Abstract
Actin cytoskeletal damage induces inactivation of the oncoprotein YAP (Yes-associated protein). It is known that the serine/threonine kinase LATS (large tumour suppressor) inactivates YAP by phosphorylating its Ser127 and Ser381 residues. However, the events downstream of actin cytoskeletal changes that are involved in the regulation of the LATS–YAP pathway and the mechanism by which LATS differentially phosphorylates YAP on Ser127 and Ser381 in vivo have remained elusive. Here, we show that cyclic AMP (cAMP)-dependent protein kinase (PKA) phosphorylates LATS and thereby enhances its activity sufficiently to phosphorylate YAP on Ser381. We also found that PKA activity is involved in all contexts previously reported to trigger the LATS–YAP pathway, including actin cytoskeletal damage, G-protein-coupled receptor activation, and engagement of the Hippo pathway. Inhibition of PKA and overexpression of YAP cooperate to transform normal cells and amplify neural progenitor pools in developing chick embryos. We also implicate neurofibromin 2 as an AKAP (A-kinase-anchoring protein) scaffold protein that facilitates the function of the cAMP/PKA–LATS–YAP pathway. Our study thus incorporates PKA as novel component of the Hippo pathway.
Keywords: actin cytoskeleton, cAMP, Hippo pathway, PKA
Introduction
The YAP (Yes-associated protein) transcriptional co-activator is a potent oncogene that drives cell proliferation and promotes survival. Overexpression of Yorkie, the Drosophila homologue of YAP, triggers massive overgrowth of fly imaginal discs (Huang et al, 2005). Similarly, transgenic mice overexpressing YAP rapidly develop tumours in multiple organs (Camargo et al, 2007; Dong et al, 2007). In humans, YAP is overexpressed as a result of genomic amplification of the 11q22 locus in several cancer types (Weber et al, 1996; Imoto et al, 2001, 2002; Dai et al, 2003; Baldwin et al, 2005; Bashyam et al, 2005; Hermsen et al, 2005; Snijders et al, 2005). YAP might also accumulate in human cancers through genomic amplification-independent mechanisms (Zhao et al, 2007). Moreover, YAP expression is able to transform normal human mammary epithelial cells in culture (Overholtzer et al, 2006). These studies establish the evolutionarily conserved role of YAP as an oncogene and underscore the importance of understanding how YAP’s activity is regulated.
The Hippo pathway has been implicated as the major negative regulator of YAP (Harvey and Tapon, 2007; Pan, 2010; Sudol and Harvey, 2010; Zhao et al, 2010a; Halder and Johnson, 2011). The core of this pathway consists of two sterile 20-like protein kinases, MST1 and MST2 (also known as STK4 and STK3, respectively), and the scaffolding protein SAV1 (salvador homologue 1). Together with SAV1, MST1/2 activate LATS (large tumour suppressor) kinases 1 and 2 by phosphorylating residues in their hydrophobic motif (HM) (Chan et al, 2005). Subsequently, with the help of Mob1A/B (mob kinase activator 1 A/B) scaffold proteins, activated LATS1/2 kinases phosphorylate and thereby inactivate YAP. Importantly, mice lacking core Hippo pathway genes have increased YAP activity and develop cancers in various epithelial tissues. For example, mice with specific deletion of Mst1/2 or Sav1 in the liver exhibit hepatomegaly followed by rapid progression to liver cancer (Zhou et al, 2009; Lee et al, 2010; Lu et al, 2010; Song et al, 2010). Likewise, deletion of Mst1/2 in the intestinal epithelium triggers colon cancer development, a phenotype that is rescued by YAP knockout (Zhou et al, 2011). Although mice deficient for intestinal Sav1 do not progress to spontaneous cancer development, they are susceptible to chronic damage-induced tumorigenesis; this phenotype is also abolished by YAP deletion (Cai et al, 2010). These studies highlight the importance of the Hippo pathway as a suppressor of YAP. In terms of events upstream of MST1/2 kinase, mounting evidence suggests that epithelial cell adhesion and polarity initiates the signalling event (Grusche et al, 2010). At the molecular level, a number of proteins, including neurofibromin 2 (NF2), Angiomotin and Kibra, have been implicated as potential upstream regulators of MST1/2 kinase (Hamaratoglu et al, 2006; Baumgartner et al, 2010; Genevet et al, 2010; Yu et al, 2010; Chan et al, 2011a, 2011b; Paramasivam et al, 2011; Wang et al, 2011; Zhao et al, 2011). However, the exact nature of the upstream event responsible for MST1/2 activation remains elusive.
LATS1/2 can potentially phosphorylate five residues in YAP that follow the LATS consensus, HxRxxS/T (Zhao et al, 2007; Lee et al, 2008). Of these five sites, two—Ser127 and Ser381—seem to be the most critical for YAP inactivation since retention of phosphorylation at either of these two serines is sufficient to abolish the transforming ability of the YAP 5SA mutant (Zhao et al, 2009). Mechanistically, Ser127 phosphorylation mediates interaction with 14-3-3 proteins, which sequester YAP in the cytosol (Dong et al, 2007; Zhao et al, 2007). On the other hand, Ser381 phosphorylation triggers successive phosphorylation on Ser384 by casein kinase-1 followed by BTRC (beta-transducin repeat-containing E3 ubiquitin protein ligase)-mediated degradation (Zhao et al, 2010b). However, in cell-free systems Ser127 serves as the primary and preferred substrate for LATS, whereas phosphorylation of Ser381 is a minor reaction (Zhao et al, 2007; Lee et al, 2008). This raises the important question of whether and how these two phosphorylations are differentially regulated in intact cells.
A recent report revealed a novel upstream regulator of YAP (Halder et al, 2012), demonstrating that, when stress fibres develop tension, YAP is imported into the nucleus and thus activated. Conversely, YAP is exported from the nucleus when stress fibres loose tension. Similarly, actin cytoskeletal damages, such as latrunculin B, cytochalasin D, or maintenance of cells in suspension, inactivates YAP (Dupont et al, 2011; Fernandez et al, 2011; Sansores-Garcia et al, 2011; Wada et al, 2011; Zhao et al, 2012). However, unlike the aforementioned canonical Hippo pathway, signalling events downstream of the actin cytoskeleton are poorly understood. Moreover, reports pertaining to the requirement of canonical Hippo pathway components in this context have been conflicting, possibly reflecting the use of siRNAs or dominant-negative approaches instead of genetically deficient cell lines. Moreover, although these studies commonly show that localization and Ser127 phosphorylation of YAP are affected by cytoskeletal damage, whether and how Ser381 participates in this signalling and which downstream components are actually involved have not been elucidated. In this study, we investigated these issues by taking advantage of genetically engineered mouse embryonic fibroblasts (MEFs) for each Hippo pathway component and a YAP phospho-Ser381-specific antibody that we generated. Our study reveals an unexpected role of cyclic AMP (cAMP)-dependent protein kinase (PKA) in promoting LATS-mediated Ser381 phosphorylation, and thus full inactivation, of YAP. Moreover, we demonstrate that NF2 plays a role as an AKAP (A-kinase-anchoring protein) in the cAMP/PKA–LATS–YAP pathway.
Results
The LATS/Mob1 complex, but not upstream canonical Hippo components, is essential for induction of YAP phosphorylation by cytoskeletal damage
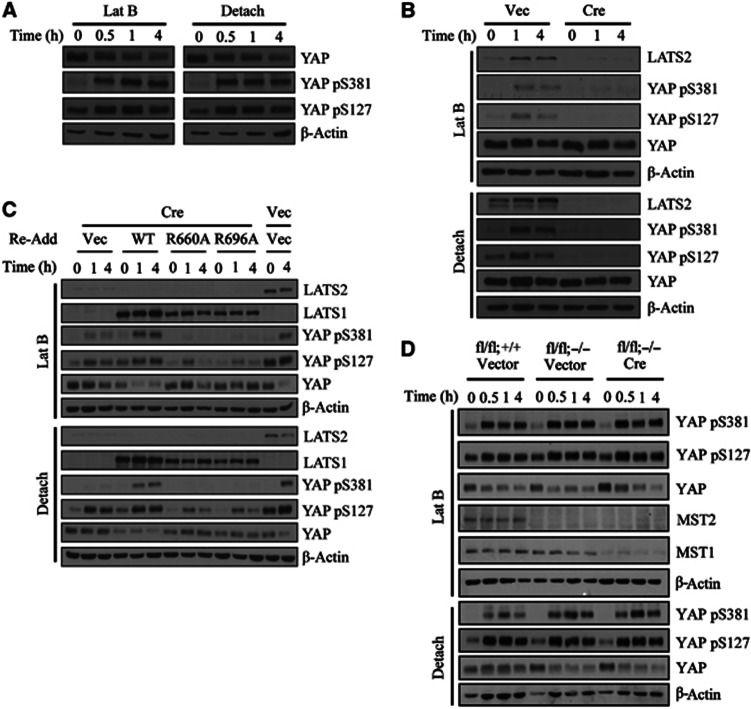
We first asked if Ser381 phosphorylation was induced by cytoskeletal damage. To do this, we first generated and confirmed the specificity of a YAP phospho-Ser381 antibody (Supplementary Figure S1). We found that YAP was rapidly phosphorylated at both Ser127 and Ser381 in NIH3T3 cells after latrunculin B treatment or cell detachment (Figure 1A). We noted that, unlike Ser127 phosphorylation, which is already abundant in unstressed cells, basal Ser381 phosphorylation was barely detectable and was induced in an almost all-or-none fashion by cytoskeletal damage. To examine whether this signalling event requires the canonical Hippo pathway, we exploited MEFs isolated from mice deficient for each pathway component. First, we generated Lats1- and Lats2-deficient MEFs by retroviral Cre infection of Lats1−/−;Lats2fl/fl MEFs. In cells deficient for all LATS isoforms, YAP was unphosphorylated under basal conditions and cytoskeletal damage was unable to induce YAP phosphorylation (Figure 1B). Next, we asked if the interaction of LATS with Mob1 was necessary for YAP phosphorylation. To overcome the problem of cellular senescence associated with multiple passaging, we immortalized Lats1−/−;Lats2fl/fl MEFs with SV40 LT. Then, either wild-type or Mob1-binding-defective versions of LATS1 were introduced followed by retroviral Cre infection (Hergovich et al, 2006). Cells reconstituted with LATS1 R690A or R696A mutants were also unable to phosphorylate YAP (Figure 1C). These results genetically confirm that an intact LATS/Mob1 complex is indispensable for both basal and cytoskeletal damage-induced YAP phosphorylation.
