Abstract
Complex I has a unique structure in plants and includes extra subunits. Here, we present a novel study to define its protein constituents. Mitochondria were isolated from Arabidopsis thaliana cell cultures, leaves, and roots. Subunits of complex I were resolved by 3D blue-native (BN)/SDS/SDS-PAGE and identified by mass spectrometry. Overall, 55 distinct proteins were found, seven of which occur in pairs of isoforms. We present evidence that Arabidopsis complex I consists of 49 distinct types of subunits, 40 of which represent homologs of bovine complex I. The nine other subunits represent special proteins absent in the animal linage of eukaryotes, most prominently a group of subunits related to bacterial gamma-type carbonic anhydrases. A GelMap http://www.gelmap.de/arabidopsis-3d-complex-i/ is presented for promoting future complex I research in Arabidopsis thaliana.
Keywords: mitochondria; OXPHOS system; respiratory chain; NADH dehydrogenase; blue-native; BN/SDS/SDS-PAGE, Arabidopsis thaliana
Introduction
The NADH dehydrogenase complex (complex I) of the Oxidative Phosphorylation (OXPHOS) system is present in the cytoplasmic membrane of aerobic bacteria and the inner mitochondrial membrane of eukaryotes. It is composed of two elongated arms: the “membrane arm,” and the so-called “peripheral arm” which protrudes into the cytoplasm of the bacterial cell or the matrix of mitochondria (reviewed in Friedrich and Böttcher, 2004; Brandt, 2006; Vogel et al., 2007; Remacle et al., 2008; Zickermann et al., 2008, 2009; Lazarou et al., 2009). The two arms form an L-like structure as originally revealed by electron microscopy (Hofhaus et al., 1991). Very recently, the structure of the entire bacterial enzyme complex has been resolved by X-ray crystallography (Baradaran et al., 2013). Complex I represents a NADH:ubiquinone oxidoreductase. Electron transfer entirely takes place within the peripheral arm and involves an electron transfer chain composed of seven FeS clusters (Hinchliffe and Sazanov, 2005). Quinone reduction takes place at the interface between the two arms and was proposed to induce an electrostatical chain reaction throughout the membrane arm which drives proton translocation across the bacterial or mitochondrial membrane (Baradaran et al., 2013).
Complex I is by far the largest complex of the OXPHOS system. In its simplest form, the bacterial complex consists of 14 subunits (seven subunits per arm) and has a molecular mass of about 500 kDa. However, in eukaryotes, complex I is much larger and consists of more than 40 subunits. Bovine complex I, which extensively was investigated with respect to its subunit composition, consists of 44 subunits, 16 of which are localized in the peripheral and 28 in the membrane arm (Carroll et al., 2006; Balsa et al., 2012). Complex I composition is remarkably conserved in different eukaryotic lineages (Cardol, 2011). However, some lineage-specific complex I subunits occur (Cardol, 2011).
Additional subunits were especially described for plants. Using electron microscopy, complex I of plants was shown to have a very unique shape (Dudkina et al., 2005; Sunderhaus et al., 2006; Peters et al., 2008; Bultema et al., 2009). It has an extra spherical domain which is attached to the membrane arm at a central position and, like the peripheral arm, protrudes into the mitochondrial matrix. It was shown to include extra subunits which resemble gamma-type carbonic anhydrases (Perales et al., 2005; Sunderhaus et al., 2006). In Arabidopsis, three carbonic anhydrase subunits form part of complex I (termed CA1, CA2, and CA3) and additionally two more derived “carbonic anhydrase-like” proteins (CAL1 and CAL2). Proteomic studies were initiated to systematically characterize complex I subunits in plants (Heazlewood et al., 2003; Cardol et al., 2004; Sunderhaus et al., 2006; Meyer et al., 2008; Klodmann et al., 2010, 2011; Klodmann and Braun, 2011; Li et al., 2013). These projects led to the identification of several proteins homologous to subunits of bovine complex I and some additional subunits specifically occurring in plants. However, resulting protein sets slightly differ between the presented studies (reviewed in Meyer, 2012).
Here, we present a new study to thoroughly characterize complex I subunits in the model plant Arabidopsis thaliana. Our study is based on a 3D gel-electrophoretic approach introduced by Meyer et al. (2008). Using mass spectrometry (MS), 55 complex I proteins were identified, seven of which occur in pairs of isoforms. We present evidence that complex I of Arabidopsis includes at least 49 types of proteins, 40 of which represent homologs of bovine complex I and 9 of which are special to plants. A 3D GelMap is presented at http://www.gelmap.de/Arabidopsis-3D-complex-I to facilitate future complex I research in Arabidopsis.
Materials and Methods
Plant material
A cell culture of Arabidopsis thaliana (Col-0) was established as described by May and Leaver (1993). Callus was maintained as suspension culture according to Sunderhaus et al. (2006). Leaves were harvested from 3 weeks old Arabidopsis thaliana (Col-0) plants grown in soil at long day conditions (16 h light, 8 h dark) at 22 °C during the day and 20 °C at night. Arabidopsis roots were cultured in liquid medium as described by Lee et al. (2011). For this approach, 50–100 seeds of Arabidopsis thaliana Col-0 were surface-sterilized in 70% ethanol for 5 min followed by 5 min incubation in 5% bleach/0.1% Tween 20. Seeds were then washed five times in sterilized water. Length of the single washing steps was increased from 10 s to finally 5 min. All incubation steps took place in a rotary shaker. After the final washing step an appropriate volume of 0.15% agarose solution was added to the seeds. The seeds immediately were carefully dispensed on a stainless steel wire mesh platform which is part of the hydroponic culture system adapted from Schlesier et al. (2003). Conditions for hydroponic culture were according to the protocol of Schlesier et al. (2003). Arabidopsis plants were grown under 16/8 h light/dark period with light intensity 100–125 μmol m−2 s−1 at 22 °C. Liquid medium was replaced with freshly made liquid medium after 2 weeks. After 4 weeks the roots were harvested, pre-washed in root culture medium [0.38% (w/v) Gamborg’s B5 salt with vitamins, 3% sucrose, pH 5.8] and transferred into Erlenmeyer flasks containing 50 ml root culture medium. The root culture was kept at 22 °C in the dark under constant agitation at 100 rpm (Lee et al., 2011). It was maintained by transferring small amounts of roots into a new culture flask containing freshly prepared sterilized root culture medium every 3 weeks.
