Abstract
AIM: To investigate HER2 expression and its correlation with clinicopathological variables between proximal and distal gastric cancers (GC) in the Chinese population.
METHODS: Immunostaining of HER2 was performed and scored on a scale of 0-3 in 957 consecutive GC cases, according to the revised scoring criteria of HercepTestTM as used in the ToGA trial. Correlations between HER2 expression and clinicopathologic variables of proximal (n = 513) and distal (n = 444) GC were investigated.
RESULTS: Our results showed that HER2 expression was significantly higher in the proximal than in distal GC (P < 0.05). Overall, HER2 expression was significantly higher in male patients (P < 0.01), the Lauren intestinal type (P < 0.001), low-grade (P < 0.001) and pM1 (P < 0.01) diseases, respectively. There was a significant difference in HER2 expression among some pTNM stages (P < 0.05). In contrast, HER2 expression in the distal GC was significantly higher in male patients (P < 0.001), low-grade histology (P < 0.001), the Lauren intestinal type(P < 0.001), and pM1 (P < 0.001). In the proximal GC, however, higher HER2 expression scores were observed only in tumors with low-grade histology (P < 0.001) and the Lauren intestinal type (P < 0.001).
CONCLUSION: HER2 over-expression in GC of Chinese patients was significantly more common in proximal than in distal GC, and significantly correlated with the Lauren intestinal type and low-grade histology in both proximal and distal GC, and with pM1 disease and male gender in distal GC.
Keywords: HER2, Gastric cancer, Immunohistochemistry, Clinicopathology
Core tip: In this study, immunostaining of HER2 was performed and scored according to the revised scoring criteria of HercepTestTM used in the ToGA trial in a very large cohort of gastric cancers (GC) patients (957 cases). Our results revealed that HER2 over-expression in GC of Chinese patients was significantly more common in proximal than in distal GC, and was significantly correlated with the Lauren intestinal type and low-grade histology in both proximal and distal GC, and with pM1 disease and male gender in distal GC.
INTRODUCTION
HER2 gene amplification and over-expression, which possibly represents a negative prognostical factor[1,2] but a potential therapeutic target[3-7], has been found in 6%-53.4% of gastric cancer (GC) in patients from Western countries[3,8]. As such, it is now recommended that all patients with GC should have their tumors tested for the HER2 status at the time of initial diagnosis[9]. At present, the tests for HER2 over-expression by immunohistochemstry (IHC) and HER2 gene amplification by fluorescence in situ hybridization (FISH) are the most common methods. As demonstrated in the phase III ToGA trial, patients with IHC 2+/FISH-positive or IHC 3+ tumors benefited from trastuzumab treatment, but cases with HER2 gene-amplified tumors, revealed by the FISH test, without HER2 over-expression (IHC 0 or 1+) did not show any survival gain[5]. The characteristics of HER2 expression in GC are different from those in breast cancer[5,10]. Therefore, in the phase III ToGA trial, the HER2 expression scoring system for breast cancer, which was proposed by the American Society of Clinical Oncology (ASCO)/College of American Pathologists (CAP), was modified for evaluation of HER2 expression in GC[11], requiring IHC for HER2 testing before use of the FISH test[9,12,13], because IHC seems to be more predictive of trastuzumab therapeutic responses than the FISH test alone in GC, compared with breast cancers[14].
In China, GC remains one of the leading cancers. Despite recent advances in treatment, the outcome of patients with advanced GCs is poor. Because there exist considerable differences in GC histopathology, environmental factors, and the Helicobacter pylori status between Western and Chinese patients[15], the need for a comprehensive investigation of the HER2 expression profile in GC of Chinese patients is urgent for better clinical management. Therefore, we carried out this study to fully investigate differences in HER2 expression and clinicopathologic features between proximal and distal GC with the same assessment criteria of IHC as used in the ToGA trial[11].
MATERIALS AND METHODS
Case selection
We retrospectively searched a prospectively established electronic pathology database for GC resection cases with HER2 immunostaining results over the period from January 2007 to August 2009 at the affiliated Nanjing Drum Tower Hospital, Nanjing University Medical School in Nanjing, China. A total of 957 consecutive cases were identified, including 513 proximal and 444 distal GC, according to the surgical resection methods of GC and gross specimen description. Because our recent research results in the same group of Chinese GC patients suggested that almost all proximal GC with esophageal involvement also included Siewert II-III GC, which are regarded as esophageal origin and stage-grouped as esophageal cancers[16,17], they might be more accurately staged as gastric rather than esophageal cancers[18,19]. Therefore, the proximal GC in this study included both GC with epicenters entirely below the gastroesophageal junction (GEJ) and those invading through the GEJ into the distal esophagus as a minor component. Distal GC was defined as tumors with epicenters in the region from the incisura angularis to the antrum-pylorus. The Lauren classification of GC was followed to subgroup all cases in histopathology. All cases were staged, according to the staging rules for GC set out in the 7th edition of the American Joint Committee on Cancer Staging[20]. The patient demographics and pathologic information were retrieved from each pathology report. The patient private identification information was deleted and the study protocol was approved by the Medical Ethics Committee of the Hospital.
