Abstract
Introduction and hypothesis
Stress urinary incontinence (SUI) is the most common type of urinary incontinence (UI) in pregnant women and is known to have detrimental effects on the quality of life in approximately 54.3 %. Pregnancy is the main risk factor for the development of SUI. This review provides details of the pathophysiology leading to SUI in pregnant women and SUI prevalence and treatment during pregnancy.
Methods
We conducted a PubMed search for English-language and human-study articles registered from January 1990 to September 2012. This search was performed for articles dealing with prevalence and treatment of SUI during pregnancy. In the intervention studies, we included studies that used a randomized controlled trial (RCT) design or studies comparing a treatment intervention to no treatment.
Results
A total of 534 articles were identified; 174 full-text articles were reviewed, and 28 of them met eligibility criteria and are reported on here. The mean prevalence of SUI during pregnancy was 41 % (18.6–60 %) and increased with gestational age. The increasing pressure of the growing uterus and fetal weight on pelvic-floor muscles (PFM) throughout pregnancy, together with pregnancy-related hormonal changes, may lead to reduced PFM strength as well as their supportive and sphincteric function. These cause mobility of the bladder neck and urethra, leading to urethral sphincter incompetence. Pelvic floor muscle exercise (PFME) is a safe and effective treatment for SUI during pregnancy, without significant adverse effects.
Conclusions
Understanding these issues can be useful for health-care professionals when informing and counseling pregnant women to help prevent SUI during pregnancy and the postpartum period.
Keywords: Stress urinary incontinence, Pregnancy, Prevalence, Pathophysiology, Treatment, Review article
Introduction
Stress urinary incontinence (SUI), the complaint of involuntary loss of urine on effort or physical exertion or sneezing or coughing [1], is the most common type of urinary incontinence (UI) in pregnant women. It is known to have detrimental effects on quality of life (QoL) in approximately 54.3 % of all pregnant women in four domains: physical activity, travel, social relationships, and emotional health [2]. Pregnant women with UI have statistically significant lower QoL during pregnancy than those without UI [3], and the QoL of incontinent pregnant women worsens with increasing gestational age to term [4]. Pregnant women with urgency (UUI) or mixed (MUI) incontinence had worse QoL scores during pregnancy than those with SUI alone. QoL scores on the physical, social, and emotional functioning domains were low, whereas scores on mobility and embarrassment domains were higher, suggesting a minimal restriction in lifestyle [5]. According to van Brummen et al. [6], the bother of lower urinary tract symptoms (LUTS) occurred most frequently at 36 weeks of pregnancy and were still a bother at 1 year after pregnancy. Interestingly, SUI occurred in the older maternal age groups, and the presence of bothersome LUTS occurred at 12 weeks of pregnancy, both of which were accounted to the predictive factor of bothersome SUI at 1 year after delivery. According to that study, cesarean section seemed to have a protective effect on bothersome SUI at 1 year after delivery.
Mascarenhas et al. [3] reported that UI negatively affects social relationships and particularly interferes with sexual relationships. Dolan et al. [2] found that UI impinges on sexual relationships. Although SUI severity increases with pregnancy progression from the first to the third trimester [7] and during 36 weeks of gestation, 16.9 % of pregnant women report having moderate to greatly bothersome UUI [6]. However, the QoL of pregnant women was found to be mildly affected by UI [8]. This may be because UI symptoms were not severe [9]. Pregnant women tend to consider this to be a common discomfort associated with pregnancy and a consequence of childbirth in the postpartum period [10]. Van de Pol et al. [4] found significantly higher rates of depressive symptoms and SUI during pregnancy than after childbirth. Another important impact of pregnancy-related UI is an increased risk of permanent incontinence in the postpartum period or later in life [11, 12]. Many studies have reported that pregnant women who had UI during pregnancy are at higher risk for postpartum UI than those without UI during pregnancy [13–15].
Pregnancy and delivery-related factors are considered to be the main risk factors for SUI development during pregnancy. The purpose of this review is to explain the pathophysiology leading to SUI in pregnant women and SUI prevalence and treatment during pregnancy. This information can be useful for health-care professionals when informing and counseling pregnant women in SUI prevention during pregnancy and the postpartum period.
Materials and methods
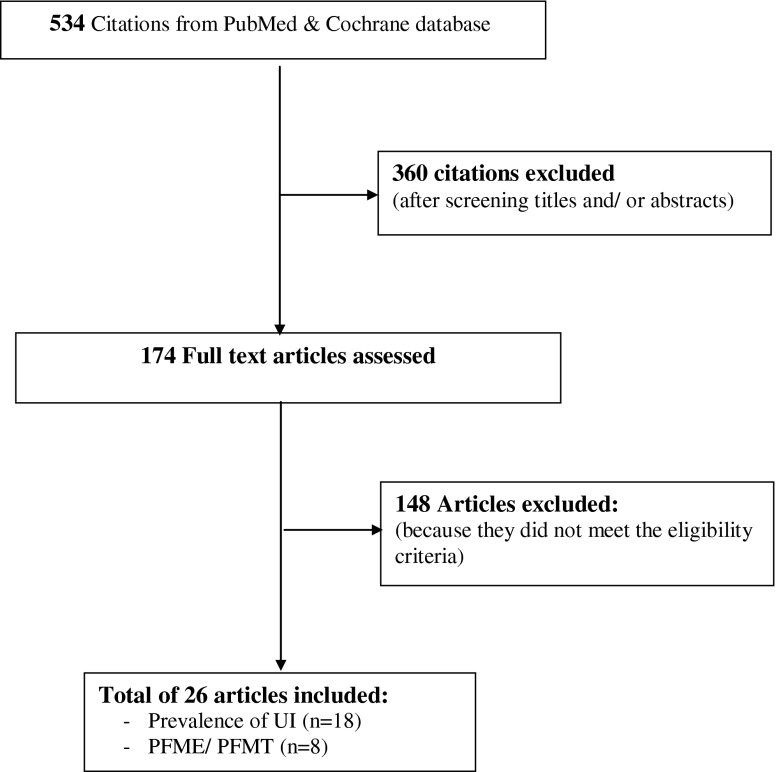
This review was performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [16]. We conducted a PubMed search for English-language and human-study articles registered from January 1990 to September 2012 using the following search terms: epidemiology, prevalence, urinary incontinence, stress urinary incontinence, pregnancy, pregnant women, pelvic-floor-muscle exercise, pelvic-floor-muscle training, and conservative treatment Conference proceedings and abstracts from the International Continence Society annual meetings were also searched. Additional relevant publications were selected from the reference lists of identified articles, and abstracts presented at international meeting were also included. No language restriction was defined. Abstracts and conventional reports were excluded. All articles were screened by title and abstract by BS and NS. Studies reporting on the prevalence of all UI that included SUI, UUI, and MUI in pregnant women both primigravidae and multigravidae were selected. Intervention studies related to pelvic-floor-muscle exercises (PFME), pelvic-floor-muscle training (PFMT), and other conservative treatments to prevent and treat UI during pregnancy and the postnatal period were included. In intervention studies, we included studies that used a randomized controlled trial (RCT) design or that compared a treatment intervention to no treatment. Noncomparative studies (i.e., pre-post studies, case report, case series) were excluded. A total of 534 publications in PubMed were identified. Titles and abstracts were screened for relevance and applicability, and 360 articles were excluded. The remaining 174 articles were screened in full text. Of these, 26 articles were included: 18 of UI prevalence and eight of UI treatment during pregnancy and the postnatal period met eligibility criteria as defined in Fig. 1.
Fig. 1.
Selection process for the review of stress urinary incontinence (SUI) prevalence and treatment during pregnancy
The prevalence of urinary incontinence in pregnancy
The most common type of UI in pregnant women is SUI. However, the true prevalence of SUI is still unknown. There are many studies on the prevalence of SUI during pregnancy, and results vary depending on study method and design, such as definitions of UI, evaluation questionnaire, and stage of pregnancy [12].
Most studies focused on European or Western counties. Only a few studies determined SUI during pregnancy in Asia, especially in Southeast Asia. Wesnes et al. [17] presented questionnaire data from the Norwegian Mother and Child Cohort Study at the Norwegian Institute of Public Health and found that the most common type of UI was SUI in high prevalence in both nulliparous and multiparous women, 31 % and 42 %, respectively. Morkved and Bø [18] from Norway found the prevalence of SUI during pregnancy was 42 %. According to the same study, SUI was 38 % 8 weeks after delivery. Other studies from Europe (Table 1), UK [19], Spain [20], Scotland [21], Germany [22], and Denmark [23] found similarly high prevalence of SUI during pregnancy.
Table 1.
