Abstract
c-Jun, the major component of the AP-1 transcription factor complex, has important functions in cellular proliferation and oncogenic transformation. The RING domain-containing protein RACO-1 functions as a c-Jun coactivator that molecularly links growth factor signalling to AP-1 transactivation. Here we demonstrate that RACO-1 is present as a nuclear dimer and that c-Jun specifically interacts with dimeric RACO-1. Moreover, RACO-1 is identified as a substrate of the arginine methyltransferase PRMT1, which methylates RACO-1 on two arginine residues. Arginine methylation of RACO-1 promotes a conformational change that stabilises RACO-1 by facilitating K63-linked ubiquitin chain formation, and enables RACO-1 dimerisation and c-Jun interaction. Abrogation of PRMT1 function impairs AP-1 activity and results in decreased expression of a large percentage of c-Jun target genes. These results demonstrate that arginine methylation of RACO-1 is required for efficient transcriptional activation by c-Jun/AP-1 and thus identify PRMT1 as an important regulator of c-Jun/AP-1 function.
Keywords: arginine methylation, c-Jun, coactivator, Prmt1, transcription
Introduction
The AP-1 transcription factor is a heterodimeric complex of various Jun, Fos and ATF-2 family members and is induced by a multitude of signals including growth factors, cytokines and extracellular stresses (Davis, 2000). Consequently AP-1 mediates diverse cellular responses ranging from cell proliferation and differentiation to tumourigenesis and cellular apoptosis (Mechta-Grigoriou et al, 2001; Shaulian and Karin, 2001; Eferl and Wagner, 2003).
c-Jun is essential for cellular proliferation and transformation by controlling the expression of cell cycle regulator genes including cyclinD1 and cdc2 (Johnson et al, 1996; Eferl et al, 1999; Wada et al, 2004). c-Jun-null fibroblasts display a severe proliferation defect, deficiency in cell cycle re-entry after serum withdrawal and an inability to undergo transformation by oncogenic Ras (Johnson et al, 1996; Schreiber et al, 1999).
We have recently described a novel c-Jun coactivator, RING domain AP-1 coactivator-1 (RACO-1), that links growth factor/oncogenic Ras signalling to AP-1 activation. Growth factor signalling stimulates RACO-1 function by increasing RACO-1 protein stability. Mechanistically, RACO-1 stability is controlled by the competition of degradative K48- and nondegradative K63-linked ubiquitylation. RACO-1 is a RING domain-containing ubiquitin E3 ligase and in unstimulated conditions RACO-1 is unstable due to K48-linked autoubiquitylation. Upon activation of the Ras/MEK/ERK pathway, K63-linked ubiquitin chains are attached to the same residues targeted for degradative ubiquitylation, thereby resulting in enhanced protein levels (Davies et al, 2010).
In mammalian cells, protein methylation occurs predominantly on lysine and arginine residues, with distinct families of methyltransferases targeting each amino acid. Despite being identified more than 30 years ago, relatively little is known about protein arginine methylation. Protein arginine methyltransferases (PRMTs) catalyse mono- and dimethylation of the guanidino group of the arginine residue using S-adenosyl methionine (SAM) as a methyl donor. Dimethylation can occur asymmetrically (ADMA) with the two methyl groups placed onto one of the terminal nitrogen atoms of the guanidine group, or symmetrically (SDMA) where one methyl group is placed onto each of the terminal nitrogen groups. ADMA methylation by PRMT1 accounts for the majority of all arginine methylation in mammalian cells (Pawlak et al, 2000). Arginine methyltransferases are a major regulator of gene expression by both direct methylation of transcription factors and coactivators, including p53, RUNX and p300/CBP, or indirectly via histone tail decoration and epigenetic modulation (reviewed in Bedford and Clarke, 2009). Thus, as more substrates for arginine methylation are being discovered, the role and importance of this modification in numerous cellular processes is only just being appreciated.
