Abstract
Background
Mesolimbic and nigrostriatal dopaminergic pathways play important roles in both the rewarding and conditioning effects of drugs. The dopamine transporter (DAT) is of central importance in regulating dopaminergic neurotransmission and in particular in activating the striatal D2-like receptors. Molecular imaging studies of the relationship between DAT availability/dopamine synthesis capacity and active cigarette smoking have shown conflicting results. Through the collaboration between 13 SPECT centres located in 10 different European countries, a database of FP-CIT-binding in healthy controls was established. We used the database to test the hypothesis that striatal DAT availability is changed in active smokers compared to non-smokers and ex-smokers.
Methods
A total of 129 healthy volunteers were included. Subjects were divided into three categories according to past and present tobacco smoking: (1) non-smokers (n = 64), (2) ex-smokers (n = 39) and (3) active smokers (n = 26). For imaging of the DAT availability, we used [123I]FP-CIT (DaTSCAN) and single photon emission computed tomography (SPECT). Data were collected in collaboration between 13 SPECT centres located in 10 different European countries. The striatal measure of DAT availability was analyzed in a multiple regression model with age, SPECT centre and smoking as predictor.
Results
There was no statistically significant difference in DAT availability between the groups of active smokers, ex-smokers and non-smokers (p = 0.34). Further, we could not demonstrate a significant association between striatal DAT and the number of cigarettes per day or total lifetime cigarette packages in smokers and ex-smokers.
Conclusion
Our results do not support the hypothesis that large differences in striatal DAT availability are present in smokers compared to ex-smokers and healthy volunteers with no history of smoking.
Keywords: Tobacco smoking, Non-smoking, SPECT, [123I]FP-CIT (DaTSCAN), Dopamine transporter
Background
The behavioural and neurobiological effects of smoking are similar to those of other addictive substances [1], and several studies have demonstrated the involvement of the mesolimbic dopaminergic system in mediating the response to cigarette smoking or nicotine intake. Nicotine-induced dopamine release has been demonstrated in rodents [2-4] and non-human primates [5-8]. These findings have been indirectly supported by several positron emission tomography (PET) studies where decreases of [11C]raclopride binding (thought to reflect increases in the extracellular concentration of dopamine) in the ventral striatum/nucleus accumbens following smoking [6,9-12] or nicotine intake [13] were observed. PET-studies, using radiopharmaceuticals for the dopamine D2/3 receptors, have demonstrated significant associations between dopamine release and reduction in craving [9,10], enhancement of pleasure [14,15] and the severity of nicotine dependence [12]. However, the reduction in D2/3 binding upon smoking compared to the baseline condition is modest (5% to 10%) in these studies compared to that following cocaine (20% to 30%) [16] and amphetamine [17-19]. Such an effect of 5% to 10% is similar to the test-retest variability of molecular imaging techniques using PET and SPECT [20-22] and may be difficult to demonstrate in PET and SPECT studies using small data samples. This may explain the failure of some PET studies to demonstrate changes in [11C]raclopride binding upon nicotine administration [14,15]. Dopamine release in the ventral striatum/nucleus accumbens has been demonstrated to be directly mediated through the binding of nicotine to α4β2 nAChRs leading to an increase in firing rate in dopamine neurons of the ventral tegmental area [23,24]. Previous human imaging studies do not provide strong support for the idea that postsynaptic dopamine D2/3 receptor availability is affected by chronic exposure to cigarette smoke. A reduced dopamine D2/3 receptor availability was demonstrated in the putamen in men [25], but not in woman [26]. Two SPECT studies failed to demonstrate changes in striatal dopamine D2/3 receptor availability in smokers compared to non-smokers [27,28].
The dopamine transporter (DAT) provides the primary mechanism through which dopamine is cleared from the extracellular fluid after its release from the presynaptic cell. However, only few studies have addressed the effect of chronic dosing of nicotine on the DAT [29]. Nicotine is not a competitor nor a substrate for DAT [30], and it does not bind to a site on the DAT protein [31]. Nicotine appears to induce changes in DAT function by indirect mechanisms which include both augmentation (enhancing amphetamine-induced reverse transport of dopamine by DAT) and reduction (increase in cell surface DAT expression) of dopaminergic neurotransmission [32]. Human imaging studies of DAT availability in relation to chronic tobacco smoking are sparse and have generated conflicting results [28,33] possibly related to methodological problems including the choice of radioligand and sample size. We studied the DAT availability using [123I]FP-CIT-SPECT in a group of 26 active smokers, 39 ex-smokers and 64 subjects with no history of smoking. We tested the hypothesis that DAT availability is changed in active smokers compared to ex-smokers and subjects with no history of tobacco smoking. Understanding the mechanisms underlying the neurobiological effects of nicotine on the regulation of DAT may have the potential to translate into new and possibly individualized treatment strategies.