Prevalence studies of urinary incontinence (UI), stress urinary incontinence (SUI), urgency urinary incontinence (UUI), and mixed urinary incontinence (MUI)
| Study | City/country | Study design | Sample | Sample size | Prevalence of UI during pregnancy |
|---|---|---|---|---|---|
| Wesnes et al. 2007; [17] | Bergen, Norway | Cohort | Nulliparous and parous women at 30 weeks’ gestation | 43,279 | SUI 31 % in nulliparous and 42 % in parous women |
| Morkved and Bo 1999; [18] | Norway | Retrospective | All women in a Norwegian community, delivering at the local hospital during a 1-year period | 144 | UI during pregnancy 42 %, and 8 weeks after delivery 38 % |
| Mason et al. 1999; [19] | UK | Prospective | Pregnant women who attended the antenatal clinic at two hospitals in northwest England. A postal questionnaire was sent to a sample of women when they reached 34 weeks’ gestation and repeated at 8 weeks postpartum. | 572 | SUI 59 % |
| Diez-Itza et al. 2009; [20] | Guipúzcoa, Spain | Observational | Primigravidae women who came to give birth at Donostia Hospital | 458 | SUI 30.3 % in primigravidae women. |
| Whitford et al. 2007; [21] | Scotland, UK | Structured cross-sectional interview survey | Nulliparous and parous women over the age of 16 years and >30 weeks’ gestation attending antenatal clinics in northeast Scotland. | 289 | SUI 54.3 % of pregnant women |
| Huebner et al. 2010; [22] | Berlin, Germany | Retrospective | Primigravidae women who delivered within 1 year (1999) at the Charité Hospital in Berlin | 610 | UI 26.3 % and significantly increased in the second half of pregnancy. |
| Viktrup et al. 1992; [23] | Denmark | Prospective | Primiparous women were interviewed repeatedly about SUI before and during pregnancy and after delivery | 305 | SUI before pregnancy 4 %, during pregnancy 32 %, after delivery 7 % |
| Zhu et al. 2012; [24] | Beijing, China | Prospective | Primiparous women from the seven regions of China ≥28 weeks’ gestation | 10,098 | SUI 18.6 %, MUI 4.3 %, UUI 2.0 % in late pregnancy |
| Liang et al. 2012; [25] | Taoyuan, Taiwan | Observational cohort | Primiparous women who delivered at ≥ 36 gestational weeks were recruited in a tertiary hospital | 1,501 | SUI 26.7 %, MUI 6.1 %, UUI 4.7 % |
| Sun et al. 2005; [26] | Changhua, Taiwan | Cross-sectional survey | Nulliparous and multiparous women attending the antenatal clinic of a medical center in central Taiwan | 799 | SUI 46.1 % , significantly higher prevalence in multiparous than in nulliparous women |
| Al-Mehaisen et al. 2009; [27] | Irbid, Jordan | Prospective | Primiparous and multiparous women at least 36 weeks’ gestation admitted in labor suite at three covering hospitals | 181 | SUI 46 %, UUI 35 % in the late third trimester |
| Sharma et al 2009; [28] | New Delhi, India | Questionnaire interview survey | Pregnant women >18 years who attended the antenatal clinic of unit III of AII India Institute of Medical Science | 240 | SUI 19.2 %, MUI 3.8 %, UUI 2.9 % during pregnancy |
| Thomason et al. 2007; [29] | Michigan, USA | Retrospective | Primiparous women who had one term vaginal delivery and no report of prepregnancy incontinence were recruited 6- to 9-months postpartum | 121 | SUI 60 % |
| Raza-Khan et al. 2006; [30] | St. Louis, MO, USA | Prospective | Nulliparous and multiparous women in third trimester receiving at Loyola University Medical Center | 113 | UI 70 % in nulliparous, 75 % in multiparous women, SUI 32 % |
| Martins et al. 2010; [31] | Sao Jose do Rio Preto, Brazil | Cross-sectional | Primigravidae and multigravidae women in third trimester who attended at 14 outpatient clinics in Sao Jose do Rio Preto | 500 | SUI 46.1 % in primigravidae, 54.0 % in multigravidae women |
| Chiarelli & Campbell 1997; [32] | New South Wales, Australia | Cross-sectional | Pregnant women in the postnatal ward of a large NSW teaching hospital were asked about any incontinence experienced in the last month of pregnancy | 304 | UI 64 % |
| Brown et al. 2010; [33] | Melbourne, Australia | Multicenter prospective cohort | Nulliparous at >18 years and <24 weeks’ gestation who gave birth at six metropolitan public hospitals | 1,507 | SUI 36.9 %, MUI 13.1 %, UUI 5.9 % |
| Bø et al. 2012; [34] | Oslo, Norway. | Population-based cross-sectional | All pregnant women in first trimester at three administrative city districts attending the Child Health Clinics | 722 | UI at 28 weeks’ gestation was 26 % for women of African origin, 36 % for women of Middle Eastern origin, 40 % for women of East-Asian origin, 43 % for women of South-Asian origin, and 45 % for women of European/North American origin |
A large population survey conducted in China [24] found a little lower prevalence of UI compare with in Europe, but it was still high among the pregnancy population: 26.7 % of pregnant women presented with UI, including 18.6 % with SUI, 4.3 % with MUI, and 2.0 % with UUI. In Taiwan, there was some discrepancy. One study found a low incidence in primiparous women: a prevalence of SUI, MUI, and UUI of 26.7 %, 6.1 %, and 4.7 %, respectively [25]. Another study found a high prevalence: SUI, 46.1 % [26]. A study conducted in Jordan also found the prevalence of SUI as high as 45 % and UUI at 35 % [27]. In India, the prevalence of SUI, MUI, and UUI during pregnancy was 19.2 %, 3.8 %, and 2.9 %, respectively [28]. The highest reported prevalence of SUI was in the USA. Thomason et al. [29] reported up to 60 %, whereas Raza-Khan et al. [30] found 70 % and 75 % of UI prevalence in nulliparous and multiparous women, respectively, and 32 % pure SUI. A high prevalence was also reported South America [31]. Results reported from Australia were comparable with those in the USA: 64 % of women reported incontinence during pregnancy [32]. The prevalence of SUI and MUI were more common during pregnancy than was UUI alone: 36.9 %, 13.1 %, and 5.9 %, respectively [33]. In a multiethnic population in Norway, Bø et al. [34] conducted their investigation in the first trimester. They investigated the prevalence rate of SUI at 28 weeks of gestation and found the lowest prevalence in women of African origin: 26 %. The prevalence in the Middle East population was 36 %, slightly higher than in women of African origin but lower than individual investigation. A prevalence of 40 % and 43 % was found in East and Southeast Asian women, which is higher than individual reports. The highest incidence was reported among women of European or North American origin: 45 %. Although the prevalence reported was different worldwide, the concordant feature was the increasing prevalence with gestational age [25, 26, 35]. The authors reported prevalence was highest in third trimester, followed by in the second and then first trimesters [25, 28, 36]. Only 13–19 % was reported in the first trimester [25, 37]. The second trimester was comparable at 19.2 %, but a significantly higher prevalence was found in third trimester: 37.5 % [25]. UI was higher with advance gestational age, as reported by Wijma et al. [36], who found the incidence of UI increased from 30 % at 28–32 weeks of gestation to 35 % at 36–38 weeks of gestation, which is similar to that reported by Kerrebroeck et al. [38]. The number of pregnant women with SUI varied according to this summary, and the prevalence of SUI ranged from 18.6 % to 60 %, UUI from 2 % to 35 %, and MUI from 3.8 % to 13.1 %, increasing with gestational age. Many studies of the prevalence of UI are shown in Table 1.
Anatomy and physiology of the lower urinary tract during pregnancy
Normal anatomy and physiology of the lower urinary tract plays an important role in the background of the knowledge of the continence mechanism. Thorough knowledge of the anatomy and physiology provides the concept of the UI. The normal anatomy, the pelvic floor musculature complex, and its normal physiology are beyond the scope of the review.
Pregnancy has significant effect on lower urinary tract function. In uncomplicated pregnancy, micturition frequency is influenced by the physiologic state of the bladder. Frequency has been described as diurnal changes, which may be up to seven times or more of normal, and slight nocturnal changes of one or more times during the night. The incidence is the same in both primigravidae and multigravidae women [39]. The first trimester is the most common time of onset. The uterine weight is the most important factor affecting frequency throughout the pregnancy. Uterine weight not only exerts pressure on the bladder but also irritates the bladder. Normal bladder capacity in the first trimester is 410 ml [39]. In late pregnancy, descent of the presenting part of fetus has an additional effect on bladder irritation. Bladder capacity in the third trimester reduces to 272 ml in conjunction with increased irritability of detrusor muscles [39]. Alternative causes include nervous and hormonal influences. Indeed, the onset of frequency in late pregnancy is a common symptom of engagement of the fetal head [39]. Approximately 80 % of pregnant women, both primigravidae and multigravidae, experience increased micturition frequency at some time during pregnancy [39]. Increased frequency usually begins in early pregnancy but can occur in the later stage; it disappears in mid pregnancy, which may be due to the increase in bladder capacity to 460 ml in the second trimester, and returns in the later weeks. [39]. Once increased frequency has occurred, it is nearly always progressive and becomes increasingly worse until term [39]. Increased frequency during pregnancy results in polyuria and is associated with increased fluid intake. However, which is the cause and which the effect remains an unsolved issue. Average daily excretion, output, and fluid intake are highest in the second trimester and lowest in the third [39].
