Abstract
AIM: To investigate the effects of proteins purified from sweet potato storage roots on human colorectal cancer cell lines.
METHODS: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, Hoechst 33258 nuclear staining and Boyden transwell chamber methods were used to determine whether purified sweet potato protein (SPP) from fresh sweet potato roots affected proliferation, migration and invasion, respectively, of human colorectal cancer SW480 cells in vitro. The inhibitory effects of SPP on growth of human colorectal cancer HCT-8 cells intraperitoneally xenografted in nude mice and spontaneous lung metastasis of murine Lewis lung carcinoma 3LL cells subcutaneously transplanted in C57 BL/6 mice were also investigated in vivo.
RESULTS: SPP inhibited the proliferation of SW480 cells in a dose-dependent manner, with an IC50 value of 38.732 μmol/L (r2 = 0.980, P = 0.003) in the MTT assay. Hoechst 33258 nuclear staining further revealed inhibition of cell viability and induction of apoptosis by SPP. The transwell assay disclosed significant reduction in migrated cells/field by 8 μmol/L SPP (8.4 ± 2.6 vs 23.3 ± 5.4, P = 0.031) and invaded cells/field through the ECMatrix by 0.8 μmol/L SPP, compared with the control (25.2 ± 5.2 vs 34.8 ± 6.1, P = 0.038). Both intraperitoneal (ip) and intragastric (ig) administration of SPP led to significant suppression of growth of intraperitoneally inoculated HCT-8 cells in nude mice to 58.0% ± 5.9% (P = 0.037) and 43.5% ± 7.1% (P = 0.004) of the controls, respectively, after 9 d treatment. Bloody ascites additionally disappeared after ip injection of trypsin inhibitor. Notably, ig and ip administration of SPP induced a significant decrease in spontaneous pulmonary metastatic nodule formation in C57 BL/6 mice (21.0 ± 12.3 and 27.3 ± 12.7 nodules/lung vs 42.5 ± 4.5 nodules/lung in controls, respectively, P < 0.05) after 25 d treatment. Moreover, the average weight of primary tumor nodules in the hind leg of mice decreased from 8.2 ± 1.3 g/mice in the control to 6.1 ± 1.4 g/mice in the ip group (P = 0.035).
CONCLUSION: SPP exerts significant antiproliferative and antimetastatic effects on human colorectal cancer cell lines, both in vitro and in vivo.
Keywords: Sweet potato protein, Colorectal cancer, Cell proliferation, Cell invasion, Metastasis
Core tip: Sweet potato protein (SPP) is a type of serine protease inhibitor that suppresses the activity of trypsin. The current study showed that SPP significantly inhibited proliferation, migration and invasion of human colorectal cancer SW480 cells in vitro. Moreover, the protein significantly suppressed the growth of intraperitoneally xenografted human colorectal cancer HCT-8 cells and volume of bloody ascites formed in nude mice. Spontaneous lung metastasis of a murine lung carcinoma cell line was significantly inhibited by SPP in mice. Notably, both intragastric infusion and intraperitoneal injection of SPP were effective in animal models.
INTRODUCTION
Colorectal cancer (CRC) is one of the most commonly diagnosed cancer types in both men and women[1]. Annually, about 49380 Americans die of CRC, accounting for 9% of all cancer-related deaths[2]. Surgical resection is currently the primary curative therapy for CRC. However, surgery alone provides a high cure rate only for patients with early-stage disease. In the later stages of CRC, the presence of clinically occult micrometastases often leads to disease recurrence and death. Numerous natural or synthetic compounds have been tested for their ability to suppress degradation of the extracellular matrix (ECM) and subsequent invasion and metastasis of cancer cells, with the aim of preventing cancer metastasis[3,4]. Among these, protease inhibitors have been increasingly shown to have significant antimetastatic effects in various cancers[5-8]. The sweet potato [Ipomoea batatas (L.) Lam] is a dicotyledonous plant that belongs to the Convolvulaceae family. Its tuberous roots contain 0.49%-2.24% crude proteins on a fresh weight basis. Proteins isolated from sweet potato can be separated into sporamin A (31 kDa) and sporamin B (22 kDa) via non-reducing sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), which merge into a single band of about 25 kDa under reducing conditions[9,10]. Previous studies have identified the sweet potato protein (SPP) as a type of Kunitz-type trypsin inhibitor (KTI)[9] with potential therapeutic effects in a variety of cancer models. For instance, Huang et al[11] reported that KTI purified from sweet potato inhibited proliferation and induced apoptosis of NB4 promyelocytic leukemia cells. Additionally, Yao et al[12] showed that SPP inhibited proliferation and induced apoptosis of human tongue carcinoma Tca8113 cells via downregulation of the Akt/glycogen synthase kinase (GSK)-3 pathway. In addition, KTIs isolated from other sources, such as human urine and soybeans, have been shown to exert antiproliferative, anti-invasion and antimetastatic activities in a variety of malignant cells, animal models and cancer patients[13-15]. The present study focused on the inhibitory effects of SPP on proliferation, migration and invasiveness of malignant cells in a number of CRC cell lines in vitro as well as in vivo.