Methods
Participants
The European Normal Control Database of DaTSCAN (ENCDAT) study is an initiative taken by the Neuroimaging Committee of the European Association of Nuclear Medicine (EANM). The database was established through the collaboration between 13 SPECT centres located in 10 different European countries. The centres were selected by EANM based on their involvement in SPECT imaging of the dopaminergic system and their high level of experience and quality of brain SPECT imaging. The protocol was approved by the medical ethical committees of all participating centers and was performed in accordance with the ethical standards of the Declaration of Helsinki. All subjects gave written informed consent to participate in the study.
In this study, 129 of the available ENCDAT healthy volunteers (all Caucasian) were included. Twelve healthy volunteers had to be excluded because no imaging data for the scatter windows data were available, and ten healthy volunteers (ex-smokers) had to be excluded because of lifetime usage of less than 60 packages of cigarettes. Our sample had a balanced male-to-female ratio (70 males and 59 females), and the age range was 20 to 83 years (>20 per decade, expected for ages between 80 to 90 years). The distribution between the participating centres was as follows: Amsterdam (n = 9), Ankara (n = 10), Copenhagen (n = 13), Genoa (n = 14), Leipzig (n = 13), Leuven (n = 16), London (n = 10), Munich (n = 11), Nice (n = 4), Southampton (n = 3), Stockholm (n = 13) and Yvoir (n = 13).
Inclusion criteria were the following:
• No history of parkinsonism in first-degree relatives
• No medication known to affect DAT binding
• Absence of psychiatric symptoms as evaluated by the following: Symptom Checklist-90-R score < 63, Beck Depression Inventory score ≤ 9 and Mini-Mental State Examination ≥ 28
• Negative urine screening for drugs (ten drugs)
• Body temperature ≤ 38.5°C on the day of scanning
• Negative pregnancy test in premenopausal females
Smoking data
All subjects were interviewed about tobacco smoking habits (as part of the inclusion criteria) and were quantified through The Copenhagen Smoking Questionnaire [34]. Data regarding the time of the last cigarette before scanning was not available. Subjects were divided into three categories according to past and present tobacco smoking habits: (1) non-smokers (n = 64), (2) ex-smokers (more than 60 total numbers of packages in lifetime, n = 39) and (3) active smokers (1 to 30 cigarettes per day, n = 26), as shown in Table 1. Ten of the active smokers used more than 15 cigarettes per day. For smokers, the cigarette use per day was registered, and for all smokers (active smokers and ex-smokers), the total number of packages in lifetime was registered.
Table 1.
Age and smoking habits for 129 healthy volunteers
| Number | Mean age ± SD (range) in years | Current average number of cigarettes per day ± SD (range) | Total number of packages in lifetime mean (range) | |
|---|---|---|---|---|
| Non-smokers |
64 (34 males) |
51.7 ± 18.5 (20 to 81) |
0 |
0 |
| Ex-smokers |
39 (21 males) |
59.2 ± 15.4 (25 to 83) |
0 |
4,497 (66 to 28,220) |
| Active smokers | 26 (15 males) | 47.2 ± 19.4 (21 to 79) | 11.2 ± 8.6 (1 to 30) | 5,991 (60 to 24,630) |
Data acquisition and reconstruction
Data acquisition was performed according to the ENCDAT protocol (EANM Research Ltd. (EARL)/European Network of Excellence for Brain Imaging) using the scanners and collimators specified in the ENCDAT protocol. All camera systems had passed elaborate quality control and phantom measurements [35]. SPECT acquisition was started 3 to 4 h after an average intravenous bolus of 180.5 MBq (range 152 to 215 MBq) of [123I]FP-CIT (GE Healthcare, Amersham, UK). Image reconstruction was carried out at a core centre using the HERMES HOSEM software (HERMES Medical Solutions, Stockholm, Sweden), using iterative reconstruction with 10 subsets and 10 iterations for 120 projections and 8 subsets and 12 iterations for 128 projections to give a similar number of EM equivalent iterations [36]. Reconstructions were performed with attenuation and scatter corrections using the triple-energy window method. After reconstruction, images were smoothed on the HERMES workstation with a 3D Butterworth filter (cut-off 1.2 cm–1, order 10).
ROI delineation and SBR calculation
We used the ratio of specifically bound radioligand to that of nondisplaceable radioligand in tissue (specific binding ratio (SBR)) calculated between 3 and 4 h after tracer injection as a measure of the DAT availability [37]. Regions of interest (ROIs) were delineated, and SBR was calculated using DATquan (Figure 1) [38]. DATquan offers a fast, accurate, and highly reproducible method for semi-automatic VOI delineation using a template-based approach.
