Abstract
At the time of writing, the Italian Parliament is debating a new law that would make it legal to practice an unproven stem cell treatment in public hospitals. The treatment, offered by a private non-medical organization, may not be safe, lacks a rationale, and violates current national laws and European regulations. This case raises multiple concerns, most prominently the urgent need to protect patients who are severely ill, exposed to significant risks, and vulnerable to exploitation. The scientific community must consider the context—social, financial, medical, legal—in which stem cell science is currently situated and the need for stringent regulation. Additional concerns are emerging. These emanate from the novel climate, created within science itself, and stem cell science in particular, by the currently prevailing model of ‘translational medicine’. Only rigorous science and rigorous regulation can ensure translation of science into effective therapies rather than into ineffective market products, and mark, at the same time, the sharp distinction between the striving for new therapies and the deceit of patients.
Unproven and unauthorized ‘stem cell therapies’ are not new (Enserink, 2006; Hyun et al, 2008; Regenberg et al, 2009). What is new is the government’s support for unproven therapies in countries, where rules set out by regulatory bodies (FDA, EMA) have so far been effective in protecting patients from serious risks associated with their indiscriminate use. This may be rapidly changing.
The Italian case (Box 1; (Abbott, 2013; Nature Editorial, 2013b)) follows two similar cases in the United States, and one in Germany (in which one patient died) that were effectively halted by relevant regulatory bodies (Nature Editorial, 2013a). In one case, the proponent was arrested. However, the Italian case is the first in which unproven ‘stem cell therapies’ may be de facto made legal, rather than being stopped by regulatory bodies and the government. Thus, this is the first case in which unproven stem cell treatments are officially recognized as a bona fide treatment, without having been tested in rigorous clinical trials, and based on flimsy and highly debated preclinical evidence, to be made part of a publicly funded, public health care system (Box 2 and Box 3).
The Italian case.
Patients with disparate, severe neurological diseases were and are being treated, and will continue to be treated in a major public hospital in Italy, by intravenous and intrathecal infusions of ‘MSCs’, purportedly prepared according to a unique, novel method of isolation in culture, and in vitro differentiation into neurons. This activity had previously been taking place in Trieste. In Brescia, an official agreement had been stipulated between the public hospital and a private foundation, whereby the foundation was granted permission to prepare cells with the purportedly proprietary method. Cells for infusion into patients were prepared within a GLP lab (not stringent enough for growing cells in culture before use in patients), intended for the handling of bone marrow and cord blood-derived haematopoietic cells. Patients were being treated in the hospital. Patient care in public hospitals in Italy is paid for by the Government.
Courts and media campaigns.
Lawsuits by multiple individual patients or families were accompanied by a web-based mass action, with protests, sit-ins and a twice-a-week campaign enacted by an entertainment TV show. A vehement campaign against scientists (portrayed as ‘incompetent, unethical and corrupt’) arguing against the lack of safety and scientific grounds, as well as against AIFA, The Ministry of Health itself and other Institutions was also conducted. This campaign was echoed and supported by part of the press, by websites, and by public statements of pop singers and movie stars, vowing the right of sick children to have access to stem cell therapy. Central to the campaign was the claim that ‘compassionate therapy’ was being denied to dying children, who had benefited from the treatment, some family members said, and would worsen or die should the treatment be interrupted. Multiple courts ruled in favour of the patients claiming their right to continue the treatment (involving multiple scheduled infusions of MSCs), and ordered the hospital to resume it immediately in spite of the ban issued by the competent Government Agency.
Government and Parliament.
Eventually, the Italian Government was forced to issue ad hoc urgent regulatory measures. These were initially intended solely for allowing completion of those individual treatments that had been initiated, provided that cells were manufactured under the more stringent GMP conditions. More general rules intended to settle the whole matter were deferred to regulations to follow rapidly. As the Health Minister decree was debated in the Senate, the forthcoming regulations, and the prescription that cells had to be manufactured under GMP conditions, were cancelled; in addition to the completion of the treatments, the treatment of an undefined number of further patients for 18 months was allowed; ‘stem cell therapies’ in individual cases were removed from the jurisdiction of the competent Drug Regulatory Agency (AIFA) and arbitrarily equated to direct transplantation of tissues and cells, thus cancelling their definition as ‘medicines.’ It was their definition as ‘medicines’ that kept them under the regulations of AIFA and EMA for concerns specifically relating to both preparation and indication for use. At the time of this writing, the Italian Senate has passed these regulations; the Chamber is expected to discuss them in the coming days. By final approval, the Italian Parliament and Government would withdraw AIFA and EMA oversight and thus patient protection from unproven stem cell therapies. This may open the way in the future to commercial entities to market unproven MSC therapies.