MATERIALS AND METHODS
Cell culture
Human colorectal cancer SW480 cells were maintained in culture medium comprising RPMI 1640 supplemented with 10% fetal bovine serum (FBS), 1 × 105 U/L penicillin and streptomycin. Cells were grown in T-75 culture flasks at 37 °C in a humidified atmosphere containing 5% CO2 and 95% air.
SPP preparation
SPP was purified from fresh sweet potato tuberous roots, as reported previously[16]. Purity of SPP exceeded 99% of the dry weight. A stock solution was freshly prepared before each experiment by dissolving SPP in culture medium, filtered through a sterile 0.22-μm membrane, and further diluted with culture medium to achieve the final concentrations, as indicated.
Protein and TI activity staining of SPP on 12% polyacrylamide gels
Purified SPP was detected using both protein and TI activity staining on 12% SDS-PAGE. For protein staining, samples were mixed with sample buffer (60 mmol/L Tris-HCl, pH 6.8, 2% SDS, 25% glycerol, and 0.1% bromophenol blue) and β-mercaptoethanol. Coomassie brilliant blue R-250 stain was used. For TI activity, native gels were stained according to the method of Huang et al[11]. Briefly, upon completion of non-denaturing PAGE, the gel was immersed and shaken twice in 25% vol/vol isopropanol in 10 mmol/L Tris buffer (pH 7.9) for 10 min each. Next, the gel was dipped into 10 mmol/L hydrogen peroxide in the same buffer for at least 30 min with gentle shaking, and washed in 10 mmol/L Tris buffer (pH 7.9) for 10 min, followed by incubation in trypsin solution (50 μg bovine trypsin/mL, 10 mmol/L Tris buffer pH 7.9) for 20 min at 37 °C. After rinsing with the same buffer to remove excess trypsin, the gel was incubated at 37 °C for at least 30 min in the dark with 160 mL substrate dye solution prepared immediately before use. The substrate dye solution consisted of 40 mg N-acetyl-D,L-phenylalanine β-naphthyl ester in 16 mL of N,N-dimethylformamide made up to 160 mL with 144 mL of 10 mmol/L Tris buffer (pH 7.9) in which 80 mg tetrazotized O-dianisidine was dissolved. The gel was destained with 10% acetic acid for at least 30 min.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay
SW480 cells were seeded into a 96-well plate (Corning, Cambridge, MA, United States) at a density of 1.5 × 104 cells/well. After achieving confluence, cells were incubated in medium containing different concentrations of SPP. Following SPP treatment, 20 μL 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (5 mg/mL) was added to each well and incubated for a further 4 h. Next, 200 μL dimethyl sulfoxide was added to cells in each well, mixed for 10 min, and optical density measured at 492 nm.
Hoechst 33258 staining
Cell viability was assayed morphologically using Hoechst 33258 (Sigma, St Louis, MO, United States) staining. SW480 cells were plated onto a 24-well plate (1.2 × 105 cells/well) and allowed to grow for 24 h. After confluence, cells were serum-starved for 16 h and treated with various concentrations of SPP for 48 h. Cells were fixed in 2% glutaraldehyde for 4 h and washed twice with 0.9% NaCl before staining with 1 μg/mL Hoechst 33258 for 30 min under ice cooling in the dark. After washing twice with 0.9% NaCl, cells were observed under a fluorescence microscope (Olympus IX71) with excitation at ultraviolet (360 nm).
Cell migration and invasion
SW480 cells were seeded into the upper chamber of a Transwell insert (Millipore, Billerica, MA, United States) in RPMI 1640 at a density of 3 × 105 cells/well. Medium containing 10% FBS was placed in the lower chamber to act as a chemoattractant. After adherence, cells were treated with various concentrations of SPP for 10 h in serum-free medium. Cells migrating through 8.0 μm polycarbonate membranes to the lower surface were stained with 0.1% crystal violet, whereas non-migratory cells remaining on the upper chambers were removed by scraping the upper surface of the membrane with a cotton swab. Migrating cells were quantified by counting stained cells under a microscope (× 200). Five random fields were selected for each well. The assay was performed in triplicate, and repeated three times. Invasion assays were performed in a similar manner to the migration assays, except that inserts were precoated with ECM substitute ECMatrix (Chemicon, Temecula, CA, United States) and cells were treated with SPP for 24 h.
In vivo experiments
Two animal experiments were conducted. In the first experiment, 2 × 105 human colorectal cancer HCT-8 cells were inoculated into the peritoneal cavity of 15 BALB/c nude mice (5 wk old, 14 ± 2 g), which were randomly divided into three groups (5 animals per group). From the second day of inoculation, SPP was administered to two of the three groups either intraperitoneally (2 μmol/L per kg/d) or intragastrically (80 μmol/L per kg/d) for 9 d, while the remaining group served as a vehicle control receiving only intraperitoneally injected physiological saline during the experiment. After treatment with SPP for the indicated times, animals were sacrificed, and the number of tumor nodules in the peritoneal cavity was compared between groups. In the second animal experiment, we examined the effects of SPP on metastatic capacity of cancer cells. Lewis lung cancer cells were inoculated subcutaneously into the hind legs of 18 female C57BL/6 mice (5 wk old, 14 ± 2 g) divided into three groups (6 animals per group). From the second day of inoculation, SPP was administered either intraperitoneally (2 μmol/L per kg/d) or intragastrically (120 μmol/L per kg/d) to animals for 25 d. After sacrifice, the number of the metastatic nodules formed in lungs of mice were counted under a stereomicroscope (× 10), and compared between the three treatment groups.