Figure 1.
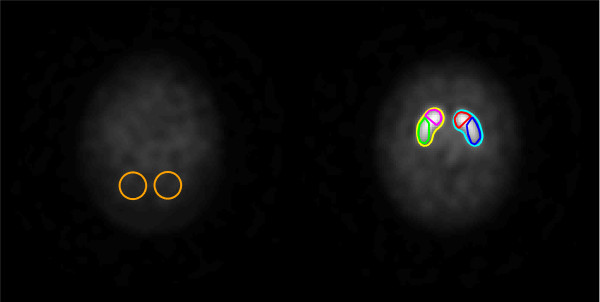
Two horizontal slices from the constructed [123I]-FP-CIT template. Illustrating exact position and configuration of the reference (left) and striatal (right) ROIs.
Results
For the study of the association between DAT measurements and smoking habits, we used a priori a linear model where we adjusted for age, which has previously been identified as an important confounder. Data were collected from several different centers, and to account for potential cluster effects, we therefore used a random intercept model.
In general, similar results were obtained from a more parsimonious model for the clustering based on marginal models with robust standard errors (i.e., a generalized estimating equation framework). Here, we only report results (maximum likelihood estimates) from the random intercept model.
Variance homogeneity of residuals across the different centers was tested using a likelihood ratio test which indicated that this assumption was reasonable (p = 0.77). In all cases, residual analysis revealed that a log transformation of the DAT measurements was favorable (all results based on the log transformation and hence parameters can be interpreted as log relative differences). However, quite similar conclusions were reached based on modeling of the data on their original scale. Linearity of the continuous predictor age was assessed using a linear mixed additive model and by inclusion of polynomial terms in the model. R version 2.15 was used for all analyses [39], http://www.R-project.org/).
Active smoking was not associated with any statistically significant effects on the striatal DAT availability compared to non-smokers (7.7% lower DAT availability, 95% confidence limits (−17.3%, 3.0%), p = 0.15); the same was true for DAT availability in caudate nucleus (95% confidence limits (−16.7%, 3.2%), p = 0.17) and putamen (95% confidence limits (−17.5%, 3.9%), p = 0.19). There was no statistically significant difference in DAT availability between the groups of active smokers, ex-smokers and non-smokers (p = 0.34) as seen in Figure 2.
Figure 2.
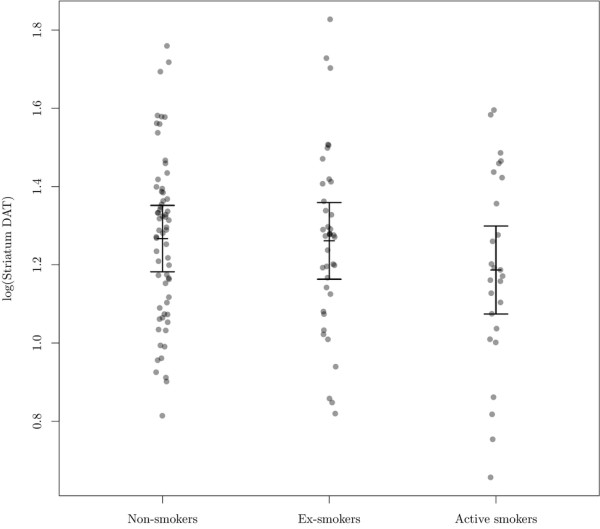
Association between striatal DAT availability and smoking status. The vertical bars shows the estimated mean within each group with 95% confidence limits as estimated by a random intercept model (with a variance component defined by the centre) adjusting for age at scan (reference, mean age = 53 years). Individual points are partial residuals (i.e., best linear unbiased predictor of the residuals plus the estimated intercept within each group).
There was a clear effect of age on DAT availability with an age decline in striatum equal to 4.6% per decade (95% confidence limits (−6.9%, −2.2%)), in line with a previous study [40]. The estimated residual standard deviation within clusters was 0.228, and the standard deviation of the random intercept (between clusters) was 0.115.
As seen in Figure 3, there was no statistically significant association between striatal DAT availability and numbers of cigarettes per day (in smokers) with an estimated 5.6% decrease in DAT availability per ten cigarettes per day ((−12.6%, 1.3%), p = 0.114); furthermore, we found no negative correlation between total cigarette packages in lifetimes and striatal DAT availability (0.6% decrease in DAT availability per 1,000 packages, 95% confidence limits (1.4%, 0.3%), p = 0.20) in caudate nucleus (p = 0.21) and putamen (p = 0.19). There was no statistically significant difference in striatal DAT availability (p = 0.906) in ex-smokers compared to non-smokers and in ex-smokers compared to active smokers (p = 0.152).