Isolation of mitochondria
Mitochondria from cell culture were isolated as described by Werhahn et al. (2001). Isolation of mitochondria from green leaves and roots was performed according to the protocol of Keech et al. (2005).
3D BN/SDS/SDS-PAGE
One-dimensional blue-native PAGE (1D BN-PAGE) was performed according to Wittig et al. (2006). Mitochondrial membranes were solubilized by digitonin at a concentration of 5 g/g mitochondrial protein (Eubel et al., 2003). The two further gel dimensions represented a 2D SDS/SDS-PAGE as originally suggested by Rais et al. (2004). Combining 1D BN-PAGE and 2D SDS/SDS-PAGE was carried out according to Meyer et al. (2008). For this approach, bands corresponding to complex I were excised from the blue-native (BN) gel. Three bands of complex I were used to build a stack on top of a SDS gel (10% polyacrylamide). Electrophoresis was carried out in the presence of 6 M urea. After end of the electrophoretic run, the lane was cut out from the second gel dimension and incubated in acidic solution (Meyer et al., 2008). The gel strip then was horizontally transferred on top of a third dimension SDS gel (16% polyacrylamide) and gel electrophoresis was carried out in the absence of urea.
Gel staining procedures
Polyacrylamide gels were stained with Coomassie Brilliant Blue G250 according to the protocol of Neuhoff et al. (1988, 1990).
Protein identification by mass spectrometry
Tryptic digestion of proteins and identification of proteins by MS were performed as described by Klodmann et al. (2010). Procedures were based on peptide separation using the EASY-nLC System (Proxeon; Thermo Scientific, Bremen, Germany) and coupled MS analyses using the MicrOTOF-Q II mass spectrometer (Bruker Bremen, Germany). MS data evaluation was carried out using ProteinScape2.1 software (Bruker, Bremen, Germany), the Mascot search engine (Matrix Science, London, UK), and (1) the Arabidopsis protein database1 as well as (2) an updated version of the complex I database used by Klodmann et al. (2010). The latter database is also based on the TAIR protein database (release 10) and includes additionally proteins known to co-migrate with complex I on Blue-native gels (like prohibitins). The following Mascot search parameters were used: enzyme, trypsin/P (up to one missed cleavage allowed); global modification, carbamidomethylation (C), variable modifications, acetyl (N), oxidation (M); precursor ion mass tolerance, 15 ppm; fragment ion mass tolerance, 0.05 Da; peptide charge, 1+, 2+, and 3+; instrument type, electrospray ionization quadrupole time of flight. Minimum ion score was 15, minimum peptide length was four amino acids, significance threshold was set to 0.05 and protein and peptide assessments were carried out if the Mascot Score was greater than 30 for proteins and 20 for peptides.
Image processing and database generation using GelMap
Coomassie-blue stained 3D BN/SDS/SDS gels of complex I were scanned using the Image Scanner III (GE Healthcare). Spot coordinates were generated using Microsoft Office Paint. The gel image and a file containing all relevant MS data including the spot coordinates were exported into the GelMap software package available at www.gelmap.de following the instructions given on the website and in Senkler and Braun (2012).
Results and Discussion
Separation of complex I subunits by 3D gel electrophoresis
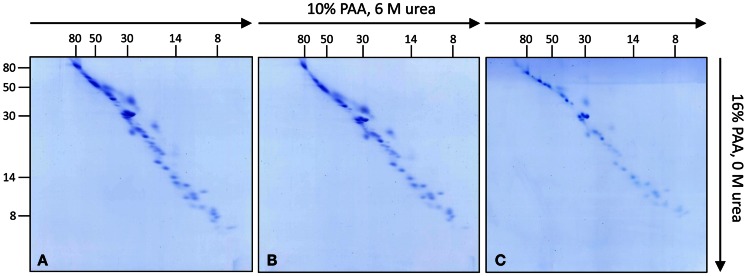
To further investigate the subunit composition of Arabidopsis complex I, isolated mitochondria from leaves, roots, and cell cultures were analyzed by 3D BN/SDS/SDS-PAGE according to Meyer et al. (2008) (Figure S1 in Supplementary Material). In the first gel dimension intact mitochondrial protein complexes are resolved by BN-PAGE. Bands representing mitochondrial complex I are cut out from the gel and staples of up to three bands are transferred onto the 2D SDS/SDS-PAGE system as published by Rais et al. (2004). The latter electrophoresis system combines the advantages of high resolution SDS-PAGE with differential resolution of hydrophilic versus hydrophobic proteins. The first SDS gel dimension contains 10% polyacrylamide (PAA) plus 6 M urea while the second SDS gel dimension contains no urea and has a PAA concentration of 16%. On the resulting SDS/SDS gels proteins are dispersed around a diagonal line. This variation in electrophoretic mobility is presumably caused by an altered interaction between SDS and proteins in the presence or absence of urea (Rais et al., 2004). Furthermore, highly hydrophobic proteins show a differential electrophoretic mobility in gels with varying PAA concentrations. In low PAA gels, hydrophobic proteins run slightly faster than hydrophilic ones and in high PPA gels the other way round. On the 2D gel system suggested by Rais et al. (2004) hydrophobic proteins run above the diagonal line. Since complex I likewise includes highly hydrophobic and hydrophilic subunits this gel system nicely allows to investigate its composition (Rais et al., 2004; Meyer et al., 2008; Angerer et al., 2011; Dröse et al., 2011). Upon optimization of protocols, 3D BN/SDS/SDS-PAGE of complex I from Arabidopsis cell culture, leaves, and roots allowed to visualize 52 protein spots per fraction based on Coomassie-staining (Figure 1; Figure S2 in Supplementary Material). Variation in subunit composition between the three Arabidopsis tissues was not observed.