Statistical analysis
Data were expressed as mean ± SD, and analyzed using analysis of variance with DPS7.55 software (Refine Information Tech Company, Hangzhou, China). Two-tailed values of P < 0.05 were considered significant.
RESULTS
SPP purification and TI activity staining
SPP was purified from fresh sweet potato tuberous roots, as reported previously[16]. Purity of SPP exceeded 99% of its dry weight. Protein (lane 1) and TI activity (lane 2) staining of purified SPP with β-mercaptoethanol treatment on 12% SDS-PAGE are depicted in Figure 1. Consistent with previous reports, extracted SPP had an average molecular weight of about 25 kDa. Both the monomeric protein and its 50-kDa dimer exhibited strong trypsin inhibitory activity in vitro.
Figure 1.
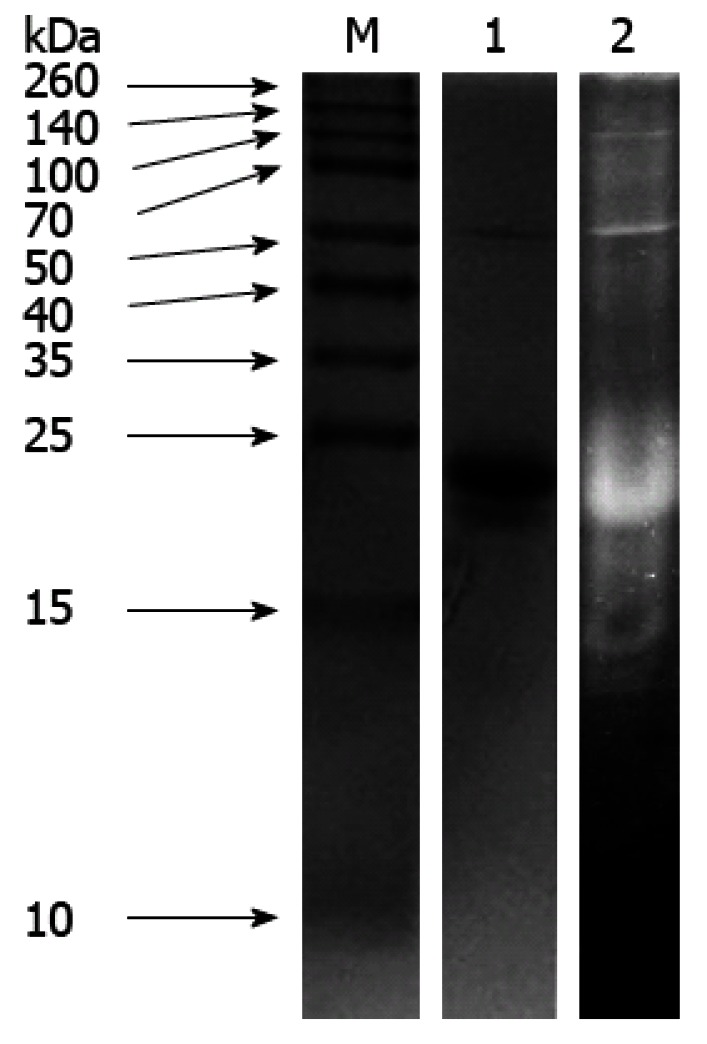
Protein (lane 1) and trypsin inhibitor activity (lane 2) staining of sweet potato protein on polyacrylamide gel electrophoresis gels (12%) with β-mercaptoethanol. A 10 μL aliquot of staining of sweet potato was loaded in each lane. M: Molecular standards.
Effect of SPP on cell proliferation
Dose-dependent inhibition of SW480 proliferation was observed upon incubation of cells with increasing doses of SPP for 48 h. Doses of 2, 4, 10, 20 and 40 μmol/L SPP reduced cell proliferation by 12% (P = 0.013), 20% (P = 0.005), 31% (P = 0.001), 34% (P = 0.001) and 49% (P = 0.001), respectively (Figure 2A). Proliferation was inhibited in a time-dependent manner upon treatment of cells with 40 μmol/L SPP (Figure 2B). After 48 h treatment with various concentrations of SPP, fluorescent Hoechst 33258 nuclear staining indicated dose-dependent suppression of cell proliferation by SPP. Specifically, Hoechst 33258 staining revealed blue, round nuclei in viable cells and condensed or fragmented nuclei in apoptotic, compared to non-apoptotic cells. Apoptotic cells were detected when the SPP concentration exceeded 10 μmol/L (Figure 2C).
Figure 2.