Figure 3.
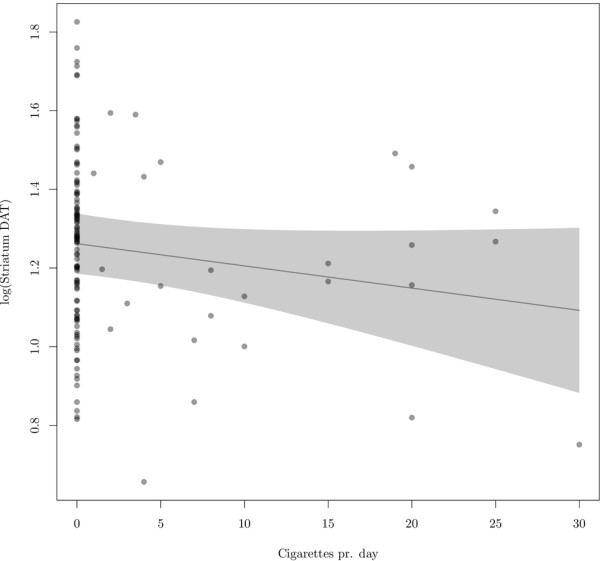
Estimated association between log (striatal DAT availability) and number of cigarettes per day (currently). With 95% pointwise confidence limits as estimated by a random intercept model adjusting for age (reference, mean age = 53 years). Individual points are the partial residuals, defined as the best linear unbiased predictor of the residuals plus the estimated cigarettes per day effect.
Discussion
In this large sample, we did not observe any statistically significant effect of tobacco smoking on striatal DAT availability as measured with [123I]FP-CIT SPECT in active smokers (n = 26) and ex-smokers (n = 39) compared to non-smokers (n = 64). In addition, a statistically significant association between striatal DAT availability and numbers of cigarettes per day or total cigarette packages in lifetimes was not found. Our results in active smokers compared to ex-smokers do not support the idea of changed striatal DAT availability upon daily nicotine administration. However, the possible acute effect of smoking on striatal DAT availability still needs to be explored.
This study is in line with the results of Staley et al. which did not demonstrate changes in striatal [123I]β-CIT binding in 21 smokers compared to 21 non-smokers [41]. [123I]β-CIT binds to both the striatal DAT and the serotonin transporter (SERT), and the non-selectivity of this tracer hampers the conclusions to be drawn from this study on the relative role of DAT compared to SERT in smoking. A recent study by Erritzoe et al. also failed to demonstrate significant differences in the binding of the PET tracer [11C]DASB to SERT in smokers compared to non-smokers [34]. Our study does not replicate the findings of a decreased striatal DAT availability in smokers [28,33] using [99mTc]TRODAT-1 SPECT. We believe that the discrepancies between the findings in the two [99mTc]TRODAT-1 SPECT studies and the results of our study and the study of Staley et al. [41] are related to the sample size (8 and 11 active smokers were included in the [99mTc]TRODAT-1 SPECT studies) and possibly the imaging properties of [99mTc]TRODAT-1 related to a small ratio between binding of tracer in striatum compared to the reference region leading to data being more susceptible to noise. In a [18F]fluorodopa PET of the striatal presynaptic dopamine activity in active smokers (n = 9) compared to non-smokers (n = 10), a significantly higher uptake of striatal [18F]fluorodopa was demonstrated in smokers compared to non-smokers [42]. A higher uptake of [18F]fluorodopa is generally interpreted as a result of an increased dopamine synthesis capacity. However, a higher uptake of [18F]fluorodopa may be tracing several independent processes including BBB transport, competition with other amino acids for transport, uptake into neurons, decarboxylation to fluorodopamine and trapping within neuronal vesicles [43]. Thus, in addition to the small sample of active smokers in the [18F]fluorodopa PET study, the outcome parameter is not directly comparable to studies using radiotracers binding to the DAT.