This makes the Italian case unique and of global concern. The protection of patients from potential fraud was the main reason why drug regulation first arose (first enacted with the Pure Food and Drug Act in 1906, applied to the historical case of ‘snake oil’ (USDA, 1917), and later evolved into the FDA). The Italian case is the first instance in the western world in which this vital regulatory barrier might be breached. Cracking regulations open is the agenda of a constellation of companies, large and small, serious or less serious, all the way to adventurers, wishing to market ‘stem cells’. Lobbying and pleading for accelerated ‘innovation’ and or ‘patients’ access to therapies’ are extensively used to this end. As in the Italian case, patients are meanwhile deceived into believing that regulations (and the prudence of scientists and physicians) are against their best interest. Breaching regulation undermines the protection of patients, paves the way for adventurers, disrupts public health care systems, destroys efforts towards sensible translation of science into medicine and wastes vital health care funds.
Safety first: testing safety in clinical trials
There is no therapy without adverse effects. Adverse effects are brought to light by proper clinical experimentation. Cell therapies are no exception.
The best current example of a stem cell therapy is bone marrow transplantation (BMT), an accepted medical practice that saves thousands of lives a year (Thomas et al, 1957; historical review in Appelbaum, 2007). But even though there is a very sound scientific rationale for this therapy, and it went on to become the standard of care for many hematological conditions, the first clinical trial of a BMT among unrelated patients led to the deaths of all patients in the trial. Donnell Thomas went back to the laboratory and spent 14 years learning why donors had to be matched to recipients during transplants. The first successful transplant between an unrelated donor and recipient was performed in 1969. This illustrates how even the simplest and most promising cell therapies must be studied in depth to be delivered safely and effectively to patients. Of course, the situation becomes much more complex in the case of therapies in which the scientific rationale for why they might help patients is unclear or untested.
In the case of systemic administration of mesenchymal stem cells (MSCs), cells are introduced into the bloodstream, which is not their natural environment. They are infused in the hope that they will reach target organs that do not normally contain MSCs. There is a wealth of knowledge about their function in their natural site (the bone marrow) and a wealth of knowledge on the properties they exhibit in a tissue culture dish. But it is not clear how exogenous MSCs will behave in the brain, kidney, or the lung. As inherently osteogenic and adipogenic cells, MSCs could generate bone or fat in the wrong organs if transplanted in sufficient numbers (Breitbach et al, 2007). MSCs can also embolize in the lungs and damage the local microcirculation. Allogeneic MSCs can trigger an adverse reaction (instant blood mediated inflammatory reaction, IBMIR; Moll et al, 2012), which leads to activation of the coagulation and complement cascades, and to the death of the infused cells. IBMIR can result occasionally in thromboembolism, but we were unaware until recently that IBMIR could be triggered by MSCs. This exemplifies why infusion of MSCs must necessarily be studied in rigorously controlled and monitored clinical trials before such therapies can be considered safe in patients.
Ensuring safety during manufacturing
MSCs are generated in sufficient quantities for infusion into patients by ex vivo culture. Ex vivo culture, expansion, or manipulation (which are not involved in transplantation of haematopoietic cells) introduces specific risks, some of which are known, and therefore subject to specific regulatory measures or controls (see Box 2; Sabatino et al, 2012). For this reason, cells intended to be administered to patients after culture are not defined by regulatory agencies as transplants. They are ‘medicines’, which need to be manufactured in highly controlled environments, with precise protocols, traceability and accountability (European Medicines Agency, 2010; http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2013/04/news_detail_001769.jsp&mid=WC0b01ac058004d5c1). What ensures that clinical-grade cells administered to patients are ‘safe’ is their definition as ‘medicines’. This puts them under regulation and vigilance by the FDA, the EMA and other equivalent national agencies.