Immunohistochemistry
A conventional immunostaining protocol was used for all cases. Briefly, paraffin-embedded tumor tissue blocks were cut at 4-μm in thickness. Sections were deparaffinized, dehydrated, subjected to the antigen retrieval procedure, and then incubated with the primary rabbit antihuman HER2 polyclonal antibody (clone A0485, dilution: 1:2500, Dako, Denmark) for one hour at 37 °C. The HER2 immunoreactivity was visualized after a brief treatment with the EnVision Plus system kit (Dako). Both positive and negative controls were included in each run.
Three experienced pathologists independently evaluated HER2-stained slides blindly without the knowledge of patient clinicopathologic information. The HER2 immunoreactivity of neoplastic cells was scored according to the revised ToGA scoring criteria of HercepTestTM for GC[5,11], which was based on the intensity of membrane staining and quantity of positive neoplastic cells on a scale of 0-3. In brief, no membranous reactivity in less than 10% of tumor cells was scored as 0; faint/barely visible complete or basolateral membranous reactivity in 10% or more of tumor cells was scored as 1+; weak-to-moderate complete or basolateral membranous reactivity in 10% or more of tumor cells was scored as 2+; strong complete or basolateral membranous reactivity in 10% or more of tumor cells was scored as 3+. A score of 0 or 1+ was considered negative while scores 2+ and 3+ were positive.
Statistical analysis
The absolute and relative frequencies of qualitative variables were calculated in percentages. χ2 tests for categorical variables were used and the differences were considered to be statistically significant if P values were less than 0.05. All analyses were performed using the SPSS version 20.0 software for Windows (SPSS Inc., Chicago, IL, United States).
RESULTS
Clinicopathologic features
The mean patient age was 63 years (range: 17-89 years). As shown in Table 1, most patients were male (75%) with a male-female ratio of 3.0; 29% of patients were older than 70 years. Most tumors were the Lauren intestine type (59%), high-grade histology (66%), and at advanced stages of pIII and pIV (61%). About 54% of GC was located in the proximal stomach (Table 2). None of the patients received neoadjuvant therapy before surgical resections.
Table 1.
HER2 expression in gastric adenocarcinoma
| n |
HER2 score |
χ2 | P |
Univariate analysis |
||||||
| 0 | 1 | 2 | 3 | rs | P | |||||
| Gender | F | 244 | 149 | 58 | 19 | 18 | 10.106 | 0.001 | - | - |
| M | 713 | 366 | 176 | 98 | 73 | |||||
| Age (yr) | ≤ 70 | 682 | 370 | 164 | 82 | 66 | 1.347 | 0.418 | - | - |
| > 70 | 275 | 145 | 70 | 35 | 25 | |||||
| pT | T1 | 51 | 28 | 14 | 6 | 3 | 7.464 | 0.070 | - | - |
| T2 | 145 | 79 | 38 | 19 | 9 | |||||
| T3 | 749 | 404 | 179 | 90 | 76 | |||||
| T4 | 12 | 4 | 3 | 2 | 3 | |||||
| pN | N0 | 243 | 138 | 60 | 27 | 18 | 13.591 | 0.335 | - | - |
| N1 | 140 | 63 | 34 | 23 | 20 | |||||
| N2 | 205 | 104 | 50 | 31 | 20 | |||||
| N3 | 369 | 210 | 90 | 36 | 33 | |||||
| pM | M0 | 935 | 506 | 232 | 114 | 83 | 19.984 | 0.001 | 0.077 | 0.020 |
| M1 | 22 | 9 | 2 | 3 | 8 | 0.105 | 0.002 | |||
| pTNM | I | 110 | 59 | 32 | 16 | 3 | 29.943 | 0.041 | 0.039 | 0.187 |
| II | 267 | 151 | 57 | 30 | 29 | |||||
| III | 558 | 296 | 143 | 68 | 51 | |||||
| IV | 22 | 9 | 2 | 3 | 8 | |||||
| G | Low | 324 | 135 | 82 | 50 | 57 | 51.360 | 0.000 | - | - |
| High | 633 | 380 | 152 | 67 | 34 | |||||
| Lauren | Intestinal | 568 | 256 | 145 | 83 | 84 | 79.548 | 0.000 | - | - |
| Diffuse/mixed | 389 | 253 | 88 | 34 | 7 | |||||
pTNM: Pathological tumor-node-metastasis; M: Male; F: Female.
Table 2.