Figure 1.
The LATS/Mob1 complex is essential for induction of YAP Ser127 and Ser381 phosphorylation by cytoskeletal damage. (A) Phosphorylation at Ser127 and Ser381 by actin cytoskeletal damage. NIH3T3 cells were treated with 5 μM latrunculin B or seeded onto poly-HEMA-coated dishes and incubated for the indicated times. (B) Indispensability of LATS1/2 for YAP phosphorylation. Lats1−/−;Lats2fl/fl MEFs were transduced with either empty or Cre retroviruses. Selected cells were treated as indicated. (C) Indispensability of intact LATS/Mob1 complex for YAP phosphorylation. SV40 LT-immortalized Lats1−/−;Lats2fl/fl MEFs were complemented with either LATS1 WT, LATS1 R660A or R696A mutants. After Cre infection, cells were treated as indicated. Lanes 13 and 14 were infected with empty virus in place of Cre to measure LATS2 deletion efficiency. (D) Dispensability of MST1/2 for YAP phosphorylation. Mst1fl/fl;Mst2+/+vector, Mst1fl/fl;Mst2−/− vector, and Mst1fl/fl;Mst2−/− Cre MEFs were infected and treated as indicated.
Mst1/2-deficient cells were obtained similarly by retroviral Cre infection into Mst1fl/fl;Mst2−/− MEFs. Mst1/2-null MEFs were fully competent to induce YAP phosphorylation, both basally and in response to cytoskeletal damage (Figure 1D), consistent with previous reports (Zhou et al, 2009; Song et al, 2010; Zhao et al, 2012). Also, YAP was normally phosphorylated in Sav1-null MEFs (Supplementary Figure S2). We conclude that the upstream components of the canonical Hippo pathway, Mst1/2 and Sav1, are dispensable for cytoskeletal damage-induced YAP phosphorylation; thus, some other upstream signal(s) is involved in this context.
PKA activity is required for Ser381 phosphorylation of YAP after cytoskeletal damage
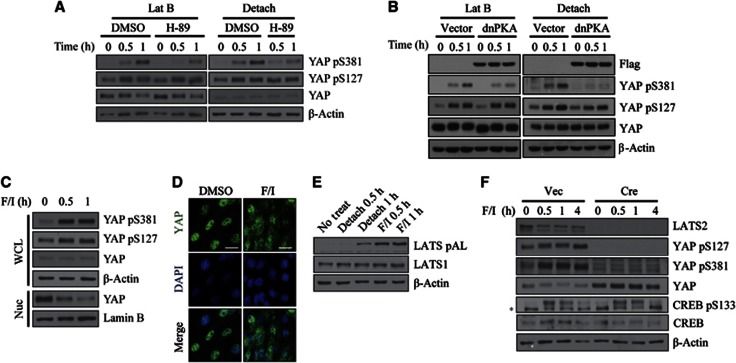
Cell detachment has been reported to increase the concentration of cAMP, which contributes to anchorage-dependent mitogenic signalling (Howe and Juliano, 2000). To determine if cAMP signalling is necessary for the induction of YAP phosphorylation by cytoskeletal damage, we pre-treated cells with the PKA inhibitor, H-89. Interestingly, PKA inhibition selectively decreased YAP Ser381 phosphorylation without affecting YAP Ser127 phosphorylation (Figure 2A). To confirm these results using an independent approach, we inhibited PKA activity using the dominant-negative PKA mutant (dnPKA), a mutant regulatory subunit 1α that is defective for cAMP binding. Overexpression of this mutant renders the PKA holoenzyme complex insensitive to cAMP stimuli, and thus inhibits downstream signalling (Clegg et al, 1987). Expression of dnPKA, like H-89, also specifically inhibited YAP Ser381 phosphorylation (Figure 2B). We further confirmed the involvement of PKA in YAP Ser381 phosphorylation by using PKI, PKA-specific peptide inhibitor (Day et al, 1989). In order to deliver PKI peptide inside the cell, we linked 11 Arg to N terminus of PKI (11R-PKI). Such polybasic peptides can be efficiently taken up by the cell (Matsushita et al, 2001). Pre-treatment of 11R-PKI also attenuated YAP Ser381 phosphorylation induced by latrunculin B (Supplementary Figure S3). Of note, the adenylate cyclase inhibitor DDA (2′,5′-dideoxyadenosine), which inhibits adenylate cyclase only when signalling via the G-protein Gs subunit is involved (Florio and Ross, 1983), failed to block YAP Ser381 phosphorylation (Supplementary Figure S4A). This result is consistent with an early study that reported that Gs does not participate in cAMP production induced by cytoskeletal damage (Watson, 1990).
Figure 2.
Requirement of cAMP/PKA signalling for induction of YAP Ser381 phosphorylation by cytoskeletal damage. (A) Effect of H-89 on YAP phosphorylation by cytoskeletal damage. NIH3T3 cells were pre-treated with 20 μM H-89 for 1 h followed by addition of latrunculin B or seeding onto poly-HEMA-coated dishes. (B) Effect of dnPKA on YAP phosphorylation by cytoskeletal damage. NIH3T3 cells were infected with Flag–dnPKA retroviruses. Selected cells were treated with latrunculin B or seeded onto poly-HEMA-coated dishes. (C, D) Effect of PKA agonist on YAP phosphorylation. NIH3T3 cells were stimulated with 20 μM forskolin and 500 μM IBMX for the indicated times. YAP phosphorylation (C) and localization (C, D) were determined. WCL, whole cell lysate; Nuc, nuclear lysate. Scale bar, 10 μm. (E) LATS activation by PKA agonist or cell detachment. LATS activation loop (AL) phosphorylation was determined in cells treated with the indicated stimuli. (F) Requirement of LATS1/2 for YAP phosphorylation by PKA agonist. Lats1−/−;Lats2fl/fl MEFs were transduced with either empty or Cre retroviruses. Selected cells were treated with forskolin/IBMX. *, nonspecific signal.
Next, we attempted to activate cAMP/PKA signalling by treating cells with the adenylate cyclase agonist, forskolin/IBMX (3-isobutyl-1-methylxanthine). This treatment induced both Ser127 and Ser381 phosphorylation of YAP and excluded YAP from the nucleus (Figures 2C and D). We noted that a 1-h treatment with cAMP agonist did not cause obvious cytoskeletal damage, ruling out secondary effects (Supplementary Figure S4B). cAMP can signal through the cAMP-activated guanine nucleotide exchange factors (GEFs) Epac1/2 in parallel with PKA (de Rooij et al, 1998). However, cells stimulated with compound 007, an Epac1/2-selective agonist, failed to induce YAP phosphorylation (Supplementary Figure S4C). Forskolin/IBMX as well as cell detachment activated LATS kinase, as evidenced by activation-loop (AL) phosphorylation (Figure 2E). YAP phosphorylation induced by activated PKA signalling was mediated by LATS kinases since forskolin/IBMX was unable to induce YAP phosphorylation in Lats1/2-null MEFs (Figure 2F). Importantly, Lats1/2-null MEFs normally showed CREB phosphorylation at Ser133, indicating that PKA is still active in the absence of Lats1/2. Taken together, these data suggest that cAMP/PKA signal to LATS1/2 to promote YAP phosphorylation, and this signalling has stronger effect on Ser381 phosphorylation.
YAP Ser127 and Ser381 phosphorylation are differentially sensitive to a reduction in LATS1/2
The observation that PKA inhibition selectively affected Ser381 phosphorylation was surprising. However, because PKA agonists were also able to increase YAP Ser127 phosphorylation, we reasoned that PKA generally enhanced LATS activity but Ser127 phosphorylation did not necessarily require PKA. In contrast, PKA might be necessary for efficient Ser381 phosphorylation by further activating LATS. This model agrees well with the biochemical observation that Ser381 is a poor substrate of LATS in cell-free systems. If so, we hypothesized that Ser381 phosphorylation might be more sensitive to a reduction in LATS expression level, whereas Ser127 phosphorylation would be relatively unaffected. To test this idea, we partially depleted LATS1/2 in RPE cells by siRNA transfection. In our experimental system, about 75% of LATS1 and 90% of LATS2 were depleted. Interestingly, latrunculin B treatment showed that Ser127 phosphorylation was almost completely unaffected by the knockdown level we achieved. However, in the same sample, Ser381 phosphorylation markedly decreased (Supplementary Figure S5). We conclude that only a trace amount of LATS is capable of inducing Ser127 phosphorylation with high efficiency in intact cells. In contrast, Ser381 phosphorylation requires sufficient LATS and PKA activity.