Uterine position is another issue that plays a significant role. The impacted retrovert gravid uterus causes fluid retention because it interferes with the obliteration of the posterior urethrovesical angle. It does not elevate the urethrovesical junction in the pelvis, nor does it elongate the urethra [39].
Pathophysiology of stress urinary incontinence during pregnancy
PFM weakness causes bladder-neck and urethral mobility, leading to urethral sphincter incompetence [40, 41]. When the pregnant woman coughs, sneezes, laughs, or moves, intra-abdominal pressure increases, and this pressure is transmitted to the bladder. When pressure inside the bladder is greater than urethral closure pressure, incorporated with weakness of the urethral sphincter, SUI is the result. Pregnancy is one of the main risk factors for the development of SUI in young women [42–44]. Studies in pregnant women with SUI have found significantly decreased PFM strength in incontinent pregnant women compared with continent pregnant women [45]. Furthermore, there is a significantly higher prevalence of SUI in pregnant women than in nonpregnant women [46–48]. Pregnancy may also be associated with reduced PFM strength, which can cause SUI. However, the exact causes of pregnancy-related SUI remain unclear [49]. Physiological changes during pregnancy may lead to reduced PFM strength and SUI development in pregnant women due to the following mechanism.
Trauma to the PFM due to maternal weight, the uterus, and the fetus
Physiological weight gain during pregnancy may lead to increased pressure on the PFM and bladder, which may result in greater urethral mobility [50, 51]. Furthermore, excess maternal weight gain may impair blood flow and innervations to the bladder and urethra [52]. Several studies show the association between obesity and SUI. Zhu et al. [24] reported that the risk of SUI increased with increasing prenatal BMI [odds ratio (OR = 1.037)]. Liang et al. [25] reported that women with a prepregnancy BMI >30 kg/m2 were at increased risk of developing SUI during pregnancy. Glazener et al. [53] found that women with UI first occurring during pregnancy had a BMI > 25 kg/m2 [OR 1.68, 95% confidence interval (CI) 1.12–2.43]. Increased maternal weight correlated with increased intra-abdominal pressure during urodynamic assessments [54, 55]. In addition, increased BMI was not only associated with UI but with pelvic organ prolapse (POP) as well. Gyhagen et al [56] found that symptomatic POP increased 3 % with each unit increase of BMI (OR 1.03; 95 % CI 1.01–1.05). They also found that women with POP more frequently had UI and UI for > 10 years compared with women without POP. Weakening of PFM is found more often in women with POP [57]. Obesity or high BMI before pregnancy is a potentially modifiable risk factor for SUI; weight reduction may be an effective treatment option [58]. Weight loss by behavioral change can significantly improve SUI [58].
The growing uterus and fetus weigh solely on PFM, which contributes to chronic stress on PFM throughout pregnancy and results in PFM weakness. Sphincter strength and its supportive function of PFM are jeopardized [41, 47, 59]. Furthermore, multigravidity causes a decrease in PFM strength at a rate of 22–35 % beginning at a gestational age of 20 weeks and lasting until 6 weeks postpartum [40]. Hilde et al. [60], in a cross-sectional study of 300 nulliparous women at 18–22 weeks’ gestational age showed that continent pregnant women had significantly higher PFM strength and endurance compared with incontinent pregnant women (p = 0.003 and p = 0.01, respectively).
Prenatal issues and childbirth can damage the pudendal nerve, caudal aspects of the levator ani muscle, fascial pelvic organ supports, and the external and internal anal sphincters [61, 62]. This damage reduces PFM strength and may lead to increased bladder-neck and urethral mobility [63, 64] and cause mobility of the urethrovesical junction [11]. Wijma et al. [65] found increased bladder-neck mobility from early pregnancy onward to near term. Jundt et al. [66] found that pregnant women with SUI had more a mobile bladder neck than continent pregnant women.
Collagen changes during pregnancy
Collagen changes included both tensile properties and number. Changes in tensile properties contribute to reduced functional support of PFM, and reduced total collagen content may result in joint laxity and stretching of pelvic ligaments [67]. Several studies have reported a decrease in the total collagen content in women with SUI [63, 68]. Keane et al. [63] showed that nulliparous women with SUI had significantly less collagen content in their tissues than did continence pregnant women (p < 0.0001). They also concluded that the etiology of SUI in these pregnant women appears due to both quantitative and qualitative reduction in collagen.
Chen et al [69] showed the extracellular matrix (ECM) component is related to increased elastase activity in vaginal tissues obtained from SUI patients. In addition, the breakdown of collagen, smooth-muscle inhibition, and inflammatory processes play an important role in contributing to SUI. Lin et al. found the relevant genes that influence cellular signaling pathways, including matrix metalloproteinases (MMPs), regulator of G-protein signaling 2 (RGS2), and SMAD2 [70]. Skorupski et al. also found more expression of 1G/2G MMP-1 and the 5A/6A MMP-3 promoter single nucleotide polymorphisms (SNPs) in women with POP than in the population with a normal PFM [71]. Genetic and environmental factors contribute to the occurrence of SUI and POP. A study of twins by Altman et al [72] on SUI and POP analyzed 3,376 monozygotic and 5,067 dizygotic female twins and found a greater similarity in outcomes in monozygotic twins. In a study of nulliparous women, Dietz et al. [73] demonstrated the heritability of bladder-neck mobility. The systematic review by Lince et al. [74] found a substantially greater likelihood of SUI in family members with women with POP compares with women without POP, indicating that genetic predisposition play an important role in the development of POP.
Hormonal changes during pregnancy
Changes in relaxin and progesterone levels during pregnancy may have a significant role in the development of SUI [75].Relaxin, which plays an important role in maintaining urinary continence during pregnancy [76], could stimulate tissue growth in the lower urinary tract and increase urethral pressure. There is a marked increase in relaxin concentration to peak at a gestational age of 10–14 weeks and then decrease to a stable level of approximately half the peak value at the 17th–24th week of pregnancy, resulting in decreased growth of the urethral epithelium [77, 78]. This may lead to a decrease in urethral pressure [67]. As pregnant women with SUI have lower urethral pressure than continent pregnant women [79], lower relaxin concentrations in late pregnancy, therefore, correlate with a higher prevalence of SUI at the second and third trimesters [76].
Progesterone increases during pregnancy from 24 ng/ml at the 8th week to 150 ng/ml at the 36th week [80]. Increased progesterone may relax smooth muscles in the urinary system [81], result in reduced ureter, bladder, and urethral tone [82]. Values urethral pressure profile parameters below the median value and defective transmission of pressure over the urethra were observed in almost all pregnant women who experienced SUI during pregnancy. These observations suggest that an inherent weakness of the urethral sphincter mechanism plays a key role in the pathogenesis of SUI [80]. However, alterations in hormone levels during pregnancy were not correlated with changes in urethral pressure profile measurements [80].
The expansion of the uterus and fetal weight
Two major factors—expansion of the uterus and increment increase in fetal weight with gestational age, especially at the third trimester—influence the incontinence mechanism. They put direct pressure on the bladder, which may lead to changing the bladder-neck position [66] and reducing bladder capacity, contributing to bladder pressure that exceeds urethral pressure [65, 83]. This results in urine leakage.
In summary, commonly encountered prenatal physiological changes such as increasing pressure of the growing uterus and fetal weight on PFM throughout pregnancy, together with pregnancy-related hormonal changes in progesterone, estrogen, and relaxin, may lead to reduced strength and supportive and sphincteric function of PFM. PFM muscle weakness causes bladder-neck and urethral mobility, leading to urethral sphincter incompetence. Hence, when intra-abdominal pressure is increased with coughing, sneezing, laughing, or moving, the pressure inside the bladder becomes greater than the urethral closure pressure and the urethral sphincter is not strong enough to maintain urethral closure. Urinary leakage will be the result. In particular, SUI is common during pregnancy and puerperium. After delivery, SUI symptoms resolve in the vast majority of cases [84]. The healing process may take some time after the delivery [85], but in a significant percentage of women, it can persist in subsequent stages of life [84]. In primiparae women, SUI symptoms tend to resolve within 3 month after delivery [86].
The relevant literature reports that the prevalence of UI decreases in the postpartum period [85, 87]. In general, UI can affect 27.4 % of parous women for 6 months postpartum, with only a small portion of women experiencing spontaneous resolution [88]. Thomason et al. [29] found that only 8 % of pregnant women who developed UI during pregnancy had resolution, but in 47 % of those who had UI, incontinence had not resolved at 6 months postpartum. Glazener et al. [53] found that 13.5 % of primigravid women had SUI 3 months after delivery, whereas Wesnes et al. [55] showed an SUI rate around 14 % at 6 months after delivery. Groutz et al. [89] found that 1 year after the first spontaneous vaginal delivery, 10.3 % of women had SUI.