Figure 1.
Investigation of complex I subunits from different tissues of Arabidopsis thaliana by 3D BN/SDS/SDS-PAGE. Total mitochondrial protein from cell culture, leaves, and roots (1200 μg each) was resolved by BN-PAGE in a first dimension. Complex I was cut out from the BN gel and used for second gel dimensions [SDS-PAGE within a 10% polyacrylamide (PAA) gel in the presence of 6 M urea]. Lanes from the second dimension gels were again cut out and transferred horizontally onto third gel dimensions (SDS-PAGE within a 16% PAA gel in the absence of urea). Gels were stained with Coomassie colloidal. (A) Complex I of cell cultures, (B) of leaves, (C) of roots. Molecular masses (in kilodaltons) are given to the left and on the top of the gels.
Analysis of complex I subunits
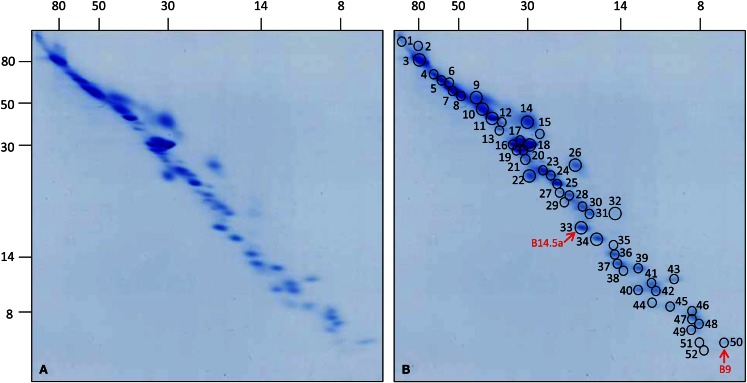
All 52 protein spots of complex I from cell culture and selected subunits of complex I from leaves and roots were analyzed by ESI MS/MS (Figure 2; Table 1; Figure S2 and Tables in Supplementary Material). Overall, 55 distinct proteins were identified. Analyses of two spots in the low-molecular-mass range did not allow identifying any proteins (spots 51 and 52 on Figure 2). Due to spot overlappings, some proteins were detected in more than one spot. The main locations of all proteins (here: highest Mascot score) as well as their secondary locations on the gel are given in Table 1. Overall, 7 out of the 55 subunits of Arabidopsis complex I occur in pairs of isoforms. This reduces the number of distinct types of subunits detected in our complex I fraction to 48. The subunit ND4L was not detected by MS in our or any previous investigation on Arabidopsis complex I which is most likely due to its extreme hydrophobicity (gravy score + 0.976). Systematic analysis of the subunit composition of complex I in the model organism Yarrowia lipolytica also did not led to the identification of this subunit (Abdrakhmanova et al., 2004). ND4L belongs to the “core” set of subunits present in all complex I particles. Its gene is localized on the mitochondrial genome in Arabidopsis, transcribed and edited (Giegé and Brennicke, 1999). We speculate that ND4L is represented by spots 51 or 52 in the 7 kDa range of our 3D gel, both of which could not be identified (Figure 2; Figure S3 in Supplementary Material). ND4L has a calculated mass of 10.9 kDa but is very hydrophobic and therefore should run at ∼7 kDa upon SDS-PAGE. We conclude that Arabidopsis complex I consists of at least 49 subunits, 48 of which were detected by our analyses, seven of which occur in pairs of isoforms.
Figure 2.
3D map of complex I from Arabidopsis thaliana cell culture. Total mitochondrial protein (1200 μg each) was resolved by 3D BN/SDS/SDS-PAGE. (A) Coomassie-stained gel, (B) same gel as in (A) indicating protein spots which have been analyzed by mass spectrometry. Numbers correspond to those given in Table 1. Red arrows indicate the newly identified subunits B14.5a and B9. Molecular masses (in kilodaltons) are given to the left and on the top of the gels.
Table 1.
Complex I subunits in Arabidopsis thaliana.