PKA phosphorylates LATS and thereby enhances its activity
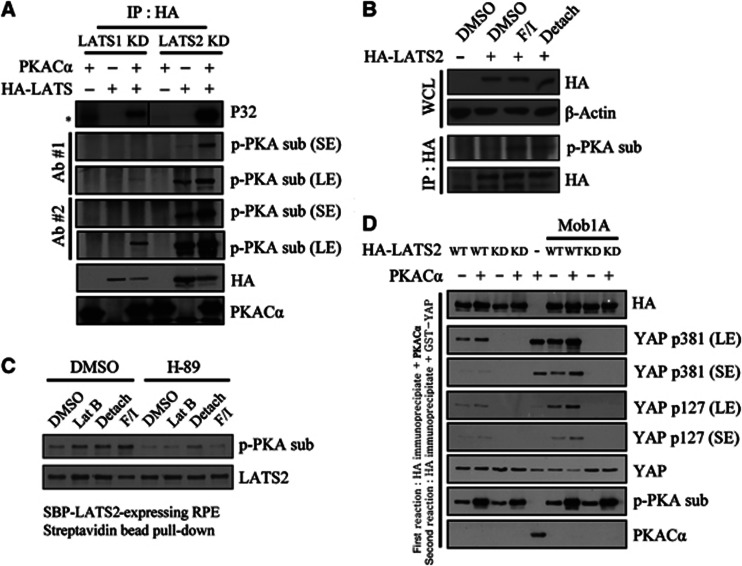
To address the question of how PKA increases the ability of LATS to phosphorylate YAP on Ser381, we tested whether PKA enhances LATS activity through direct phosphorylation. Using a cell-free system, we found that purified PKACα catalytic subunits phosphorylated immunoprecipitated LATS1 and LATS2 as monitored using either radioisotope labelling or antibodies against phosphorylated PKA substrate (Figure 3A). To determine if PKA phosphorylates LATS in intact cells, we transfected NIH3T3 cells with HA-tagged LATS2 and treated transfected cells with cytoskeletal-damaging agents or forskolin/IBMX followed by immunoprecipitation with an anti-HA antibody. Western blotting of HA immunoprecipitates with an antibody against a phosphorylated PKA substrate revealed that LATS2 was phosphorylated (Figure 3B). We also obtained similar results using a stable RPE clone expressing SBP (streptavidin-Flag-S tag)-fused LATS2 (Figure 3C, lanes 1–4). H-89 treatment abolished the increase in the signal of the phosphorylated PKA substrate antibody, confirming that this signal was indeed due to PKA activity (Figure 3C, lanes 5–8). Importantly, H-89 did not affect AL phosphorylation and even increased phosphorylation in the HM of LATS (Supplementary Figure S6). It is not yet clear why PKA inhibition increased phosphorylation of the LATS HM. It may be that PKA affects the activity of a phosphatase or kinase that targets this motif.
Figure 3.
Phosphorylation of LATS1/2 by PKA enhances LATS kinase activity in cell-free systems and in intact cells. (A) PKA phosphorylates LATS in cell-free system. HA–LATS1 KD (kinase dead) or HA–LATS2 KD was immunoprecipitated from transfected 293T cells. Immunoprecipitated beads were incubated with purified PKACα and radiolabelled ATP. Reaction products were analysed isotopically or by western blotting with phospho-PKA substrate antibodies. *, nonspecific signal. (B) PKA phosphorylates LATS in intact cells. HA–LATS2-transfected NIH3T3 cells were treated with the indicated stimuli for 1 h. After immunoprecipitation with anti-HA antibody, phosphorylation by PKA was examined using a phospho-PKA substrate antibody. WCL, whole cell lysate. (C) RPE cells stably expressing SBP-LATS2 were pre-treated with 20 μM H-89 for 1 h, followed by an additional 1-h treatment with the indicated stimuli. LATS2 was pulled down using Streptavidin agarose bead and assayed as in panel (B). (D) LATS2 pre-incubated with PKA has increased kinase activity. HA–LATS2 WT or KD mutant was immunoprecipitated from 293T cells and reacted with PKACα. PKACα was extensively washed out, followed by incubation with 1 μg GST–YAP (full-length) protein and 200 μM unlabelled ATP. Reaction products were analysed by SDS–PAGE and immunoblotting. In lanes 6–9, HA–Mob1A was co-transfected to increase overall kinase activity. In lane 5, GST–YAP was reacted with PKACα to examine possible background phosphorylation of YAP by PKA. SE, short exposure; LE, long exposure.
We then tested if PKA-mediated LATS phosphorylation increased LATS enzymatic activity using a sequential ‘cold’ kinase assay. The first kinase reaction was carried out using PKA and immunoprecipitated LATS2. After washing out recombinant PKA, the second kinase reaction was run using a GST-fusion protein of recombinant, full-length YAP as a substrate. Pre-incubation with PKA increased LATS2 kinase activity towards GST–YAP, monitored using antibodies against YAP phospho-Ser127 and phospho-Ser381 (Figure 3D). Overall activity was enhanced by co-transfection of Mob1A. Using purified PKA and YAP in in vitro kinase assays, we noted that PKA induced robust YAP Ser381 phosphorylation (Figure 3D, lane 5). However, we ruled out the possibility that residual PKA was responsible for the additional phosphorylation since PKA pre-incubation also enhanced activity towards Ser127, which was poorly phosphorylated by PKA alone. In addition, PKA pre-incubation increased LATS kinase activity even when we added PKI (5-24) in the second kinase reaction buffer to block residual PKA activity (Supplementary Figure S7). This result suggests that PKA increases LATS activity through direct phosphorylation of LATS.
PKA phosphorylates LATS2 at (R/K)(R/K)xS/T motifs, thereby mediating cytoskeletal damage-induced YAP phosphorylation
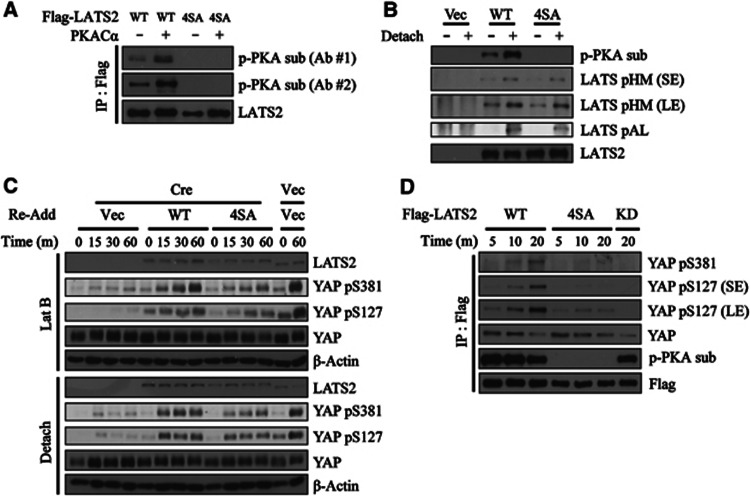
We then tried to identify the PKA target residues on LATS. PKA optimally phosphorylates RRxS/T motifs whereas Arg can be replaced with Lys in some instances. We noticed that LATS2 have four such sequences; Ser172 and Ser380 belonging to the optimal RRxS/T motif and Ser592 and Ser598 belonging to weaker consensus. Thus, we generated LATS2 mutant bearing alanine substitutions in all four serine residues (LATS2 4SA). This mutant was not phosphorylated in vitro by PKA as examined by phospho-PKA substrate western blot (Figure 4A). Signals from both antibodies, one raised against phosphorylated RRxS/T motifs (Ab #2) and another raised against phosphorylated RxxS/T motifs (Ab #1), were absent for LATS2 4SA. More importantly, LATS2 4SA was not phosphorylated by PKA in intact cells after cell detachment, while AL and HM were normally phosphorylated in LATS2 4SA (Figure 4B). LATS2 4SA interacted with Mob1 with comparable affinity to LATS2 WT (Supplementary Figure S8). These results confirm that LATS2 4SA mutant is specifically defective in phosphorylation by PKA while retaining other known regulations.
Figure 4.
Identification of PKA target sites on LATS2 and their contribution to cytoskeletal damage-induced YAP phosphorylation. (A) LATS2 4SA mutant is not phosphorylated by PKA in vitro. Flag–LATS2 WT or LATS2 4SA were prepared by immunoprecipitation from transfected 293T cells. Flag immunoprecipiates were incubated with cold ATP and PKACα. Reaction products were analysed by two antibodies against phosphorylated PKA substrate. (B) LATS2 4SA mutant is not phosphorylated by cell detachment. NIH3T3 cells were transfected with empty vector, Flag–LATS2 WT, or LATS2 4SA. Transfected cells were suspended for 1 h followed by Flag immunoprecipiation. Flag immunoprocipiates were fractionated by SDS–PAGE and analysed with indicated phospho-specific antibodies. SE, short exposure; LE, long exposure. (C) LATS2 4SA-reconstituted cells attenuate YAP phosphorylation. SV40 LT-immortalized Lats1−/−;Lats2fl/fl MEFs were complemented with either LATS2 WT or LATS2 4SA mutant. After Cre infection, cells were treated as indicated. Lanes 13 and 14 were infected with empty virus in place of Cre to measure LATS2 deletion efficiency. The different mobility of human LATS2 (complemented products) and murine Lats2 (endogenous product before deletion) ensures efficient excision of Lats2 in lanes 1–12. (D) Reduced kinase activity of LATS2 4SA mutant. 293T cells were transfected with indicated Flag-tagged LATS2 constructs. LATS2 was immunoprecipiated by Flag antibody followed by time-course kinase assay as indicated.
To test whether PKA-mediated LATS2 phosphorylation is required for actin cytoskeletal damage-induced YAP phosphorylation, we reconstituted immortalized Lats1/2-null MEFs with either LATS2 WT or LATS2 4SA mutant followed by Cre infection. These cells were harvested at early time points (15 min, 30 min and 1 h) after latrunclin B treatment or detachment. Remarkably, YAP phosphorylation was attenuated in cells reconstituted with LATS2 4SA (Figure 4C). Of note, in this experimental setup where only LATS2 4SA mutant is expressed in cell, Ser127 as well as Ser381 phosphorylation was affected. Nevertheless, Ser127 phosphorylation level eventually catches up to that of LATS2 WT-reconstituted cells. In contrast, Ser381 phosphorylation was markedly reduced at all time points examined. Lastly, we performed kinase assay using immunoprecipitated LATS2 WT or 4SA mutant, and found that LATS2 4SA mutant have significantly lower activity (Figure 4D). These results prove that PKA-mediated LATS phosphorylation is required for full kinase activity and efficient YAP phosphorylation induced by actin cytoskeletal damages.