Comparison of HER2 expression between proximal and distal gastric adenocarcinoma
| Group | n |
HER2 score |
χ2 | P | |||
| 0 | 1 | 2 | 3 | ||||
| Proximal | 513 | 260 | 126 | 72 | 55 | 6.691 | 0.011 |
| Distal | 444 | 255 | 108 | 45 | 36 | ||
Overall HER2 immunoreactivity in GC
In general, HER2 immunoreactivity of neoplastic cells in GC was characterized by basolateral, lateral, and/or circumferential membranous, heterogeneous or diffuse, staining patterns (Figures 1-3). HER2 over-expression with score 3, and scores 2 and 3 were found in 9.5% (91/957) and 21.73% (208/957) of cases, respectively. Overall, HER2 expression with score 3 was significantly higher in male patients (10.24% vs 7.38% in females, P < 0.01), the proximal GC (8.11% vs 10.72% in distal GC, P < 0.05), the Lauren intestine type (14.79% vs 1.8% in the Lauren diffuse/mixed type, P < 0.001), histological low-grade histology (17.6% vs 5.37% in high-grade histology, P < 0.001), and pM1 (36.36% vs 8.88% in pM0, P < 0.01). Although there was a significant difference in HER2 expression between advanced and early GC, especially in stage pIV (pI: 2.7%; pII: 10.86%; pIII: 9.12%; pIV: 38.1%; P < 0.05), the correlation between HER2 and pTNM stage was not statistically significant (P > 0.05) (Table 1).
Figure 1.
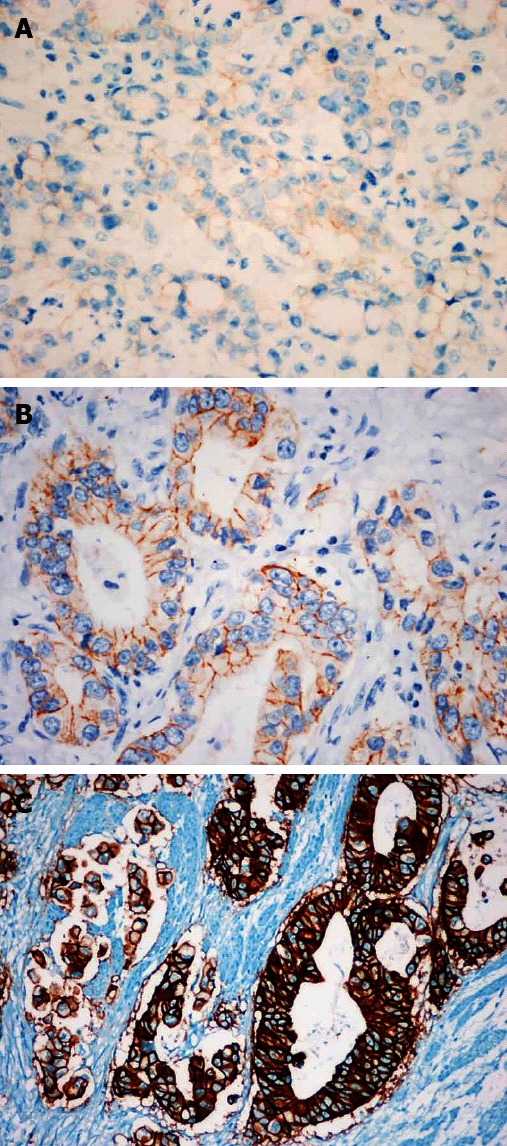
HER2 immunostaining in gastric cancers is scored as 1+, 2+ and 3+ in A, B and C, respectively. EnVision immunohistochemistry stain, × 400.
Figure 3.
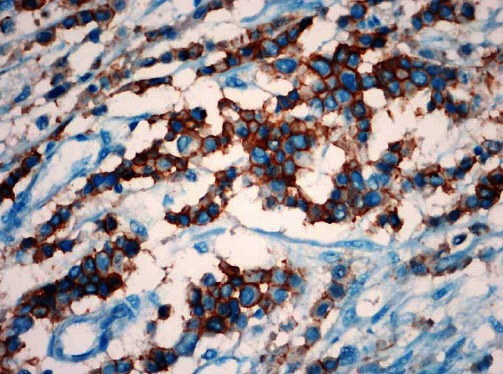
A rare diffuse and strong positive pattern of HER2 expression in the Lauren diffuse type gastric cancers. EnVision immunohistochemistry stain, × 400.
Figure 2.