| Subunit1 |
Accession2 | Spot3 |
Organ4 | ||
|---|---|---|---|---|---|
| Plant subunit | Bovine homolog | Main spot | Further spots | ||
| Membrane arm | |||||
| 15 kDa-1 | 15 kDa | At3g62790 | 41 | c | |
| 15 kDa-2 | 15 kDa | At2g47690 | 41 | c | |
| AGGG | AGGG | At1g76200 | 47 | c | |
| ASHI | ASHI | At5g47570 | 44 | c | |
| B9 | B9 | At2g46540 | 50 | c | |
| B12-1 | B12 | At1g14450 | 45 | c, l | |
| B12-2 | B12 | At2g02510 | 45 | c, l | |
| B14 | B14 | At3g12260 | 36 | c | |
| B14.5b | B14.5b | At4g20150 | 47 | c, l | |
| B14.7 | B14.7 | At2g42210 | 33 | c, l | |
| B15 | B15 | At2g31490 | 46 | c, l | |
| B16.6-1 | B16.6 | At1g04630 | 34 | c, l | |
| B16.6-2 | B16.6 | At2g33220 | 34 | c | |
| B18 | B18 | At2g02050 | 38 | 37 | c, l, r |
| B22 | B22 | At4g34700 | 35 | c | |
| ESSS-1 | ESSS | At2g42310 | 42 | c | |
| ESSS-2 | ESSS | At3g57785 | 42 | c | |
| KFYI | KFYI | At4g00585 | 41 | 32 | c |
| MNLL | MNLL | At4g16450 | 40 | c, l, r | |
| MWFE | MWFE | At3g08610 | 49 | c, l | |
| ND1 | ND1 | AtMg00516/AtMg01120/AtMg012755 | 26 | c, l | |
| ND2 | ND2 | AtMg00285/AtMg013205 | 14 | c, l, r | |
| ND3 | ND3 | AtMg00990 | 43 | c | |
| ND4 | ND4 | AtMg00580 | 14 | c | |
| ND4L | ND4L | AtMg00650 | – | – | |
| ND5 | ND5 | AtMg00060/AtMg00513/AtMg006655 | 9 | 2 | c, l |
| ND6 | ND6 | AtMg00270 | 15 | c | |
| PDSW-2 | PDSW | At1g49140 | 36 | c, l, r | |
| PDSW-1 | PDSW | At3g18410 | 36 | c, l, r | |
| PGIV-1 | PGIV | At3g06310 | 37 | c | |
| PGIV-2 | PGIV | At5g18800 | 38 | 37 | c |
| GLDH | – | At3g47930 | 4 | c | |
| P1 | – | At1g67350 | 39 | c, l | |
| P2 | – | At2g27730 | 37 | c, l, r | |
| At1g18320 | – | At1g18320 | 29 | c | |
| Carbonic anhydrase domain (membrane arm) | |||||
| CA1 | – | At1g19580 | 16 | 17, 18, 19, 20 | c, l |
| CA2 | – | At1g47260 | 16 | 13, 17, 18, 19, 20 | c, l |
| CA3 | – | At5g66510 | 21 | 19, 20 | c, l |
| CAL1 | – | At5g63510 | 23 | c, l | |
| CAL2 | – | At3g48680 | 23 | 22, 24 | c, l, r |
| Peripheral arm | |||||
| 13 kDa | 13 kDa | At3g03070 | 44 | c | |
| 18 kDa | 18 kDa | At5g67590 | 33 | c, l | |
| 24 kDa | 24 kDa | At4g02580 | 21 | c, l, r | |
| 39 kDa | 39 kDa | At2g20360 | 11 | 12 | c, l, r |
| 51 kDa | 51 kDa | At5g08530 | 8 | 5, 6, 7, 9 | c, l |
| 75 kDa | 75 kDa | At5g37510 | 3 | 1, 2, 4 | c, l |
| B8 | B8 | At5g47890 | 42 | 41 | c, l |
| B13 | B13 | At5g52840 | 28 | 27, 29 | c, l, r |
| B14.5a | B14.5a | At5g08060 | 33 | c | |
| B17.2 | B17.2 | At3g03100 | 31 | c | |
| ND7 | 49 kDa | AtMg00510 | 10 | c, l | |
| ND9 | 30 kDa | AtMg00070 | 25 | c, l, r | |
| PSST | PSST | At5g11770 | 30 | c, l, r | |
| SGDH | SGDH | At1g67785 | 48 | c, l | |
| TYKY-1 | TYKY | At1g79010 | 22 | c, l | |
| TYKY-2 | TYKY | At1g16700 | 22 | c, l | |
1Subunits of complex I from Arabidopsis were named according to their homologs in bovine complex I (40 homologous subunits). Exceptions: Arabidopsis homologs to the 30 and 49 kDa subunits of bovine complex I are designated ND7 and ND9 because the corresponding proteins are encoded by the mitochondrial genome in plants. Seven subunits occur in pairs of isoforms in Arabidopsis. The names of these proteins were extended by “−1” and “−2.” Arabidopsis complex I includes nine additional subunits absent in bovine complex I. These proteins are named in accordance to the literature: CA1, CA2, CA3, CAL1, CAL2, GLDH (L-galactone 1-4 lactone dehydrogenase), P1, P2, and At1g18320.
2Accession numbers as given by TAIR http://www.arabidopsis.org.
3Spot number in accordance with Figure 2.
4Organ/culture in which the subunit was identified; c, cell culture; l, leaf; r, root.
5Two to three accession numbers are given for the ND1, ND2, and ND5 proteins because they are encoded by a corresponding number of gene fragments on the mitochondrial genome in Arabidopsis. Transcripts encoding the complete proteins are generated by trans-splicing (Knoop et al., 1991; Knoop and Brennicke, 1993; Lippok et al., 1996).
For a limited number of subunits, MS analysis also was carried out for the Arabidopsis leaves and roots fractions (Table 1; Table S1 in Supplementary Material). Identifications confirm the results obtained for the Arabidopsis cell culture. However, in some cases the main locations of corresponding subunits slightly vary between the fractions. It cannot be excluded that these differences are caused by minor gel to gel variations which in some cases made it difficult to precisely assign spots between different fractions. Possible variations in complex I subunit composition between different Arabidopsis fractions should be further addressed by future studies.
Based on previous topological investigations for Arabidopsis and other model organisms (Carroll, 2003; Hunte et al., 2010; Klodmann et al., 2010; Angerer et al., 2011; Cardol, 2011; Dröse et al., 2011), all 49 subunits can be assigned to the membrane or the peripheral arm of complex I. The peripheral arm consists of 15 subunits, the membrane arm of 34 subunits (Table 1). Five subunits of the membrane arm form part of the so-called carbonic anhydrase (CA/CAL) domain, which is absent in mitochondria of opisthokonts (animals and fungi; Gawryluk and Gray, 2010; Cardol, 2011). Of the 49 subunits, 40 represent homologs of subunits present in bovine complex I (Table 1). Two of these proteins (subunits B14.5a and B9) were identified for the first time in Arabidopsis but previously predicted to form part of complex I by genome analyses (Cardol, 2011). The high number of homologs in bovine and Arabidopsis complex I underlines the remarkable conservation of this protein complex in Eukaryotes (Cardol, 2011). Bovine complex I consists of 44 subunits (Carroll et al., 2006; Balsa et al., 2012), only four of which were not found in Arabidopsis (10 kDa, 42 kDa, SDAP, and B17 subunits; Meyer, 2012). On the contrary, Arabidopsis complex I includes nine subunits absent in the bovine complex (for summary, see Figure S4 in Supplementary Material).