PKA inhibition and YAP cooperate to confer resistance to anoikis and serum starvation
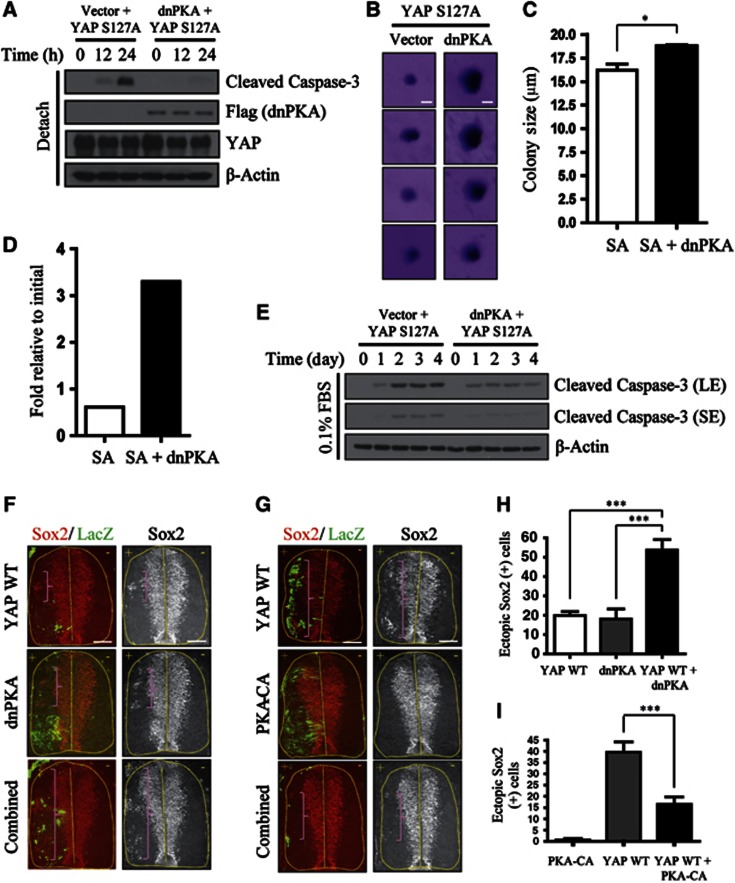
Next, we asked if PKA inhibition and YAP can functionally cooperate. Because PKA promoted YAP inhibition by cytoskeletal damage, the first phenotype associated with YAP hyperactivation that we assessed was resistance to anoikis, a type of cell death caused by deprivation of cell anchorage that, when dysregulated, supports the initial stages of cancer metastasis (Simpson et al, 2008; Kim et al, 2012). Because PKA activity had a greater impact on Ser381 phosphorylation, and the YAP S127/381A double-mutation (YAP 2SA) was necessary for anoikis resistance, we hypothesized that PKA inhibition would effectively synergize with the YAP S127A mutant. To test this, we infected NIH3T3 cells with dnPKA, YAP WT (wild-type), or YAP S127A alone, or YAP (WT or mutant) in combination with dnPKA. Interestingly, dnPKA cooperated with the YAP S127A mutant such that cells co-expressing YAP S127A and dnPKA underwent less anoikis compared to cells expressing YAP S127A alone (Figure 5A). dnPKA conferred no advantage in vector- or YAP WT-infected backgrounds (Supplementary Figure S9A). In soft-agar assays, which allow monitoring of long-term survival of anchorage-deprived cells, dnPKA addition increased the size of colonies but did not increase colony numbers (Figures 5B and C). We noted that dnPKA/YAP S127A-infected cells ultimately underwent anoikis at later time points; hence, the size of these colonies was still very small compared to those formed by fully transformed cells. Nevertheless, YAP and PKA inhibition together conferred partial anoikis resistance.
Figure 5.
Functional cooperation between PKA and YAP. (A) YAP S127A and dnPKA cooperate to resist anoikis. NIH3T3 cells expressing Flag–YAP S127A alone or Flag–YAP S127A plus Flag–dnPKA were seeded onto poly-HEMA-coated dishes and incubated for the indicated times. The apoptosis index was measured by immunoblotting for cleaved caspase-3. (B) Cells from (A) were also grown in soft agar for 15 days. Colonies were stained with crystal violet and imaged with a dissecting microscope. Scale bar, 10 μm. (C) Quantification of average colony size for the results in (B). The result was quantified from three independent experiments with triplicates in each experiment. Error bar indicates s.e.m. (two-tailed Student’s t-test). *P<0.05. (D) YAP S127A and dnPKA cooperate to resist serum starvation-induced cell death. Approximately 5 × 103 cells from (A) were seeded onto 6-well plates. One day later, media were changed to DMEM containing 0.1% FBS and cells were maintained for 6 days. Cell numbers were counted and expressed as fold induction relative to the original number of cells (5 × 103). The graph shown is a representative result from two independent experiments. (E) Cells incubated in 0.1% FBS for the indicated number of days were analysed by immunoblotting for caspase-3 cleavage. SE, short exposure; LE, long exposure. (F) YAP WT and dnPKA cooperate to generate ectopic neural progenitors in developing chick. Immunohistochemical analyses of ectopic neural progenitor formation in chick spinal cords electroporated with YAP WT, dnPKA, or YAP WT plus dnPKA, along with LacZ (left) to mark the transfected side. Neural progenitors were detected by immunostaining for Sox2. Brackets indicate ectopic Sox2+ neural progenitor cells. +, electroporated side. Scale bar, 75 μm. (G) PKA-CA suppresses YAP-induced generation of ectopic neural progenitors. Similar procedure as in (F) using YAP WT, PKA-CA, and YAP WT plus PKA-CA. Scale bar, 75 μm. (H) The number of ectopic neural progenitors from (F) were quantified. ***P<0.001. (I) Quantification of results in (G). ***P<0.001. Error bars indicate s.e.m.’s (two-tailed Student’s t-test).
To examine the functional cooperation of YAP and PKA inhibition in another assay, we serum-starved infected cells by incubating with 0.1% fetal bovine serum (FBS). Six days after starvation, ∼6-fold more dnPKA/YAP S127A-infected cells survived compared to cells infected with YAP S127A alone (Figure 5D). An examination of caspase-3 cleavage also indicated prominent protection from serum starvation in dnPKA/YAP S127A-infected cells (Figure 5E). Again, dnPKA conferred no advantage on vector- or YAP WT-expressing cells (Supplementary Figure S9B). Collectively, these data indicate that PKA inhibition strengthened YAP overexpression phenotypes in cell culture systems.
PKA inhibition and YAP cooperate to regulate neural progenitor pools
We next sought to examine the cooperation between PKA inhibition and YAP in vivo. For this, we turned to the developing chick embryo as a model system. YAP overexpression in the developing chick embryo generates ectopic neural progenitors, whereas its inhibition promotes spontaneous neural progenitor death (Cao et al, 2008). During mammalian neurogenesis, YAP inhibition is also critical for cell cycle exit and differentiation on-set of progenitor cells (Zhang et al, 2012). We first tested the cooperation between dnPKA and YAP WT. Co-transfection of dnPKA and YAP WT induced an increase in the generation of Sox2 (SRY box 2)-positive neural progenitors that was more than additive, indicating a synergistic effect (Figures 5F and H). Ectopic proliferation was observed by staining for phospho-H3 (Supplementary Figure S10). We then asked if constitutively active PKA could suppress phenotypes associated with YAP overexpression alone. Constitutive PKA activity can be achieved by expressing PKA-CA, a PKACα subunit defective for interactions with regulatory subunits (Orellana and Mcknight, 1992). The induction of ectopic neural progenitors by YAP was abolished by co-transfection of PKA-CA (Figures 5G and I). Using YAP 2SA, we observed a dramatic induction of ectopic neural progenitors that was not rescued by PKA-CA (Supplementary Figure S11). Thus, PKA-CA inhibits YAP activity by inducing phosphorylation at these residues. Of note, we often observed ectopic neural progenitors in untransfected cells (LacZ negative), suggesting existence of cell non-autonomous effect of YAP. Cell-extrinsic effect of YAP on stem/progenitor cells have also been noticed by other studies (Zhang et al, 2009; Staley and Irvine, 2010; Ohsawa et al, 2012; Zhang et al, 2012). Taken together, these results demonstrate a functional interaction between PKA and YAP in regulating stem/progenitor cell proliferation.
PKA activity is required for induction of YAP phosphorylation by upstream Hippo pathway components or GPCR stimulation
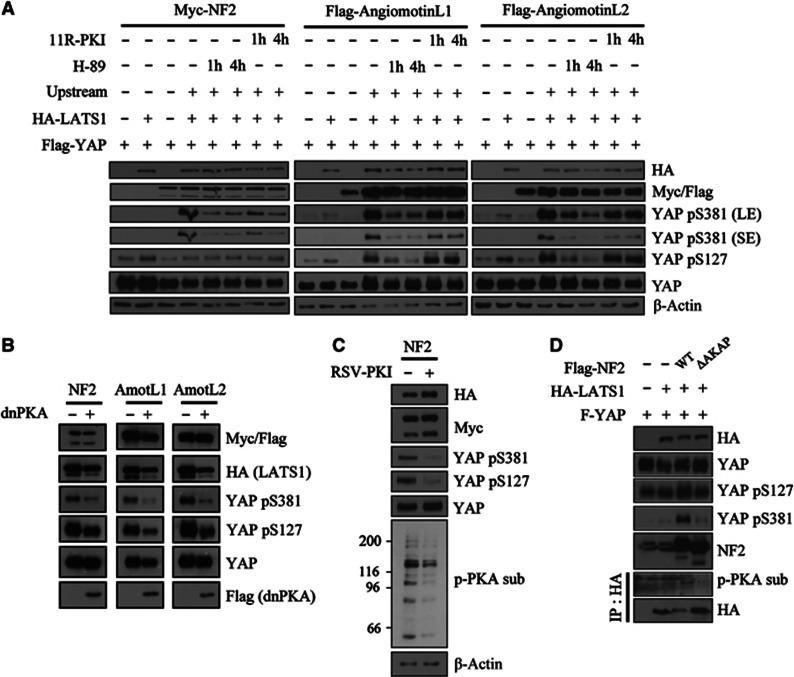
We wondered if the requirement of PKA activity in suppressing YAP could be generalized to other known contexts that activate LATS–YAP pathway. Overexpression of upstream components of the canonical Hippo pathway in 293T induced both YAP Ser127 and Ser381 phosphorylation in a LATS-dependent manner (Figure 6A). Similar to actin cytoskeletal damage, Ser381 phosphorylation was induced in an all-or-none fashion only in the presence of LATS and upstream components. In contrast, significant levels of Ser127-phosphorylated YAP were present under basal condition. To examine the impact of inhibiting PKA activity in this experimental system, we used LATS1 specifically because LATS2 alone (i.e., without any upstream components) resulted in too strong basal YAP phosphorylation. Incubation of cells expressing all Hippo components with H-89 caused an abrupt disappearance of YAP phosphorylation signals (Figure 6A, lanes 5 and 6). Incubation of 11R-PKI also reduced the pre-established YAP phosphorylation, although the effect was weaker than H-89 (Figure 6A, lanes 7 and 8). Co-expression of dnPKA also abolished YAP phosphorylation induced by these upstream components (Figure 6B). Co-transfecting RSV-PKI (PKI expression driven by RSV promoter) also reduced YAP phosphorylation induced by NF2 and LATS1 (Figure 6C). Anti-phospho-PKA substrate western blot from the cell lysates confirmed the inhibition of PKA activity in PKI-transfected cells. Unlike the effect of PKA inhibition on cytoskeletal damage-induced YAP phosphorylation, both phosphorylation sites were affected in these experiments. This is likely because YAP phosphorylation is saturated in overexpression conditions; thus, most YAP proteins are doubly phosphorylated and Ser127-phosphorylated species are co-degraded together with Ser381-phosphorylated species. Our results thus indicate that PKA activity also likely functions in the canonical Hippo pathway.