Despite this clear association, the mechanism involved in SUI remains unclear. Similarly, the pathophysiology of the development of SUI during pregnancy is not clearly understood. However, it has been suggested that it could be caused by both hormonal and mechanical changes that occur during this period [90, 91]. After pregnancy, most women recover their prepregnancy hormonal levels, and pressure of the enlarged uterus on the bladder and PFM resolves. Postnatal remission of SUI may be explained by the resolution of hormonal and metabolic changes associated with pregnancy and spontaneous healing of traumatic lesions due to vaginal childbirth [92]. The compensation may be due to PFM, which play a urethral support role, and it may be aided by pelvic floor training [92]. Improvement in PFM strength in women during the postpartum period showed that pregnancy, with its hormonal and mechanic effects, is a very important risk factor for UI during pregnancy [87].
Treatment for stress urinary incontinence during pregnancy
During pregnancy and postpartum, conservative therapy or perineal rehabilitation by PFME is the first-line intervention to treat SUI [35]. Pregnant women are often instructed to perform PFME to prevent the development and treat symptoms of SUI during pregnancy [93]. Other treatments, whether medical or surgical, must not be proposed at as the first intervention during pregnancy or during the immediate postpartum period [35].
Pelvic floor muscle exercise
PFME, the repetitively selective voluntary contraction and relaxation of specific PFM [1], also known as Kegel exercises, are the most popular method of treatment for SUI. In the late 1940s, an American gynecologist named Arnold Kegel first introduced this method for treating postpartum UI and improving the function and tone of PFM following childbirth. PFME have been successful because postpartum women who have performed these exercises have reported cure rates as high as 84 % and improvement rates as high as 100 % [94]. PFME is the most commonly recommended conservative therapy for pregnant women with SUI. PFME under the direction of a therapist (re-educator or midwife) reduces the prevalence of UI in the short term compared with simple advice about individual PFME [35]. Many investigators have assessed the effectiveness of PFME, as shown in Table 2.
Table 2.
Details of studies of pelvic floor muscle exercise (PFME) to prevent and treat stress urinary incontinence (SUI) during pregnancy and the postpartum period
| Study | Study design | Sample | Sample size | Intervention | Timing and method of assessment | Findings |
|---|---|---|---|---|---|---|
| Dinc et al. 2009; [87] | Prospective, randomized trial | Pregnant women with UI at gestational age 20–34 weeks | 80 | Intervention group:3 sets of PFME, each including contracting and relaxing PFME repeated 10 times ; control group: usual care | Urine leakage episodes, number of voidings, pad test and examiner-assessed PFM strength at 36–38 weeks’ gestational age and 6 to 8 weeks postpartum | Decrease in the number of UI episodes, amount of urine in the pad test during pregnancy in the intervention group; PFM strength increased to a larger extent. |
| Sangsawang and Serisathien 2012; [96] | Quasiexperimental with comparison group | Pregnant women with SUI at gestational age 20–30 weeks | 66 | Intervention group: 6-week PFME program with weekly training session led by a nurse; control group: usual care | Severity of SUI: frequency, amount of urine leakage, and VAS score of perceived SUI severity after the intervention period | Lower frequency, amount of urine leakage, and score of perceived SUI in the intervention group |
| Sampselle et al. 1998; [98] | Prospective randomized trial | Primigravid women with US at gestational age 20 weeks | 72 | Intervention group: standardized instruction in PFME; control group: usual care | UI symptoms by questionnaire, PFM strength by speculum at 20 and 35 weeks’ gestation and 6 weeks, 6 months, and 12 months postpartum | Decreased UI symptoms in the intervention group at 35 weeks’ gestation (p = .043), 6 weeks postpartum (p = .032), and 6 months postpartum (p = .044); greater PFM strength in the intervention group at 6 weeks and at 6 months postpartum |
| Morkved et al. 2003; [99] | Single-blind RCT | Nulliparous women with UI at gestational age 18 weeks | 301 | Intervention group: 12-week intensive PFMT with weekly training session led by a physiotherapist; control group: usual care | Self-report of UI and examiner-assessed PFM strength at 36 weeks’ gestational age and 3 months postpartum | At 36 weeks’ gestational age: prevalence of UI 32 % in the intervention group; 48 % in the control group (p = .007); lower number of urinary leakage in the intervention group (p = .014); higher PFM strength in the intervention group (p = .008) and 3 months after delivery (p = .048) |
| Ko et al. 2011; [100] | RCT | Nulliparous women with UI at gestational ages 16–24 weeks | 300 | Intervention group: 12- week intensive PFME with weekly training session for 45 min led by a physiotherapist; control group: usual care | Urinary symptoms measured by UDI-6, IIQ-7, and self-reported UI | Lower total UDI-6, IIQ-7 scores and self-report rate of UI in the intervention group during late pregnancy and postpartum period |
| Stafne et al. 2012; [101] | RCT | Pregnant women with UI at gestational age 20 weeks | 855 | Intervention group: 12-week PFMT, conducted between 20 and 36 weeks of gestation, with one weekly training session led by a physiotherapist and home exercises at least twice a week.; control group: usual care | Self-reported UI and FI after the intervention period | Fewer reported any weekly UI and FI in the intervention group (p = 0.004 and p = 0.18, respectively) |
| Glazener et al. 2001; [102] | RCT | Women with UI 3 months after childbirth | 747 | Intervention group: advice on PFME and bladder training at 5, 7, and 9 months after delivery by nurses; control group: usual care | Persistence and severity of UI at 12 months after delivery, performance of PFME, FI, well-being, anxiety, and depression | Lower UI in the intervention group of both UI and severe UI (p < 0.037 and p < 0.002, respectively). Lower severity of UI and less FI in intervention group (p = .007 and p = 0.012, respectively). |
| Reilly et al. 2002; [103] | Single-blind RCT | Primigravid women with UI at gestational age 20 weeks with bladder-neck mobility | 268 | Intervention group: antenatal PFME at a 20 weeks’ gestation until delivery, with monthly training session led by a physiotherapist; control group: usual care | Self-report of UI, pad test, and examiner-assessed PFM strength and bladder-neck mobility at 3 months postpartum | Prevalence of UI at 3 months: 19.2 % in intervention group; 32.7 % in control group (p = 0.023). No difference in pad test, PFM strength, and bladder-neck mobility |
RCT randomized controlled trial; UI urinary incontinence; SUI stress urinary incontinence; FI fecal incontinence; PFME pelvic floor muscle exercise; PFMT pelvic floor muscle training; PFM pelvic floor muscle; VAS visual analog scale; UDI-6 Urogenital Distress Inventory-6; IIQ-7 Incontinence Impact Questionnaire-7
PFME is used to strengthen the pelvic floor and periurethral muscles to improve the continence mechanism [44, 87, 95]. As a result, this method can either cure or relieve SUI by reducing incontinence severity, including frequency and volume of urine leakage [45, 96]. PFME prescribed during pregnancy improves pregnancy UI and reduces the prevalence of UI in late pregnancy and early postpartum [35, 97]. Dinc et al. [87] found that pregnant women with PFME had a significant decrease in UI episodes during pregnancy and in the postpartum period. Undoubtedly, their PFM strength increased to a larger extent. Sangsawang and Serisathien [96] investigated the effects of a PFME on the severity of SUI in 66 pregnant women who had SUI at gestational ages of 20–30 weeks. After the PFME program, women in the experimental group had significantly reduced frequency, volume of urine leakage, and score of perceived SUI severity compared with those in the control group. They concluded that the 6-week PFME program was able to decrease symptom severity in pregnant women with SUI. Sampselle et al. [98] studied the effects of PFME on SUI and PFM strength during pregnancy and postpartum in 72 primigravid women with gestational ages of 20 weeks. This study suggested that PFME can reduce the incidence of SUI at gestational ages of 35 weeks and at 6 weeks and 6 months postpartum by significantly increasing PFM strength compared with women who did not perform PFME. Morkved et al. [99] found that pregnant women who performed PFME reported 32 % UI at 36 weeks of gestational ages compared to 48 % in the control group (p = .007). The number of urinary leakage episodes at gestational age 36 weeks was also significantly lower in the experimental group (p = .014). Furthermore, PFM strength was significantly higher in women with PFME at gestational age 36 weeks and 3 months after delivery than in the control group (p = .008 and p = .048, respectively). During late pregnancy and the postpartum period, Ko et al. [100] found that women in the PFME group had significantly lower total Urogenital Distress Inventory-6 (UDI-6) and Incontinence Impact Questionnaire-7 (IIQ-7) scores; their self-report rate of UI was also less than in the control group. This study concluded that PFME applied in pregnancy was effective in treating and preventing UI during pregnancy, and this effect may persist to postpartum period.
