Abstract
Bariatric surgery is now widely accepted for treatment of morbid obesity. This study compared the effects of laparoscopic sleeve gastrectomy (LSG) and laparoscopic adjustable gastric banding (LAGB) on excess weight loss (EWL) and type 2 diabetes mellitus (T2DM). PubMed and Embase were searched for publications concerning LAGB and LSG from 2000 to 2012, with the last search on August 17, 2012. EWL and T2DM improvement over 6 and 12 months were pooled and compared by meta-analysis. Odds ratios (ORs) and mean differences were calculated with 95 % confidence intervals (CIs). Eleven studies involving 1,004 patients met the inclusion criteria. Compared with LAGB, LSG achieved greater EWL. The mean percentage EWL for LAGB was 33.9 % after 6 months in six studies and 37.8 % after 12 months in four studies; for LSG, EWL was 50.6 % after 6 months and 51.8 % after 12 months in the same studies. LSG was also superior to LAGB in treating T2DM. In five studies, T2DM was improved in 42 of 68 (61.8 %) patients after LAGB and 66 of 80 (82.5 %) after LSG, representing a pooled OR of 0.34 (95 % CI 0.16–0.73) and pooled mean differences of −12.55 (95 % CI −15.66 to −9.43) and −4.97 (95 % CI −7.58 to −8.36), respectively. LSG is more effective than LAGB in morbid obesity, with higher percentage EWL and greater improvement in T2DM.
Keywords: Laparoscopic sleeve gastrectomy, Laparoscopic adjustable gastric banding, Type 2 diabetes mellitus, Morbid obesity, Bariatric surgery
Introduction
Morbid obesity is increasing worldwide. Its prevalence is higher in developed countries such as the USA and Western European nations than in developing countries [1], but it is also increasing in Asia and other regions due to lack of exercise, lifestyle changes, and greater stress. In China, morbid obesity is strongly associated with type 2 diabetes mellitus (T2DM), which has become a serious problem in modern Chinese society [2]. Over 90 million patients suffer from diabetes in China, 90 % of whom have T2DM [4]. Morbid obesity is also linked with hypertension, obstructive sleep apnea, and other conditions that cause distress to patients and reduce their quality of life. Obesity also increases the risk of heart attack and death.
People who are slightly overweight may find medical treatments to lose weight effective. However, pharmacologic and behavioral treatments for morbid obesity usually do not work in the long term, with patients regaining the weight they lost. Similarly, common medical treatments cannot always resolve diabetes mellitus and hypertension. According to a National Institutes of Health consensus statement in 1991, bariatric surgery is the only way to guarantee substantial weight loss and maintain weight at a reasonable level [3]. Bariatric surgery can also improve or even resolve obesity-related comorbidities, especially T2DM.
Three types of bariatric surgery are performed widely and proven effective: laparoscopic Roux-en-Y gastric bypass, laparoscopic sleeve gastrectomy (LSG) and laparoscopic adjustable gastric banding (LAGB). LAGB is a simple and safe procedure that restricts food intake using an adjustable band, which is placed around the upper stomach to create a gastric pouch that is distended during meals and connected to a reservoir under the skin to change its diameter [4]. LSG is a comparatively new technique that is safe and effective especially in super-obese patients. It involves resection of two thirds of the stomach, including the fundus, while the remaining part from the gastroesophageal junction to the pylorus along the great curvature is used to form a “sleeve”. This procedure decreases the volume of the stomach to about 100 ml, which is easier to fill and thus leads to less food intake [5]. Although comparatively new, LSG is reported to be superior to LAGB in its effects on morbid obesity. In this meta-analysis, we aimed to compare the outcomes of LAGB and LSG in terms of excess weight loss (EWL) and improvement of T2DM.
Materials and Methods
Search Strategies
Two databases (PubMed and Embase) were searched for all relevant literature, including references in articles, published between 2000 and 2012. The medical subject headings and keywords searched for individually and in combination were as follows: “laparoscopic adjustable gastric banding”, “laparoscopic sleeve gastrectomy”, “type 2 diabetes mellitus”, “bariatric surgery”, and “morbid obesity”. The last search was conducted on August 17, 2012.