Figure 6.
Requirement for PKA activity in mediating YAP phosphorylation induced by engagement of the canonical Hippo pathway. (A) YAP phosphorylation induced by Hippo pathway upstream components is opposed by PKA inhibition. 293T cells were transfected with Flag–YAP, HA–LATS1, and Myc–NF2 or Flag-angiomotin-like 1/2 as indicated. Forty-eight hours after transfection, cells were incubated with 20 μM H-89 or 50 μM 11R-PKI for the indicated times. SE, short exposure; LE, long exposure. (B) 293T cells were transfected as in (A) with or without dnPKA co-transfection. Lysates were analysed 48 h after transfection. (C) 293T cells were transfected as in (A) with or without RSV-PKI co-transfection. Transfection of RSV-PKI was confirmed by western blotting the whole transferred membrane against phospho-PKA substrate antibody. (D) NF2 mutant that is defective in AKAP function do not induce YAP Ser381 and PKA-mediated LATS phosphorylation. 293T cells were transfected with Flag–YAP, HA–LATS1, and Flag–NF2 WT or Flag–NF2 ΔAKAP mutant. HA–LATS1 was immunoprecipitated and analysed for phosphorylation by PKA.
Recently, diverse GPCR agonists have been reported to regulate YAP (Yu et al, 2012). Those that activate Gs-coupled GPCRs were shown to induce YAP phosphorylation. However, the signalling mechanism linking Gs-coupled GPCRs to LATS activation was not elucidated. We speculate that Gs-coupled GPCRs accomplish this, in part, by promoting direct phosphorylation of LATS via PKA. Indeed, we observed phosphorylation of LATS by PKA in epinephrine-stimulated MDA-MB231 cells (Supplementary Figure S12A). Moreover, dnPKA abolished YAP phosphorylation induced by epinephrine or dopamine stimulation (Supplementary Figure S12B and C). Taken together, PKA is a general activator of LATS–YAP pathway.
NF2 functions as an AKAP to induce YAP Ser381 phosphorylation
AKAPs are crucial mediators of cAMP/PKA signalling (Wong and Scott, 2004). AKAP proteins pre-anchor PKA holoenzyme and associated signalling proteins in specific subcellular localizations, enabling cells to rapidly respond to incoming stimuli in a spatially confined area. AKAP proteins directly bind through their amphipathic α-helix to the cavity formed by regulatory subunit dimers (Carr et al, 1991; Newlon et al, 1997, 2001). AKAPs are classified into Type I, Type II, or dual specificity depending on the regulatory subunits they interact with. Although we have failed to detect endogenous interaction, we observed that LATS associated with PKA regulatory subunits in overexpression system (Supplementary Figure S13A). Combinatorial pre-treatment of RIAD-11R and 11R-SuperAKAP-IS peptides, competitive inhibitors of type I or type II AKAP, respectively (Carlson et al, 2006; Gold et al, 2006), inhibited latrunculin B-induced YAP phosphorylation (Supplementary Figure S13B) as well as PKA-mediated LATS2 phosphorylation (Supplementary Figure S13C). Pre-treating RIAD-11R or 11R-SuperAKAP-IS alone had no inhibitory effect on YAP phosphorylation (Supplementary Figure S13B). These results implicate possible existence of AKAP(s) in PKA–LATS–YAP signalling and that they are likely to be dual-specificity AKAP.
Interestingly, NF2 has been shown to function as an AKAP (Gronholm et al, 2003). Although Gronholm et al suggested that NF2 is a Type I AKAP, we observed that NF2 equally interacted with Type II regulatory subunits as well (Supplementary Figure S14). To determine if the AKAP function of NF2 is necessary for NF2-induced YAP phosphorylation, we transfected 293T cells with LATS1 and wild-type NF2 or an NF2 deletion mutant lacking the amphipathic helix (amino acids 462–480), which is known to constitute the AKAP domain. NF2 ΔAKAP mutant failed to induce YAP Ser381 phosphorylation and, importantly, abolished PKA-mediated LATS phosphorylation (Figure 6D). This result indicates that the AKAP function of NF2 is necessary for efficient Ser381 phosphorylation of YAP by LATS kinases.
Discussion
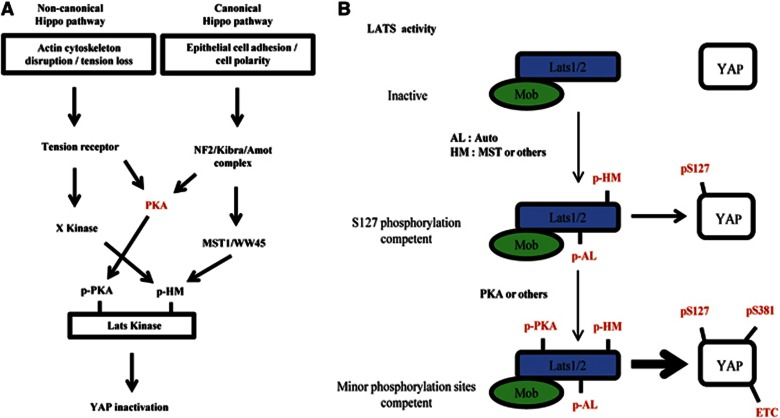
In this study, we presented findings that incorporate cAMP/PKA signalling into LATS–YAP pathway (Figure 7A). PKA was necessary to fully inactivate YAP in all known contexts that activate LATS. We provided evidences that direct phosphorylation of LATS by PKA enhance LATS kinase activity, enabling LATS to phosphorylate otherwise inefficient substrate residues including Ser381. PKA directly phosphorylated LATS2 at its (R/K)(R/K)xS/T motifs, and non-phosphorylatable LATS2 was inefficient in inducing YAP phosphorylation when reconstituted in Lats1/2-null cell. In general, inactivation of YAP is crucial for cell cycle exit of progenitor cells and differentiation (Camargo et al, 2007; Lee et al, 2008; Zhang et al, 2012). Thus, our results suggest a role of cAMP/PKA in stem/progenitor cell regulation. In this aspect, neurogenesis is particularly interesting since PKA activation can promote neuronal differentiation in various systems (Cox et al, 2000; Suh et al, 2001; Vaudry et al, 2002; Kim et al, 2005; Li et al, 2007). It is likely that neuronal-differentiation-inducing GPCR agonists such as PACAP or proneural genes (e.g., Ascl1 or Neurogenin2) act at least in part by inhibiting YAP. Further investigation of the interaction between PKA and YAP during mammalian neurogenesis is an interesting future topic.
Figure 7.
Model depicting the refined Hippo pathway and step-wise LATS activation. (A) Signalling scheme of the Hippo pathway. PKA serves a common function in both non-canonical and canonical Hippo pathways. See the text for further details. (B) LATS1/2 is activated in two steps. The first step involves Mob1 binding and phosphorylation of the AL and HM. These events are sufficient for YAP Ser127 phosphorylation, which is a good LATS substrate. The second step involves further activation by PKA (or others kinases). Only this fully activated LATS can effectively phosphorylate poor substrates, including YAP Ser38.
Although PKA inhibition in LATS1/2-proficient cells resulted in strong reduction of YAP Ser381 phosphorylation with only mild effect on Ser127 phosphorylation, we think that PKA phosphorylation of LATS increases its general kinase activity, that is, it does not confer specific advantage towards phosphorylating Ser381. Two main observations support this view. First, the sequential kinase assay (Figure 3D) shows increase of both Ser127 and Ser381 phosphorylations by PKA pre-incubation. Second, in Figure 4C, we show that LATS2 4SA-reconstituted cells attenuate both phosphorylations. Since Ser127 is a biochemically efficient substrate for LATS, PKA inhibition had weaker inhibitory effect on Ser127 phosphorylation compared to Ser381 phosphorylation. Thus, our study provides important insight on how otherwise inefficient substrate residues on YAP can be fully phosphorylated in intact cells. On the basis of these observations, we propose a model of step-wise increases in LATS activity (Figure 7B). Mob1 binding, and AL and HM phosphorylations are minimal requirements for LATS activity. If these conditions are satisfied, LATS is competent to phosphorylate its preferred substrate, YAP Ser127. However, in order to efficiently phosphorylate other poor target residues, including Ser381, LATS should acquire a further activating event, such as PKA-mediated phosphorylation. Yet, we do not understand mechanistically how LATS phosphorylation by PKA confers enhanced activity. We notice that the extent of stimulatory effect of PKA in vitro is weaker compared to its inhibition in intact cells. This implicates missing/limiting component(s) in the in vitro assay. Therefore, we speculate that LATS phosphorylation by PKA mediates interaction with yet unidentified component(s), which enhances its activity towards YAP. Proteomic analysis of interacting partners using LATS2 WT or 4SA as bait might identify the missing component(s).