Stafne et al. [101] assessed the effect of a 12-week pelvic floor muscle training (PFMT) to reduce self-reported UI and fecal incontinence (FI) in late pregnancy in 855 women. After the intervention, the authors found that fewer women in the intervention group reported any weekly UI and FI (11 % vs. 19 %, P = 0.004 and 3 % vs. 5 %, P = 0.18, respectively). They indicated that pregnant women who practice PFMT can prevent and treat UI in late pregnancy. Glazener et al. [102] studied the effects of PFME on the severity of UI at 12 months after delivery in 747 mothers who still had UI 3 months postpartum. They found that mothers in the intervention group had significantly less UI and severe UI than the control group (59.9 % vs 69.0 %, p < 0.037 and 19.7 % vs 31.8 %, p < 0.002, respectively). FI was also less common in the intervention group than the control group (4.4 % vs 10.5 %, p = 0.012). The authors concluded that PFMT provided by nurses seems to reduce UI and FI that persists for 12 months postpartum. Reilly et al. [103] studied the effects of antenatal PFME at a gestational age of 20 weeks until delivery for the prevention of postpartum SUI in 268 primigravid women with bladder-neck mobility. The authors suggested that antenatal PFME are effective in reducing the risk of postpartum SUI in primigravid women with bladder-neck mobility at 3 months postpartum. However, there were no changes in bladder-neck mobility and no difference in PFM strength between groups after exercise.
PFME is a specific exercise for PFM and is different from exercise of other muscles in the body. Thus, PFME requires a strong commitment from women. Moreover, this form of exercise requires dedication, endurance, and effort on the part of women in order to result in effective SUI treatment [104].
Women without symptoms of SUI may not want to invest their time in PFME [93]. Women with moderate to severe symptoms of SUI may achieve more improvement in their incontinence than women with mild symptoms. Alewijnse et al. [105] found that women with severe symptoms were more likely to maintain a higher adherence to PFME than women with mild to moderate symptoms. Burns et al. [106] argued that women with mild symptoms may not perceive their incontinence as a serious problem, whereas patients with severe symptoms recognized the incontinence as an ongoing problem and view adherence to exercise as positive training to affect a cure. Findings of that study are in accordance with the study of Sangsawang and Serisathien [96], who found that more than half (54.3 %) and nearly half (45.7 %) of pregnant women in the intervention group had severe and moderate symptoms of SUI, respectively, before participating in the program. Thus, pregnant women may have perceived their incontinence symptoms as a serious problem that could affect their QoL. After completing the program at the sixth week, 100 % of pregnant women in the intervention group were able to perform PFME every day during participated in the program [96]. Women with SUI symptoms were encouraged to perform this exercise continually, resulting in alleviation of SUI symptoms and contributing to the continence mechanism. Morkved et al. [99] found that 19 % of pregnant women had some noncompliance (i.e., skipping classes) in the training schedules of a 12-week antepartum PFME, which is a high rate compared with compliance in drug trials of similar duration. Goode et al. [107] found that 11 % of women dropped out of the 8-week PFME class because the treatment required too much effort and time. In a 15-year follow-up, long-term adherence to PFME was found to be low, with no difference between intensive and home training programs [108].
In summary, PFME is the first-line conservative treatment for women with SUI before consideration of other treatments [97]. PFME is more a effective treatment for SUI during pregnancy because it is a safe treatment without complications, inexpensive, simple to perform, does not require instruments, and can be done anywhere and anytime [97, 109].
Conclusions
The most common type of UI in pregnant women is SUI. The number of pregnant women with SUI in our literature review was variable, with prevalence ranging from 18.6 % to 75 % [16–32, 34] and increasing with gestational age. The increasing pressure of the growing uterus and fetal weight on PFM throughout pregnancy, together with pregnancy-related hormonal changes, may lead to reduced strength of the supportive and sphincteric function of PFM. PFM weakness causes bladder-neck and urethral mobility, thus leading to urethral sphincter incompetence. Hence, when intra-abdominal pressure increases, pressure inside the bladder becomes greater than the urethral closure pressure, and the urethral sphincter is not strong enough to close the urethra, resulting in urine leakage. PFME is an effective treatment for SUI during pregnancy and has no significant adverse effects. Continence can be improved when incontinent pregnant women adequately perform PFME.
Acknowledgments
The authors thank Dr. Denchai Laiwattana, M.D., for valuable critique of the manuscript.
Conflicts of interest
None.
Footnotes
A related article can be found at doi:10.1007/s00192-012-2017-3.
References
- 1.Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int Urogynecol J. 2010;21(1):5–26. doi: 10.1007/s00192-009-0976-9. [DOI] [PubMed] [Google Scholar]
- 2.Dolan LM, Walsh D, Hamilton S, Marshall K, Thompson K, Ashe RG. A study of quality of life in primigravidae with urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2004;15:160–164. doi: 10.1007/s00192-004-1128-x. [DOI] [PubMed] [Google Scholar]
- 3.Mascarenhas T, Coelho R, Oliveira M, Patricio B (2003) Impact of urinary incontinence on quality of life during pregnancy and after childbirth. Paper presented at the 33th annual meeting of the International Continence Society, Florence, Italy, October 9, 2003
- 4.van de Pol GG, Van Brummen HJ, Bruinse HW, Heintz AP, van der Vaart CH. Is there an association between depressive and urinary symptoms during and after pregnancy? Int Urogynecol J Pelvic Floor Dysfunct. 2007;18:1409–1415. doi: 10.1007/s00192-007-0371-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Brummen HJ, Bruinse HW, Van de Pol G, Heintz AP, Van der Vaart CH. What is the effect of overactive bladder symptoms on woman’s quality of life during and after first pregnancy? BJU Int. 2006;97(2):296–300. doi: 10.1111/j.1464-410X.2006.05936.x. [DOI] [PubMed] [Google Scholar]
- 6.van Brummen HJ, Bruinse HW, van de Pol G, Heintz AP, van der Vaart CH. Bothersome lower urinary tract symptoms 1 year after first delivery: prevalence and the effect of childbirth. BJU Int. 2006;98(1):89–95. doi: 10.1111/j.1464-410X.2006.06211.x. [DOI] [PubMed] [Google Scholar]
- 7.Milsom I, Altman D, Lapitan MC, Nelson R, Sillén U, Thom D. Epidemiology of urinary (UI) and faecal (FI) incontinence and pelvic organ prolapse (POP) In: Abrams P, Cardozo L, Khoury S, Wein A, editors. Incontinence: 4th International Consultation on Incontinence. Paris: Health Publication Ltd; 2009. pp. 35–112. [Google Scholar]
- 8.Kocaöz S, Talas MS, Atabekoğlu CS. Urinary incontinence in pregnant women and their quality of life. J Clin Nurs. 2010;19(23–24):3314–3323. doi: 10.1111/j.1365-2702.2010.03421.x. [DOI] [PubMed] [Google Scholar]
- 9.Adaji SE, Shittu OS, Bature SB, Nasir S, Olatunji O. Suffering in silence: pregnant women’s experience of urinary incontinence in Zaria, Nigeria. Eur J Obstet Gynecol Reprod Biol. 2010;150(1):19–23. doi: 10.1016/j.ejogrb.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 10.Marecki M, Seo JY. Perinatal urinary and fecal incontinence: suffering in silence. J Perinat Neonatal Nurs. 2010;24(4):330–340. doi: 10.1097/JPN.0b013e3181ec0d9b. [DOI] [PubMed] [Google Scholar]
- 11.King JK, Freeman RM. Is antenatal bladder neck mobility a risk factor for postpartum stress urinary incontinence? Br J Obstet Gynaecol. 1998;105:1300–1307. doi: 10.1111/j.1471-0528.1998.tb10009.x. [DOI] [PubMed] [Google Scholar]