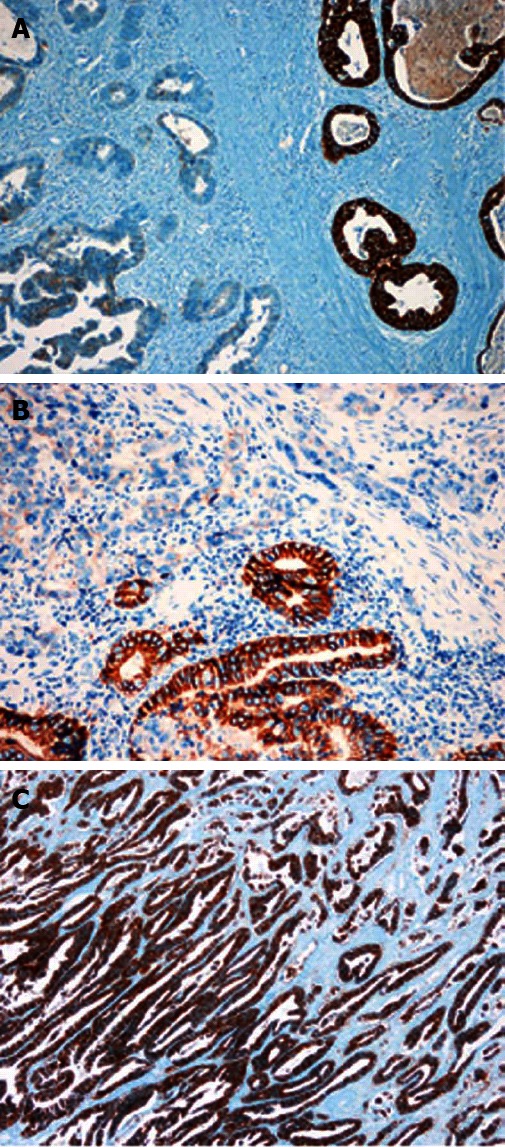
Heterogeneous (A and B, × 200) or diffuse (C, × 100) expression pattern of HER2 protein is discovered in gastric cancers. EnVision immunohistochemistry stain.
Differences in HER2 immunoreactivity between proximal and distal GCs
HER2 expression was higher in proximal GC than in distal GC (10.7% vs 8.1% with score 3; 25% vs 18.2% with scores 2 and 3; P < 0.05) (Table 2). In the proximal GC, higher expression of HER2 with score 3 was found only in tumors with low-grade histology (17.24% vs 6.45%, P < 0.001), the Lauren intestine type (15.88% vs 0.58%, P < 0.001) than those with high-grade histology and the Lauren diffuse/mixed type (Table 3). In the distal GC, however, HER2 over-expression with score 3 was significantly higher in male patients (9.09% vs 5.88%, P < 0.001), distant metastasis (50% vs 6.94%, P < 0.001), histological low-grade (18.18% vs 4.33%, P < 0.001), the Lauren intestine type (13.16% vs 2.87%, P < 0.001) GC than in female patients, pM0, high-grade histology, and the Lauren diffuse/mixed type (Table 4).
Table 3.
HER2 expression in proximal gastric adenocarcinoma
| n |
HER2 score |
χ2 | P |
Univariate analysis |
||||||
| 0 | 1 | 2 | 3 | rs | P | |||||
| Gender | F | 108 | 57 | 28 | 13 | 10 | 0.884 | 0.232 | - | - |
| M | 405 | 203 | 98 | 59 | 45 | |||||
| Age (yr) | ≤ 70 | 371 | 189 | 92 | 48 | 42 | 1.647 | 0.476 | - | - |
| > 70 | 142 | 71 | 34 | 24 | 13 | |||||
| pT | T1 | 10 | 4 | 3 | 1 | 2 | 15.026 | 0.472 | - | - |
| T2 | 61 | 30 | 16 | 11 | 4 | |||||
| T3 | 439 | 226 | 107 | 59 | 47 | |||||
| T4 | 3 | 0 | 0 | 1 | 2 | |||||
| pN | N0 | 132 | 71 | 34 | 15 | 12 | 6.071 | 0.524 | - | - |
| N1 | 70 | 31 | 16 | 13 | 10 | |||||
| N2 | 124 | 60 | 29 | 22 | 13 | |||||
| N3 | 187 | 98 | 47 | 22 | 20 | |||||
| pM | M0 | 503 | 255 | 125 | 70 | 53 | 1.959 | 0.269 | - | - |
| M1 | 10 | 5 | 1 | 2 | 2 | |||||
| pTNM | I | 43 | 20 | 14 | 6 | 3 | 6.790 | 0.133 | - | - |
| II | 129 | 74 | 26 | 16 | 13 | |||||
| III | 331 | 161 | 85 | 48 | 37 | |||||
| IV | 10 | 5 | 1 | 2 | 2 | |||||
| G | Low | 203 | 84 | 52 | 32 | 35 | 19.924 | 0.000 | -0.179 | 0.000 |
| High | 310 | 176 | 74 | 40 | 20 | |||||
| Lauren | Intestinal | 340 | 150 | 85 | 51 | 54 | 39.351 | 0.000 | -0.210 | 0.000 |
| Diffuse/mixed | 173 | 110 | 41 | 21 | 1 | |||||
pTNM: Pathological tumor-node-metastasis; M: Male; F: Female.
Table 4.