Of the nine extra subunits in plants, five represent members of the CA/CAL family. Since deletion of single CA or CAL genes does not cause complete loss of intact complex I (Perales et al., 2005; Sunderhaus et al., 2006; Meyer et al., 2011; Wang et al., 2012a) it cannot be excluded that they present isoforms which alternatively are present in complex I particles. However, deletion of the ca2 gene leads to highly reduced levels of complex I (Perales et al., 2005) indicating that CA2 cannot easily be replaced by CA1 or CA3. Sequence identity between CA1, CA2, and CA3 is in the range of 75%. In contrast, sequences of the CAL1 and CAL2 subunits of Arabidopsis are very similar (90% sequence identity), possibly indicating that these proteins represent isoforms. Indeed, deletion of the cal1 or cal2 gene in Arabidopsis does not visibly affect Arabidopsis development but the double mutant is not viable (Wang et al., 2012a). Considering the size of the CA/CAL domain upon single particle EM of Arabidopsis complex I it was concluded that it consists of at least three copies of CA/CAL proteins (Sunderhaus et al., 2006). Further experiments have to be carried out in order to clarify the number of CA/CAL subunits per individual complex I particles.
The plant-specific GLDH subunit binds to three complex I assembly intermediates of 420, 480, and 850 kDa (Schertl et al., 2012) but so far was not detected in preparations of intact complex I. Our data point to the possibility that GLDH also binds to the intact complex. However, it cannot be excluded that the 1000 kDa complex I band excised from the BN gel also included small amounts of the band representing the 850 kDa subcomplex. Three further plant-specific subunits were detected on our 3D gels: P1, P2, and a protein encoded by At1g18320. The P1 and P2 proteins were consistently detected in complex I fractions from plants (Meyer, 2012). Both form part of the membrane arm (Sunderhaus et al., 2006). The At1g18320 protein was previously found to co-migrate with complex I on a BN/SDS gel (Klodmann et al., 2011). However, its status representing an integral complex I subunit in Arabidopsis should be further investigated.
Further complex I subunits in plants?
In previous investigations based on BN-PAGE six additional complex I proteins were identified in Arabidopsis (summarized in Meyer, 2012): At5g14105 (Klodmann et al., 2010; Klodmann and Braun, 2011), At1g68680 (Meyer et al., 2008), At1g72170, At3g10110 and At2g28430 (Klodmann et al., 2011), and At1g72750 (Wang et al., 2012b) (Table 2). However, detection of these proteins is not consistent. It currently cannot be excluded that these proteins co-migrate with complex I on blue-native gels but form part of separate complexes. Interestingly, some of these proteins are known components of the pre-protein translocase of the inner mitochondrial membrane, the TIM complex (At1g72750 and At3g10110; the latter protein represents an isoform of At1g18320 which was identified in the course of our current study; Table 1). It recently has been suggested that complex I and the TIM complex are physically linked in plant mitochondria (Murcha et al., 2012).
Table 2.
Candidates of additional complex I subunits in Arabidopsis thaliana.
| Accession | Evidence | Remark |
|---|---|---|
| At5g14105 | Klodmann et al. (2010), Klodmann and Braun (2011) | Subunit P31 |
| At1g68680 | Meyer et al. (2008) | |
| At3g10110 | Klodmann et al. (2011) | Similar to TIM22 |
| At1g72170 | Klodmann et al. (2011) | |
| At2g28430 | Klodmann et al. (2011) | |
| At1g72750 | Wang et al. (2012b) | Similar to TIM23 |
1At5g14105 was suggested to be named P3 in Meyer (2012).
3D reference map of complex I
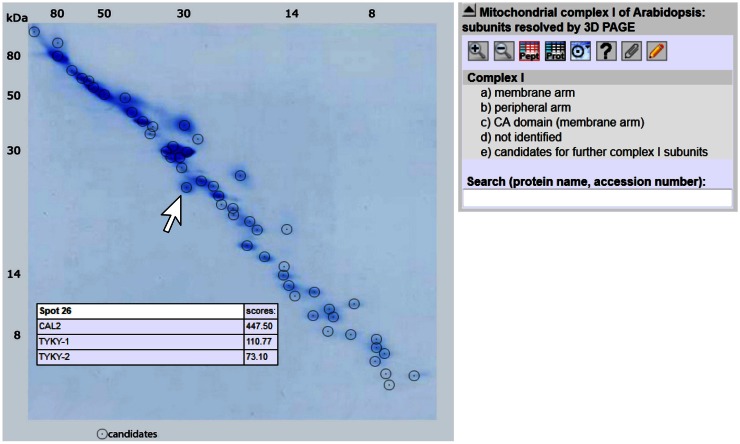
To facilitate identifying complex I subunits upon 3D BN/SDS/SDS-PAGE, a GelMap was generated for the MS dataset of the gel presented in Figure 2. GelMap is a software tool for the building and presentation of proteome reference maps (www.gelmap.de; Senkler and Braun, 2012). In contrast to alternative software packages, it allows assignment of multiple proteins per protein spot and at the same time functional annotation of all proteins. By clicking onto protein spots, widespread information is offered. Several GelMaps on Arabidopsis mitochondria are presented at the GelMap homepage, including a map on SDS-induced complex I subcomplexes2.
For the 3D GelMap of Arabidopsis complex I, the 55 identified proteins are grouped into functional categories according to their localization within the peripheral arm, the membrane arm, or the carbonic anhydrase domain attached to the membrane arm (Figure 3; http://www.gelmap.de/arabidopsis-3d-complex-i/). Furthermore, the six candidates for additional complex I subunits are given in another category. The proteins of the latter category are linked to an “extra” spot below the gel. By clicking onto any protein spot on the map, all included proteins are displayed. Proteins are sorted according to their MASCOT scores. Upon clicking onto an individual protein, a tooltip opens which includes additional information. Extensive further information on each protein is offered by links to several external databases. The new GelMap is intended to be a helpful tool for future complex I research in Arabidopsis.