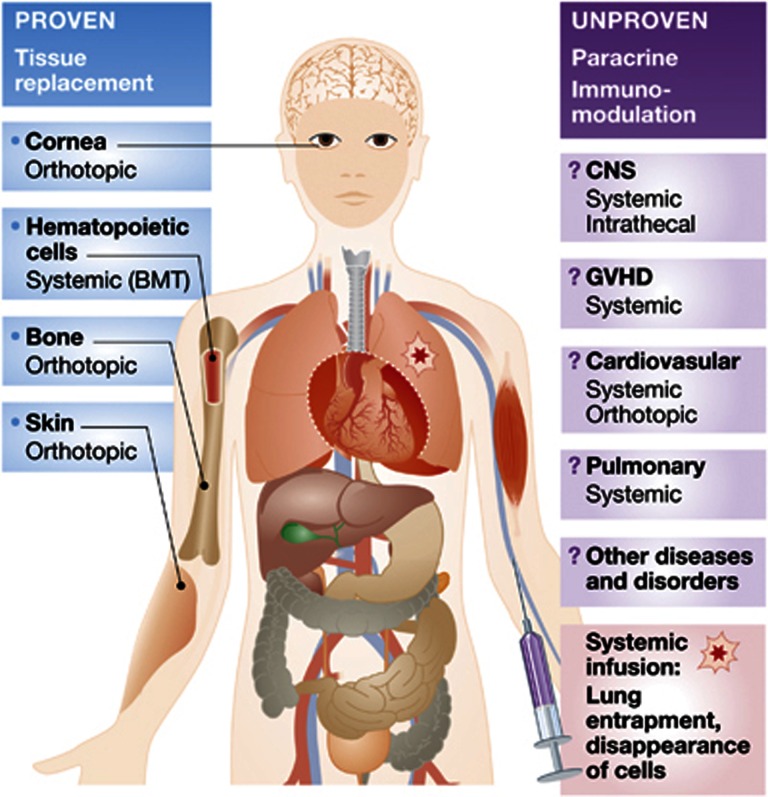
The dangers of failing to regulate the manufacture of medicines do not apply solely to novel, or stem cell-based, therapies. Patients can be harmed by medicines that are not manufactured in a highly regulated way, even when the medicines themselves are widely known to be safe and effective, and in routine commercial use. In the Italian case, the proponents of the purported ‘therapy’ have argued that their ex vivo-expanded MSCs should not be regulated as a medicine, but rather should be considered a ‘transplant’, which is exempt from regulations designed to ensure the safety of the manufacturing process and the need for formal trials (Box 3). The reason why transplanted tissues are exempt from these regulations is that they are not cultured ex vivo. Regulatory agencies should oppose efforts to end the regulation of cultured cell products. In addition, inravenously infused MSCs rapidly disappear from the body and do not engraft: even in a biological sense, infusion of MSCs is not a transplant (Figure 1).
Figure 1.
To date, there are very few examples of proven stem cell therapies. These therapies include BMT with populations that contain haematopoietic stem cells, corneal resurfacing with populations that contain limbal stem cells and skin regeneration with populations that contain epidermal stem cells. There is also strong preclinical evidence and case reports for bone regeneration using bone marrow stromal cells (BMSCs), a subset of which are skeletal stem cells. This type of regenerative medicine is dependent on the presence of stem cells (left). A number of therapies have been envisioned for the treatment of diverse disorders and diseases, such as diseases of the CNS, GVHD, cardiovasular diseases, pulmonary diseases and many more, using primarily bone marrow-derived ‘MSCs’, a term commonly used to mean cultures of BMSC, which does not equate to a population of stem cells (the skeletal stem cells are a subset of BMSCs). It is now clear that these cells do not transdifferentiate into cells outside of the skeletal lineage (bone, cartilage, haematopoiesis supportive stroma and marrow adipocytes). However, it is thought, but as yet unproven, that they may exert paracrine, immunomodulatory and immunoregulatory effects on endogenous tissues upon systemic infusion or direct injection. It is not clear that these cells display these properties in vivo, and if so, by what mechanisms. Upon intravenous injection, these cells accumulate in the lungs, and are then rapidly removed from the body. Thus, they neither transdifferentiate nor engraft, making the putative paracrine/immunomodulatory feature a property of the population as a whole, and not of the stem cell subset within it.
Taking cell therapies out of the jurisdiction of drug-regulating agencies is sometimes invoked by companies wishing to market unapproved cell therapies. This is claimed to be a necessary measure to speed up the development of therapies, fostering both innovation and patient care. The FDA and the EMA, which are the prime barrier protecting patients from fraud, are viewed or portrayed by some as the main obstacle to the development of innovation and medical advance. Governments around the world are being lobbied by companies and are pressured to favour marketing of MSCs before their efficacy can be proven through Phase II and III trials. This position is not tenable. Cell therapies must remain under strict vigilance of the FDA, EMA and other equivalent national agencies. Formal clinical trials remain the only way to learn about new therapies, to do good to patients and to do no harm. The EMA and the European Union should carefully scrutinize and monitor the Italian case. They should intervene in the event that the Italian Parliament would infringe European regulations and classify intravenous injections of MSCs as ‘transplants’ of cells or tissues, taking them out of the vigilance of the AIFA (the Italian Drug Authority).