Inclusion and Exclusion Criteria
The inclusion criteria were as follows: (1) studies including randomized controlled trials and non-randomized studies that compared LAGB with LSG, (2) studies that provided information on at least one of the outcome measures, and (3) studies published in English. When a study reporting the same patient cohort was included in several publications, only the most recent or complete study was selected. The exclusion criteria were as follows: (1) case reports, (2) articles that were not full text or non-comparative studies, and (3) open operations, not by laparoscopic surgery.
Data Extraction
All included studies were assessed for the quality of their methodology and relevance to the objective of our meta-analysis. Conduct and reporting were in accordance with the QUOROM statement. Data on EWL and improvement of T2DM from each trial were extracted and compared independently by the two investigators.
Statistical Analysis
Statistical analysis was conducted using Review Manager software version 5.0.0 (Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark). Pooled odds ratios (ORs) and pooled mean differences with 95 % confidence intervals (CIs) were used to assess the outcomes of the studies; statistical heterogeneity was tested by the chi-square test. According to forest plots, heterogeneity was limited; thus, we used the Mantel–Haenszel fixed effect model. The significance of the pooled ORs was determined by Z-test, and statistical significance was set at p < 0.05.
Results
Study Characteristics
There were 11 studies in this meta-analysis [1, 3, 5–13] (Tables 1 and 2). All were published in or after 2005 and most were published during the last 3 years. The meta-analysis included 1,004 patients from these studies, of whom 616 underwent LAGB and 388 underwent LSG. Some other studies also reported the use of LSG or LAGB for morbid obesity or comparisons between the two [14–25] but were excluded because they did not provide the information we needed for analysis or their data did not meet our criteria.
Table 1.
Characteristics of the studies included in the meta-analysis
| Author | Year | Number of cases | Mean BMI (kg/m2) | Mean age (years) | Male/female | ||||
|---|---|---|---|---|---|---|---|---|---|
| LAGB | LSG | LAGB | LSG | LAGB | LSG | LAGB | LSG | ||
| Simon KH Wong et al. | 2009 | 57 | 30 | 40 ± 7 | 45 ± 8 | 41 ± 9 | 33 ± 7 | 24:33 | 9:21 |
| B Breznikar et al. | 2009 | 180 | 30 | 42.6 (29.4–50.0) | 51.6 (40.9–67.5) | 41.2(17.2–68.8) | 43.5 (22.8–60.4) | 21:159 | 10:20 |
| Juan J. Omana et al. | 2010 | 74 | 49 | 44 ± 5 | 52 ± 11 | 41 ± 14 | 45 ± 12 | 16:58 | 13:36 |
| Joshua B. Alley et al. | 2012 | 39 | 69 | 41.9 ±5.2 | 42.7 ± 5.0 | 47.0 ± 9.5 | 49.6 ± 10.7 | 7:32 | 15:54 |
| Kazunori Kasama et al. | 2008 | 13 | 23 | 37.5 ± 4 | 49.1 ± 12 | 43¡À10 | 38 ± 10 | 5:8 | 17:6 |
| Paul Brunault et al. | 2011 | 102 | 29 | 48.1 ± 6.1 | 54.3 ± 10.6 | 39.3 ± 9.6 | 41.0 ± 10.6 | 17:85 | 7:22 |
| Susan S. H. Gan et al. | 2007 | 12 | 21 | 45.6 | 52.8 | N | N | 2:10 | 8:13 |
| W. K. Fenske et al. | 2012 | 13 | 11 | 44.6 ± 9 | N | 35–54 | 35–54 | N | N |
| F. B. Langer et al. | 2005 | 10 | 10 | 46.7 ± 35 (45–54) | 48.3 ± 5.7 (41–56) | 38.5 ± 13.6 | 39.3 ± 11.7 | 1:9 | 1:9 |
| M. A. Kueper et al. | 2008 | 16 | 16 | 44.9 (41–65) | 49.1 (43–68) | 43.9 (27–62) | 42.8 (24–68) | 7:9 | 7:9 |
| H. R. Hady et al. | 2012 | 100 | 100 | 45.21 ± 3.96 | 52.15 ± 8.5 | 37.0 ± 12.6/39.18 ± 12.77 | 47.93 ± 9.24/44.19 ± 9.33 | 34:66 | 48:52 |
Table 2.