Figure 3.
GelMap of complex I as resolved by 3D BN/SDS/SDS-PAGE (http://www.gelmap.de/arabidopsis-3d-complex-i/). Upon hovering with the cursor over a spot, a tooltip including information on all included proteins is opened. In the example given on the figure, the indicated spot includes the CAL2 protein and two isoforms of the TYKY subunit. Upon clicking into the spot the protein names are converted into stable links which can be used to obtain further information. Protein information also can be obtained by clicking into the menu given to the right or by entering protein names or accessions into the search field below the menu.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: http://www.frontiersin.org/Plant_Proteomics/10.3389/fpls.2013.00153/abstract
Principle of 3D BN/SDS/SDS-PAGE.
Replicates of 3D BN/SDS/SDS gels for complex I from cell cultures of Arabidopsis.
Regions on 3D BN/SDS/SDS gels showing the smallest complex I subunits. Gels were Coomassie stained (left, middle) or silver stained (right). The three smallest proteins (corresponding to spots 50, 51, and 52 on Figure 2) only become clearly visible upon silver staining. Spot 50 represents the B9 subunit. Spot 51 might represent subunit ND4L. Spot 52 could not be identified.
Species specific complex I subunits in B. taurus and A. thaliana.
Identity of complex I subunits of Arabidopsis upon analysis by 3D BN/SDS/SDS PAGE.
Protein table of the GelMap (http://www.gelmap.de/arabidopsis-3d-complex-i/).
Protein table of complex I subunits in leaves of Arabidopsis thaliana.
Protein table of complex I subunits in roots of Arabidopsis thaliana.
Acknowledgments
Katrin Peters was supported by the “Wege in die Forschung II” program offered by Leibniz University Hannover. We acknowledge support by Deutsche Forschungsgemeinschaft (DFG) and Open Access Publishing Fund of Leibniz Universität Hannover.
Footnotes
1www.Arabidopsis.org; release TAIR 10
References
- Abdrakhmanova A., Zickermann V., Bostina M., Radermacher M., Schägger H., Kerscher S., et al. (2004). Subunit composition of mitochondrial complex I from the yeast Yarrowia lipolytica. Biochim. Biophys. Acta 1658, 148–156 10.1016/j.bbabio.2004.04.019 [DOI] [PubMed] [Google Scholar]
- Angerer H., Zwicker K., Wumaier Z., Sokolova L., Heide H. (2011). A scaffold of accessory subunits links the peripheral arm and the distal proton-pumping module of mitochondrial complex I. Biochem. J. 437, 279–288 10.1042/BJ20110359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsa E., Marco R., Perales-Clemente E., Szklarczyk R., Calvo E., Landazuri M. O., et al. (2012). NDUFA4 is a subunit of complex IV of the mammalian electron transport chain. Cell Metab. 16, 378–386 10.1016/j.cmet.2012.07.015 [DOI] [PubMed] [Google Scholar]
- Baradaran R., Berrisford J. M., Minhas G. S., Sazanov L. A. (2013). Crystal structure of the entire respiratory complex I. Nature 494, 443–448 10.1038/nature11871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt U. (2006). Energy converting NADH:quinone oxidoreductase (complex I). Annu. Rev. Biochem. 75, 69–92 10.1146/annurev.biochem.75.103004.142539 [DOI] [PubMed] [Google Scholar]
- Bultema J., Braun H.-P., Boekema E., Kouril R. (2009). Megacomplex organization of the oxidative phosphorylation system by structural analysis of respiratory supercomplexes from potato. Biochim. Biophys. Acta 1787, 60–67 10.1016/j.bbabio.2008.10.010 [DOI] [PubMed] [Google Scholar]
- Cardol P. (2011). Mitochondrial NADH:ubiquinone oxidoreductase (complex I) in eukaryotes: a highly conserved subunit composition highlighted by mining of protein databases. Biochim. Biophys. Acta 1807, 1390–1397 10.1016/j.bbabio.2011.06.015 [DOI] [PubMed] [Google Scholar]
- Cardol P., Vanrobaeys F., Devreese B., van Beeumen J., Matagne R. F., Remacle C. (2004). Higher plant-like subunit composition of mitochondrial complex I from Chlamydomonas reinhardtii: 31 conserved components among eukaryotes. Biochim. Biophys. Acta 1658, 212–224 10.1016/j.bbabio.2004.06.001 [DOI] [PubMed] [Google Scholar]
- Carroll J. (2003). Analysis of the subunit composition of complex I from bovine heart mitochondria. Mol. Cell. Proteomics 2, 117–126 [DOI] [PubMed] [Google Scholar]
- Carroll J., Fearnley I. M., Skehel J. M., Shannon R. J., Hirst J., Walker J. E. (2006). Bovine complex I is a complex of 45 different subunits. J. Biol. Chem. 281, 32724–32727 10.1074/jbc.M607135200 [DOI] [PubMed] [Google Scholar]
- Dröse S., Krack S., Sokolova L., Zwicker K., Barth H. D., Morgner N., et al. (2011). Functional dissection of the proton pumping modules of mitochondrial complex I. PLoS Biol. 9:e1001128. 10.1371/journal.pbio.1001128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudkina N. V., Eubel H., Keegstra W., Boekema E. J., Braun H.-P. (2005). Structure of a mitochondrial supercomplex formed by respiratory-chain complexes I and III. Proc. Natl. Acad. Sci. U.S.A. 102, 3225–3229 10.1073/pnas.0408870102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eubel H., Jänsch L., Braun H.-P. (2003). New insights into the respiratory chain of plant mitochondria. Supercomplexes and a unique composition of complex II. Plant Physiol. 133, 274–286 10.1104/pp.103.024620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich T., Böttcher B. (2004). The gross structure of the respiratory complex I: a Lego System. Biochim. Biophys. Acta 1608, 1–9 10.1016/j.bbabio.2003.10.002 [DOI] [PubMed] [Google Scholar]