- 12.Schytt E, Lindmark G, Waldenstrom U. Symptoms of stress incontinence 1 year after childbirth: prevalence and predictors in a national Swedish sample. Acta Obstet Gynecol Scand. 2004;83:928–936. doi: 10.1111/j.0001-6349.2004.00431.x. [DOI] [PubMed] [Google Scholar]
- 13.Burgio KL, Zyczynski H, Locher JL, Richter HE, Redden DT, Wright KC. Urinary incontinence in the 12-month postpartum period. Obstet Gynecol. 2003;102:1291–1298. doi: 10.1016/j.obstetgynecol.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 14.Hvidman L, Foldspang A, Mommsen S, Nielsen JB. Postpartum urinary incontinence. Acta Obstet Gynecol Scand. 2003;82:556–563. doi: 10.1034/j.1600-0412.2003.00132.x. [DOI] [PubMed] [Google Scholar]
- 15.Liang CC, Wu MP, Lin SJ, Lin YJ, Chang SD, Wang HH. Clinical impact of and contributing factors to urinary incontinence in women 5 years after first delivery. Int Urogynecol J. 2013;24(1):99–104. doi: 10.1007/s00192-012-1855-3. [DOI] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 21;339:b2535 [PMC free article] [PubMed]
- 17.Wesnes SL, Rortveit G, Bø K, Hunskaar S. Urinary incontinence during pregnancy. Obstet Gynecol. 2007;109(4):922–928. doi: 10.1097/01.AOG.0000257120.23260.00. [DOI] [PubMed] [Google Scholar]
- 18.Mørkved S, Bø K. Prevalence of urinary incontinence during pregnancy and postpartum. Int Urogynecol J Pelvic Floor Dysfunct. 1999;10:394–398. doi: 10.1007/s001920050067. [DOI] [PubMed] [Google Scholar]
- 19.Mason L, Glenn S, Walton I, Appletion C. The prevalence of stress incontinence during pregnancy and following delivery. Midwifery. 1999;15(2):120–128. doi: 10.1016/s0266-6138(99)90008-6. [DOI] [PubMed] [Google Scholar]
- 20.Diez-Itza I, Ibañez L, Arrue M, Paredes J, Murgiondo A, Sarasqueta C. Influence of maternal weight on the new onset of stress urinary incontinence in pregnant women. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20(10):1259–1263. doi: 10.1007/s00192-009-0923-9. [DOI] [PubMed] [Google Scholar]
- 21.Whitford HM, Alder B, Jones M. A cross-sectional study of knowledge and practice of pelvic floor exercises during pregnancy and associated symptoms of stress urinary incontinence in North-East Scotland. Midwifery. 2007;23(2):204–217. doi: 10.1016/j.midw.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 22.Huebner M, Antolic A, Tunn R. The impact of pregnancy and vaginal delivery on urinary incontinence. Int J Gynecol Obstet. 2010;110(3):249–251. doi: 10.1016/j.ijgo.2010.04.019. [DOI] [PubMed] [Google Scholar]
- 23.Viktrup L, Lose G, Rolff M, Barford K. The symptom of stress incontinence caused by pregnancy or delivery in primiparas. Obstet Gynecol. 1992;79:945–949. [PubMed] [Google Scholar]
- 24.Zhu L, Li L, Lang JH, Xu T. Prevalence and risk factors for peri- and postpartum urinary incontinence in primiparous women in China: a prospective longitudinal study. Int Urogynecol J. 2012;23(5):563–572. doi: 10.1007/s00192-011-1640-8. [DOI] [PubMed] [Google Scholar]
- 25.Liang CC, Chang SD, Lin SJ, Lin YJ. Lower urinary tract symptoms in primiparous women before and during pregnancy. Arch Gynecol Obstet. 2012;285(5):1205–1210. doi: 10.1007/s00404-011-2124-2. [DOI] [PubMed] [Google Scholar]
- 26.Sun MJ, Chen GD, Chang SY, Lin KC, Chen SY. Prevalence of lower urinary tract symptoms during pregnancy in Taiwan. J Formos Med Assoc. 2005;104(3):185–189. [PubMed] [Google Scholar]
- 27.Al-Mehaisen LM, Al-Kuran O, Lataifeh IM, et al. Prevalence and frequency of severity of urinary incontinence symptoms in late pregnancy: a prospective study in the north of Jordan. Arch Gynecol Obstet. 2009;279(4):499–503. doi: 10.1007/s00404-008-0720-6. [DOI] [PubMed] [Google Scholar]
- 28.Sharma JB, Aggarwal S, Singhal S, Kumar S, Roy KK. Prevalence of urinary incontinence and other urological problems during pregnancy: a questionnaire based study. Arch Gynecol Obstet. 2008;279(6):845–851. doi: 10.1007/s00404-008-0831-0. [DOI] [PubMed] [Google Scholar]
- 29.Thomason AD, Miller JM, Delancey JO. Urinary incontinence symptoms during and after pregnancy in continent and incontinent primiparas. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(2):147–151. doi: 10.1007/s00192-006-0124-8. [DOI] [PubMed] [Google Scholar]
- 30.Raza-Khan F, Graziano S, Kenton K, Shott S, Brubaker L. Peripartum urinary incontinence in a racially diverse obstetrical population. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17(5):525–530. doi: 10.1007/s00192-005-0061-y. [DOI] [PubMed] [Google Scholar]
- 31.Martins G, Soler ZA, Cordeiro JA, Amaro JL, Moore KN. Prevalence and risk factors for urinary incontinence in healthy pregnant Brazilian women. Int Urogynecol J. 2010;21(10):1271–1277. doi: 10.1007/s00192-010-1185-2. [DOI] [PubMed] [Google Scholar]
- 32.Chiarelli P, Campbell E. Incontinence during pregnancy. Prevalence and opportunities for continence promotion. Aust N Z J Obstet Gynaecol. 1997;37(1):66–73. doi: 10.1111/j.1479-828x.1997.tb02220.x. [DOI] [PubMed] [Google Scholar]
- 33.Brown SJ, Donath S, MacArthur C, McDonald EA, Krastev AH. Urinary incontinence in nulliparous women before and during pregnancy: prevalence, incidence, and associated risk factors. Int Urogynecol J. 2010;21(2):193–202. doi: 10.1007/s00192-009-1011-x. [DOI] [PubMed] [Google Scholar]
- 34.Bø K, Pauck Øglund G, Sletner L, Mørkrid K, Jenum A. The prevalence of urinary incontinence in pregnancy among a multi-ethnic population resident in Norway. Br J Obstet Gynaecol. 2012;119(11):1354–1360. doi: 10.1111/j.1471-0528.2012.03435.x. [DOI] [PubMed] [Google Scholar]
- 35.Fritel X, Fauconnier A, Bader G, et al. Diagnosis and management of adult female stress urinary incontinence: guidelines for clinical practice from the French College of Gynaecologists and Obstetricians. Eur J Obstet Gynecol Reprod Biol. 2010;151(1):14–19. doi: 10.1016/j.ejogrb.2010.02.041. [DOI] [PubMed] [Google Scholar]
- 36.Wijma J, Weis Potters AE, Tinga DJ, Aarnoudse JG. The diagnostic strength of the 24-h pad test for self-reported symptoms of urinary incontinence in pregnancy and after childbirth. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(4):525–530. doi: 10.1007/s00192-007-0472-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thorp JM, Norton PA, Wall LL, Kuller JA, Eucker B, Wells E. Urinary incontinence in pregnancy and the puerperium: a prospective study. Am J Obstet Gynecol. 1999;181:266–273. doi: 10.1016/s0002-9378(99)70546-6. [DOI] [PubMed] [Google Scholar]
- 38.van Kerrebroeck P, Abrams P, Chaikin D, et al. The standardisation of terminology in nocturia: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn. 2002;21(2):179–183. doi: 10.1002/nau.10053. [DOI] [PubMed] [Google Scholar]
- 39.Francis WJ. Disturbances of bladder function in relation to pregnancy. J Obstet Gynaecol Br Emp. 1960;67:353–366. doi: 10.1111/j.1471-0528.1960.tb07013.x. [DOI] [PubMed] [Google Scholar]
- 40.Davis K, Kumar D. Pelvic floor dysfunction: a conceptual framework for collaborative patient-centred care. J Adv Nurs. 2003;43(6):555–568. doi: 10.1046/j.1365-2648.2003.02754.x. [DOI] [PubMed] [Google Scholar]
- 41.Morkved S, Bo K, Schei B, Salvesen KA. Pelvic floor muscle training during pregnancy to prevent urinary incontinence: a single-blind randomized controlled trial. Obstet Gynecol. 2003;101(2):313–319. doi: 10.1016/s0029-7844(02)02711-4. [DOI] [PubMed] [Google Scholar]
- 42.FitzGerald MP, Graziano S. Anatomic and functional changes of the lower urinary tract during pregnancy. Urol Clin N Am. 2007;34(1):7–12. doi: 10.1016/j.ucl.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 43.McKinnie V, Swift SE, Wang W, et al. The effect of pregnancy and mode of delivery on the prevalence of urinary and fecal incontinence. Am J Obstet Gynecol. 2005;193(2):512–518. doi: 10.1016/j.ajog.2005.03.056. [DOI] [PubMed] [Google Scholar]