Here, we demonstrate that RACO-1 is arginine methylated by PRMT1 and that this stabilises the protein and facilitates dimerisation of RACO-1, enabling c-Jun binding. Thus, our data suggest that arginine methylation is an important regulator of c-Jun/AP-1-mediated gene transcription.
Results
c-Jun binds to nuclear dimeric RACO-1
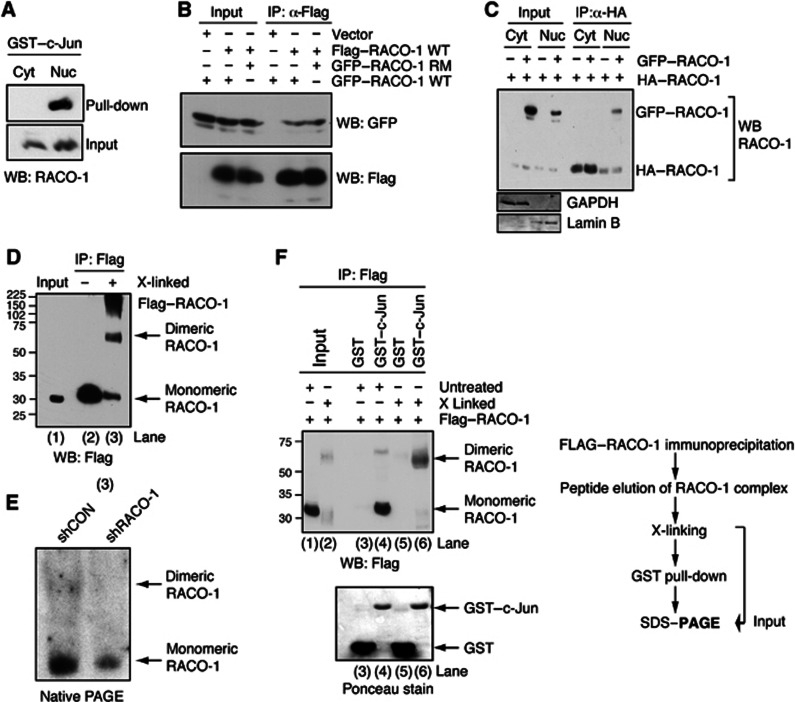
RACO-1 is expressed ubiquitously throughout the cell and is present in both the cytoplasm and nucleus (Davies et al, 2010). To test whether these two pools of RACO-1 protein may show functional differences, we performed immunoprecipitation (IP) experiments using GST–c-Jun. Notably, recombinant c-Jun specifically interacted with nuclear RACO-1 but not with cytoplasmic RACO-1 protein (Figure 1A). We therefore sought to identify functional differences between cytoplasmic and nuclear pools of RACO-1 protein. In addition to the analysis of RACO-1 post-translational modifications (see below), we also determined whether RACO-1 was capable of dimerisation. Cells were transfected with both Flag- and GFP-tagged RACO-1 followed by Flag IPs. GFP–RACO-1 was co-immunoprecipitated with Flag–RACO-1, implying that dimerisation does occur (Figure 1B). Point mutations of the first two cysteine residues of the RACO-1 RING domain (RACO-1 RM) destroy RING integrity (Davies et al, 2010); however, dimer formation was not affected. Thus, the RING domain is dispensable for dimerisation (Figure 1B). Furthermore, we investigated whether RACO-1 dimerisation was influenced by subcellular localisation, and found that RACO-1 dimerisation occurred specifically within the nucleus (Figure 1C). Next, we investigated RACO-1 dimerisation by chemically crosslinking RACO-1 complexes after immunoprecipitation. Denaturing SDS–polyacrylamide gel electrophoresis (PAGE) followed by immunoblotting for RACO-1 showed that a substantial proportion of RACO-1 migrated at the molecular weight predicted for dimeric RACO-1 after crosslinking (Figure 1D, lane 3). In agreement with this, nondenaturing native gel analysis showed that endogenous RACO-1 protein migrated in two different forms. In addition to the monomeric protein, a second RACO-1 protein form migrating at higher molecular weight was detected. Importantly, depletion of RACO-1 by stable integration of shRNA construct against RACO-1 reduced both RACO-1 protein forms (Figure 1E).