- Gawryluk R. M., Gray M. W. (2010). Evidence for an early evolutionary emergence of gamma-type carbonic anhydrase as components of mitochondrial respiratory complex I. BMC Evol. Biol. 10:176 10.1186/1471-2148-10-176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giegé P., Brennicke A. (1999). RNA editing in Arabidopsis mitochondria effects 441 C to U changes in ORFs. Proc. Natl. Acad. Sci. U.S.A. 96, 15324–15329 10.1073/pnas.96.26.15324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heazlewood J. L., Howell K. A., Millar A. H. (2003). Mitochondrial complex I from Arabidopsis and rice: orthologs of mammalian and fungal components coupled with plant-specific subunits. Biochim. Biophys. Acta 1604, 159–169 10.1016/S0005-2728(03)00045-8 [DOI] [PubMed] [Google Scholar]
- Hinchliffe P., Sazanov L. A. (2005). Organization of iron-sulfur clusters in respiratory complex I. Science 309, 771–774 10.1126/science.1113988 [DOI] [PubMed] [Google Scholar]
- Hofhaus G., Weiss H., Leonard K. (1991). Electron microscopic analysis of the peripheral and membrane parts of mitochondrial NADH dehydrogenase (complex I). J. Mol. Biol. 221, 1027–1043 10.1016/0022-2836(91)80190-6 [DOI] [PubMed] [Google Scholar]
- Hunte C., Zickermann V., Brandt U. (2010). Functional modules and structural basis of conformational coupling in mitochondrial complex I. Science 329, 448–451 10.1126/science.1191046 [DOI] [PubMed] [Google Scholar]
- Keech O., Dizengremel P., Gardeström P. (2005). Preparation of leaf mitochondria from Arabidopsis thaliana. Physiol. Plant 124, 403–409 10.1111/j.1399-3054.2005.00521.x [DOI] [Google Scholar]
- Klodmann J., Braun H.-P. (2011). Proteomic approach to characterize mitochondrial complex I from plants. Phytochemistry 72, 1071–1080 10.1016/j.phytochem.2010.11.012 [DOI] [PubMed] [Google Scholar]
- Klodmann J., Senkler M., Rode C., Braun H.-P. (2011). Defining the protein complex proteome of plant mitochondria. Plant Physiol. 157, 587–598 10.1104/pp.111.182352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klodmann J., Sunderhaus S., Nimtz M., Jansch L., Braun H.-P. (2010). Internal architecture of mitochondrial complex I from Arabidopsis thaliana. Plant Cell 22, 797–810 10.1105/tpc.109.073726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoop V., Brennicke A. (1993). “Group II introns in plant mitochondria – trans-splicing, RNA editing, evolution and promiscuity,” in Plant Mitochondria, eds Brennicke A., Kück U. (Weinheim: VCH Verlagsgesellschaft; ), 221–232 [Google Scholar]
- Knoop V., Schuster W., Wissinger B., Brennicke A. (1991). Trans splicing integrates an exon of 22 nucleotides into the nad5 mRNA in higher plant mitochondria. EMBO J. 10, 3483–3493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarou M., Thorburn D. R., Ryan M. T., McKenzie M. (2009). Assembly of mitochondrial complex I and defects in disease. Biochim. Biophys. Acta 1793, 78–88 10.1016/j.bbamcr.2008.04.015 [DOI] [PubMed] [Google Scholar]
- Lee C. P., Eubel H., O’Toole N., Millar A. H. (2011). Combining proteomics of root and shoot mitochondria and transcript analysis to define constitutive and variable components in plant mitochondria. Phytochemistry 72, 1092–1108 10.1016/j.phytochem.2010.12.004 [DOI] [PubMed] [Google Scholar]
- Li L., Nelson C. J., Carrie C., Gawryluk R. M., Solheim C., Gray M. W., et al. (2013). Subcomplexes of ancestral respiratory complex I subunits rapidly turn over in vivo as productive assembly intermediates in Arabidopsis. J. Biol. Chem. 288, 5707–5717 10.1074/jbc.M112.432070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippok B., Brennicke A., Unseld M. (1996). The rps4-gene is encoded upstream of the nad2-gene in Arabidopsis mitochondria. Biol. Chem. Hoppe-Seyler 377, 251–257 10.1515/bchm3.1996.377.4.251 [DOI] [PubMed] [Google Scholar]
- May M. J., Leaver C. J. (1993). Oxidative stimulation of glutathione synthesis in Arabidopsis thaliana suspension cultures. Plant Physiol. 103, 621–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer E. H. (2012). Proteomic investigations of complex I composition: how to define a subunit? Front. Plant Sci. 3:106 10.3389/fpls.2012.00106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer E. H., Solheim C., Tanz S. K., Bonnard G., Millar A. H. (2011). Insights into the composition and assembly of the membrane arm of plant complex I through analysis of subcomplexes in Arabidopsis mutant lines. J. Biol. Chem. 286, 26081–26092 10.1074/jbc.M110.209601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer E. H., Taylor N. L., Millar A. H. (2008). Resolving and identifying protein components of plant mitochondrial respiratory complexes using three dimensions of gel electrophoresis. J. Proteome Res. 7, 786–794 10.1021/pr700595p [DOI] [PubMed] [Google Scholar]
- Murcha M. W., Wang Y., Whelan J. (2012). A molecular link between mitochondrial preprotein transporters and respiratory chain complexes. Plant Signal. Behav. 7, 1594–1597 10.4161/psb.22250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhoff V., Stamm R., Eibl H. (1988). Clear background and highly sensitive protein staining with coomassie blue dyes in polyacrylamide gels: a systematic analysis. Electrophoresis 11, 427–448 [Google Scholar]