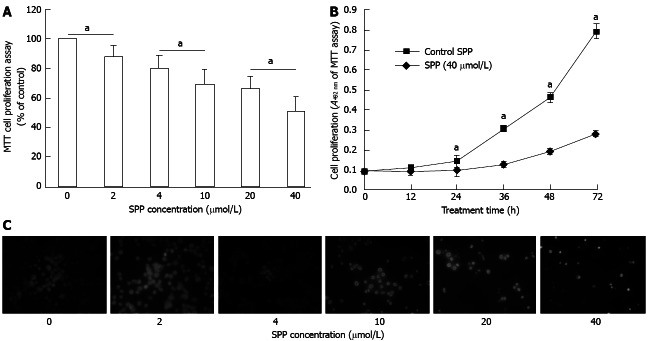
Anti-proliferative effect of sweet potato protein on human colorectal cancer SW480 cells. A: Effects of various concentrations of sweet potato protein (SPP) on SW480 cell proliferation. We observed dose-dependent inhibition by SPP. aP < 0.05 between groups; B: Effect of various treatment times with 40 μmol/L SPP on SW480 cell proliferation. Time-dependent inhibition was observed. aP < 0.05 vs control; C: Hoechst 33258 nuclear staining. SW480 cells were incubated in the absence or presence of various concentrations of SPP for 48 h, stained with Hoechst 33258 dye, and observed under a fluorescent microscope (magnification, × 400). Images are representative of at least two independent experiments, with similar results.
Effect of SPP on cell migration
Migration of SW480 cells through the 8-μm polycarbonate membrane of Transwell inserts was significantly decreased following treatment with SPP for 10 h. Doses of 0.8, 8 and 40 μmol/L SPP induced reduction of migrated cells/field from 23.3 ± 5.4 in the control group to 21.5 ± 3.9 (P = 0.068), 8.4 ± 2.6 (P = 0.031) and 6.1 ± 2.1 (P = 0.017), respectively (Figure 3A).
Figure 3.
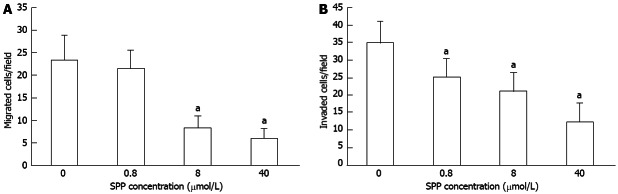
Effects of various concentrations of sweet potato protein on migration (A) and invasion (B) of SW480 cells in the Transwell assay. Inhibition of cell migration was observed with 0.8, 8 and 40 μmol/L sweet potato protein (SPP), aP < 0.05 vs control.
Effect of SPP on cell invasion
Invasiveness of SW480 cells through the artificial basement membrane ECMatrix of Transwell inserts was significantly decreased upon incubation with SPP for 24 h. Doses of 0.8, 8 and 40 μmol/L SPP reduced the invaded cells/field from 34.8 ± 6.1 in the control group to 25.2 ± 5.2 (P = 0.038), 12.5 ± 4.9 (P = 0.024) and 6.1 ± 2.1 (P = 0.005), respectively (Figure 3B).
Effects of SPP on malignant cell growth and metastasis in vivo
The growth of intraperitoneally inoculated human colorectal cancer HCT-8 cells was significantly suppressed by intraperitoneal (ip) or intragastric (ig) administration of SPP into nude mice (Figure 4). After 9 d inoculation, a significant number of tumor nodules formed in the vehicle group, which was markedly reduced to 58.0% ± 5.9% (P = 0.037) and 43.5% ± 7.1% (P = 0.004), respectively, compared to the vehicle, with ig infusion and ip injection of SPP (Figure 4B). Upon sacrifice, bloody ascites were found in the vehicle group, which were collected with a microsyringe, and the volumes compared between groups. The volume of ascities was significantly decreased following administration of SPP (Figure 4C). In the ig group, the volume decreased to 41.2 ± 10% of that of vehicle (P = 0.002), and ascites was barely detectable in the ip group.
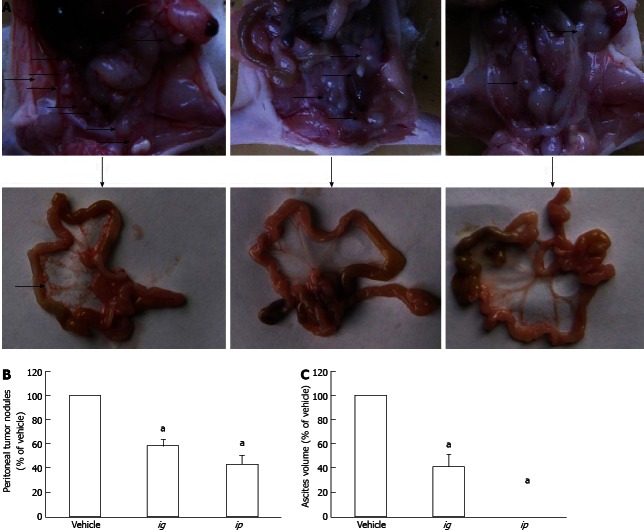
Figure 4.
Effect of sweet potato protein on growth of intraperitoneally inoculated human colorectal cancer HCT-8 cells in nude mice. A: Representative images of growth status of tumor nodules in the peritoneal cavity of nude mice after treatment with intraperitoneally injected or intragastically infused sweet potato protein (SPP); B: Comparison of the number of tumor nodules formed in the peritoneal cavity of nude mice between different treatment groups; C: Volume of bloody ascites generated in the peritoneal cavity of nude mice. Arrows indicate the nodules formed. aP < 0.05 vs vehicle.