- 44.Joanna Briggs Institute The Joanna Briggs Institute best practice information sheet: the effectiveness of pelvic floor muscle exercises on urinary incontinence in women following childbirth. Nurs Health Sci. 2011;13(3):378–381. doi: 10.1111/j.1442-2018.2011.00617.x. [DOI] [PubMed] [Google Scholar]
- 45.Morkved S, Salvesen KA, Bo K, Eik-Nes S. Pelvic floor muscle strength and thickness in continent and incontinent nulliparous pregnant women. Int Urogynecol J Pelvic Floor Dysfunct. 2004;15:384–390. doi: 10.1007/s00192-004-1194-0. [DOI] [PubMed] [Google Scholar]
- 46.Hojberg KE, Salvig JD, Winslow NA, Lose G, Secher NJ. Urinary incontinence: prevalence and risk factors at 16 weeks of gestation. Br J Obstet Gynecol. 1999;106:842–850. doi: 10.1111/j.1471-0528.1999.tb08407.x. [DOI] [PubMed] [Google Scholar]
- 47.MacLennan AH, Taylor AW, Wilson DH, Wilson D. The prevalence of pelvic floor disorder and their relationship to gender, age, parity and mode of delivery. Br J Obstet Gynecol. 2000;107:1460–1470. doi: 10.1111/j.1471-0528.2000.tb11669.x. [DOI] [PubMed] [Google Scholar]
- 48.Peyrat L, Haillot O, Bruyere F, Boutin JM, Bertrand P, Lanson Y. Prevalence and risk factors of urinary incontinence in young and middle-aged women. Br J Urol. 2002;89:61–66. doi: 10.1046/j.1464-4096.2001.01813.x. [DOI] [PubMed] [Google Scholar]
- 49.Viktrup L. The risk of urinary tract symptom five years after the first delivery. Neurourol Urodyn. 2002;21(1):2–29. doi: 10.1002/nau.2198. [DOI] [PubMed] [Google Scholar]
- 50.Pregazzi R, Sartore A, Troiano L, et al. Postpartum Urinary symptoms: prevalence and risk factors. Obstet Gynecol. 2002;103(2):179–182. doi: 10.1016/s0301-2115(02)00045-3. [DOI] [PubMed] [Google Scholar]
- 51.Greer WJ, Richter HE, Bartolucci AA, Burgio KL. Obesity and pelvic floor disorders: a systematic review. Obstet Gynecol. 2008;112(2 Pt 1):341–349. doi: 10.1097/AOG.0b013e31817cfdde. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bump RC, Sugerman H, Fantl JA, McClish DM. Obesity and lower urinary tract function in women: effect of surgically induced weight loss. Am J Obstet Gynecol. 1992;166:392–399. doi: 10.1016/s0002-9378(11)91418-5. [DOI] [PubMed] [Google Scholar]
- 53.Glazener CM, Herbison GP, MacArthur C, et al. New postnatal urinary incontinence: obstetric and other risk factors in primiparae. Br J Obstet Gynaecol. 2006;113(2):208–217. doi: 10.1111/j.1471-0528.2005.00840.x. [DOI] [PubMed] [Google Scholar]
- 54.Van Kessel K, Reed S, Newton K, Meier A, Lentz G. The second stage of labor and stress urinary incontinence. Am J Obstet Gynecol. 2011;184(7):1571–1575. doi: 10.1067/mob.2001.114856. [DOI] [PubMed] [Google Scholar]
- 55.Wesnes SL, Hunskaar S, Bo K, Rortveit G. The effect of urinary incontinence status during pregnancy and delivery mode on incontinence postpartum. A cohort study. Br J Obstet Gynaecol. 2009;116(5):700–707. doi: 10.1111/j.1471-0528.2008.02107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gyhagen M, Bullarbo M, Nielsen T, Milsom I. Prevalence and risk factors for pelvic organ prolapse 20 years after childbirth: a national cohort study in singleton primiparae after vaginal or caesarean delivery. Br J Obstet Gynaecol. 2013;120(2):152–160. doi: 10.1111/1471-0528.12020. [DOI] [PubMed] [Google Scholar]
- 57.Singh K, Jakab M, Reid WM, Berger LA, Hoyte L. Three-dimensional magnetic resonance imaging assessment of levator ani morphologic features in different grades of prolapse. Am J Obstet Gynecol. 2003;188:910–915. doi: 10.1067/mob.2003.254. [DOI] [PubMed] [Google Scholar]
- 58.Stothers L, Friedman B. Risk factors for the development of stress urinary incontinence in women. Curr Urol Rep. 2011;12(5):363–369. doi: 10.1007/s11934-011-0215-z. [DOI] [PubMed] [Google Scholar]
- 59.Wijma J, Weis Potters AE, van der Mark TW, Tinga DJ, Aarnoudse JG. Displacement and recovery of the vesical neck position during pregnancy and after childbirth. Neurourol Urodyn. 2007;26(3):372–376. doi: 10.1002/nau.20354. [DOI] [PubMed] [Google Scholar]
- 60.Hilde G, Stær-Jensen J, Ellström Engh M, Brækken IH, Bø K. Continence and pelvic floor status in nulliparous women at midterm pregnancy. Int Urogynecol J. 2012;23(9):1257–1263. doi: 10.1007/s00192-012-1716-0. [DOI] [PubMed] [Google Scholar]
- 61.Dietz HP, Wilson PD. Childbirth and pelvic floor trauma. Best Pract Res Clin Obstet Gynaecol. 2005;19(6):913–924. doi: 10.1016/j.bpobgyn.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 62.Dietz HP, Schierlitz L. Pelvic floor trauma in childbirth—myth or reality? Aust N Z J Obstet Gynaecol. 2005;45(1):3–11. doi: 10.1111/j.1479-828X.2005.00363.x. [DOI] [PubMed] [Google Scholar]
- 63.Keane DP, Sims TJ, Abrams P, Bailey AJ. Analysis of collagen status in premenopausal nulliparous women with genuine stress incontinence. Br J Obstet Gynaecol. 1997;104(9):994–998. doi: 10.1111/j.1471-0528.1997.tb12055.x. [DOI] [PubMed] [Google Scholar]
- 64.Dietz HP, Eldridge A, Grace M, Clarke B. Does pregnancy affect pelvic organ mobility? Aust N Z J Obstet Gynaecol. 2004;44:517–520. doi: 10.1111/j.1479-828X.2004.00301.x. [DOI] [PubMed] [Google Scholar]
- 65.Wijma J, Weis Potters AE, de Wolf BTHM, Tinga DJ, Aarnoudse JG. Anatomical and functional changes in the lower urinary tract during pregnancy. Br J Obstet Gynaecol. 2001;108:726–732. doi: 10.1111/j.1471-0528.2001.00123.x. [DOI] [PubMed] [Google Scholar]
- 66.Jundt K, Scheer I, Schiessl B, Karl K, Friese K, Peschers UM. Incontinence, bladder neck mobility, and sphincter ruptures in primiparous women. Eur J Med Res. 2010;15(6):246–252. doi: 10.1186/2047-783X-15-6-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chaliha C, Kalia V, Stanton SL, Monga A, Sultan AH. Antenatal prediction of postpartum urinary and fecal incontinence. Obstet Gynecol. 1999;94:689–694. doi: 10.1016/s0029-7844(99)00364-6. [DOI] [PubMed] [Google Scholar]
- 68.Falconer C, Ekman G, Malmstrom A, Ulmsten U. Decreased collagen synthesis in stress-incontinence women. Obstet Gynecol. 1994;84:583–586. [PubMed] [Google Scholar]
- 69.Chen B, Wen Y, Yu X, Polan ML. The role of neutrophil elastase in elastin metabolism of pelvic tissues from women with stress urinary incontinence. Neurourol Urodyn. 2007;26(2):274–279. doi: 10.1002/nau.20347. [DOI] [PubMed] [Google Scholar]
- 70.Lin G, Shindel AW, Banie L, et al. Molecular mechanisms related to parturition-induced stress urinary incontinence. Eur Urol. 2009;55(5):1213–1222. doi: 10.1016/j.eururo.2008.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Skorupski P, Jankiewicz K, Miotła P et al (2012) The polymorphisms of the MMP-1 and the MMP-3 genes and the risk of pelvic organ prolapse. Int Urogynecol J. doi:10.1007/s00192-012-1970-1 [DOI] [PMC free article] [PubMed]
- 72.Altman D, Forsman M, Falconer C, Lichtenstein P. Genetic influence on stress urinary incontinence and pelvic organ prolapse. Eur Urol. 2008;54(4):918–922. doi: 10.1016/j.eururo.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 73.Dietz HP, Hansell NK, Grace ME, Eldridge AM, Clarke B, Martin NG. Bladder neck mobility is a heritable trait. Br J Obstet Gynaecol. 2005;112(3):334–339. doi: 10.1111/j.1471-0528.2004.00428.x. [DOI] [PubMed] [Google Scholar]
- 74.Lince SL, van Kempen LC, Vierhout ME, Kluivers KB. A systematic review of clinical studies on hereditary factors in pelvic organ prolapse. Int Urogynecol J. 2012;23(10):1327–1336. doi: 10.1007/s00192-012-1704-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hilton P, Dolan LM. Pathophysiology of urinary incontinence and pelvic organ prolapse. Br J Obstet Gynaecol. 2004;111(sup.1):5–9. doi: 10.1111/j.1471-0528.2004.00458.x. [DOI] [PubMed] [Google Scholar]
- 76.Kristiansson P, Samuelsson E, Schoultz B, Svardsudd K. Reproductive hormones and stress urinary incontinence in pregnancy. Acta Obstet Gynecol Scand. 2001;80:1125–1130. doi: 10.1034/j.1600-0412.2001.801209.x. [DOI] [PubMed] [Google Scholar]