This study has some limitations. (1) As seen in Table 1, the numbers of cigarettes smoked per day range from 1 to 30 cigarettes, and 10 of the subjects smoked more than 15 cigarettes per day. Thus, the group of heavy smokers is small. However, the DAT availability in the group smoking more than 15 cigarettes a day was not significantly different from ex-smokers and non-smokers. Furthermore, a statistical significant association between cigarettes smoked per day and DAT availability was not demonstrated (Figure 3), and according to our data, we would expect a very small decrease in striatal DAT of 0.6% if an individual consumed an additional 1,000 packages. (2) We did not control for passive smoking. However, based upon our dose–response data, we find it unlikely that passive smoking results in detectable changes in DAT availability in any of the three groups. (3) Though our data sample is larger than the previous studies on DAT availability in smoking, the study is still underpowered to detect subtle changes in DAT availability related to its variation between subjects and a [123I]FP-CIT SPECT test-retest variability of approximately 10%. Though not statistically significant, a trend toward a decrease in DAT availability (5.6% decrease in DAT availability per ten cigarettes per day, p = 0.114) was found. Our study may be underpowered to demonstrate a small effect. (4) In this multi-centre study, information regarding time of last cigarette smoked before injection of the radiotracer and information regarding nicotine dependence, smoking urges and passive smoking was not available. Future studies must address the acute effects of cigarette smoking and thus explain whether DAT binding predisposes to current smoking or whether current smoking influences DAT binding. In contrast to the current study using cross-sectional design, future studies could benefit from a longitudinal design testing DAT availability in patients before and after smoking cessation.
Conclusion
No statistically significant effect of chronic tobacco smoking on striatal DAT availability or changes in DAT availability in previous smokers compared to subjects with no history of smoking was seen with [123I]FP-CIT SPECT. With the limitations underlined in the discussion, our data do not suggest that changes in the dopaminergic system resulting from smoking and/or nicotine administration involve any regulatory changes in DAT. Further studies are needed to address whether the DAT availability is susceptible to acute smoking or nicotine administration.
Competing interests
Prof. Jan Booij is a consultant for GE Healthcare. All other authors have no competing interests.
Authors’ contributions
All authors contributed to the design of the ENCDAT study. The authors MZ, GMK, SA, JB, JD, JCD, OLK, FN, OS, KT, LTB, KVL, TVB and MP recruited the subjects, interviewed the subjects about tobacco smoking habits and acquired SPECT data on the subjects. GT, PSJ, MZ, KKH, JCD, TS, LTB, AV and LHP analyzed the data. GT and LHP drafted the manuscript. All authors read and approved the final manuscript.
Contributor Information
Gerda Thomsen, Email: gerda@nru.dk.
Gitte Moos Knudsen, Email: gitte@nru.dk.
Peter S Jensen, Email: peje@nru.dk.
Morten Ziebell, Email: zeibell@nru.dk.
Klaus K Holst, Email: kkho@biostat.ku.dk.
Susanne Asenbaum, Email: susanne.asenbaum@meduniwien.ac.at.
Jan Booij, Email: j.booij@amc.uva.nl.
Jacques Darcourt, Email: darcourt@unice.fr.
John C Dickson, Email: john.dickson@uclh.nhs.uk.
Özlem L Kapucu, Email: kapucu@gazi.edu.tr.
Flavio Nobili, Email: flaviomariano.nobili@hsanmartino.it.
Osama Sabri, Email: osama.sabri@medizin.uni-leipzig.de.
Terez Sera, Email: sera.terez@med.u-szeged.hu.
Klaus Tatsch, Email: klaus.tatsch@klinikum-karlsruhe.de.
Livia Tossici-Bolt, Email: livia.bolt@suht.swest.nhs.uk.
Koen Van Laere, Email: koen.vanlaere@uz.kuleuven.ac.be.
Thierry Vander Borght, Email: thierry.vanderborght@uclouvain.be.
Andrea Varrone, Email: andrea.varrone@ki.se.
Marco Pagani, Email: marcopagani2@yahoo.it.
Lars Hageman Pinborg, Email: pinborg@nru.dk.
Acknowledgements
The authors thank all the members of the European Network of Excellence Initiative in Neuroimaging. This investigation was part of a project launched by EANM Research Ltd. (EARL)/European Network of Excellence for Brain Imaging whose aim was to create a European Normal Control Database of DaTSCAN (ENCDAT). This work was supported by Rigshospitalet, the Lundbeck Foundation and the Toyota Foundation. The authors also thank Svitlana Olsen and Glenna Skouboe for the expert technical assistance.