To investigate the inhibitory effect of SPP on the metastatic capacity of malignant cells, murine Lewis lung carcinoma 3LL cells were inoculated subcutaneously into the hind legs of C57BL/6 mice, and primary tumor growth and formation of spontaneous lung metastatic colonies examined after 25 d of SPP treatment. As shown in Figure 5, after 25 d, apparent spontaneous pulmonary metastasis was evident in the vehicle-treated group (42.5 ± 4.5 nodules/lung), which was significantly inhibited following ig and ip administration of SPP into mice (21.0 ± 12.3 nodules/lung and 27.3 ± 12.7 nodules/lung, respectively, P < 0.05). The antimetastatic effect was more significant in the ig than ip group (Figure 5B). A possible explanation for this phenomenon is that orally administered SPP is absorbed into the blood circulation and functions in the lung. However, this hypothesis requires further validation. The weights of primary tumor nodules in the hind leg of mice were additionally examined. The weight was 8.2 ± 1.3 g/mouse in the control group, which decreased to 6.1 ± 1.4 g in the ip group (P = 0.035; Figure 5C). The observed reduction in the ig group (7.1 ± 1.5 g/mouse, P > 0.05) was not statistically significant, suggesting that ip administration had a more significant effect than ig administration.
Figure 5.
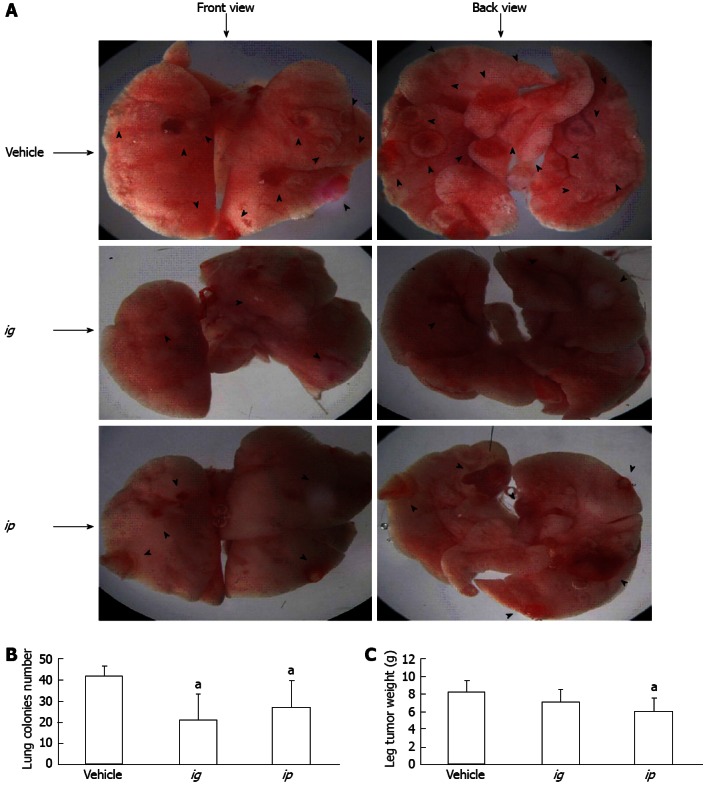
Effect of sweet potato protein on spontaneous metastasis of murine Lewis lung cancer 3LL cells in C57BL/6 mice. A: Representative images of lungs of mice after inoculation of cancer cells for 25 d; B: Number of spontaneous lung metastatic colonies formed after 25 d of inoculation; C: Weight of subcutaneously inoculated tumor nodules after 25 d. Arrowheads indicate the nodules formed. aP < 0.05 vs vehicle.
In both animal experiments, changes in the body weight of mice were monitored. After tumor cell transplantation, body weight stopped increasing. However, SPP treatment did not significantly affect the growth of mice during the experimental period, compared with the vehicle group (Table 1). Moreover, no apparent adverse effects of SPP were observed.
Table 1.
Body weights of mice before and after sweet potato protein treatment
|
BALB/c nude (g) |
C57BL/6 (g) |
|||
| Before | After | Before | After | |
| Vehicle | 14.3 ± 0.9 | 14.6 ± 1.0 | 13.9 ± 0.6 | 14.1 ± 0.7 |
| ig | 14.6 ± 0.6 | 14.8 ± 0.6 | 13.4 ± 0.5 | 13.9 ± 0.7 |
| ip | 14.5 ± 0.5 | 14.7 ± 0.6 | 13.8 ± 0.5 | 13.9 ± 0.6 |
DISCUSSION
The present study showed that SPP promotes dose- and time-dependent inhibition of human colorectal cancer SW480 cell proliferation, migration and invasion. The antiproliferative and antimetastatic effects of SPP were subsequently confirmed in nude and C57BL/6 mice in vivo.