HER2 expression in distal gastric adenocarcinoma
| n |
HER2 score |
χ2 | P |
Univariate analysis |
||||||
| 0 | 1 | 2 | 3 | rs | P | |||||
| Gender | F | 136 | 92 | 30 | 6 | 8 | 11.510 | 0.000 | 0.148 | 0.000 |
| M | 308 | 163 | 78 | 39 | 28 | |||||
| Age (yr) | ≤ 70 | 311 | 181 | 72 | 34 | 24 | 2.443 | 0.446 | - | - |
| > 70 | 133 | 74 | 36 | 11 | 12 | |||||
| pT | T1 | 41 | 24 | 11 | 5 | 1 | 3.983 | 0.131 | - | - |
| T2 | 84 | 49 | 22 | 8 | 5 | |||||
| T3 | 310 | 178 | 72 | 31 | 29 | |||||
| T4 | 9 | 4 | 3 | 1 | 1 | |||||
| pN | N0 | 111 | 67 | 26 | 12 | 6 | 9.645 | 0.255 | - | - |
| N1 | 70 | 32 | 18 | 10 | 10 | |||||
| N2 | 81 | 44 | 21 | 9 | 7 | |||||
| N3 | 182 | 112 | 43 | 14 | 13 | |||||
| pM | 0 | 432 | 251 | 107 | 44 | 30 | 29.728 | 0.000 | 0.133 | 0.000 |
| 1 | 12 | 4 | 1 | 1 | 6 | |||||
| pTNM | I | 67 | 39 | 18 | 10 | 0 | 39.702 | 0.164 | - | - |
| II | 138 | 77 | 31 | 14 | 16 | |||||
| III | 227 | 135 | 58 | 20 | 14 | |||||
| IV | 12 | 4 | 1 | 1 | 6 | |||||
| G | Low | 121 | 51 | 30 | 18 | 22 | 31.286 | 0.000 | -0.231 | 0.000 |
| High | 323 | 204 | 78 | 27 | 14 | |||||
| Lauren | Intestinal | 228 | 106 | 60 | 32 | 30 | 40.352 | 0.000 | -0.209 | 0.000 |
| Diffuse/mixed | 209 | 143 | 47 | 13 | 6 | |||||
pTNM: Pathological tumor-node-metastasis; M: Male; F: Female.
DISCUSSION
In this retrospective study using the revised IHC scoring criteria of HercepTestTM for HER2 expression in GC of Chinese patients, we compared HER2 protein expression profiles along with clinicopathologic features between 513 proximal and 444 distal GC in Chinese patients. The data showed that HER2 over-expression was significantly more common in proximal GC than in distal GC, and significantly correlated with the Lauren intestine type and low-grade histology in both proximal and distal GC, and with pM1 disease and male gender in distal GC.
In the recent literature, the frequency of HER2 over-expression in GC, determined by IHC, ranges widely from 6.8% to 26.8%[21]. In this study, HER2 over-expression with score 2 and 3 was found in 21.73% of cases, which was higher than that (15.9%) in another recent study using the same antibody and scoring criteria in Korean patients[8]. The discrepancy appears to result from the differences in tissue preparations because they used tissue microarray for HER2 immunostaining, which has a much lower sensitivity for HER2 immunoreactivity because of the well-known heterogeneous expression of HER2 in GC[14,21-24]. In addition, different primary antibody clones, various immunostaining protocols, and diverse immunoreactivity scoring schemes may also contribute to variations described among recent studies[8,12,25]. This inconsistency indicates an urgent need for standardized HER2 immunostaining in GC for better reporting and comparison of HER2 IHC results among different centers.
HER2 expression is known to differ among various clinicopathologic factors, such as patient gender, age, ethnicity, tumor location, type, and differentiation, etc., as shown in this and previous studies[26-28]. Our data showed high HER2 expression in GC with low-grade histology and advanced pTNM stages. Similar to our results, a recent study in Japanese patients also described higher HER2 expression in male patients, tumors with the Lauren intestine type, and pM1 stage[26]. With a multivariate analysis, Janjigian et al[29] reported a significantly higher frequency of HER2 immunopositivity in GC with liver metastasis and the Lauren intestine histology, but did not find significant differences in HER2 immunopositivity between resections and biopsies, or primaries and metastases. In that study, approximately 20% (78/381) of distant metastatic GC or GEJ cancers in Western patients were HER2-immunopositive (score 3+ or FISH-positive)[29], a figure which is much lower than ours (36.4%, 8/22). However, the number of GC cases with distant metastasis was limited in this study and our results should be verified in studies with more qualified cases. Nonetheless, our data suggested that HER2 over-expression was more often seen in GC with the Lauren intestine type, low-grade histology, advanced pTNM stage, and in male patients.
In this study, we found a significantly higher frequency of HER2 over-expression in proximal GC than in distal GC. The result is similar to those reported recently in most other studies[30]. It must be pointed out that the vast majority of GEJ cancers in Chinese patients are not Barrett’s esophagus-related and originate primarily in the proximal stomach, invading into the distal esophagus with clinicopathologic features of GC, as we reported previously[31]. Despite similar HER2 over-expression characteristics between Western GEJ cancers and Chinese proximal GC, there exist a number of differences in HER2 over-expression features between proximal and distal GC of Chinese patients in the present study. For example, in distal rather than proximal GC, HER2 over-expression in GC was more common in male patients and in tumors staged at pM1 than in female patients and in pM0 cases, respectively.