There can be no compassion without safety and efficacy
The argument was offered in the Italian case that safety is not a concern in the face of severely ill children or adults, for whom there are no therapeutic alternatives (see Box 4). However, the terminally ill need extra safety and protection, not less. Exposing the weakest people to unknown risks is ethically unacceptable. Recourse to unproven and unsafe therapy is said to be ‘compassion’, or to fall into an arbitrary category of ‘compassionate treatment’. This is not the case at all. Compassion only applies when one offers a safe and potentially effective remedy. That a remedy is effective must be supported by published clinical data. If such data are not available, there is no legitimate assumption of effectiveness in the individual patient, and therefore no ‘compassion’.
A ‘unique method’.
There is no retrievable, scientifically published account of the method used for preparing MSCs. The method, said to be developed by two Ukranian scientists who apparently cannot be tracked at this time, is described in two patent applications submitted under their own name by the Italian proponents (Molino and Vannoni, 2010a, 2010b) to the European and US Patent Offices, not approved, and published by the latter. Treatment was proposed by the President of a private Foundation, an expert in persuasive communication holding an academic degree in humanities and serving as an Associate Professor of Psychology in a State University. Treatment was presented to the public as intended for ‘compassionate use’ in a regulatory sense (see below) and motivated by compassion in an emotional and ethical sense. Nonetheless, the operation is supported by a commercial firm, previously engaged in cosmetic medicine (anti-cellulite treatments). The term ‘compassionate use’ describes the use of a treatment unapproved, but tested as safe, and with preliminary evidence of potential efficacy, in the absence of a sound therapeutic alternative to treat a single case outside of a formal clinical trial. ‘Compassionate use’ is considered as feasible by regulations. In Europe, the EMA defers for specific regulations to individual member countries. An insufficient set of rules intended to provide guidance in these cases was issued by the Italian government in 2006, which was seen as room for the unauthorized unproven treatment of multiple diseases; however, multiple violations of the same rules (as to cell preparation, facilities, required expertise, informed consent and other matters) were detected by the relevant Italian regulatory body (AIFA), which had ordered the practice to be stopped in 2012. AIFA and the Armed Forces officers who inspected the lab in Brescia officially asked two scientific labs, one in the Italian National Institute of Health and one in the University of Modena, to determine the content of frozen vials containing the stem cell preparations to be infused to patients. The results apparently did not support the manufacturer’s claims as to cell identity, purity and properties.
Regenerative medicine must be medicine
Stem cells are not a homogeneous class of cells; ‘stem cells’ are not one-size-fits-all cures. There are different kinds of stem cells in different tissues, and even when the appropriate stem cell is selected for an indication it takes years of research to learn how to administer the stem cell safely and effectively, as demonstrated by the decades of research that was required to transplant bone marrow safely and effectively. The use of stem cells in medicine must remain cognizant not only of the true biological nature of the type of cells considered for use, but also of the biology of the diseases being targeted. Treating patients with disparate neurological diseases with intravenous or intrathecal infusion of MSCs, which is being done in Italy, has no medical rationale. The range of diseases being tackled in the Italian case include Spinal Muscular Atrophy, lysosomal storage diseases, such as Krabbe’s disease or metachromatic Leukodystrophy, Parkinson’s disease and other kinds of irreversible brain or spinal damage (Figure 1). The diseases being tackled differ strikingly from one another with respect to cause, mechanism and natural history. It is unclear if the properties of MSCs to be specifically harnessed for treatment of such diseases would reside in their nature as progenitor cells or in their non-progenitor properties—that is, their ability to exert ‘immune modulating, anti-inflammatory, trophic effects’ through the release of an unknown range of paracrine factors. There is, therefore, no visible rationale justifying expectation of a therapeutic effect.
We remain largely ignorant about how to turn cells into medicines. First, we only know how to regenerate tissues and organs that are lost through disease processes or by accident (e.g., burned skin or corneas), or that are removed through medical measures (e.g., myeloablation). We do not know how to regenerate organs and tissues that are damaged, but remain in situ in the body and undergo reactive changes. We cannot remove a dystrophic muscle, a gliotic brain or a dysplastic skeleton. Second, we only know how to regenerate tissues with a high turnover and a minimal spatial complexity (blood, which is fluid, and epithelia, which are macroscopically two-dimensional). It is for these reasons that the only known instances of successful regenerative medicine involves haematopoietic tissues, skin and cornea (reviewed in Bianco et al, 2013). Third, the route of administration of cells or tissues for regenerative purposes is critical. Haematopoiesis is regenerated by infusion of stem cells in the bloodstream solely because the circulation is integral to the development and physiology of haematopoiesis (Wright et al, 2001); skin and cornea are regenerated by local transplantation of tissue generated ex vivo from stem cells, but infusion of MSCs to treat brain disorders lacks such a logic (Figure 1).
Therapy from MSCs as progenitors?