SPP isolated from sweet potato had a strong trypsin inhibitory effect, as shown in Figure 1, consistent with previous findings[9]. In recent years, several families of proteases, including matrix metalloproteinases and serine and cysteine proteases, have been shown to play important roles in tumor invasion and metastasis, because formation of metastasis requires degradation of ECM[17]. The initial hypothesis that the inhibitors function in suppressing cancer invasion and metastasis was confirmed in subsequent studies. For instance, urokinase-type plasminogen activator (uPA), a serine protease, plays a critical role in cancer cell migration, ECM invasion, and metastasis in a variety of tumors[18-21]. In contrast, several serine protease inhibitors, including urinary KTI bikunin and soybean KTI, have been shown to suppress the expression of uPA and its receptor, uPAR, at both the gene and protein levels[22,23]. Bikunin is reported to suppress invasiveness in a number of malignant cell lines by directly inhibiting tumor-cell-associated serine protease plasmin activity, as well as uPA and uPAR expression[24,25]. Notably, the therapeutic efficacy of orally administered bikunin against human ovarian cancer HRA cells growing in the peritoneum of nude mice and human cancer has been demonstrated[26]. Specifically, bikunin (30 mg/kg per day) induced a 40% decrease in tumor load in mice. In cancer patients, bikunin exhibited biological activity, as the post-treatment uPA levels in uterine cervical carcinoma tissue specimens were significantly decreased[26]. Analogous to SPP in the present study, soybean KTI is a natural trypsin inhibitor that has a similar effect as bikunin on the uPA signaling cascade and cancer cell invasion[27]. Dietary supplementation with soybean KTI (15 and 50 g/kg) also significantly reduced the tumor burden in an in vivo spontaneous metastasis assay in C57BL/6 mice[28]. KTIs isolated from sweet potato clearly have an anticancer effect. For example, Huang et al[11] observed growth inhibition and apoptotic effects of TI from sweet potato in NB4 promyelocytic leukemia cells. More recently, Yao et al[12] reported a significant antiproliferative effect of SPP in human tongue carcinoma Tca8113 cells. The group used the same method to isolate SPP from sweet potato roots as that used in the present study. The anticancer effects of SPP reported by our group are therefore consistent with earlier findings.
Several mechanisms have been proposed to explain the mechanism of action of SPP. First, as a serine protease inhibitor, SPP may directly suppress degradation of the ECM by tumor cells, similar to bikunin and soybean KTI. Second, it may exert its role indirectly by suppressing signaling pathways involved in the proliferation and invasion of cancer cells[12,15,23]. Yao et al[12] have suggested that the effect of SPP results partly from induction of apoptosis of tongue cancer cells via downregulation of the Akt/GSK-3 pathway. Huang et al[11] have demonstrated that sweet potato TI induces apoptosis in NB4 cells through a mitochondria-dependent pathway, associated with activation of the caspase-3 and -8 cascades. Moreover, an anti-inflammatory effect of KTIs has been reported, including suppression of proinflammatory cytokines, such as tumor necrosis factor-α, interleukin (IL)-1β and IL-6[29]. This may additionally, in part, explain their effects on tumor development. Bikunin therapy leads to significant inhibition of angiogenesis-related molecules (vascular endothelial growth factor and fibroblast growth factor-β), and this antiangiogenic effect also partly explains the effect of KTIs in cancer cells[30]. Shakiba et al[27] have reported that soybean KTI has a strong inhibitory effect on human umbilical vein endothelial cell migration and tubulogenesis in fibrin matrix. These findings collectively indicate that KTIs present multiple mechanisms of action that are cell type dependent. The results from the current study clearly show that SPP suppresses growth of cancer cells, both in vitro and in vivo. Moreover, the invasiveness of cancer cells is inhibited by SPP. However, the specific mechanisms of action involved in the anticancer activity of SPP remain to be established.
Another particularly interesting finding of the present study was that although ip injection of SPP had a more significant suppressive effect on primary tumors (Figure 5C), the antimetastatic effect against spontaneous lung metastasis of cancer cells was better in the ig than ip group (Figure 5B). One possible explanation for this phenomenon is that orally administered SPP is absorbed into the blood circulation and exerts its activity in the lung. This theory is supported by the finding that dietary supplementation of soybean and urinary KTIs is effective[26,28]. It is also possible that the SPP is digested in the gastrointestinal tract and its active peptides absorbed into the circulation. However, this hypothesis needs validation.
In summary, our results demonstrate that the SPP markedly inhibits proliferation, migration and invasion of human colorectal cancer SW480 cells in vitro. Moreover, SPP administered both orally and intraperitoneally exerts its effect on cancer cells in vivo. The antiproliferative activity of SPP may be triggered through induction of apoptosis of malignant cells, and anti-invasive activity through inhibition of the uPA signaling pathways. Antiangiogenic and anti-inflammatory activities may, at least in part, explain its effects on malignant cells in tumor-bearing animals. However, further studies are required to elucidate the mechanisms underlying the protective effects of SPP in CRC.
COMMENTS
Background
Colorectal cancer (CRC) is one of the major contributors to cancer-induced mortality worldwide. Sweet potato protein (SPP), a type of trypsin inhibitor (TI) that induces apoptosis and suppresses growth of various malignant cells, is a potentially effective anticancer agent for CRC.
Research frontiers
Previous studies suggest that SPP inhibits growth and induces apoptosis in promyelocytic leukemia and human tongue carcinoma cells. A similar TI extracted from wampee (Clausena lansium) seeds has a growth inhibitory effect in human leukemia and hepatoma cells. However, the issue of whether SPP affects the growth and metastasis of CRC cells has remained unclear until now. In the current study, the antiproliferative and antimetastatic effects of SPP on human colorectal cancer cells were investigated in vitro and in vivo.
Innovations and breakthroughs
Several recent studies have demonstrated that SPP affects the growth of malignant cancer cells, although the issue of whether SPP plays a role in CRC is yet to be established. The current study is believed to be the first thorough investigation focusing on the antiproliferative and antimetastatic effects of SPP on CRC cell lines, both in vitro and in vivo.