- 77.Kristiansson P, Svardsudd K, Schoultz B. Serum relaxin, symphyseal pain, and back pain during pregnancy. Am J Obstet Gynecol. 1996;175:1342–1347. doi: 10.1016/s0002-9378(96)70052-2. [DOI] [PubMed] [Google Scholar]
- 78.Harvey MA, Johnston SL, Davies GA. Mid-trimester serum relaxin concentrations and post-partum pelvic floor dysfunction. Acta Obstet Gynecol Scand. 2008;87(12):1315–1321. doi: 10.1080/00016340802460321. [DOI] [PubMed] [Google Scholar]
- 79.Chaliha C, Stanton SL. Urological problems in pregnancy. Br J Urol. 2002;89:469–476. doi: 10.1046/j.1464-410x.2002.02657.x. [DOI] [PubMed] [Google Scholar]
- 80.van Geelen JM, Lemmens WA, Eskes TK, Martin CB., Jr The urethral pressure profile in pregnancy and after delivery in healthy nulliparous women. Am J Obstet Gynecol. 1982;144(6):636–649. doi: 10.1016/0002-9378(82)90431-8. [DOI] [PubMed] [Google Scholar]
- 81.Swift SE, Ostergard DR. Effects of progesterone on the urinary tract. Int Urogynecol J. 1993;4(4):232–236. [Google Scholar]
- 82.Tincello DG, Teare J, Fraser WD. Second trimester concentration of relaxin and pregnancy related incontinence. Eur J Obstet Gynecol Reprod Biol. 2003;106:237–238. doi: 10.1016/s0301-2115(02)00360-3. [DOI] [PubMed] [Google Scholar]
- 83.Chaliha C, Bland JM, Monga A, Stanton SL, Sultan AH. Pregnancy and derivery: a urodynamic viewpoint. Br J Obstet Gynaecol. 2000;107:1354–1359. doi: 10.1111/j.1471-0528.2000.tb11647.x. [DOI] [PubMed] [Google Scholar]
- 84.Viktrup L, Rortveit G, Lose G. Risk of stress urinary incontinence twelve years after the first pregnancy and delivery. Obstet Gynecol. 2006;108(2):248–254. doi: 10.1097/01.AOG.0000226860.01127.0e. [DOI] [PubMed] [Google Scholar]
- 85.Thom DH, Rortveit G. Prevalence of postpartum urinary incontinence: a systematic review. Acta Obstet Gynecol Scand. 2010;89(12):1511–1522. doi: 10.3109/00016349.2010.526188. [DOI] [PubMed] [Google Scholar]
- 86.Viktrup L, Lose G, Rolf M, Barfoed K. The frequency of urinary symptoms during pregnancy and puerperium in the primipara. Int Urogynecol J. 1993;4(1):27–30. [Google Scholar]
- 87.Dinc A, Kizilkaya Beji N, Yalcin O. Effect of pelvic floor muscle exercises in the treatment of urinary incontinence during pregnancy and the postpartum period. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20(10):1223–1231. doi: 10.1007/s00192-009-0929-3. [DOI] [PubMed] [Google Scholar]
- 88.Serati M, Salvatore S, Khullar V, et al. Prospective study to assess risk factors for pelvic floor dysfunction after delivery. Acta Obstet Gynecol Scand. 2008;87(3):313–318. doi: 10.1080/00016340801899008. [DOI] [PubMed] [Google Scholar]
- 89.Groutz A, Rimon E, Peled S, et al. Cesarean section: does it really prevent the development of postpartum stress urinary incontinence? A prospective study of 363 women one year after their first delivery. Neurourol Urodyn. 2004;23(1):2–6. doi: 10.1002/nau.10166. [DOI] [PubMed] [Google Scholar]
- 90.Arrue M, Ibañez L, Paredes J, et al. Stress urinary incontinence six months after first vaginal delivery. Eur J Obstet Gynecol Reprod Biol. 2010;150(2):210–214. doi: 10.1016/j.ejogrb.2010.02.039. [DOI] [PubMed] [Google Scholar]
- 91.Hvidman L, Foldspang A, Mommsen S, Bugge Nielsen J. Correlates of urinary incontinence in pregnancy. Int Urogynecol J Pelvic Floor Dysfunct. 2002;13(5):278–283. doi: 10.1007/s001920200061. [DOI] [PubMed] [Google Scholar]
- 92.Fritel X, Ringa V, Quiboeuf E, Fauconnier A. Female urinary incontinence, from pregnancy to menopause: a review of epidemiological and pathophysiological findings. Acta Obstet Gynecol Scand. 2012;91(8):901–910. doi: 10.1111/j.1600-0412.2012.01419.x. [DOI] [PubMed] [Google Scholar]
- 93.Brostrøm S, Lose G. Pelvic floor muscle training in the prevention and treatment of urinary incontinence in women—what is the evidence? Acta Obstet Gynecol Scand. 2008;87(4):384–402. doi: 10.1080/00016340801938806. [DOI] [PubMed] [Google Scholar]
- 94.Kegel N. Progressive resistance exercise in the functional retroration of the perineal muscle. Am J Obstet Gynaecol. 1948;56:238–248. doi: 10.1016/0002-9378(48)90266-x. [DOI] [PubMed] [Google Scholar]
- 95.Hay-Smith J, Herbison P, Mørkved S (2002) Physical therapies for prevention of urinary and faecal incontinence in adults. Cochrane Database Syst Rev (2):CD003191 [DOI] [PubMed]
- 96.Sangsawang B, Serisathien Y. Effect of pelvic floor muscle exercise programme on stress urinary incontinence among pregnant women. J Adv Nurs. 2012;68(9):1997–2007. doi: 10.1111/j.1365-2648.2011.05890.x. [DOI] [PubMed] [Google Scholar]
- 97.Price N, Dawood R, Jackson SR. Pelvic floor exercise for urinary incontinence: a systematic literature review. Maturitas. 2010;67(4):309–315. doi: 10.1016/j.maturitas.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 98.Sampselle CM, Miller JM, Mims BL, Delancey JOL, Ashton-Miller JA, Antonakos CL. Effect of pelvic muscle exercise on transient incontinence during pregnancy and after birth. Obstet Gynecol. 1998;91(3):406–412. doi: 10.1016/s0029-7844(97)00672-8. [DOI] [PubMed] [Google Scholar]
- 99.Morkved S, Bo K, Schei B, Salvesen KA. Pelvic floor muscle training during pregnancy to prevent urinary incontinence: a single-blind randomized controlled trial. Obstet Gynecol. 2003;101(2):313–319. doi: 10.1016/s0029-7844(02)02711-4. [DOI] [PubMed] [Google Scholar]
- 100.Ko PC, Liang CC, Chang SD, Lee JT, Chao AS, Cheng PJ. A randomized controlled trial of antenatal pelvic floor exercises to prevent and treat urinary incontinence. Int Urogynecol J. 2011;22(1):17–22. doi: 10.1007/s00192-010-1248-4. [DOI] [PubMed] [Google Scholar]
- 101.Stafne S, Salvesen K, Romundstad P, Torjusen I, Mørkved S. Does regular exercise including pelvic floor muscle training prevent urinary and anal incontinence during pregnancy? A randomised controlled trial. Br J Obstet Gynaecol. 2012;119(10):1270–1280. doi: 10.1111/j.1471-0528.2012.03426.x. [DOI] [PubMed] [Google Scholar]
- 102.Glazener CM, Herbison GP, Wilson PD, et al. Conservative management of persistent postnatal urinary and faecal incontinence: randomised controlled trial. BMJ. 2001;323(7313):593–596. doi: 10.1136/bmj.323.7313.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Reilly ET, Freeman RM, Waterfield MR, Waterfield AE, Steggles P, Pedlar F. Prevention of postpartum stress incontinence in primigravidae with increased bladder neck mobility: a randomised controlled trial of antenatal pelvic floor exercises. Br J Obstet Gynaecol. 2002;109(1):68–76. doi: 10.1111/j.1471-0528.2002.t01-1-01116.x. [DOI] [PubMed] [Google Scholar]
- 104.Newman DK. Conservative management of urinary incontinence in women. Prim Care Updat Obstet Gynecol. 2001;8(4):153–162. doi: 10.1016/s1068-607x(01)00076-2. [DOI] [PubMed] [Google Scholar]
- 105.Alewijnse D, Mesters I, Metsemakers J, van den Borne B. Predictors of long-term adherence to pelvic floor muscle exercise therapy among women with urinary incontinence. Heal Educ Res. 2003;18(5):511–524. doi: 10.1093/her/cyf043. [DOI] [PubMed] [Google Scholar]
- 106.Burns PA, Pranikoff K, Nochajski TH, Hadley EC, Levy KJ, Ory MG. A comparison of effectiveness of biofeedback and pelvic muscle exercise treatment of stress incontinence in older community-dwelling women. J Gerontol. 1993;48:167–174. doi: 10.1093/geronj/48.4.m167. [DOI] [PubMed] [Google Scholar]
- 107.Goode PS, Burgio KL, Locher JL, et al. Effect of behavioral training with or without pelvic floor electrical stimulation on stress incontinence in women: a randomized controlled trial. JAMA. 2003;290(3):345–352. doi: 10.1001/jama.290.3.345. [DOI] [PubMed] [Google Scholar]
- 108.Bø K, Kvarstein B, Nygaard I. Lower urinary tract symptoms and pelvic floor muscle exercise adherence after 15 years. Obstet Gynecol. 2005;105(5 Pt 1):999–1005. doi: 10.1097/01.AOG.0000157207.95680.6d. [DOI] [PubMed] [Google Scholar]
- 109.Allahdin S, Kambhampati L. Stress urinary incontinence in continent primigravidas. J Obstet Gynaecol. 2012;32(1):2–5. doi: 10.3109/01443615.2011.626542. [DOI] [PubMed] [Google Scholar]