A major limitation in this observational study is the absence of the confirmatory FISH test for HER2 gene amplification. However, unlike in breast cancer, HER2 expression with an IHC score of 0 or 1+ but with FISH positivity in GC tumors does not play a statistically significant role with regard to trastuzumab therapy[5]. GC with IHC 3+ HER2 status responds well to this treatment. Thus, the ToGA trial recommended testing HER2 gene amplification by the FISH method only in GC cases with an IHC score of 2+[5]. Therefore, a conformation FISH test might not be performed in GC with IHC 0, 1+ or 3+. However, our cohort is large with 957 GC resection cases and differences in HER2 expression are dramatically significant in many important clinicopathologic parameters. Therefore, the validity of our results should be reasonably sound.
In summary, our data showed a significantly higher frequency of HER2 over-expression in proximal GC than in distal GC. HER2 over-expression was significantly correlated with low-grade histology and the Lauren intestine type in both proximal and distal GC, and with male gender and distant metastasis in distal GC.
ACKNOWLEDGMENTS
This work was presented as a poster in an abstract form at the annual meeting of United States and Canadian Academy of Pathology in 2011 and Digestive Disease Week in 2011.
COMMENTS
Background
HER2 gene amplification and over-expression is a potential therapeutic target and is found in 6%-53.4% of gastric cancer (GC) in patients from Western countries. As such, it is now recommended that all patients with GC should have their tumors tested for HER2 status at the time of initial diagnosis. In China, GC remains one of the leading cancers. Because there exist considerable differences in GC between Western and Chinese patients, the need for a comprehensive investigation of the HER2 expression profile in GC of Chinese patients is urgent for better clinical management.
Research frontiers
The purpose of the present study was to investigate HER2 expression with the same assessment criteria of IHC as used in the ToGA trial and its correlation with clinicopathological variables between proximal and distal GC in the Chinese population.
Innovations and breakthroughs
Our study represented a very large cohort of GC. In this study, HER2 expression in the overall GC was significantly higher in male patients, the Lauren intestinal type, low-grade and pM1 diseases. There was a significant difference in HER2 expression among some pTNM stages. Similar to some Western study results, our data showed that HER2 expression was significantly higher in proximal GC than in distal GC. Also, HER2 expression in the distal GC was significantly higher in male patients, low-grade histology, the Lauren intestinal type, and pM1. In the proximal GC, however, higher HER2 expression scores were observed only in tumors with low-grade histology and the Lauren intestinal type.
Applications
Our data, derived from a comprehensive investigation of HER2 expression profile with the same ToGA criteria in GC resection specimens in a large cohort of homogeneous Chinese patients, provide pathologists and oncologists with more accurate study results than tissue microarray regarding HER2 over-expression characteristics in both proximal and distal GC, which is essential for better clinical management of patients with GC in the Chinese population.
Terminology
ToGA trial: An international, open-label, randomized, controlled, phase III trial of Herceptin (Trastuzumab) in combination with chemotherapy compared with chemotherapy alone in patients with HER2-positive advanced gastric cancer, which was undertaken in 122 centers in 24 countries; Lauren classification: the Lauren classification is based on the histological features of gastric adenocarcinomas, and divides gastric adenocarcinomas into 3 types: intestinal type (the tumor consists of neoplastic glands arranged in tubules, acini, and papillae), diffuse type (the tumor cells are discohesive and many show the signet-ring morphology) and mixed type.
Peer review
The authors reported statistically more frequent HER2 over-expression in proximal than distal GC. HER2 over-expression was also associated with some clinicopathological characteristics, such as gender, the Lauren intestinal type, low-grade dysplasia, and pM1 diseases in GC. This is a valuable paper which represents a very large cohort of cancers (957 cases). This provides some real strength and confidence in the HER2 expression values. The data are consistent with but extend (for the Chinese population) the information already available.