If expected from a putative ‘progenitor function’ of MSCs, any hoped for effect would imply efficient replacement of either neurons or glial cells by infused MSCs. In the past, several papers had claimed the unexpected ability of MSCs to be turned into these cell types upon various kinds of empirical doctoring (e.g., Woodbury et al, 2000). While these data raised immediate interest, the prevailing consensus at this time, however, is that MSCs are system-specific, committed progenitors. They spontaneously generate, upon local transplantation, skeletal tissues, but not tissues that are derived from other germ layers. Most, if not all, prior data suggesting an actual neural differentiation are currently regarded either as the result of artificial manipulation, or as not robust enough to warrant use in the clinic to regenerate neural cells and tissues. MSCs are not pluripotent cells, unless reprogrammed via defined sets of genes (Takahashi et al, 2007); their inherent differentiation potential does not extend across germ-layer boundaries and in vitro doctoring does not signify a therapeutic potential to regenerate neural tissues (Bianco et al, 2013).
Regardless of differentiation potential, different types of neural cells (dopaminergic neurons, spinal motor neurons, oligodendrocytes) would have to be regenerated in diseases that are very disparate from one another, such as Parkinson’s disease, spinal muscular atrophy and metachromatic leukodystrophy. Nothing in the procedure as applied gives any clue as to how this could occur. How would cells differentiate into ‘fully mature neurons’ in vitro (after 2 h of treatment with ethanol-dissolved retinoic acid; Molino and Vannoni, 2010a, 2010b), and then be turned into myelin-making oligodendrocytes? How many cells would actually be needed to restore enough neurons or glial cells in these diseases is also unknown. How could any cells engraft in the proper position after intrathecal infusion, or how would they reach the brain in any significant number after traversing the lung filter following intravenous infusion? How would the gliosis (glial ‘scarring’) that characterizes brain changes in virtually all of the targeted diseases be effectively cleared to permit local regeneration? All of these considerations, obvious to scientists and physicians, are hard to convey to the public, but are the kinds of issues that are considered when new cell therapies work their way through the regulatory process. Patients, of course, are primarily concerned with perceivable improvement. But perceived or real improvement in a single patient may not at all relate to the treatment. Perceived or even real improvement may be due to an oscillating course of the disease, which is common in the diseases being ‘treated’ in Italy. It may be due to the placebo effect, which is known to be particularly strong in certain diseases, including Parkinson’s, or in children. In the absence of a controlled study, improvements or benefit over existing (albeit palliative) treatment can neither be assessed nor claimed.
Therapy from non-progenitor functions of MSCs?
Alternatively, MSCs are said to be potentially effective not as progenitors of specific cell types but through their ‘trophic, immune modulating, anti-inflammatory effects’. This statement is vague and the putative mechanisms of these effects are so generic as to appear scientifically and clinically meaningless. In the best-case scenario, these claimed mechanisms are worthy of investigation but do not warrant translation to the clinic at this time and stage. Indeed, any putative paracrine factor involved should be identified and then developed into a drug, substituting for the infusion of cells that cannot stably engraft. Thus, the endless repetition of trials and claims based on assumptions of such effects without a prior definition of the effects being pursued is unwarranted. The very fact that such trials are being conducted is often cited as an argument supporting the effectiveness of the treatment, but this is what the trials should be designed to prove in the first instance. Thus, it is wholly misleading to offer MSC treatments directly to patients as though they are grounded in scientific rationale and with proven clinical efficacy. They are not.
Bogus MSC therapies reflects the vagaries of some of the peer-reviewed literature on MSCs. That intravenous infusions of MSCs can ‘mute or cure’ autism, ALS, spinal transection, contusion and so on can even be read in top journals (Caplan and Correa, 2011). Problematically, a significant proportion of ‘scientific’ activity and publications in the MSC field comes from companies with a direct interest in commercializing MSCs. These activities and publications disseminate notions and beliefs that are far from conclusive (and therefore far from amenable to translation), and in some cases just plain wrong. We view with considerable scepticism recent data or claims on MSC biology and therapeutic potential that have been driven by commercial interests and, thus, may not reflect an unbiased appreciation of MSCs as an object of the natural world—relevant to biology and medicine, as much good science shows.
Rare diseases
Incurable diseases, including rare genetic diseases, are a target of stem cell science. What we hope for in these diseases is that science can provide solutions, not palliation, that may involve stem cell-based therapies. Providing a solution for just one such disease would justify the efforts of the entire stem cell field worldwide. However, hundreds of different genetic diseases are without a cure, and large gaps in our understanding of genotype–phenotype correlations are perhaps the main obstacles to developing therapeutic approaches in these diseases. In this context, stem cell science offers a novel way of understanding genetic diseases at the cell and tissue level (e.g., Bianco et al, 1998). It also offers perspectives of general significance, such as gene correction in the case of patient’s own pluripotent cells obtained through reprogramming (Takahashi et al, 2007). These are entirely new paradigms and challenges, far from immediate clinical translation.