Figure 1.
c-Jun binds to nuclear dimeric RACO-1. (A) Nuclear but not cytoplasmic RACO-1 interacts with recombinant c-Jun. RACO-1-transfected cells were fractionated into cytoplasmic/nuclear extracts prior to incubation with GST–c-Jun. (B) RACO-1 homodimerises independently of the RING domain. HEK293T cells were transfected with Flag–RACO-1 and GFP–RACO-1 wild-type (WT) or RING mutant (RM). Flag–RACO-1 was immunoprecipitated from cell lysates and associated GFP–RACO-1 detected by immunoblotting. (C) Nuclear but not cytoplasmic RACO-1 forms dimers. Transfected cells were fractionated into cytoplasmic/nuclear extracts prior to immunoprecipitation with α-HA-agarose beads. Associated GFP–RACO-1 was detected by immunoblotting. Immunoblotting for GAPDH and Lamin B validates the purity of subcellular fractionation. (D) RACO-1 forms dimers in vivo. Flag–RACO-1-transfected cells were immunoprecipitated, peptide eluted and chemically crosslinked with glutaraldehyde (X-linked) before resolving by SDS–PAGE and immunoblotting with Flag antibody. (E) Endogenous RACO-1 forms a homodimer. Cell extracts from H727 cells, with stably integrated shLuciferase or shRACO-1, were analysed in nondenaturing PAGE and immunoblotted with RACO-1 antibody. (F) Recombinant c-Jun preferentially interacts with dimeric RACO-1. Cells were experimentally prepared as in (D). After chemical crosslinking, one-tenth of the eluate was removed to assess efficiency of crosslinking (lanes 1 and 2), and the remaining elution was either incubated with GST–c-Jun or GST as control (lanes 3–6). RACO-1 protein associated with GST–c-Jun was resolved by SDS–PAGE and immunoblotted.
Source data for this figure is available on the online supplementary information page.
To test the functional significance of the RACO-1 dimer, RACO-1 was chemically crosslinked, which resulted in the formation of covalent RACO-1 dimers with approximately 50% efficiency, that is, roughly half of the total RACO-1 protein was monomeric and the other half was present as a dimer (Figure 1F, lane 2). GST–c-Jun pull-downs showed that recombinant c-Jun bound to dimeric crosslinked RACO-1 with much higher efficiency compared to monomeric RACO-1 (Figure 1F). Taken together, these findings imply that the RACO-1 dimer is a physiologically relevant binding partner of c-Jun.
Analysis of RACO-1 dimerisation
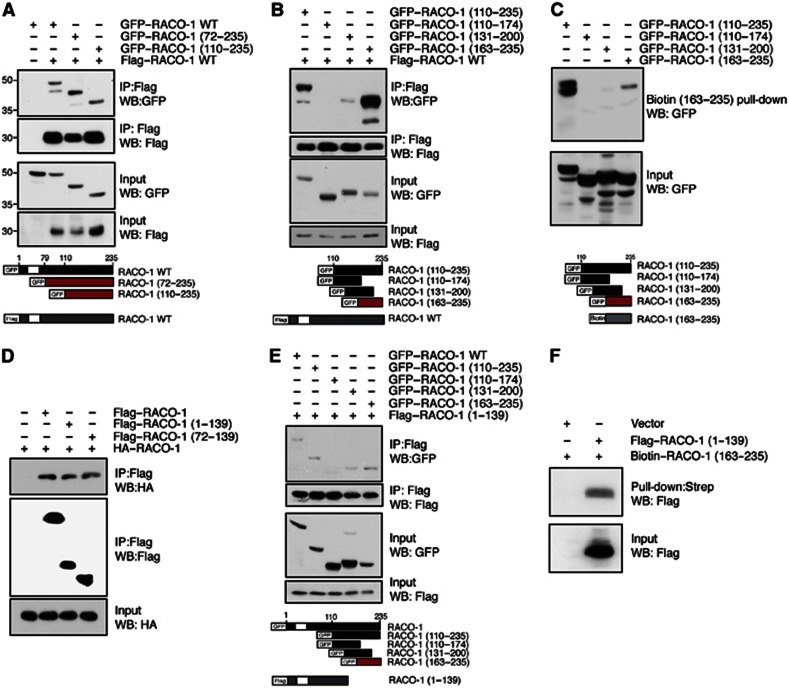
Next, we investigated RACO-1 dimerisation. Immunoprecipitated full-length Flag–RACO-1 interacted with two sequential N-terminal deletion constructs (RACO-1 (72–235) and RACO-1 (110–235)) as efficiently as wild-type protein (Figure 2A), implying that the dimer interface resides within the C-terminal portion of the protein. Fine mapping of this C-terminal region using overlapping 60–70 amino acid protein fragments revealed that the C-terminal 72 amino acids (RACO-1 (163–235)) were sufficient for dimer formation with full-length RACO-1 protein (Figure 2B). Biotin–RACO-1 (163–235) strongly interacted with a long C-terminal fragment (GFP–RACO-1 (110–235)) and a shorter protein corresponding to residues 163–235 of RACO-1 (Figure 2C). Thus, RACO-1 dimerisation is mediated by a homodimerisation domain located in the very C-terminus of the protein.
