Abstract
Following the sexual phase of its life cycle, the hypotrichous ciliate Oxytricha nova transforms a copy of its chromosomal micronucleus into a macronucleus containing short, linear DNA molecules with an average size of 2.2 kilobase pairs. In addition, more than 90% of the DNA sequences in the micronuclear genome are eliminated during this process. We have examined the organization of macronuclear DNA molecules in the micronuclear chromosomes. Macronuclear DNA molecules were found to be clustered and separated by less than 550 base pairs in two cloned segments of micronuclear DNA. Recombinant clones of two macronuclear DNA molecules that are adjacent in the micronucleus were also isolated and examined by DNA sequencing. The two macronuclear DNA molecules were found to be separated by only 90 base pairs in the micronuclear genome.
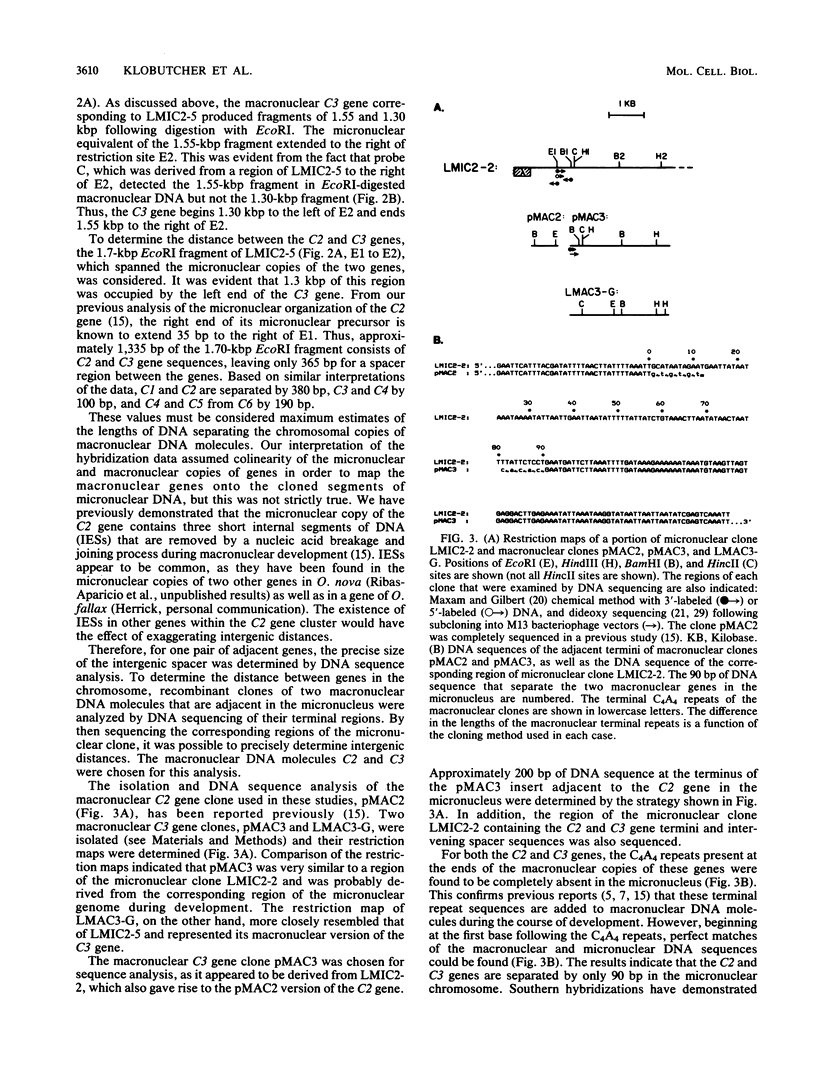
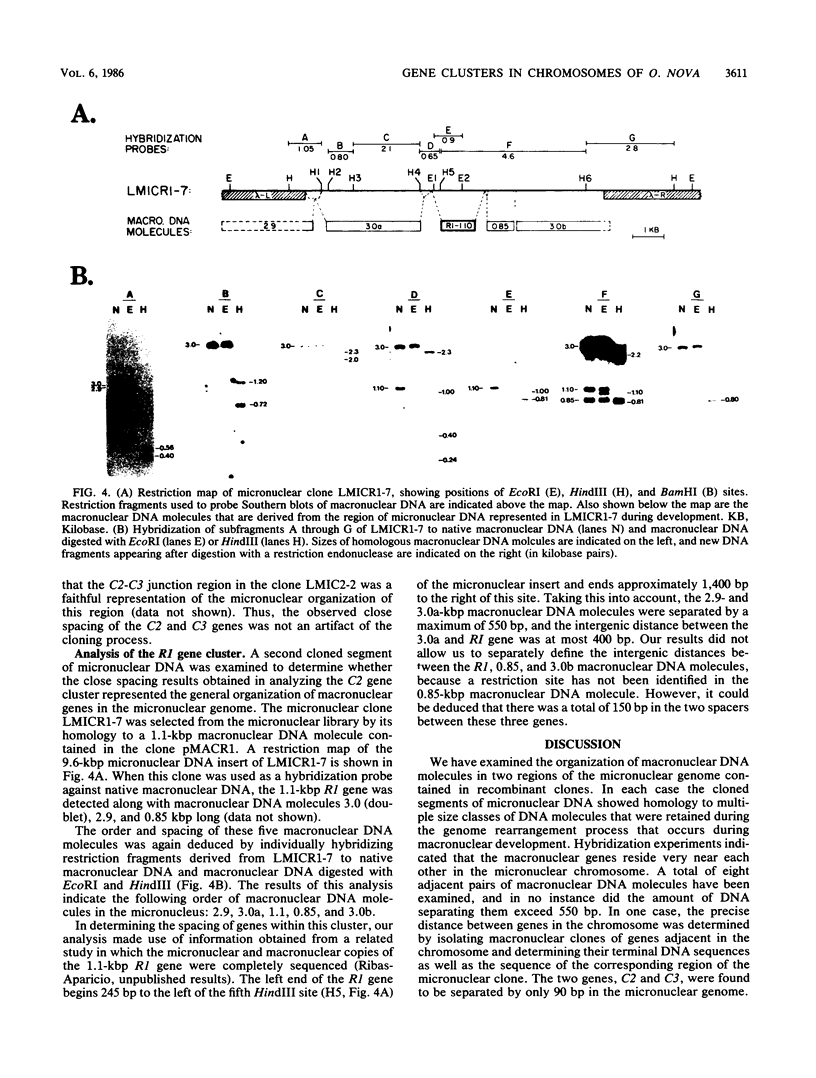
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ammermann D. Morphology and development of the macronuclei of the ciliates Stylonychia mytilus and Euplotes aediculatus. Chromosoma. 1971;33(2):209–238. doi: 10.1007/BF00285634. [DOI] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Bolivar F. Construction and characterization of new cloning vehicles. III. Derivatives of plasmid pBR322 carrying unique Eco RI sites for selection of Eco RI generated recombinant DNA molecules. Gene. 1978 Oct;4(2):121–136. doi: 10.1016/0378-1119(78)90025-2. [DOI] [PubMed] [Google Scholar]
- Boswell R. E., Jahn C. L., Greslin A. F., Prescott D. M. Organization of gene and non-gene sequences in micronuclear DNA of Oxytricha nova. Nucleic Acids Res. 1983 Jun 11;11(11):3651–3663. doi: 10.1093/nar/11.11.3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boswell R. E., Klobutcher L. A., Prescott D. M. Inverted terminal repeats are added to genes during macronuclear development in Oxytricha nova. Proc Natl Acad Sci U S A. 1982 May;79(10):3255–3259. doi: 10.1073/pnas.79.10.3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartinhour S. W., Herrick G. A. Three different macronuclear DNAs in Oxytricha fallax share a common sequence block. Mol Cell Biol. 1984 May;4(5):931–938. doi: 10.1128/mcb.4.5.