Rare diseases present specific regulatory challenges. The rarity of a particular disease itself is an obstacle to finding enough patients to undertake large efficacy trials, and controlled trial can be difficult to design. However, creative ways of conducting double-blind trials, the gold standard of clinical experimentation, can be devised even in rare and lethal diseases. Severity of disease and lack of an alternative therapeutic approach often presents additional challenges. For these reasons, rare diseases represent a specific case in which access to patient treatment may appear justified, despite the lack of formal clinical trials. This, however, does not obviate the need for rigour. Substantial preclinical data, a plausible mechanism of action, a rational route of administration, a stringent definition of outcomes and a transparent evaluation thereof are even more stringently required when testing therapies for rare diseases, specifically because conducting large trials is more difficult than with common diseases. Treatment of single patients (so-called compassionate use) is also conditional on having a rational basis and putting in place all the necessary safety measures with respect to cell preparation. The reiterative use of the same kind of cell populations in the same way in hundreds of single patients (i.e., outside of a formal trial) for vastly different kinds of disease unrelated to one another, based on the loose assumption of generic ‘stem cell effects,’ is not acceptable and should be prevented. Regulatory agencies have a key role in preventing this.
It must also be realized that by their nature, rare diseases may represent a specific market. The difficulty in completing phase III trials may be used, and is used, by commercial entities to invoke authorization of marketing after phase I trials. Again, the danger in this should not elude attention of regulatory bodies. Worldwide, a constellation of commercial interests (industries, small companies and also adventurers) has risen, aiming at rapid marketing of stem cell therapies before any sound proof is given of either their rationale or expected clinical benefits.
MSCs—‘Most Suspicious Cells’?
Cells that have become known as ‘MSCs’ are locally transplantable, system-specific and self-renewing perivascular progenitors of skeletal tissues, including the haematopoietic microenvironment (Sacchetti et al, 2007). They are found in the bone marrow and have significant potential in medicine and unique biological appeal. It is therefore perhaps not surprising that multiple cases of unauthorized stem cell treatments being offered directly to the public before any approval or evidence of efficacy are centred on the use of the so-called MSCs. Lack of scientific rigour, although not unique to the MSC subfield, has flourished therein. Loose definitions and poor assays have disseminated across the scientific community as ‘gold standards’ (Dominici et al, 2006), creating huge confusion and opening the way to the completely erroneous belief that any culture of cells from any kind of connective tissue is a culture of stem cells. The apparent ease of isolation and culture, and the conceptual confusion between a stem cell as a physical and functional entity, and a culture of cells originating from stem cells ex vivo (Caplan and Correa, 2011), have contributed to the widespread use of such cells worldwide. Their nature as ‘adult’ stem cells has granted license and exemption from unwanted ethical controversy. Pressure towards development of therapies from all funding bodies around the world, a general climate dominated by the need to develop treatments (‘translational medicine’, Zerhouni (2005)) and the very existence of multiple companies ready to commercialize ‘MSCs’ have contributed, in turn, to making this particular biological object prone to misuse in the clinic and user-friendly for ill-intentioned salesmen. At this time, hundreds of clinical trials (see http://www.clinicaltrials.gov/) are being conducted worldwide with infusions of MSCs for treating a vast array of disorders. Of course, safety and reliability of outcome monitoring are less of an issue in a regular trial than they are with unauthorized use of MSCs in patients. However, in many of these trials the medical and scientific rationale is weak and untenable.
Error and trial
Empiricism as a productive approach in medicine is often invoked as a reason to conduct trials with MSCs, blind of any putative mechanism of action (Caplan and Correa, 2011). Conducting formal, regulated, transparent clinical trials using MSCs for a variety of non-skeletal ailments can be legitimate, even if based on a partial or weak rationale. It is also, however, expensive and highly likely to be uninformative. Clinical trials can be essential in revealing unexpected obstacles in translating a scientifically robust approach into an effective therapy. But the robust approach must come from science. It must be robust before one embarks on a clinical trial. Again, there are only three cases so far of overt success of regenerative medicine, successfully transferred to the clinic: BMT, regeneration of skin and regeneration of cornea. All of them are based on a robust preclinical rationale and clear, sound evidence of efficacy in preclinical models. In the case of BMT, it was the strength of the rationale that warranted persistence in the clinic, in the wake of initial failure. If it were based on the outcome of the initial trials, BMT would have been abandoned. It was only because of multiple different lines of preclinical evidence and animal models that persistence was justified and ultimately rewarded (reviewed in Bianco et al (2013)). Clinical trials are not reproducible experiments, but aim at providing the tools whereby to predict, in probabilistic terms, the outcome of a therapy when routinely used in the clinic. But there are two theoretical ways of doing this. One is to master completely the mechanism of action of a drug. In that case, one can make an absolutely accurate prediction of the probability that the drug will work. The other is to ignore completely the mechanism of action and to conduct trials blindly with the hope that some effect will be seen by chance. Commercial entities interested in marketing MSCs often cite the mere existence of ongoing trials as an argument for supporting their clinical use. An ongoing trial is essential for product development and as a token of the commercial value of a company; scientifically and medically, an ongoing trial has no meaning. Trials are only meaningful when they are concluded and provide clear information; while in progress they can only add economic value to a company, via communication to the lay public and financial leverage.