References
- Pontieri FE, Tanda G, Orzi F, Di CG. Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs. Nature. 1996;3:255–257. doi: 10.1038/382255a0. [DOI] [PubMed] [Google Scholar]
- Di CG, Imperato A. Opposite effects of mu and kappa opiate agonists on dopamine release in the nucleus accumbens and in the dorsal caudate of freely moving rats. J Pharmacol Exp Ther. 1988;3:1067–1080. [PubMed] [Google Scholar]
- Damsma G, Day J, Fibiger HC. Lack of tolerance to nicotine-induced dopamine release in the nucleus accumbens. Eur J Pharmacol. 1989;3:363–368. doi: 10.1016/0014-2999(89)90798-X. [DOI] [PubMed] [Google Scholar]
- Sziraki LMN, Hashim A, Sershen H, Allen D, Cooper T, Czobor P, Lajtha A. Differences in nicotine-induced dopamine release and nicotine pharmacokinetics between Lewis and Fischer 344 rats. Neurochem Res. 2001;3:609–617. doi: 10.1023/A:1010979018217. [DOI] [PubMed] [Google Scholar]
- Dewey SL, Brodie JD, Gerasimov M, Horan B, Gardner EL, Ashby CR Jr. A pharmacologic strategy for the treatment of nicotine addiction. Synapse. 1999;3:76–86. doi: 10.1002/(SICI)1098-2396(199901)31:1<76::AID-SYN10>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Tsukada H, Miyasato K, Kakiuchi T, Nishiyama S, Harada N, Domino EF. Comparative effects of methamphetamine and nicotine on the striatal [(11)C]raclopride binding in unanesthetized monkeys. Synapse. 2002;3:207–212. doi: 10.1002/syn.10102. [DOI] [PubMed] [Google Scholar]
- Cumming P, Rosa-Neto P, Watanabe H, Smith D, Bender D, Clarke PB, Gjedde A. Effects of acute nicotine on hemodynamics and binding of [11C]raclopride to dopamine D2,3 receptors in pig brain. Neuroimage. 2003;3:1127–1136. doi: 10.1016/S1053-8119(03)00079-X. [DOI] [PubMed] [Google Scholar]
- Marenco S, Carson RE, Berman KF, Herscovitch P, Weinberger DR. Nicotine-induced dopamine release in primates measured with [11C]raclopride PET. Neuropsychopharmacology. 2004;3:259–268. doi: 10.1038/sj.npp.1300287. [DOI] [PubMed] [Google Scholar]
- Brody AL, Olmstead RE, London ED, Farahi J, Meyer JH, Grossman P, Lee GS, Huang J, Hahn EL, Mandelkern MA. Smoking-induced ventral striatum dopamine release. Am J Psychiatry. 2004;3:1211–1218. doi: 10.1176/appi.ajp.161.7.1211. [DOI] [PubMed] [Google Scholar]
- Brody AL. Functional brain imaging of tobacco use and dependence. J Psychiatr Res. 2006;3:404–418. doi: 10.1016/j.jpsychires.2005.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Olmstead RE, Len-Martinez Z, Scheibal D, Abrams AL, Costello MR, Farahi J, Saxena S, Monterosso J, London ED. Ventral striatal dopamine release in response to smoking a regular vs a denicotinized cigarette. Neuropsychopharmacology. 2009;3:282–289. doi: 10.1038/npp.2008.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott DJ, Domino EF, Heitzeg MM, Koeppe RA, Ni L, Guthrie S, Zubieta JK. Smoking modulation of mu-opioid and dopamine D2 receptor-mediated neurotransmission in humans. Neuropsychopharmacology. 2007;3:450–457. doi: 10.1038/sj.npp.1301238. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Fujimura Y, Hayashi M, Takano H, Kato M, Okubo Y, Kanno I, Ito H, Suhara T. Enhanced dopamine release by nicotine in cigarette smokers: a double-blind, randomized, placebo-controlled pilot study. Int J Neuropsychopharmacol. 2008;3:413–417. doi: 10.1017/S1461145707008103. [DOI] [PubMed] [Google Scholar]
- Barrett SP, Boileau I, Okker J, Pihl RO, Dagher A. The hedonic response to cigarette smoking is proportional to dopamine release in the human striatum as measured by positron emission tomography and [11C]raclopride. Synapse. 2004;3:65–71. doi: 10.1002/syn.20066. [DOI] [PubMed] [Google Scholar]