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson D., Herrick G. Rare internal C4A4 repeats in the micronuclear genome of Oxytricha fallax. Mol Cell Biol. 1984 Dec;4(12):2661–2667. doi: 10.1128/mcb.4.12.2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Godson G. N., Vapnek D. A simple method of preparing large amounts of phiX174 RF 1 supercoiled DNA. Biochim Biophys Acta. 1973 Apr 11;299(4):516–520. doi: 10.1016/0005-2787(73)90223-2. [DOI] [PubMed] [Google Scholar]
- Helftenbein E. Nucleotide sequence of a macronuclear DNA molecule coding for alpha-tubulin from the ciliate Stylonychia lemnae. Special codon usage: TAA is not a translation termination codon. Nucleic Acids Res. 1985 Jan 25;13(2):415–433. doi: 10.1093/nar/13.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren R., Livak K., Morimoto R., Freund R., Meselson M. Studies of cloned sequences from four Drosophila heat shock loci. Cell. 1979 Dec;18(4):1359–1370. doi: 10.1016/0092-8674(79)90246-0. [DOI] [PubMed] [Google Scholar]
- Judd B. H., Young M. W. An examination of the one cistron: one chromomere concept. Cold Spring Harb Symp Quant Biol. 1974;38:573–579. doi: 10.1101/sqb.1974.038.01.061. [DOI] [PubMed] [Google Scholar]
- Klobutcher L. A., Jahn C. L., Prescott D. M. Internal sequences are eliminated from genes during macronuclear development in the ciliated protozoan Oxytricha nova. Cell. 1984 Apr;36(4):1045–1055. doi: 10.1016/0092-8674(84)90054-0. [DOI] [PubMed] [Google Scholar]
- Kühn S., Fritz H. J., Starlinger P. Close vicinity of IS1 integration sites in the leader sequence of the gal operon of E. coli. Mol Gen Genet. 1979 Jan 2;167(3):235–241. doi: 10.1007/BF00267414. [DOI] [PubMed] [Google Scholar]
- Lauth M. R., Spear B. B., Heumann J., Prescott D. M. DNA of ciliated protozoa: DNA sequence diminution during macronuclear development of Oxytricha. Cell. 1976 Jan;7(1):67–74. doi: 10.1016/0092-8674(76)90256-7. [DOI] [PubMed] [Google Scholar]