We also emphasize that, although we have focused on Ser381 phosphorylation in this study, PKA might promote phosphorylation of other minor residues in YAP. Indeed, we have found that the YAP 5SA mutant exhibits stronger transcriptional activity than the YAP 2SA mutant (data not shown), indicating that one or more of the remaining three residues also contribute to suppressing YAP activity. Future characterization of additional phosphorylation sites in intact cells is necessary to completely understand YAP regulation.
Incorporation of PKA into the LATS–YAP pathway made identification of relevant AKAPs an immediate issue. Role of AKAP in the PKA–LATS–YAP signalling is supported by our findings that LATS and regulatory subunits associate in intact cells and, more importantly, that AKAP inhibitors inhibited signalling by latrunculin B. Here, we showed that one Hippo pathway protein, NF2, can function as an AKAP. Deletion of AKAP domain in NF2 abrogated its ability to induce YAP- and PKA-mediated LATS phosphorylation. We speculate that NF2 is the universal AKAP that facilitates PKA–LATS–YAP signalling. Angiomotin and NF2 physically interact and cooperate at cell junctions (Yi et al, 2011), suggesting that angiomotin proteins might work with NF2 to bring about PKA-mediated LATS phosphorylation. In regards of cytoskeletal damage signalling, it has been shown that NF2 is dephosphorylated and becomes activated by cell detachment (Shaw et al, 1998), implicating NF2 as candidate AKAP during cytoskeletal damage-induced signalling. However, depletion of NF2 in RPE cells failed to attenuate YAP phosphorylation by latrunclin B treatment (Supplementary Figure S15). The failure of NF2 depletion to inhibit cytoskeletal damage-induced YAP regulation may reflect the presence of other proteins with redundant functions. Importantly, many FERM-domain-containing proteins in addition to NF2 also possess AKAP function (Dransfield et al, 1997; Neisch and Fehon, 2011). In fact, it has been shown that NF2 functions redundantly with another FERM-domain protein, expanded, in Drosophila (Hamaratoglu et al, 2006), and mammalian ‘expanded’ also activates the Hippo pathway in cultured cells (Angus et al, 2012). These results suggest the possibility that mammalian ‘expanded’ and other FERM-domain proteins may also redundantly carry out AKAP function and cooperate with NF2 to transduce actin cytoskeletal damage signals. However, at the same time, we still open the possibility that other protein might function as AKAP to facilitate PKA–LATS–YAP signalling during cytoskeletal damage.
Because cAMP/PKA signalling has pleotropic effects on diverse cellular physiologies, manipulating PKA activity to interfere with YAP activity would have obvious drawbacks. However, understanding the mechanistic basis of step-wise LATS activation will support the development of LATS agonists as potential anti-cancer therapeutics.
Materials and methods
Cell culture and MEF isolation
NIH3T3, MDA-MB231, and U2OS cell lines were maintained in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% FBS. RPE cells were maintained in DMEM/F12 with 10% FBS. For MEFs, 2 mM L-glutamine was added to DMEM/10% FBS.
MEFs were isolated from E12.5−13.5 pregnant mice of the indicated genotypes. After sacrificing mice, brains and livers were removed from embryos and the remaining embryonic tissue was trypsinized to a single-cell suspension and plated on a 100-mm dish. This initial plating was designated passage 0. All experiments with primary MEFs were performed at passage 3 or 4.
All treatments (Latrunculin B, detachment, Forskolin/IBMX, and GPCR stimulation) in this study were performed in 70–80% confluent cells.
Western blotting, immunoprecipitation, and kinase assay
Cells were washed once with ice-cold phosphate-buffered saline (PBS) and harvested with 1 mM EDTA (ethylenediaminetetraacetic acid) in PBS. For western blot analysis, cells were lysed with NP-40 buffer (1% NP-40, 50 mM Tris–Cl pH 7.5, 150 mM NaCl, 1 mM EDTA, and 1 mM MgCl2) or RIPA buffer (NP-40 buffer containing 0.5% sodium deoxycholate and 0.1% sodium dodecyl sulphate (SDS)). Lysates were cleared by microcentrifugation for 20 min at 13 000 r.p.m. and protein concentration was measured by BCA or Bradford methods. Lysates were fractionated by SDS–PAGE (polyacrylamide gel electrophoresis), and proteins were transferred to a nitrocellulose membrane. Membranes were blocked in 5% non-fat dry milk for 1 h and incubated with primary antibodies diluted in 5% non-fat dry milk or bovine serum albumin (BSA). After incubating with horseradish peroxidase-linked secondary antibodies (Jackson Laboratory) diluted 1:10 000 in 5% non-fat dry milk, blots were developed using an enhanced chemiluminescence kit (Amersham).
For immunoprecipitation experiments involving immunoblotting phospho-PKA substrate, RPE cells were lysed with NP-40 buffer, and 293T and NIH3T3 cells were lysed with RIPA buffer. For RPE, lysates containing 1 mg protein were incubated with 20 μl S-protein agarose beads (Novagen). For 293T and NIH3T3 cells, lysates (1 mg protein) were incubated with 3 μg anti-HA antibody followed by incubation with 20 μl protein A/G-agarose beads (GeneDepot). Beads were washed three times with lysis buffers and boiled.
For kinase assays, cells were lysed with 0.5% NP-40 buffer and processed as described for immunoprecipitations. After the last wash, beads were washed with 1X kinase assay buffer. The buffer for PKA assays was 50 mM Tris–Cl (pH 7.5) and 10 mM MgCl2, and that for LATS kinase assays was 25 mM HEPES (pH 7.4), 50 mM NaCl, 10 mM MgCl2, and 1 mM dithiothreitol (DTT). For ‘cold’ kinase assays, 200 μM unlabelled ATP was used; for ‘hot’ kinase assays, 1 μCi γP32-labelled ATP and 10 μM unlabelled ATP were used. Reactions were allowed to proceed for 30 min at 30°C
Generation of Lats2-floxed (Lats2 fl/fl) mice
The Lats2-floxed embryonic stem (ES) cell line was generated by targeting mouse Lats2 exon 4 with a fragment of DNA containing Lats2 exon 4 flanked by two LoxP sites. ES cell culture, electroporation, chimera generation, germline transmission, and deletion of FRT were performed as described (Jeon et al, 2011). See Supplementary Figure S16 for detailed targeting schemes and Southern blot validation results.
Retrovirus generation
pMSCV-puro vector/Cre plasmids were used to infect Lats1/2 primary MEFs. pMSCV-neo vector/Cre plasmids were used to infect Mst1/2 primary MEFs. pBABE-puro SV40 LT plasmid was used for immortalization. For LATS1/2-null MEF complementation experiments, LATS1 was cloned into pMSCV-hygro plasmids and the Lats2 allele was excised using pBABE-zeocin Cre. For experiments in NIH3T3 cells, dnPKA was cloned into pMSCV-puro, and YAP WT or S127A was cloned into pMSCV-neo. pBABE-puro SV40 LT and pBABE-Zeocin Cre plasmids were purchased from Addgene.
Retroviruses used to infect murine cell lines were generated by co-transfecting 293T cells with 6 μg retroviral plasmids and 3 μg pCLEGO helper virus plasmid using the calcium phosphate precipitation method. Viral supernatants were collected for 48 h beginning 1 day after transfection. Retroviral supernatants were supplemented with 6 μg/ml polybrene and added to target cells. On the next day, cells were selected by growing in media supplemented with 3 μg puromycin, 400 μg/ml G418, or 200 μg/ml hygromycin.
Anoikis and soft-agar assay
Poly-HEMA (2-hydroxyethyl methacrylate; Sigma) was dissolved in 95% ethanol. Culture dishes were coated with 10 mg/ml poly-HEMA overnight and extensively washed with PBS before use. After trypsinization, 3 × 105 cells were seeded onto 60-mm poly-HEMA-coated dishes and incubated for the indicated times.
For soft-agar assays, 1 ml of 0.5% bottom agar in DMEM was solidified in 6-well plates. Cells (5 × 103) were resuspended in 0.4% top agar in DMEM and overlain onto bottom agar. Growth medium (500 μl) was added on top to prevent drying and was replenished every 4 days. After 15 days, colonies were stained with 0.1% crystal violet followed by extensive washing with distilled water. Stained colonies were examined under a dissecting microscope.
In ovo electroporation
In ovo electroporation assays were performed as described (Thaler et al, 2002; Joshi et al, 2009). In chick electroporation assays, DNAs were injected into a Hamburger and Hamilton (HH) stage-13 chick neural tube. The embryos were harvested 3 days post electroporation and fixed in 4% paraformaldehyde, embedded in OCT, and cryosectioned (12 μm thickness) for immunohistochemistry assays. Each set of chick electroporation experiments was repeated independently more than three times with at least three embryos injected with the same combination of plasmids in each experimental set. Representative sets of images from reproducible results are presented.
Statistical analysis
Graphs were drawn using Graph Prism software. Statistical analyses were done by unpaired two-tailed Student’s t-test with a 95% confidence interval.
Supplementary Material
Acknowledgments
We thank Stanley McKnight for providing dnPKA, PKA-CA, RSV-PKI, and regulatory subunit plasmids; Tatsuo Kinashi for providing the Mst1-floxed mice; and Cheolju Lee for providing the SBP plasmid. This research was supported by grants from the National Creative Research Program (20120001228), to D-SL; the Research Institute of Pharmaceutical Sciences, Research Settlement Fund for the new faculty of SNU, POSCO TJ Park Science Fellowship, Basic Science Research Program of National Research Foundation of Korea (NRF) (2012R1A1A1001749) and National R&D Program for Cancer Control, Ministry of Health and Welfare, Republic of Korea (1220120), to S. Lee; and the NIH/NINDS (R01 NS054941), March of Dimes Foundation, and Christopher and Dana Reeve Foundation, to S.-K. Lee.