Applications
Elucidation of the effects of SPP on CRC cell lines in vitro and in vivo may present an effective strategy for prevention or treatment of CRC in the clinic.
Terminology
TIs are a group of peptides present in various sources, such as soybean, egg white and sweet potato, which mask or inhibit the active site of the trypsin molecule. Storage proteins are biological reserves of metal ions and amino acids used by organisms. Amino acids of storage proteins are used during the embryonic development of animals or plants.
Peer review
This was a good experimental study in which authors investigated the effects of SPP on malignant cancer cells in different cell lines and animal models. The results are interesting, and support the utility of SPP as a potential therapeutic agent for preventing and treating CRC.
Footnotes
Supported by The Earmarked Fund for China Agriculture Research System, No. CARS-11-B-19; “Technique of Processing and Utilization for Plant Proteins” from China-Argentina Science and Technology Cooperation Program, No. 2010DFA32690; Grant from the Capital Medical University, No. 2009ZR03 and No. 2012ZR17; the Importation and Development of High-Caliber Talents Project of Beijing Municipal Institutions
P- Reviewer Zhang J S- Editor Zhai HH L- Editor Kerr C E- Editor Li JY
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 3.Half E, Arber N. Colon cancer: preventive agents and the present status of chemoprevention. Expert Opin Pharmacother. 2009;10:211–219. doi: 10.1517/14656560802560153. [DOI] [PubMed] [Google Scholar]
- 4.Lee M, Fridman R, Mobashery S. Extracellular proteases as targets for treatment of cancer metastases. Chem Soc Rev. 2004;33:401–409. doi: 10.1039/b209224g. [DOI] [PubMed] [Google Scholar]
- 5.Joanitti GA, Azevedo RB, Freitas SM. Apoptosis and lysosome membrane permeabilization induction on breast cancer cells by an anticarcinogenic Bowman-Birk protease inhibitor from Vigna unguiculata seeds. Cancer Lett. 2010;293:73–81. doi: 10.1016/j.canlet.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 6.Hsieh CC, Hernández-Ledesma B, Jeong HJ, Park JH, de Lumen BO. Complementary roles in cancer prevention: protease inhibitor makes the cancer preventive peptide lunasin bioavailable. PLoS One. 2010;5:e8890. doi: 10.1371/journal.pone.0008890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brandi G, Tavolari S, De Rosa F, Di Girolamo S, Agostini V, Barbera MA, Frega G, Biasco G. Antitumoral efficacy of the protease inhibitor gabexate mesilate in colon cancer cells harbouring KRAS, BRAF and PIK3CA mutations. PLoS One. 2012;7:e41347. doi: 10.1371/journal.pone.0041347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gocho T, Uwagawa T, Furukawa K, Haruki K, Fujiwara Y, Iwase R, Misawa T, Ohashi T, Yanaga K. Combination chemotherapy of serine protease inhibitor nafamostat mesilate with oxaliplatin targeting NF-κB activation for pancreatic cancer. Cancer Lett. 2013;333:89–95. doi: 10.1016/j.canlet.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 9.Maeshima M, Sasaki T, Asahi T. Characterization of major proteins in sweet potato tuberous roots. Phytochemistry. 1985;166:515–523. [Google Scholar]
- 10.Li PG, Mu TH. Recovery of sporamin from naturally fermented sweet potato starch slurry by foam fractionation. Int J Food Sci Tech. 2012;47:1889–1895. [Google Scholar]
- 11.Huang GJ, Sheu MJ, Chen HJ, Chang YS, Lin YH. Growth inhibition and induction of apoptosis in NB4 promyelocytic leukemia cells by trypsin inhibitor from sweet potato storage roots. J Agric Food Chem. 2007;55:2548–2553. doi: 10.1021/jf063008m. [DOI] [PubMed] [Google Scholar]
- 12.Yao J, Qian C. Sporamin induce apoptosis in human tongue carcinoma cells by down-regulating Akt/GSK-3 signaling. Fundam Clin Pharmacol. 2011;25:229–236. doi: 10.1111/j.1472-8206.2010.00830.x. [DOI] [PubMed] [Google Scholar]
- 13.Gao F, Sun Z, Sun X, Zhang Y, Wang H, Zhong B, Luo J, Zhao X. Ulinastatin exerts synergistic effects with taxotere and inhibits invasion and metastasis of breast cancer by blocking angiogenesis and the epithelial-mesenchymal transition. Cancer Biother Radiopharm. 2013;28:218–225. doi: 10.1089/cbr.2011.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamauchi Y, Izumi Y, Inoue M, Sugiura H, Goto T, Anraku M, Ohtsuka T, Kohno M, Soejima K, Nomori H. Safety of postoperative administration of human urinary trypsin inhibitor in lung cancer patients with idiopathic pulmonary fibrosis. PLoS One. 2011;6:e29053. doi: 10.1371/journal.pone.0029053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kobayashi H, Suzuki M, Kanayama N, Terao T. A soybean Kunitz trypsin inhibitor suppresses ovarian cancer cell invasion by blocking urokinase upregulation. Clin Exp Metastasis. 2004;21:159–166. doi: 10.1023/b:clin.0000024751.73174.c2. [DOI] [PubMed] [Google Scholar]