Why we need to understand
Complementary to empirical clinical trials, a number of studies have been conducted, claiming ‘beneficial effects’ of systemically infused MSCs in animal models. The conceptual design of these studies as ‘clinical trials in a mouse’ is often flawed, detracting from the power of such studies to highlight a robust rationale for subsequent clinical use. Typically, a pharmacological effect is measured, without measuring the dose, kinetics and dynamics of the active principle, unknown at the outset. A putative active principle is often identified ex post through ex vivo reductionistic experiments. These arbitrarily single out a putative ad hoc molecular mechanism out of a maze of possible, pleiotropic, interlocking mechanisms. An arbitrary hypothesis that fits the results is pursued and alternative hypotheses are ignored. In vivo effects are often interpreted in a biased way (e.g., in studies on ischaemic heart disease, size of post-infarct scars is equated to extent of necrosis; effects of cardiac remodelling are ignored). Even though these studies sometimes specifically record the vanishing of infused cells, they fail to relate any claimed effect to kinetics of cell survival. ‘Clinical data in the mouse’ are descriptive and insufficient to offer mechanistic insight. Mechanistic insight is not a dispensable intellectual luxury. It is specifically required to develop effective therapies. It is to this end that we need mechanisms and rationale. MSCs are thought to have a role in treating GvHD and arthritis alike (Keating, 2012), owing to their generic ‘known immune modulatory effects’. The specific immune modulatory effects are not known. We have no way to model and measure them effectively in vitro or in vivo. We have, therefore, no way to distinguish those operating in GvHD from those operating in arthritis, or to tell whether they are the same or different. We have no way to tell whether these effects are unique to MSCs or shared with other kinds of cells, and which ones. While potentially crucial to advance therapies that harness immune modulation, these issues are mostly neglected. Meanwhile repetitive, expensive, small, uncontrolled phase I–II trials with i.v. infusions of MSCs continue to be pursued as useful.
Rethinking a model of ‘translational medicine’
A model of ‘translational medicine’ has been subliminally accepted by many scientists. The scheme is driven by the pressure to effect the rapid translation of data from the bench. Translation cannot be aimed for a priori; not everything can, or needs to, be translated. ‘Translation at all costs, and quick’ can only be unselective and premature. Unselective and premature development of compounds into potential drugs is exactly the paradigm that inflated the cost of developing new drugs in the pharmaceutical industry, leading to crisis. Ninety-five per cent of potential drugs entering the drug development process are unsafe and/or ineffective, and fail to reach the market. However, they burden the cost of drugs that make it to the market.
As exported to the entire scientific community, now pressured to engage in rapid development of therapies, this model of translation cannot work any better—scientifically, medically or financially. Use of public funds to accelerate translation must be prudent and conditional to scientific rigour. Premature translation of provisional data and concepts in the stem cell field, in conjunction with loosened regulation, can perhaps bring to the market products, but cannot provide solutions for diseases. The Italian case suggests that pressure for premature translation of inconclusive science can also encourage, albeit indirectly, practices that are destructive for patients. Stem cells have already shaped contemporary medicine. They may continue to do so, but only if they are properly evaluated in the lab and translated to the clinic at such time as the data supports this, and then only in trials that have rigour and proper regulatory oversight.