Footnotes
Supported by The National Natural Science Foundation of China, No. 81101815; the Science and Technology Development Project of Medicine in Nanjing, No. YKK08064; Jiangsu Health International Exchange Program and Young Talents Training Project of Health in Nanjing
P- Reviewers Langdon S, Zhang HT S- Editor Wen LL L- Editor Logan S E- Editor Zhang DN
References
- 1.Jørgensen JT, Hersom M. HER2 as a Prognostic Marker in Gastric Cancer - A Systematic Analysis of Data from the Literature. J Cancer. 2012;3:137–144. doi: 10.7150/jca.4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chua TC, Merrett ND. Clinicopathologic factors associated with HER2-positive gastric cancer and its impact on survival outcomes--a systematic review. Int J Cancer. 2012;130:2845–2856. doi: 10.1002/ijc.26292. [DOI] [PubMed] [Google Scholar]
- 3.Bang YJ. Advances in the management of HER2-positive advanced gastric and gastroesophageal junction cancer. J Clin Gastroenterol. 2012;46:637–648. doi: 10.1097/MCG.0b013e3182557307. [DOI] [PubMed] [Google Scholar]
- 4.Hicks DG, Whitney-Miller C. HER2 testing in gastric and gastroesophageal junction cancers: a new therapeutic target and diagnostic challenge. Appl Immunohistochem Mol Morphol. 2011;19:506–508. doi: 10.1097/PAI.0b013e31822c3a0f. [DOI] [PubMed] [Google Scholar]
- 5.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 6.Yamashita-Kashima Y, Iijima S, Yorozu K, Furugaki K, Kurasawa M, Ohta M, Fujimoto-Ouchi K. Pertuzumab in combination with trastuzumab shows significantly enhanced antitumor activity in HER2-positive human gastric cancer xenograft models. Clin Cancer Res. 2011;17:5060–5070. doi: 10.1158/1078-0432.CCR-10-2927. [DOI] [PubMed] [Google Scholar]
- 7.Lordick F. Trastuzumab: a new treatment option for HER2-positive metastatic gastric and gastroesophageal junction cancer. Future Oncol. 2011;7:187–199. doi: 10.2217/fon.10.178. [DOI] [PubMed] [Google Scholar]
- 8.Cho EY, Srivastava A, Park K, Kim J, Lee MH, Do I, Lee J, Kim KM, Sohn TS, Kang WK, et al. Comparison of four immunohistochemical tests and FISH for measuring HER2 expression in gastric carcinomas. Pathology. 2012;44:216–220. doi: 10.1097/PAT.0b013e3283513e8b. [DOI] [PubMed] [Google Scholar]
- 9.Rüschoff J, Hanna W, Bilous M, Hofmann M, Osamura RY, Penault-Llorca F, van de Vijver M, Viale G. HER2 testing in gastric cancer: a practical approach. Mod Pathol. 2012;25:637–650. doi: 10.1038/modpathol.2011.198. [DOI] [PubMed] [Google Scholar]
- 10.Rüschoff J, Nagelmeier I, Baretton G, Dietel M, Höfler H, Schildhaus HU, Büttner R, Schlake W, Stoss O, Kreipe HH. Her2 testing in gastric cancer. What is different in comparison to breast cancer? Pathologe. 2010;31:208–217. doi: 10.1007/s00292-010-1278-1. [DOI] [PubMed] [Google Scholar]
- 11.Hofmann M, Stoss O, Shi D, Büttner R, van de Vijver M, Kim W, Ochiai A, Rüschoff J, Henkel T. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology. 2008;52:797–805. doi: 10.1111/j.1365-2559.2008.03028.x. [DOI] [PubMed] [Google Scholar]
- 12.Park YS, Hwang HS, Park HJ, Ryu MH, Chang HM, Yook JH, Kim BS, Jang SJ, Kang YK. Comprehensive analysis of HER2 expression and gene amplification in gastric cancers using immunohistochemistry and in situ hybridization: which scoring system should we use? Hum Pathol. 2012;43:413–422. doi: 10.1016/j.humpath.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 13.Fox SB, Kumarasinghe MP, Armes JE, Bilous M, Cummings MC, Farshid G, Fitzpatrick N, Francis GD, McCloud PI, Raymond W, et al. Gastric HER2 Testing Study (GaTHER): an evaluation of gastric/gastroesophageal junction cancer testing accuracy in Australia. Am J Surg Pathol. 2012;36:577–582. doi: 10.1097/PAS.0b013e318244adbb. [DOI] [PubMed] [Google Scholar]
- 14.Albarello L, Pecciarini L, Doglioni C. HER2 testing in gastric cancer. Adv Anat Pathol. 2011;18:53–59. doi: 10.1097/PAP.0b013e3182026d72. [DOI] [PubMed] [Google Scholar]
- 15.Bickenbach K, Strong VE. Comparisons of Gastric Cancer Treatments: East vs. West. J Gastric Cancer. 2012;12:55–62. doi: 10.5230/jgc.2012.12.2.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors . AJCC cancer staging handbook. 7th ed. New York: Springer; 2009. pp. 129–144. [Google Scholar]
- 17.Odze J-FF RD, Boffetta P, Hofler H, Montgomery E. Spchler: Tumours of the oesophagogastric junction. In: Fred T, Bosman FC, Ralph HH, Neil DT, editors. World Health Organization Classification of Tumours of the Digestive System. Lyon: IARC Press; 2010. pp. 40–44. [Google Scholar]