Author contributions: Minchul Kim and D-SL designed the experiment and wrote the paper. Minchul Kim and Miju Kim performed the experiments. Seunghee Lee and Sookyung Lee performed the chicken electroporation experiments. HL generated the Lats2-floxed mice. SK and HS generated the Lats1-knockout mice.
Footnotes
The authors declare that they have no conflict of interest.
References
- Angus L, Moleirinho S, Herron L, Sinha A, Zhang X, Niestrata M, Dholakia K, Prystowsky MB, Harvey KF, Reynolds PA, Gunn-Moore FJ (2012) Willin/FRMD6 expression activates the Hippo signaling pathway kinases in mammals and antagonizes oncogenic YAP. Oncogene 31: 238–250 [DOI] [PubMed] [Google Scholar]
- Baldwin C, Garnis C, Zhang LW, Rosin MP, Lam WL (2005) Multiple microalterations detected at high frequency in oral cancer. Cancer Res 65: 7561–7567 [DOI] [PubMed] [Google Scholar]
- Bashyam MD, Bair R, Kim YH, Wang P, Hernandez-Boussard T, Karikari CA, Tibshirani R, Maitra A, Pollack JR (2005) Array-based comparative genomic hybridization identifies localized DNA amplifications and homozygous deletions in pancreatic cancer. Neoplasia 7: 556–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner R, Poernbacher I, Buser N, Hafen E, Stocker H (2010) The WW domain protein kibra acts upstream of Hippo in Drosophila. Developmental Cell 18: 309–316 [DOI] [PubMed] [Google Scholar]
- Cai J, Zhang N, Zheng Y, de Wilde RF, Maitra A, Pan D (2010) The Hippo signaling pathway restricts the oncogenic potential of an intestinal regeneration program. Genes Dev 24: 2383–2388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camargo FD, Gokhale S, Johnnidis JB, Fu D, Bell GW, Jaenisch R, Brummelkamp TR (2007) YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol 17: 2054–2060 [DOI] [PubMed] [Google Scholar]
- Cao X, Pfaff SL, Gage FH (2008) YAP regulates neural progenitor cell number via the TEA domain transcription factor. Genes Dev 22: 3320–3334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson CR, Lygren B, Berge T, Hoshi N, Wong W, Tasken K, Scott JD (2006) Delineation of type I protein kinase A-selective signaling events using an RI anchoring disruptor. J Biol Chem 281: 21535–21545 [DOI] [PubMed] [Google Scholar]
- Carr DW, Stofko-Hahn RE, Fraser ID, Bishop SM, Acott TS, Brennan RG, Scott JD (1991) Interaction of the regulatory subunit (RII) of cAMP-dependent protein kinase with RII-anchoring proteins occurs through an amphipathic helix binding motif. J Biol Chem 266: 14188–14192 [PubMed] [Google Scholar]
- Chan EH, Nousiainen M, Chalamalasetty RB, Schafer A, Nigg EA, Sillje HH (2005) The Ste20-like kinase Mst2 activates the human large tumor suppressor kinase Lats1. Oncogene 24: 2076–2086 [DOI] [PubMed] [Google Scholar]
- Chan SW, Lim CJ, Chong YF, Pobbati AV, Huang C, Hong W (2011a) Hippo pathway-independent restriction of TAZ and YAP by angiomotin. J Biol Chem 286: 7018–7026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SW, Lim CJ, Chong YF, Pobbati AV, Huang CX, Hong WJ (2011b) Hippo pathway-independent restriction of TAZ and YAP by angiomotin. J Biol Chem 286: 7018–7026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg CH, Correll LA, Cadd GG, McKnight GS (1987) Inhibition of intracellular cAMP-dependent protein kinase using mutant genes of the regulatory type I subunit. J Biol Chem 262: 13111–13119 [PubMed] [Google Scholar]
- Cox ME, Deeble PD, Bissonette EA, Parsons SJ (2000) Activated 3′,5 ′-cyclic AMP-dependent protein kinase is sufficient to induce neuroendocrine-like differentiation of the LNCaP prostate tumor cell line. J Biol Chem 275: 13812–13818 [DOI] [PubMed] [Google Scholar]
- Dai ZY, Zhu WG, Morrison CD, Brena RM, Smiraglia DJ, Raval A, Wu YZ, Rush LJ, Ross P, Molina JR, Otterson GA, Plass C (2003) A comprehensive search for DNA amplification in lung cancer identifies inhibitors of apoptosis cIAP1 and cIAP2 as candidate oncogenes. Hum Mol Genet 12: 791–801 [DOI] [PubMed] [Google Scholar]
- Day RN, Walder JA, Maurer RA (1989) A protein-kinase inhibitor gene reduces both basal and multihormone-stimulated prolactin gene-transcription. J Biol Chem 264: 431–436 [PubMed] [Google Scholar]
- De Rooij J, Zwartkruis FJT, Verheijen MHG, Cool RH, Nijman SMB, Wittinghofer A, Bos JL (1998) Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature 396: 474–477 [DOI] [PubMed] [Google Scholar]
- Dong J, Feldmann G, Huang J, Wu S, Zhang N, Comerford SA, Gayyed MF, Anders RA, Maitra A, Pan D (2007) Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell 130: 1120–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dransfield DT, Bradford AJ, Smith J, Martin M, Roy C, Mangeat PH, Goldenring JR (1997) Ezrin is a cyclic AMP-dependent protein kinase anchoring protein. EMBO J 16: 35–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, Elvassore N, Piccolo S (2011) Role of YAP/TAZ in mechanotransduction. Nature 474: 179–183 [DOI] [PubMed] [Google Scholar]
- Fernandez BG, Gaspar P, Bras-Pereira C, Jezowska B, Rebelo SR, Janody F (2011) Actin-capping protein and the Hippo pathway regulate F-actin and tissue growth in Drosophila. Development 138: 2337–2346 [DOI] [PubMed] [Google Scholar]
- Florio VA, Ross EM (1983) Regulation of the catalytic component of adenylate cyclase. Potentiative interaction of stimulatory ligands and 2′,5′-dideoxyadenosine. Mol Pharmacol 24: 195–202 [PubMed] [Google Scholar]
- Genevet A, Wehr MC, Brain R, Thompson BJ, Tapon N (2010) Kibra is a regulator of the Salvador/Warts/Hippo signaling network. Dev Cell 18: 300–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold MG, Lygren B, Dokurno P, Hoshi N, McConnachie G, Tasken K, Carlson CR, Scott JD, Barford D (2006) Molecular basis of AKAP specificity for PKA regulatory subunits. Mol Cell 24: 383–395 [DOI] [PubMed] [Google Scholar]
- Gronholm M, Vossebein L, Carlson CR, Kuja-Panula J, Teesalu T, Alfthan K, Vaheri A, Rauvala H, Herberg FW, Tasken K, Carpen O (2003) Merlin links to the cAMP neuronal signaling pathway by anchoring the RIbeta subunit of protein kinase A. J Biol Chem 278: 41167–41172 [DOI] [PubMed] [Google Scholar]
- Grusche FA, Richardson HE, Harvey KF (2010) Upstream regulation of the hippo size control pathway. Curr Biol 20: R574–R582 [DOI] [PubMed] [Google Scholar]
- Halder G, Dupont S, Piccolo S (2012) Transduction of mechanical and cytoskeletal cues by YAP and TAZ. Nat Rev Mol Cell Biol 13: 591–600 [DOI] [PubMed] [Google Scholar]
- Halder G, Johnson RL (2011) Hippo signaling: growth control and beyond. Development 138: 9–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaratoglu F, Willecke M, Kango-Singh M, Nolo R, Hyun E, Tao CY, Jafar-Nejad H, Halder G (2006) The tumour-suppressor genes NF2/Merlin and Expanded act through Hippo signalling to regulate cell proliferation and apoptosis. Nat Cell Biol 8: 27–U29 [DOI] [PubMed] [Google Scholar]
- Harvey K, Tapon N (2007) The Salvador-Warts-Hippo pathway—an emerging tumour-suppressor network. Nat Rev Cancer 7: 182–191 [DOI] [PubMed] [Google Scholar]
- Hergovich A, Schmitz D, Hemmings BA (2006) The human tumour suppressor LATS1 is activated by human MOB1 at the membrane. Biochem Biophys Res Co 345: 50–58 [DOI] [PubMed] [Google Scholar]
- Hermsen M, Guervos MA, Meijer G, van Diest P, Nieto CS, Marcos CA, Sampedro A (2005) Chromosomal changes in relation to clinical outcome in larynx and pharynx squamous cell carcinoma. Cell Oncol 27: 191–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe AK, Juliano RL (2000) Regulation of anchorage-dependent signal transduction by protein kinase A and p21-activated kinase. Nat Cell Biol 2: 593–600 [DOI] [PubMed] [Google Scholar]
- Huang J, Wu S, Barrera J, Matthews K, Pan D (2005) The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell 122: 421–434 [DOI] [PubMed] [Google Scholar]
- Imoto I, Tsuda H, Hirasawa A, Miura M, Sakamoto M, Hirohashi S, Inazawa J (2002) Expression of cIAP1, a target for 11q22 amplification, correlates with resistance of cervical cancers to radiotherapy. Cancer Res 62: 4860–4866 [PubMed] [Google Scholar]
- Imoto I, Yang ZQ, Pimkhaokham A, Tsuda H, Shimada Y, Imamura M, Ohki M, Inazawa J (2001) Identification of cIAP1 as a candidate target gene within an amplicon at 11q22 in esophageal squamous cell carcinomas. Cancer Res 61: 6629–6634 [PubMed] [Google Scholar]
- Jeon Y, Ko E, Lee KY, Ko MJ, Park SY, Kang J, Jeon CH, Lee H, Hwang DS (2011) TopBP1 deficiency causes an early embryonic lethality and induces cellular senescence in primary cells. J Biol Chem 286: 5414–5422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi K, Lee S, Lee B, Lee JW, Lee SK (2009) LMO4 controls the balance between excitatory and inhibitory spinal V2 interneurons. Mech Develop 126: S139–S139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SS, Choi JM, Kim JW, Ham DS, Ghil SH, Kim MK, Yunhee KK, Hong SY, Ahn SC, Kim SU, Lee YD, Haeyoung SK (2005) cAMP induces neuronal differentiation of mesenchymal stem cells via activation of extracellular signal-regulated kinase/MAPK. Neuroreport 16: 1357–1361 [DOI] [PubMed] [Google Scholar]