References
- Abbott A (2013) Stem-cell ruling riles researchers. Nature 495: 418–419 [DOI] [PubMed] [Google Scholar]
- Appelbaum FR (2007) Hematopoietic-cell transplantation at 50. N Engl J Med 357: 1472–1475 [DOI] [PubMed] [Google Scholar]
- Bianco P, Cao X, Frenette PS, Mao JJ, Robey PG, Simmons PJ, Wang CY (2013) The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat Med 19: 35–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco P, Kuznetsov SA, Riminucci M, Fisher LW, Spiegel AM, Robey PG (1998) Reproduction of human fibrous dysplasia of bone in immunocompromised mice by transplanted mosaics of normal and Gsalpha-mutated skeletal progenitor cells. J Clin Invest 101: 1737–1744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitbach M, Bostani T, Roell W, Xia Y, Dewald O, Nygren JM, Fries JW, Tiemann K, Bohlen H, Hescheler J, Welz A, Bloch W, Jacobsen SE, Fleischmann BK (2007) Potential risks of bone marrow cell transplantation into infarcted hearts. Blood 110: 1362–1369 [DOI] [PubMed] [Google Scholar]
- Caplan AI, Correa D (2011) The MSC: an injury drugstore. Cell Stem Cell 9: 11–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8: 315–317 [DOI] [PubMed] [Google Scholar]
- Enserink M (2006) Selling the stem cell dream. Science 313: 160–163 [DOI] [PubMed] [Google Scholar]
- European Medicines Agency Committee for Advanced Therapies and CAT Scientific Secretariat. (2010) Correspondence: Use of unregulated stem-cell based medicinal products. Lancet 376: 514. [DOI] [PubMed] [Google Scholar]
- European Medicines Agency. (2011) Reflection paper on stem cell-based medicinal products. Committee for Advanced Therapies EMA CAT/571134/09, pp 1–14 [Google Scholar]
- Hyun I, Lindvall O, Ahrlund-Richter L, Cattaneo E, Cavazzana-Calvo M, Cossu G, De Luca M, Fox IJ, Gerstle C, Goldstein RA, Hermeren G, High KA, Kim HO, Lee HP, Levy-Lahad E, Li L, Lo B, Marshak DR, McNab A, Munsie M et al. (2008) New ISSCR guidelines underscore major principles for responsible translational stem cell research. Cell Stem Cell 3: 607–609 [DOI] [PubMed] [Google Scholar]
- Keating A (2012) Mesenchymal stromal cells: new directions. Cell Stem Cell 10: 709–716 [DOI] [PubMed] [Google Scholar]
- Molino E, Vannoni D (2010a) Differentiation process of mesenchymal stem cells and therapeutic use thereof. Patent Application US 2012/0149009 A1, pp 1–9(submitted 4 December 2010)
- Molino E, Vannoni D (2010b) Extraction of process for mesenchymal stromal stem cells. Patent Application US 2012/0149098 A1, pp 1–9(submitted 4 December 2010)
- Moll G, Rasmusson-Duprez I, von Bahr L, Connolly-Andersen AM, Elgue G, Funke L, Hamad OA, Lonnies H, Magnusson PU, Sanchez J, Teramura Y, Nilsson-Ekdahl K, Ringden O, Korsgren O, Nilsson B, Le Blanc K (2012) Are therapeutic human mesenchymal stromal cells compatible with human blood? Stem Cells 30: 1565–1574 [DOI] [PubMed] [Google Scholar]
- Nature Editorial. (2013a) Preventative therapy. Nature 494: 147–148 [Google Scholar]
- Nature Editorial. (2013b) Smoke and mirrors. Nature 496: 269–270 [DOI] [PubMed] [Google Scholar]
- Regenberg AC, Hutchinson LA, Schanker B, Mathews DJ (2009) Medicine on the fringe: stem cell-based interventions in advance of evidence. Stem Cells 27: 2312–2319 [DOI] [PubMed] [Google Scholar]
- Sabatino M, Ren J, David-Ocampo V, England L, McGann M, Tran M, Kuznetsov SA, Khuu H, Balakumaran A, Klein HG, Robey PG, Stroncek DF (2012) The establishment of a bank of stored clinical bone marrow stromal cell products. J Transl Med 10: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, Tagliafico E, Ferrari S, Robey PG, Riminucci M, Bianco P (2007) Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell 131: 324–336 [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131: 861–872 [DOI] [PubMed] [Google Scholar]
- Thomas ED, Lochte HL Jr, Lu WC, Ferrebee JW. (1957) Intravenous infusion of bone marrow in patients receiving radiation and chemotherapy. N Engl J Med 1957;257 491–496 [DOI] [PubMed] [Google Scholar]
- United States Department of Agriculture. (1917) Bureau of Chemistry. Notice 4944, 17 October 1917. Service and Regulatory Announcements, Suppl 29, p 592.
- Woodbury D, Schwarz EJ, Prockop DJ, Black IB (2000) Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res 61: 364–370 [DOI] [PubMed] [Google Scholar]
- Wright DE, Wagers AJ, Gulati AP, Johnson FL, Weissman IL (2001) Physiological migration of hematopoietic stem and progenitor cells. Science 294: 1933–1936 [DOI] [PubMed] [Google Scholar]
- Zerhouni EA (2005) Translational and clinical science--time for a new vision. N Engl J Med 353: 1621–1623 [DOI] [PubMed] [Google Scholar]