- 18.Zhang YF, Shi J, Yu HP, Feng AN, Fan XS, Lauwers GY, Mashimo H, Gold JS, Chen G, Huang Q. Factors predicting survival in patients with proximal gastric carcinoma involving the esophagus. World J Gastroenterol. 2012;18:3602–3609. doi: 10.3748/wjg.v18.i27.3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang Q, Shi J, Feng A, Fan X, Zhang L, Mashimo H, Cohen D, Lauwers G. Gastric cardiac carcinomas involving the esophagus are more adequately staged as gastric cancers by the 7th edition of the American Joint Commission on Cancer Staging System. Mod Pathol. 2011;24:138–146. doi: 10.1038/modpathol.2010.183. [DOI] [PubMed] [Google Scholar]
- 20.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Handbook. 7th ed. New York: Springer; 2009. pp. 145–152. [Google Scholar]
- 21.Kunz PL, Mojtahed A, Fisher GA, Ford JM, Chang DT, Balise RR, Bangs CD, Cherry AM, Pai RK. HER2 expression in gastric and gastroesophageal junction adenocarcinoma in a US population: clinicopathologic analysis with proposed approach to HER2 assessment. Appl Immunohistochem Mol Morphol. 2012;20:13–24. doi: 10.1097/PAI.0b013e31821c821c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim KC, Koh YW, Chang HM, Kim TH, Yook JH, Kim BS, Jang SJ, Park YS. Evaluation of HER2 protein expression in gastric carcinomas: comparative analysis of 1,414 cases of whole-tissue sections and 595 cases of tissue microarrays. Ann Surg Oncol. 2011;18:2833–2840. doi: 10.1245/s10434-011-1695-2. [DOI] [PubMed] [Google Scholar]
- 23.Yang J, Luo H, Li Y, Li J, Cai Z, Su X, Dai D, Du W, Chen T, Chen M. Intratumoral heterogeneity determines discordant results of diagnostic tests for human epidermal growth factor receptor (HER) 2 in gastric cancer specimens. Cell Biochem Biophys. 2012;62:221–228. doi: 10.1007/s12013-011-9286-1. [DOI] [PubMed] [Google Scholar]
- 24.Fusco N, Rocco EG, Del Conte C, Pellegrini C, Bulfamante G, Di Nuovo F, Romagnoli S, Bosari S. HER2 in gastric cancer: a digital image analysis in pre-neoplastic, primary and metastatic lesions. Mod Pathol. 2013:Epub ahead of print. doi: 10.1038/modpathol.2012.228. [DOI] [PubMed] [Google Scholar]
- 25.Atkinson R, Mollerup J, Laenkholm AV, Verardo M, Hawes D, Commins D, Engvad B, Correa A, Ehlers CC, Nielsen KV. Effects of the change in cutoff values for human epidermal growth factor receptor 2 status by immunohistochemistry and fluorescence in situ hybridization: a study comparing conventional brightfield microscopy, image analysis-assisted microscopy, and interobserver variation. Arch Pathol Lab Med. 2011;135:1010–1016. doi: 10.5858/2010-0462-OAR. [DOI] [PubMed] [Google Scholar]
- 26.Kataoka Y, Okabe H, Yoshizawa A, Minamiguchi S, Yoshimura K, Haga H, Sakai Y. HER2 expression and its clinicopathological features in resectable gastric cancer. Gastric Cancer. 2013;16:84–93. doi: 10.1007/s10120-012-0150-9. [DOI] [PubMed] [Google Scholar]
- 27.Tafe LJ, Janjigian YY, Zaidinski M, Hedvat CV, Hameed MR, Tang LH, Hicks JB, Shah MA, Barbashina V. Human epidermal growth factor receptor 2 testing in gastroesophageal cancer: correlation between immunohistochemistry and fluorescence in situ hybridization. Arch Pathol Lab Med. 2011;135:1460–1465. doi: 10.5858/arpa.2010-0541-OA. [DOI] [PubMed] [Google Scholar]
- 28.Im SA, Kim JW, Kim JS, Kim MA, Jordan B, Pickl M, Han SW, Oh DY, Lee HJ, Kim TY, et al. Clinicopathologic characteristics of patients with stage III/IV (M(0)) advanced gastric cancer, according to HER2 status assessed by immunohistochemistry and fluorescence in situ hybridization. Diagn Mol Pathol. 2011;20:94–100. doi: 10.1097/PDM.0b013e3181fc02b7. [DOI] [PubMed] [Google Scholar]
- 29.Janjigian YY, Werner D, Pauligk C, Steinmetz K, Kelsen DP, Jäger E, Altmannsberger HM, Robinson E, Tafe LJ, Tang LH, et al. Prognosis of metastatic gastric and gastroesophageal junction cancer by HER2 status: a European and USA International collaborative analysis. Ann Oncol. 2012;23:2656–2662. doi: 10.1093/annonc/mds104. [DOI] [PubMed] [Google Scholar]
- 30.Lee S, de Boer WB, Fermoyle S, Platten M, Kumarasinghe MP. Human epidermal growth factor receptor 2 testing in gastric carcinoma: issues related to heterogeneity in biopsies and resections. Histopathology. 2011;59:832–840. doi: 10.1111/j.1365-2559.2011.04017.x. [DOI] [PubMed] [Google Scholar]
- 31.Huang Q, Fan X, Agoston AT, Feng A, Yu H, Lauwers G, Zhang L, Odze RD. Comparison of gastro-oesophageal junction carcinomas in Chinese versus American patients. Histopathology. 2011;59:188–197. doi: 10.1111/j.1365-2559.2011.03924.x. [DOI] [PubMed] [Google Scholar]