Main outcomes of the 12 studies included in the meta-analysis
| Author | Improve or resolve diabetes mellitus | EWL% (6 months) | EWL% (12 months) | |||
|---|---|---|---|---|---|---|
| LAGB | LSG | LAGB | LSG | LAGB | LSG | |
| Simon KH Wong et al. | N | N | 27 ± 26 | 63 ± 33 | 31 ± 24 | 65 ± 32 |
| B Breznikar et al. | 16/22 | 6/8 | N | N | 52.4 (−2.0–145.3) | 57.9 (7.6–92.3) |
| Juan J. Omana et al. | 6/13 | 14/14 | 25.2 ± 12 | 39.5 ± 16 | 40.3 ± 19 | 50.6 ± 19 |
| Joshua B. Alley et al. | 11/17 | 22/31 | N | N | 29.5 ± 16.7 | 47.2 ± 11.9 |
| Kazunori Kasama et al. | 3/4 | 4/6 | N | N | N | N |
| Paul Brunault et al. | N | N | 34.8 ± 18.4 | 43.8 ± 17.8 | 34.8 ± 18.4 | 43.8 ± 17.8 |
| Susan S. H. Gan | 6/12 | 20/21 | N | N | 34.2 | 35.9 |
| W. K. Fenske et al. | N | N | N | N | 45.0 ± 2.4 | 47.8 ± 4.5 |
| S. K. H. Wong | N | N | N | N | 25.4 ± 20.2 | 68.6 ± 39.6 |
| F. B. Langer et al. | N | N | 28.1 ± 10.6 | 61.4 ± 16.3 | N | N |
| M. A. Kueper et al. | N | N | 39.1 ± 19.1 | 33.0 ± 10 | N | N |
| H. R. Hady et al. | 4/8 | 18/39 | 48.98 ± 6.58 | 62.71 ± 21.17 | N | N |
EWL excess weight loss
Meta-analysis Results
According to our meta-analysis, LSG had a greater effect than LAGB on EWL at 6 and 12 months. For LAGB, the mean percentage EWL was 33.9 % after 6 months from six studies and 37.8 % after 12 months from four studies; for LSG, EWL was 50.6 % after 6 months and 51.8 % after 12 months from the same studies. After 6 and 12 months, the mean percentage EWL was higher for LSG than for LAGB by 33.0 and 27.0 %, respectively, indicating that, at these time points, LSG had a greater effect on weight loss than LAGB. LSG was also superior to LAGB in treating T2DM. According to five studies, 42 of 68 (61.8 %) T2DM patients experienced improvement of their diabetes after LAGB, whereas 66 of 80 (82.5 %) T2DM patients improved after LSG, an increase of 20.7 %. It can be concluded that LSG was a more effective procedure than LAGB, with a pooled OR of 0.34 (95 % CI 0.16–0.73; Fig. 1) and pooled mean differences of −12.55 (95 % CI −15.66 to −9.43; Fig. 2) and −4.97 (95 % CI −7.58 to −8.36; Fig. 3), respectively.
Fig. 1.
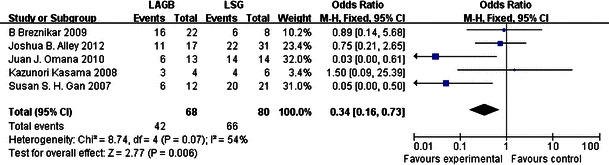
Forest plot of comparison: (1) LAGB vs LSG in terms of short-term results, outcome: (1.1) resolution of diabetes. Odds ratios are shown with 95 % CIs
Fig. 2.
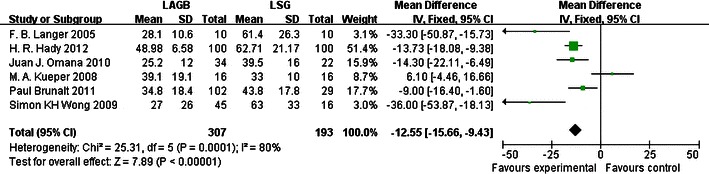
Forest plot of comparison: (1) LAGB vs LSG in terms of short-term results, outcome: (1.2) EWL% (6 months). Mean differences are shown with 95 % CIs
Fig. 3.