- Montgomery AJ, Lingford-Hughes AR, Egerton A, Nutt DJ, Grasby PM. The effect of nicotine on striatal dopamine release in man: a [11C]raclopride PET study. Synapse. 2007;3:637–645. doi: 10.1002/syn.20419. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Gatley SJ, Dewey SL, Wang GJ, Logan J, Ding YS, Franceschi D, Gifford A, Morgan A, Pappas N, King P. Comparable changes in synaptic dopamine induced by methylphenidate and by cocaine in the baboon brain. Synapse. 1999;3:59–66. doi: 10.1002/(SICI)1098-2396(199901)31:1<59::AID-SYN8>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Carson RE, Breier A, De BA, Saunders RC, Su TP, Schmall B, Der MG, Pickar D, Eckelman WC. Quantification of amphetamine-induced changes in [11C]raclopride binding with continuous infusion. J Cereb Blood Flow Metab. 1997;3:437–447. doi: 10.1097/00004647-199704000-00009. [DOI] [PubMed] [Google Scholar]
- Hartvig P, Torstenson R, Tedroff J, Watanabe Y, Fasth KJ, Bjurling P, Langstrom B. Amphetamine effects on dopamine release and synthesis rate studied in the Rhesus monkey brain by positron emission tomography. J Neural Transm. 1997;3:329–339. doi: 10.1007/BF01277655. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JC, Kupfer DJ, Kinahan PE, Lopresti B, Holt D, Mathis C. PET measures of amphetamine-induced dopamine release in ventral versus dorsal striatum. Neuropsychopharmacology. 1999;3:694–709. doi: 10.1016/S0893-133X(99)00079-2. [DOI] [PubMed] [Google Scholar]
- Ziebell M, Thomsen G, Knudsen GM, De NR, Svarer C, Wagner A, Pinborg LH. Reproducibility of [123I]PE2I binding to dopamine transporters with SPECT. Eur J Nucl Med Mol Imaging. 2007;3:101–109. doi: 10.1007/s00259-006-0161-z. [DOI] [PubMed] [Google Scholar]
- Booij J, Habraken JB, Bergmans P, Tissingh G, Winogrodzka A, Wolters EC, Janssen AG, Stoof JC, van Royen EA. Imaging of dopamine transporters with iodine-123-FP-CIT SPECT in healthy controls and patients with Parkinson's disease. J Nucl Med. 1998;3:1879–1884. [PubMed] [Google Scholar]
- DeLorenzo C, Kumar JS, Zanderigo F, Mann JJ, Parsey RV. Modeling considerations for in vivo quantification of the dopamine transporter using [(11)C]PE2I and positron emission tomography. J Cereb Blood Flow Metab. 2009;3:1332–1345. doi: 10.1038/jcbfm.2009.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Pich EM, Fuxe K, Changeux JP. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;3:173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- Chen Y, Sharples TJ, Phillips KG, Benedetti G, Broad LM, Zwart R, Sher E. The nicotinic alpha 4 beta 2 receptor selective agonist, TC-2559, increases dopamine neuronal activity in the ventral tegmental area of rat midbrain slices. Neuropharmacology. 2003;3:334–344. doi: 10.1016/S0028-3908(03)00189-8. [DOI] [PubMed] [Google Scholar]
- Fehr C, Yakushev I, Hohmann N, Buchholz HG, Landvogt C, Deckers H, Eberhardt A, Klager M, Smolka MN, Scheurich A, Dielentheis T, Schmidt LG, Rosch F, Bartenstein P, Grunder G, Schreckenberger M. Association of low striatal dopamine d2 receptor availability with nicotine dependence similar to that seen with other drugs of abuse. Am J Psychiatry. 2008;3:507–514. doi: 10.1176/appi.ajp.2007.07020352. [DOI] [PubMed] [Google Scholar]
- Brown AK, Mandelkern MA, Farahi J, Robertson C, Ghahremani DG, Sumerel B, Moallem N, London ED. Sex differences in striatal dopamine D2/D3 receptor availability in smokers and non-smokers. Int J Neuropsychopharmacol. 2012;3:989–994. doi: 10.1017/S1461145711001957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YK, Yao WJ, McEvoy JP, Chu CL, Lee IH, Chen PS, Yeh TL, Chiu NT. Striatal dopamine D2/D3 receptor availability in male smokers. Psychiatry Res. 2006;3:87–90. doi: 10.1016/j.pscychresns.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Yang YK, Yao WJ, Yeh TL, Lee IH, Chen PS, Lu RB, Chiu NT. Decreased dopamine transporter availability in male smokers – a dual isotope SPECT study. Prog Neuropsychopharmacol Biol Psychiatry. 2008;3:274–279. doi: 10.1016/j.pnpbp.2007.08.018. [DOI] [PubMed] [Google Scholar]
- Danielson K, Truman P, Kivell BM. The effects of nicotine and cigarette smoke on the monoamine transporters. Synapse. 2011;3:866–879. doi: 10.1002/syn.20914. [DOI] [PubMed] [Google Scholar]
