hürdağ C, Ayanoğlu-Dülger G. Protective effect of famotidine, omeprazole, and melatonin against acetylsalicylic acid-induced gastric damage in rats. Dig Dis Sci. 2001;46:318–330. doi: 10.1023/a:1005652815921. [DOI] [PubMed] [Google Scholar]
- 73.Hernández-Muñoz R, Montiel-Ruíz C, Vázquez-Martínez O. Gastric mucosal cell proliferation in ethanol-induced chronic mucosal injury is related to oxidative stress and lipid peroxidation in rats. Lab Invest. 2000;80:1161–1169. doi: 10.1038/labinvest.3780124. [DOI] [PubMed] [Google Scholar]
- 74.Lu N, Chen W, Peng YY. Effects of glutathione, Trolox and desferrioxamine on hemoglobin-induced protein oxidative damage: anti-oxidant or pro-oxidant? Eur J Pharmacol. 2011;659:95–101. doi: 10.1016/j.ejphar.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 75.Alayash AI. Oxidative mechanisms of hemoglobin-based blood substitutes. Artif Cells Blood Substit Immobil Biotechnol. 2001;29:415–425. doi: 10.1081/bio-100108547. [DOI] [PubMed] [Google Scholar]
- 76.Kobert R. Beitrage zur Kenntniss der Methamoglobine. Pflugers Arch. Gesamte Physiol. Menschen Tiere. 1900;82:603–630. [Google Scholar]
- 77.Wu H. Studies on hemoglobin. III. An ultra-micro-method for the determination of hemoglobin as a peroxidase. J Biochem. 1923;2:189–194. [Google Scholar]
- 78.Allentoff AJ, Bolton JL, Wils A, Thompson JA, Ortiz de Montellano PR. Heterolytic versus hemolytic peroxide bond cleavage by sperm whale myoglobin and myoglobin mutants. J Am Chem Soc. 1992;114:9744–9749. [Google Scholar]
- 79.Gutteridge JM. Iron promoters of the Fenton reaction and lipid peroxidation can be released from haemoglobin by peroxides. FEBS Lett. 1986;201:291–295. doi: 10.1016/0014-5793(86)80626-3. [DOI] [PubMed] [Google Scholar]
- 80.Giulivi C, Cadenas E. Heme protein radicals: formation, fate, and biological consequences. Free Radic Biol Med. 1998;24:269–279. doi: 10.1016/s0891-5849(97)00226-8. [DOI] [PubMed] [Google Scholar]
- 81.Giulivi C, Davies KJ. A novel antioxidant role for hemoglobin. The comproportionation of ferrylhemoglobin with oxyhemoglobin. J Biol Chem. 1990;265:19453–19460. [PubMed] [Google Scholar]
- 82.D’Agnillo F, Alayash AI. Interactions of hemoglobin with hydrogen peroxide alters thiol levels and course of endothelial cell death. Am J Physiol Heart Circ Physiol. 2000;279:H1880–H1889. doi: 10.1152/ajpheart.2000.279.4.H1880. [DOI] [PubMed] [Google Scholar]
- 83.Kapturczak MH, Wasserfall C, Brusko T, Campbell-Thompson M, Ellis TM, Atkinson MA, Agarwal A. Heme oxygenase-1 modulates early inflammatory responses: evidence from the heme oxygenase-1-deficient mouse. Am J Pathol. 2004;165:1045–1053. doi: 10.1016/S0002-9440(10)63365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nath KA, Haggard JJ, Croatt AJ, Grande JP, Poss KD, Alam J. The indispensability of heme oxygenase-1 in protecting against acute heme protein-induced toxicity in vivo. Am J Pathol. 2000;156:1527–1535. doi: 10.1016/S0002-9440(10)65024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ueda K, Ueyama T, Yoshida K, Kimura H, Ito T, Shimizu Y, Oka M, Tsuruo Y, Ichinose M. Adaptive HNE-Nrf2-HO-1 pathway against oxidative stress is associated with acute gastric mucosal lesions. Am J Physiol Gastrointest Liver Physiol. 2008;295:G460–G469. doi: 10.1152/ajpgi.00204.2007. [DOI] [PubMed] [Google Scholar]