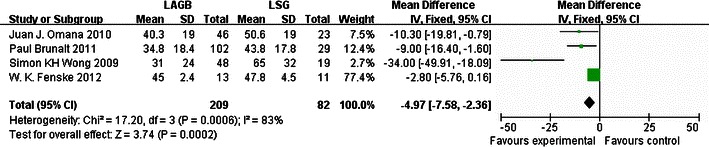
Forest plot of comparison: (1) LAGB vs LSG in terms of short-term results, outcome: (1.3) EWL% (12 months). Mean differences are shown with 95 % CIs
Publication Bias
Funnel plots were created to access the publication bias of the literature. The shapes of the plots did not reveal any evidence of obvious asymmetry (Figs. 4, 5, and 6).
Fig. 4.
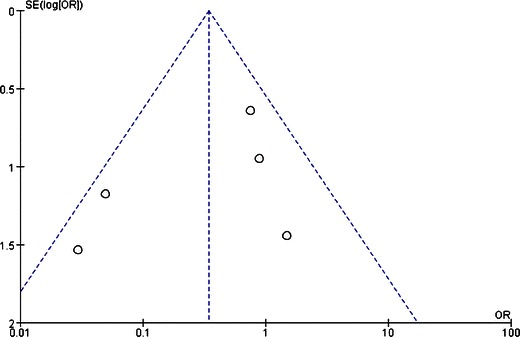
Funnel plot of comparison: (1) LAGB vs LSG in terms of short-term results, outcome: (1.1) resolution of diabetes. OR odds ratio
Fig. 5.
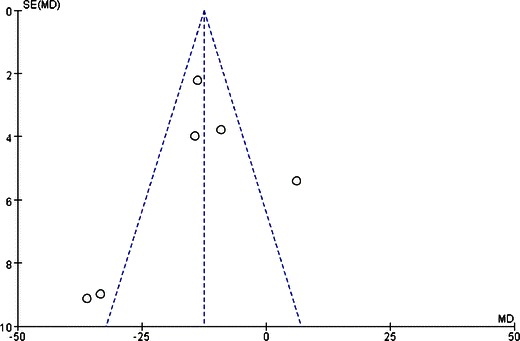
Funnel plot of comparison: (1) LAGB vs LSG in terms of short-term results, outcome: (1.2) EWL% (6 months). Mean difference
Fig. 6.
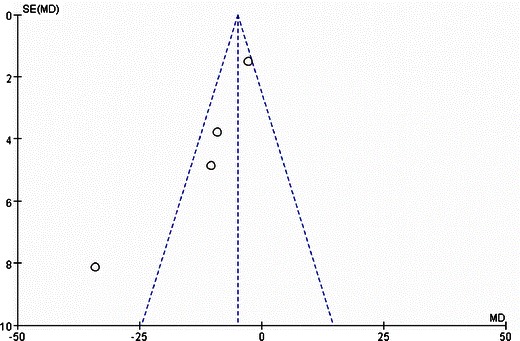
Forest plot of comparison: (1) LAGB vs LSG in terms of short-term results, outcome: (1.3) EWL% (12 months). Mean difference
Discussion
Although LAGB can significantly reduce weight, LSG had a greater effect on morbid obesity in terms of EWL and improvement of T2DM. Both LAGB and LSG are restrictive procedures that achieve EWL by decreasing the volume of the stomach to reduce food intake to comparatively low levels. It is widely accepted that the effects of these procedures are associated with levels of ghrelin, a 28-amino-acid acylated peptide primarily produced in endocrine A/X cells located in the fundus of the stomach [26]. Secretion of ghrelin increases appetite and promotes gastric emptying and intestinal mobility, causing feelings of hunger. Langer et al. [12] reported that ghrelin levels remained unchanged immediately after LAGB but had increased after 1 and 6 months, whereas ghrelin was decreased both immediately and at 1 and 6 months after LSG. This is because the fundus of the stomach, where most ghrelin is produced, is resected in LSG, though ghrelin levels can be maintained by up to 45 % after gastrectomy due to secretion from other sites such as the upper small intestine [26]. Patients who undergo LSG thus have less appetite for food and a longer-lasting and stronger sensation of fullness, leading to less food intake and, ultimately, weight loss. However, it is reported that patients continue to lose weight regardless of ghrelin levels, which suggest a complex relationship between this hormone and weight loss that requires further study [11].