- Kim YN, Koo KH, Sung JY, Yun UJ, Kim H (2012) Anoikis resistance: an essential prerequisite for tumor metastasis. Int J Cell Biol 2012: 306879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Kim TS, Yang TH, Koo BK, Oh SP, Lee KP, Oh HJ, Lee SH, Kong YY, Kim JM, Lim DS (2008) A crucial role of WW45 in developing epithelial tissues in the mouse. EMBO J 27: 1231–1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KP, Lee JH, Kim TS, Kim TH, Park HD, Byun JS, Kim MC, Jeong WI, Calvisi DF, Kim JM, Lim DS (2010) The Hippo-Salvador pathway restrains hepatic oval cell proliferation, liver size, and liver tumorigenesis. Proc Natl Acad Sci USA 107: 8248–8253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Yin W, Wang X, Zhu WB, Huang YJ, Yan GM (2007) Cholera toxin induces malignant glioma cell differentiation via the PKA/CREB pathway. Proc Natl Acad Sci USA 104: 13438–13443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Li Y, Kim SM, Bossuyt W, Liu P, Qiu Q, Wang Y, Halder G, Finegold MJ, Lee JS, Johnson RL (2010) Hippo signaling is a potent in vivo growth and tumor suppressor pathway in the mammalian liver. Proc Natl Acad Sci USA 107: 1437–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita M, Tomizawa K, Moriwaki A, Li ST, Terada H, Matsui H (2001) A high-efficiency protein transduction system demonstrating the role of PKA in long-lasting long-term potentiation. J Neurosci 21: 6000–6007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neisch AL, Fehon RG (2011) Ezrin, radixin and moesin: key regulators of membrane-cortex interactions and signaling. Curr Opin Cell Biol 23: 377–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newlon MG, Roy M, Hausken ZE, Scott JD, Jennings PA (1997) The A-kinase anchoring domain of type IIalpha cAMP-dependent protein kinase is highly helical. J Biol Chem 272: 23637–23644 [DOI] [PubMed] [Google Scholar]
- Newlon MG, Roy M, Morikis D, Carr DW, Westphal R, Scott JD, Jennings PA (2001) A novel mechanism of PKA anchoring revealed by solution structures of anchoring complexes. EMBO J 20: 1651–1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsawa S, Sato Y, Enomoto M, Nakamura M, Betsumiya A, Igaki T (2012) Mitochondrial defect drives non-autonomous tumour progression through Hippo signalling in Drosophila. Nature 490: 547–551 [DOI] [PubMed] [Google Scholar]
- Orellana SA, Mcknight GS (1992) Mutations in the catalytic subunit of camp-dependent protein-kinase result in unregulated biological-activity. Proc Natl Acad Sci USA 89: 4726–4730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overholtzer M, Zhang J, Smolen GA, Muir B, Li W, Sgroi DC, Deng CX, Brugge JS, Haber DA (2006) Transforming properties of YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proc Natl Acad Sci USA 103: 12405–12410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan DJ (2010) The Hippo signaling pathway in development and cancer. Dev Cell 19: 491–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paramasivam M, Sarkeshik A, Yates JR 3rd, Fernandes MJ, McCollum D (2011) Angiomotin family proteins are novel activators of the LATS2 kinase tumor suppressor. Mol Biol Cell 22: 3725–3733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansores-Garcia L, Bossuyt W, Wada K, Yonemura S, Tao C, Sasaki H, Halder G (2011) Modulating F-actin organization induces organ growth by affecting the Hippo pathway. EMBO J 30: 2325–2335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw RJ, McClatchey AI, Jacks T (1998) Regulation of the neurofibromatosis type 2 tumor suppressor protein, Merlin, by adhesion and growth arrest stimuli. J Biol Chem 273: 7757–7764 [DOI] [PubMed] [Google Scholar]
- Simpson CD, Anyiwe K, Schimmer AD (2008) Anoikis resistance and tumor metastasis. Cancer Lett 272: 177–185 [DOI] [PubMed] [Google Scholar]
- Snijders AM, Schmidt BL, Fridlyand J, Dekker N, Pinkel D, Jordan RCK, Albertson DG (2005) Rare amplicons implicate frequent deregulation of cell fate specification pathways in oral squamous cell carcinoma. Oncogene 24: 4232–4242 [DOI] [PubMed] [Google Scholar]
- Song H, Mak KK, Topol L, Yun K, Hu J, Garrett L, Chen Y, Park O, Chang J, Simpson RM, Wang CY, Gao B, Jiang J, Yang Y (2010) Mammalian Mst1 and Mst2 kinases play essential roles in organ size control and tumor suppression. Proc Natl Acad Sci USA 107: 1431–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley BK, Irvine KD (2010) Warts and yorkie mediate intestinal regeneration by influencing stem cell proliferation. Curr Biol 20: 1580–1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudol M, Harvey KF (2010) Modularity in the Hippo signaling pathway. Trends Biochem Sci 35: 627–633 [DOI] [PubMed] [Google Scholar]
- Suh JH, Lu NR, Nicot A, Tatsuno I, DiCicco-Bloom E (2001) PACAP is an anti-mitogenic signal in developing cerebral cortex. Nat Neurosci 4: 123–124 [DOI] [PubMed] [Google Scholar]
- Thaler JP, Lee SK, Jurata LW, Gill GN, Pfaff SL (2002) LIM factor Lhx3 contributes to the specification of motor neuron and interneuron identity through cell-type-specific protein-protein interactions. Cell 110: 237–249 [DOI] [PubMed] [Google Scholar]
- Vaudry D, Stork PJS, Lazarovici P, Eiden LE (2002) Signaling pathways for PC12 cell differentiation: making the right connections. Science 296: 1648–1649 [DOI] [PubMed] [Google Scholar]
- Wada KI, Itoga K, Okano T, Yonemura S, Sasaki H (2011) Hippo pathway regulation by cell morphology and stress fibers. Development 138: 3907–3914 [DOI] [PubMed] [Google Scholar]
- Wang W, Huang J, Chen J (2011) Angiomotin-like proteins associate with and negatively regulate YAP1. J Biol Chem 286: 4364–4370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson PA (1990) Direct stimulation of adenylate cyclase by mechanical forces in S49 mouse lymphoma cells during hyposmotic swelling. J Biol Chem 265: 6569–6575 [PubMed] [Google Scholar]
- Weber RG, Sommer C, Albert FK, Kiessling M, Cremer T (1996) Clinically distinct subgroups of glioblastoma multiforme studied by comparative genomic hybridization. Lab Invest 74: 108–119 [PubMed] [Google Scholar]
- Wong W, Scott JD (2004) AKAP signalling complexes: focal points in space and time. Nat Rev Mol Cell Biol 5: 959–970 [DOI] [PubMed] [Google Scholar]
- Yi CL, Troutman S, Fera D, Stemmer-Rachamimov A, Avila JL, Christian N, Persson NL, Shimono A, Speicher DW, Marmorstein R, Holmgren L, Kissil JL (2011) A tight junction-associated merlin-angiomotin complex mediates merlin's regulation of mitogenic signaling and tumor suppressive functions. Cancer Cell 19: 527–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FX, Zhao B, Panupinthu N, Jewell JL, Lian I, Wang LH, Zhao J, Yuan H, Tumaneng K, Li H, Fu XD, Mills GB, Guan KL (2012) Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell 150: 780–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Zheng Y, Dong J, Klusza S, Deng WM, Pan D (2010) Kibra functions as a tumor suppressor protein that regulates Hippo signaling in conjunction with Merlin and Expanded. Dev Cell 18: 288–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HQ, Deo M, Thompson RC, Uhler MD, Turner DL (2012) Negative regulation of Yap during neuronal differentiation. Dev Biol 361: 103–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JM, Ji JY, Yu M, Overholtzer M, Smolen GA, Wang R, Brugge JS, Dyson NJ, Haber DA (2009) YAP-dependent induction of amphiregulin identifies a non-cell-autonomous component of the Hippo pathway. Nat Cell Biol 11: 1444–U1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Kim J, Ye X, Lai ZC, Guan KL (2009) Both TEAD-binding and WW domains are required for the growth stimulation and oncogenic transformation activity of Yes-associated protein. Cancer Res 69: 1089–1098 [DOI] [PubMed] [Google Scholar]
- Zhao B, Li L, Lei Q, Guan KL (2010a) The Hippo-YAP pathway in organ size control and tumorigenesis: an updated version. Genes Dev 24: 862–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Li L, Lu Q, Wang LH, Liu CY, Lei QY, Guan KL (2011) Angiomotin is a novel Hippo pathway component that inhibits YAP oncoprotein. Gene Dev 25: 51–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Li L, Tumaneng K, Wang CY, Guan KL (2010b) A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP). Genes Dev 24: 72–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Li L, Wang L, Wang CY, Yu J, Guan KL (2012) Cell detachment activates the Hippo pathway via cytoskeleton reorganization to induce anoikis. Genes Dev 26: 54–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, Xie J, Ikenoue T, Yu J, Li L, Zheng P, Ye K, Chinnaiyan A, Halder G, Lai ZC, Guan KL (2007) Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev 21: 2747–2761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Conrad C, Xia F, Park JS, Payer B, Yin Y, Lauwers GY, Thasler W, Lee JT, Avruch J, Bardeesy N (2009) Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell 16: 425–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Zhang Y, Wu H, Barry E, Yin Y, Lawrence E, Dawson D, Willis JE, Markowitz SD, Camargo FD, Avruch J (2011) Mst1 and Mst2 protein kinases restrain intestinal stem cell proliferation and colonic tumorigenesis by inhibition of Yes-associated protein (Yap) overabundance. Proc Natl Acad Sci USA 108: E1312–E1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.