- Neuhoff V., Stamm R., Pardowitz I., Arold N., Ehrhardt W., Taube D. (1990). Essential problems in quantification of proteins following colloidal staining with Coomassie brilliant blue dyes in polyacrylamide gels, and their solution. Electrophoresis 11, 101–117 10.1002/elps.1150110202 [DOI] [PubMed] [Google Scholar]
- Perales M., Eubel H., Heinemeyer J., Colaneri A., Zabaleta E., Braun H.-P. (2005). Disruption of a nuclear gene encoding a mitochondrial gamma carbonic anhydrase reduces complex I and supercomplex I+III2 levels and alters mitochondrial physiology in Arabidopsis. J. Mol. Biol. 350, 263–277 10.1016/j.jmb.2005.04.062 [DOI] [PubMed] [Google Scholar]
- Peters K., Dudkina N. V., Jänsch L., Braun H.-P., Boekema E. J. (2008). A structural investigation of complex I and I+III2 supercomplex from Zea mays at 11-13 A resolution: assignment of the carbonic anhydrase domain and evidence for structural heterogeneity within complex I. Biochim. Biophys. Acta 1777, 84–93 10.1016/j.bbabio.2007.10.012 [DOI] [PubMed] [Google Scholar]
- Rais I., Karas M., Schägger H. (2004). Two-dimensional electrophoresis for the isolation of integral membrane proteins and mass spectrometric identification. Proteomics 4, 2567–2571 10.1002/pmic.200400829 [DOI] [PubMed] [Google Scholar]
- Remacle C., Barbieri M. R., Cardol P., Hamel P. P. (2008). Eukaryotic complex I: functional diversity and experimental systems to unravel the assembly process. Mol. Genet. Genomics 280, 93–110 10.1007/s00438-008-0350-5 [DOI] [PubMed] [Google Scholar]
- Schertl P., Sunderhaus S., Klodmann J., Grozeff G. E. G., Bartoli C. G., Braun H.-P. (2012). L-galactono-1,4-lactone dehydrogenase (GLDH) forms part of three subcomplexes of mitochondrial complex I in Arabidopsis thaliana. J. Biol. Chem. 287, 14412–14419 10.1074/jbc.M111.305144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesier B., Bréton F., Mock H.-P. (2003). A hydroponic culture system for growing Arabidopsis thaliana plantlets under sterile conditions. Plant Mol. Biol. Rep. 21, 449–456 10.1007/BF02772594 [DOI] [Google Scholar]
- Senkler M., Braun H.-P. (2012). Functional annotation of 2D protein maps: the GelMap portal. Front. Plant Sci. 3:87 10.3389/fpls.2012.00087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunderhaus S., Dudkina N., Jansch L., Klodmann J., Heinemeyer J., Perales M., et al. (2006). Carbonic anhydrase subunits form a matrix-exposed domain attached to the membrane arm of mitochondrial complex I in plants. J. Biol. Chem. 281, 6482–6488 10.1074/jbc.M511542200 [DOI] [PubMed] [Google Scholar]
- Vogel R. O., Smeitink J. A. M., Nijtmans L. G. J. (2007). Human mitochondrial complex I assembly: a dynamic and versatile process. Biochim. Biophys. Acta 1767, 1215–1227 10.1016/j.bbabio.2007.07.008 [DOI] [PubMed] [Google Scholar]
- Wang Q., Fristedt R., Yu X., Chen Z., Liu H., Lee Y., et al. (2012a). The gamma-carbonic anhydrase subcomplex of mitochondrial complex I is essential for development and important for photomorphogenesis of Arabidopsis. Plant Physiol. 160, 1373–1383 10.1104/pp.112.204339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Carrie C., Giraud E., Elhafez D., Narsai R., Duncan O., et al. (2012b). Dual location of the mitochondrial preprotein transporters B14.7 and Tim23-2 in complex I and the TIM17:23 complex in Arabidopsis links mitochondrial activity and biogenesis. Plant Cell 24, 2675–2695 10.1105/tpc.112.098731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werhahn W., Niemeyer A., Jansch L., Kruft V., Schmitz U. K., Braun H. (2001). Purification and characterization of the preprotein translocase of the outer mitochondrial membrane from Arabidopsis. Identification of multiple forms of TOM20. Plant Physiol. 125, 943–954 10.1104/pp.125.2.943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittig I., Braun H.-P., Schägger H. (2006). Blue native PAGE. Nat. Protoc. 1, 418–428 10.1038/nprot.2006.62 [DOI] [PubMed] [Google Scholar]
- Zickermann V., Drose S., Tocilescu M. A., Zwicker K., Kerscher S., Brandt U. (2008). Challenges in elucidating structure and mechanism of proton pumping NADH:ubiquinone oxidoreductase (complex I). J. Bioenerg. Biomembr. 40, 475–483 10.1007/s10863-008-9171-9 [DOI] [PubMed] [Google Scholar]
- Zickermann V., Kerscher S., Zwicker K., Tocilescu M. A., Radermacher M., Brandt U. (2009). Architecture of complex I and its implications for electron transfer and proton pumping. Biochim. Biophys. Acta 1787, 574–583 10.1016/j.bbabio.2009.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Principle of 3D BN/SDS/SDS-PAGE.
Replicates of 3D BN/SDS/SDS gels for complex I from cell cultures of Arabidopsis.
Regions on 3D BN/SDS/SDS gels showing the smallest complex I subunits. Gels were Coomassie stained (left, middle) or silver stained (right). The three smallest proteins (corresponding to spots 50, 51, and 52 on Figure 2) only become clearly visible upon silver staining. Spot 50 represents the B9 subunit. Spot 51 might represent subunit ND4L. Spot 52 could not be identified.
Species specific complex I subunits in B. taurus and A. thaliana.
Identity of complex I subunits of Arabidopsis upon analysis by 3D BN/SDS/SDS PAGE.
Protein table of the GelMap (http://www.gelmap.de/arabidopsis-3d-complex-i/).
Protein table of complex I subunits in leaves of Arabidopsis thaliana.
Protein table of complex I subunits in roots of Arabidopsis thaliana.