Another gastrointestinal hormone, peptide tyrosine–tyrosine (PYY), must also be considered. This 36-amino-acid peptide is produced by endocrine L cells in the distal ileum and colon and is secreted before lipids reach the distal ileum. PYY is associated with appetite and has a hunger-reducing effect. Xanthakos [26] reported that, after intravenous infusion of PYY in 12 obese and 12 lean human subjects, single meal intake decreased by 30 % in both obese and lean individuals. Moreover, PYY reduced levels of ghrelin, enhancing its hunger-reducing effect. Some reports indicate that, after LSG, PYY increases following a test meal [27], whereas PYY does not increase after LAGB; this increases the weight loss effect of LSG. Overall, both restriction of stomach volume and hormonal changes contribute to the greater weight loss achieved by LSG.
Insulin is important in the treatment of diabetes, and it is believed that insulin resistance is key to the association between morbid obesity and T2DM. Improved control of T2DM can be achieved by countering insulin resistance with increased insulin sensitivity. All surgical interventions that lead to weight loss will increase insulin sensitivity and therefore improve T2DM [14]. Improvement of T2DM after LAGB is directly related to weight loss; that is, the more weight lost, the more T2DM is improved or even resolved. However, for LSG, different studies draw differing conclusions. Abbatini [14] reported a statistically significant reduction in body mass index 3 months after LSG in a patient in whom T2DM was not resolved. These data suggest that improvement of T2DM after LSG may be unrelated to weight loss. However, Ding concluded that improvement of T2DM is largely related to weight loss after both LAGB and LSG and not a consequence of the surgical procedure used as Hady et al. did [11]. Patients who did not achieve a good rate of EWL showed little improvement in their diabetes [2]. Further studies are needed to clarify these discrepancies.
Incretins, including glucagon-like peptide 1 (GLP-1), also play an important role. GLP-1 is secreted by intestinal endocrine L cells in the ileum and colon and is released into ingested food, especially that rich in lipids and glucose or other carbohydrates; it increases pancreatic secretion of insulin in response to oral glucose ingestion to decrease the level of glucose in the blood [26]. No increase of GLP-1 has been reported after LAGB, whereas it is speculated that GLP-1 increases following LSG, which further helps improve T2DM. The incretin gastric inhibitory peptide is also released as a response to food rich in lipids and glucose. These hormones not only improve T2DM but also help reduce weight.
All of the above factors may account for the weight loss observed after LSG and LABG and explain why LSG has a greater effect on EWL and improvement of T2DM. However, LAGB has advantages over LSG including easier technique, shorter operative time, and fewer early complications [28]. Furthermore, LAGB is less invasive than LSG and is totally reversible. These factors contribute to the popularity of LAGB among obese patients, but in terms of effect we consider LSG a better option.
There are limitations to this meta-analysis to which we must pay attention. First, the sample size of some of the studies was quite low, as was the number of studies included in our meta-analysis; this may have biased the results. Second, not all of the included trials were randomized because of a lack of qualified randomized studies providing the required details. Third, we did not compare improvements in other comorbidities following LAGB and LSG, and these factors may be important in assessing and recommending a procedure. Because LSG is a comparatively new procedure that has become popular in recent years, there is also concern about the long-term results; the follow-up periods in most reports are 6 or 12 months, and the studies analyzed here provided relatively short-term findings. Some studies that reported 3-year results were not included in this meta-analysis because of insufficient data, but their numbers are low. There are few reports with a follow-up period of 5 years or more. However, we believe that, with greater awareness and the increasing popularity of bariatric surgery, more long-term follow-up reports will be published.
In summary, this meta-analysis showed LSG to be a more effective procedure for morbid obesity than LABG, with a greater effect on EWL and improvement of T2DM. Larger, randomized, and long-term follow-up studies need to be conducted to compare the efficacy of LSG, LAGB, and laparoscopic Roux-en-Y gastric bypass.