- 16.Xiong ZD, Li PG, Mu TH. The Differentiation- and Proliferation-Inhibitory Effects of Sporamin from Sweet Potato in 3T3-L1 Preadipocytes. Zhongguo Nongye Kexve. 2009;8:671–677. [Google Scholar]
- 17.Rowe RG, Weiss SJ. Navigating ECM barriers at the invasive front: the cancer cell-stroma interface. Annu Rev Cell Dev Biol. 2009;25:567–595. doi: 10.1146/annurev.cellbio.24.110707.175315. [DOI] [PubMed] [Google Scholar]
- 18.Zhang W, Ling D, Tan J, Zhang J, Li L. Expression of urokinase plasminogen activator and plasminogen activator inhibitor type-1 in ovarian cancer and its clinical significance. Oncol Rep. 2013;29:637–645. doi: 10.3892/or.2012.2148. [DOI] [PubMed] [Google Scholar]
- 19.Zheng W, Zhang Y, Ma D, Shi Y, Liu C, Wang P. (±)Equol inhibits invasion in prostate cancer DU145 cells possibly via down-regulation of matrix metalloproteinase-9, matrix metalloproteinase-2 and urokinase-type plasminogen activator by antioxidant activity. J Clin Biochem Nutr. 2012;51:61–67. doi: 10.3164/jcbn.11-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zong H, Wang F, Fan QX, Wang LX. Curcumin inhibits metastatic progression of breast cancer cell through suppression of urokinase-type plasminogen activator by NF-kappa B signaling pathways. Mol Biol Rep. 2012;39:4803–4808. doi: 10.1007/s11033-011-1273-5. [DOI] [PubMed] [Google Scholar]
- 21.Huang WS, Chin CC, Chen CN, Kuo YH, Chen TC, Yu HR, Tung SY, Shen CH, Hsieh YY, Guo SE, et al. Stromal cell-derived factor-1/CXC receptor 4 and β1 integrin interaction regulates urokinase-type plasminogen activator expression in human colorectal cancer cells. J Cell Physiol. 2012;227:1114–1122. doi: 10.1002/jcp.22831. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi H, Suzuki M, Kanayama N, Terao T. Genetic down-regulation of phosphoinositide 3-kinase by bikunin correlates with suppression of invasion and metastasis in human ovarian cancer HRA cells. J Biol Chem. 2004;279:6371–6379. doi: 10.1074/jbc.M305749200. [DOI] [PubMed] [Google Scholar]
- 23.Inagaki K, Kobayashi H, Yoshida R, Kanada Y, Fukuda Y, Yagyu T, Kondo T, Kurita N, Kitanaka T, Yamada Y, et al. Suppression of urokinase expression and invasion by a soybean Kunitz trypsin inhibitor are mediated through inhibition of Src-dependent signaling pathways. J Biol Chem. 2005;280:31428–31437. doi: 10.1074/jbc.M501406200. [DOI] [PubMed] [Google Scholar]
- 24.Gorantla B, Asuthkar S, Rao JS, Patel J, Gondi CS. Suppression of the uPAR-uPA system retards angiogenesis, invasion, and in vivo tumor development in pancreatic cancer cells. Mol Cancer Res. 2011;9:377–389. doi: 10.1158/1541-7786.MCR-10-0452. [DOI] [PubMed] [Google Scholar]
- 25.Kapoor S. Bikunin and its emerging role in the modulation of tumor invasion and metastasis. J Surg Res. 2012:Nov 2; Epub ahead of print. doi: 10.1016/j.jss.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 26.Kobayashi H, Yagyu T, Inagaki K, Kondo T, Suzuki M, Kanayama N, Terao T. Therapeutic efficacy of once-daily oral administration of a Kunitz-type protease inhibitor, bikunin, in a mouse model and in human cancer. Cancer. 2004;100:869–877. doi: 10.1002/cncr.20034. [DOI] [PubMed] [Google Scholar]
- 27.Shakiba Y, Mansouri K, Mostafaie A. Anti-angiogenic effect of soybean kunitz trypsin inhibitor on human umbilical vein endothelial cells. Fitoterapia. 2007;78:587–589. doi: 10.1016/j.fitote.2007.03.027. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi H, Yoshida R, Kanada Y, Fukuda Y, Yagyu T, Inagaki K, Kondo T, Kurita N, Suzuki M, Kanayama N, et al. Dietary supplementation of soybean kunitz trypsin inhibitor reduces lipopolysaccharide-induced lethality in mouse model. Shock. 2005;23:441–447. doi: 10.1097/01.shk.0000160940.16008.a5. [DOI] [PubMed] [Google Scholar]
- 29.Kanayama S, Yamada Y, Onogi A, Shigetomi H, Ueda S, Tsuji Y, Haruta S, Kawaguchi R, Yoshida S, Sakata M, et al. Molecular structure and function analysis of bikunin on down-regulation of tumor necrosis factor-alpha expression in activated neutrophils. Cytokine. 2008;42:191–197. doi: 10.1016/j.cyto.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 30.Kobayashi H, Yagyu T, Inagaki K, Kondo T, Suzuki M, Kanayama N, Terao T. Bikunin plus paclitaxel markedly reduces tumor burden and ascites in mouse model of ovarian cancer. Int J Cancer. 2004;110:134–139. doi: 10.1002/ijc.20082. [DOI] [PubMed] [Google Scholar]