- Loenen W. A., Brammar W. J. A bacteriophage lambda vector for cloning large DNA fragments made with several restriction enzymes. Gene. 1980 Aug;10(3):249–259. doi: 10.1016/0378-1119(80)90054-2. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerowitz E. M., Hogness D. S. Molecular organization of a Drosophila puff site that responds to ecdysone. Cell. 1982 Jan;28(1):165–176. doi: 10.1016/0092-8674(82)90386-5. [DOI] [PubMed] [Google Scholar]
- Nock A. RNA and macronuclear transcription in the ciliate Stylonychia mytilus. Chromosoma. 1981;83(2):209–220. doi: 10.1007/BF00286790. [DOI] [PubMed] [Google Scholar]
- Pluta A. F., Dani G. M., Spear B. B., Zakian V. A. Elaboration of telomeres in yeast: recognition and modification of termini from Oxytricha macronuclear DNA. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1475–1479. doi: 10.1073/pnas.81.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott D. M., Murti K. G., Bostock C. J. Genetic apparatus of Stylonychia sp. Nature. 1973 Apr 27;242(5400):576, 597-600. doi: 10.1038/242576a0. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Roth M., Prescott D. M. DNA intermediates and telomere addition during genome reorganization in Euplotes crassus. Cell. 1985 Jun;41(2):411–417. doi: 10.1016/s0092-8674(85)80014-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spear B. B., Lauth M. R. Polytene chromosomes of Oxytricha: biochemical and morphological changes during macronuclear development in a ciliated protozoan. Chromosoma. 1976 Jan 27;54(1):1–13. doi: 10.1007/BF00331828. [DOI] [PubMed] [Google Scholar]
- Swanton M. T., Greslin A. F., Prescott D. M. Arrangement of coding and non-coding sequences in the DNA molecules coding for rRNAs in Oxytricha sp. DNA of ciliated protozoa. VII. Chromosoma. 1980;77(2):203–215. doi: 10.1007/BF00329545. [DOI] [PubMed] [Google Scholar]
- Swanton M. T., Heumann J. M., Prescott D. M. Gene-sized DNA molecules of the macronuclei in three species of hypotrichs: size distributions and absence of nicks. DNA of ciliated protozoa. VIII. Chromosoma. 1980;77(2):217–227. doi: 10.1007/BF00329546. [DOI] [PubMed] [Google Scholar]