Abbreviations
- LSG
Laparoscopic sleeve gastrectomy
- LAGB
Laparoscopic adjustable gastric banding
- T2DM
Type 2 diabetes mellitus
- EWL
Excess weight loss
- LRYGB
Laparoscopic Roux-en-Y gastric bypass
- BPD/DS
Biliopancreatic diversion with duodenal switch
- PYY
Peptide tyrosine–tyrosine
- GLP-1
Glucagon-like peptide 1
References
- 1.Kasama K, et al. Has laparoscopic bariatric surgery been accepted in Japan? The experience of a single surgeon. Obes Surg. 2008;18(11):1473–8. doi: 10.1007/s11695-008-9492-0. [DOI] [PubMed] [Google Scholar]
- 2.Ding D, et al. Outcomes after laparoscopic surgery for 219 patients with obesity. Zhonghua Wei Chang Wai Ke Za Zhi. 2011;14(2):128–31. [PubMed] [Google Scholar]
- 3.Wong SK, et al. Laparoscopic bariatric surgery: a five-year review. Hong Kong Med J. 2009;15(2):100–9. [PubMed] [Google Scholar]
- 4.Nocca D. Laparoscopic adjustable gastric banding and laparoscopic sleeve gastrectomy: which has a place in the treatment of diabetes in morbidly obese patients? Diabetes Metab. 2009;35(6 Pt 2):524–7. doi: 10.1016/S1262-3636(09)73460-3. [DOI] [PubMed] [Google Scholar]
- 5.Kueper MA, et al. Laparoscopic sleeve gastrectomy: standardized technique of a potential stand-alone bariatric procedure in morbidly obese patients. World J Surg. 2008;32(7):1462–5. doi: 10.1007/s00268-008-9548-2. [DOI] [PubMed] [Google Scholar]
- 6.Alley JB, et al. Quality of life after sleeve gastrectomy and adjustable gastric banding. Surg Obes Relat Dis. 2012;8(1):31–40. doi: 10.1016/j.soard.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 7.Breznikar B, Dinevski D. Bariatric surgery for morbid obesity: pre-operative assessment, surgical techniques and post-operative monitoring. J Int Med Res. 2009;37(5):1632–45. doi: 10.1177/147323000903700543. [DOI] [PubMed] [Google Scholar]
- 8.Brunault P, et al. Observations regarding ‘quality of life’ and ‘comfort with food’ after bariatric surgery: comparison between laparoscopic adjustable gastric banding and sleeve gastrectomy. Obes Surg. 2011;21(8):1225–31. doi: 10.1007/s11695-011-0411-4. [DOI] [PubMed] [Google Scholar]
- 9.Fenske WK et al. Effect of bariatric surgery-induced weight loss on renal and systemic inflammation and blood pressure: a 12-month prospective study. Surg Obes Relat Dis. 2012. [DOI] [PubMed]
- 10.Gan SS, Talbot ML, Jorgensen JO. Efficacy of surgery in the management of obesity-related type 2 diabetes mellitus. ANZ J Surg. 2007;77(11):958–62. doi: 10.1111/j.1445-2197.2007.04290.x. [DOI] [PubMed] [Google Scholar]
- 11.Hady HR, et al. The influence of laparoscopic adjustable gastric banding and laparoscopic sleeve gastrectomy on weight loss, plasma ghrelin, insulin, glucose and lipids. Folia Histochem Cytobiol. 2012;50(2):15785. doi: 10.5603/FHC.2012.0039. [DOI] [PubMed] [Google Scholar]
- 12.Langer FB, et al. Sleeve gastrectomy and gastric banding: effects on plasma ghrelin levels. Obes Surg. 2005;15(7):1024–9. doi: 10.1381/0960892054621125. [DOI] [PubMed] [Google Scholar]
- 13.Omana JJ, et al. Comparison of comorbidity resolution and improvement between laparoscopic sleeve gastrectomy and laparoscopic adjustable gastric banding. Surg Endosc. 2010;24(10):2513–7. doi: 10.1007/s00464-010-0995-0. [DOI] [PubMed] [Google Scholar]
- 14.Abbatini F, et al. Long-term effects of laparoscopic sleeve gastrectomy, gastric bypass, and adjustable gastric banding on type 2 diabetes. Surg Endosc. 2010;24(5):1005–10. doi: 10.1007/s00464-009-0715-9. [DOI] [PubMed] [Google Scholar]