- Carr LA, Rowell PP, Pierce WM Jr. Effects of subchronic nicotine administration on central dopaminergic mechanisms in the rat. Neurochem Res. 1989;3:511–515. doi: 10.1007/BF00964911. [DOI] [PubMed] [Google Scholar]
- Yamashita H, Kitayama S, Zhang YX, Takahashi T, Dohi T, Nakamura S. Effect of nicotine on dopamine uptake in COS cells possessing the rat dopamine transporter and in PC12 cells. Biochem Pharmacol. 1995;3:742–745. doi: 10.1016/0006-2952(94)00422-I. [DOI] [PubMed] [Google Scholar]
- Zhu J, Reith ME. Role of the dopamine transporter in the action of psychostimulants, nicotine, and other drugs of abuse. CNS Neurol Disord Drug Targets. 2008;3:393–409. doi: 10.2174/187152708786927877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newberg A, Lerman C, Wintering N, Ploessl K, Mozley PD. Dopamine transporter binding in smokers and nonsmokers. Clin Nucl Med. 2007;3:452–455. doi: 10.1097/01.rlu.0000262980.98342.dd. [DOI] [PubMed] [Google Scholar]
- Erritzoe D, Frokjaer VG, Haugbol S, Marner L, Svarer C, Holst K, Baare WF, Rasmussen PM, Madsen J, Paulson OB, Knudsen GM. Brain serotonin 2A receptor binding: relations to body mass index, tobacco and alcohol use. Neuroimage. 2009;3:23–30. doi: 10.1016/j.neuroimage.2009.01.050. [DOI] [PubMed] [Google Scholar]
- Tossici-Bolt L, Dickson JC, Sera T, De NR, Bagnara MC, Jonsson C, Scheepers E, Zito F, Seese A, Koulibaly PM, Kapucu OL, Koole M, Raith M, George J, Lonsdale MN, Munzing W, Tatsch K, Varrone A. Calibration of gamma camera systems for a multicentre European (1)(2)(3)I-FP-CIT SPECT normal database. Eur J Nucl Med Mol Imaging. 2011;3:1529–1540. doi: 10.1007/s00259-011-1801-5. [DOI] [PubMed] [Google Scholar]
- Dickson JC, Tossici-Bolt L, Sera T, Erlandsson K, Varrone A, Tatsch K, Hutton BF. The impact of reconstruction method on the quantification of DaTSCAN images. Eur J Nucl Med Mol Imaging. 2010;3:23–35. doi: 10.1007/s00259-009-1212-z. [DOI] [PubMed] [Google Scholar]
- Dickson JC, Tossici-Bolt L, Sera T, De NR, Booij J, Bagnara MC, Seese A, Koulibaly PM, Akdemir UO, Jonsson C, Koole M, Raith M, Lonsdale MN, George J, Zito F, Tatsch K. Proposal for the standardisation of multi-centre trials in nuclear medicine imaging: prerequisites for a European 123I-FP-CIT SPECT database. Eur J Nucl Med Mol Imaging. 2012;3:188–197. doi: 10.1007/s00259-011-1884-z. [DOI] [PubMed] [Google Scholar]
- Jensen PS, Ziebell M, Skouboe G, Khalid U, De NR, Thomsen G, Knudsen GM, Svarer C. Validation of a method for accurate and highly reproducible quantification of brain dopamine transporter SPECT studies. J Nucl Med Technol. 2011;3:271–278. doi: 10.2967/jnmt.111.090324. [DOI] [PubMed] [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2012. [Google Scholar]
- van Dyck CH, Seibyl JP, Malison RT, Laruelle M, Zoghbi SS, Baldwin RM, Innis RB. Age-related decline in dopamine transporters: analysis of striatal subregions, nonlinear effects, and hemispheric asymmetries. Am J Geriatr Psychiatry. 2002;3:36–43. [PubMed] [Google Scholar]
- Staley JK, Krishnan-Sarin S, Zoghbi S, Tamagnan G, Fujita M, Seibyl JP, Maciejewski PK, O'Malley S, Innis RB. Sex differences in [123I]beta-CIT SPECT measures of dopamine and serotonin transporter availability in healthy smokers and nonsmokers. Synapse. 2001;3:275–284. doi: 10.1002/syn.1084. [DOI] [PubMed] [Google Scholar]
- Salokangas RK, Vilkman H, Ilonen T, Taiminen T, Bergman J, Haaparanta M, Solin O, Alanen A, Syvalahti E, Hietala J. High levels of dopamine activity in the basal ganglia of cigarette smokers. Am J Psychiatry. 2000;3:632–634. doi: 10.1176/appi.ajp.157.4.632. [DOI] [PubMed] [Google Scholar]
- Gjedde A, Reith J, Dyve S, Leger G, Guttman M, Diksic M, Evans A, Kuwabara H. Dopa decarboxylase activity of the living human brain. Proc Natl Acad Sci U S A. 1991;3:2721–2725. doi: 10.1073/pnas.88.7.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]