- 15.Brockmeyer JR, et al. Upper gastrointestinal swallow study following bariatric surgery: institutional review and review of the literature. Obes Surg. 2012;22(7):1039–43. doi: 10.1007/s11695-012-0658-4. [DOI] [PubMed] [Google Scholar]
- 16.Farrell TM, et al. Clinical application of laparoscopic bariatric surgery: an evidence-based review. Surg Endosc. 2009;23(5):930–49. doi: 10.1007/s00464-008-0217-1. [DOI] [PubMed] [Google Scholar]
- 17.Franco JV, et al. A review of studies comparing three laparoscopic procedures in bariatric surgery: sleeve gastrectomy, Roux-en-Y gastric bypass and adjustable gastric banding. Obes Surg. 2011;21(9):1458–68. doi: 10.1007/s11695-011-0390-5. [DOI] [PubMed] [Google Scholar]
- 18.Heinberg LJ, Keating K, Simonelli L. Discrepancy between ideal and realistic goal weights in three bariatric procedures: who is likely to be unrealistic? Obes Surg. 2010;20(2):148–53. doi: 10.1007/s11695-009-9982-8. [DOI] [PubMed] [Google Scholar]
- 19.Himpens J, Dapri G, Cadiere GB. A prospective randomized study between laparoscopic gastric banding and laparoscopic isolated sleeve gastrectomy: results after 1 and 3 years. Obes Surg. 2006;16(11):1450–6. doi: 10.1381/096089206778869933. [DOI] [PubMed] [Google Scholar]
- 20.Himpens J, De Schepper M, Dapri G. Laparoscopic conversion of adjustable gastric banding to sleeve gastrectomy: a feasibility study. Surg Laparosc Endosc Percutan Tech. 2010;20(3):162–5. doi: 10.1097/SLE.0b013e3181e31fa9. [DOI] [PubMed] [Google Scholar]
- 21.Kasza J, et al. Analysis of poor outcomes after laparoscopic adjustable gastric banding. Surg Endosc. 2011;25(1):41–7. doi: 10.1007/s00464-010-1126-7. [DOI] [PubMed] [Google Scholar]
- 22.Li VK, et al. Predictors of gallstone formation after bariatric surgery: a multivariate analysis of risk factors comparing gastric bypass, gastric banding, and sleeve gastrectomy. Surg Endosc. 2009;23(7):1640–4. doi: 10.1007/s00464-008-0204-6. [DOI] [PubMed] [Google Scholar]
- 23.Lomanto D, et al. Bariatric surgery in Asia in the last 5 years (2005–2009) Obes Surg. 2012;22(3):502–6. doi: 10.1007/s11695-011-0547-2. [DOI] [PubMed] [Google Scholar]
- 24.Schroeder R, Garrison JM, Jr, Johnson MS. Treatment of adult obesity with bariatric surgery. Am Fam Physician. 2011;84(7):805–14. [PubMed] [Google Scholar]
- 25.Zundel N, Hernandez JD. Revisional surgery after restrictive procedures for morbid obesity. Surg Laparosc Endosc Percutan Tech. 2010;20(5):338–43. doi: 10.1097/SLE.0b013e3181f6287a. [DOI] [PubMed] [Google Scholar]
- 26.Xanthakos SA. Bariatric surgery for extreme adolescent obesity: indications, outcomes, and physiologic effects on the gut–brain axis. Pathophysiology. 2008;15(2):135–46. doi: 10.1016/j.pathophys.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karamanakos SN, et al. Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide-YY levels after Roux-en-Y gastric bypass and sleeve gastrectomy: a prospective, double blind study. Ann Surg. 2008;247(3):401–7. doi: 10.1097/SLA.0b013e318156f012. [DOI] [PubMed] [Google Scholar]
- 28.Chakravarty PD, et al. Comparison of laparoscopic adjustable gastric banding (LAGB) with other bariatric procedures; a systematic review of the randomised controlled trials. Surgeon. 2012;10(3):172–82. doi: 10.1016/j.surge.2012.02.001. [DOI] [PubMed] [Google Scholar]