Figure 2.
The C-terminus of RACO-1 is required for dimerisation and intramolecular interactions. (A, B). Mapping of the RACO-1 dimerisation domain. Lysates from HEK293T cells coexpressing Flag-tagged full-length RACO-1 with various N-terminal GFP-tagged truncated proteins were immunoprecipitated with α-Flag antibodies and immunoblotted for associated GFP-tagged RACO-1 proteins. Schematic below represents constructs used. White box represents RING domain. (C) RACO-1 dimerises in a tail-to-tail orientation. The minimal region required for interaction with full-length protein (RACO-1 (163–235)) was generated as a synthetic biotinylated peptide and used for pull-down experiments with cell lysates expressing overlapping GFP-fused RACO-1 fragments encompassing the C-terminal domain (110–235). Schematic below represents constructs used. (D) RACO-1 lacking the dimerisation domain (RACO-1 (1–139)) and the RING domain (Flag–RACO-1 (72–139)) interacts with full-length RACO-1. (E) Mapping of additional RACO-1 protein interaction. Lysates from HEK293T cells coexpressing Flag-tagged dimerisation domain deleted RACO-1 (RACO-1 (1–139) with full-length and various N-terminal GFP-tagged truncated proteins were immunoprecipitated with α-FLAG antibodies and immunoblotted for associated GFP-tagged RACO-1. Schematic below represents constructs used. (F) Flag–RACO-1 (1–139) interacts with a biotinylated RACO-1 peptide corresponding to residues 163–235. Peptide-associated RACO-1 (1–139) was resolved by SDS–PAGE and immunoblotted with α-Flag antibody.
Source data for this figure is available on the online supplementary information page.
However, RACO-1 molecular interactions turned out to be more complex. RACO-1 (1–139), which lacks the C-terminal homodimerisation domain, and RACO-1 (72–139), which in addition lacks the RING domain, were able to interact with full-length RACO-1 as effectively as wild-type protein (Figure 2D). This suggested the presence of a second interaction domain within the RACO-1 N-terminus. Further fine mapping revealed that the region of RACO-1 identified as the homodimerisation interface (residues 163–235) was also able to interact with the RACO-1 N-terminus (Figure 2E). A biotinylated peptide corresponding to residues 163–235 of RACO-1 was sufficient to interact with Flag–RACO-1 (1–139) (Figure 2F). Therefore, the very C-terminus of RACO-1 is able to interact with two independent domains within RACO-1: a homotypic C-terminal interaction that mediates dimerisation and a second intramolecular interaction between the RACO-1 C- and N- termini.
RACO-1 is methylated on two arginine residues
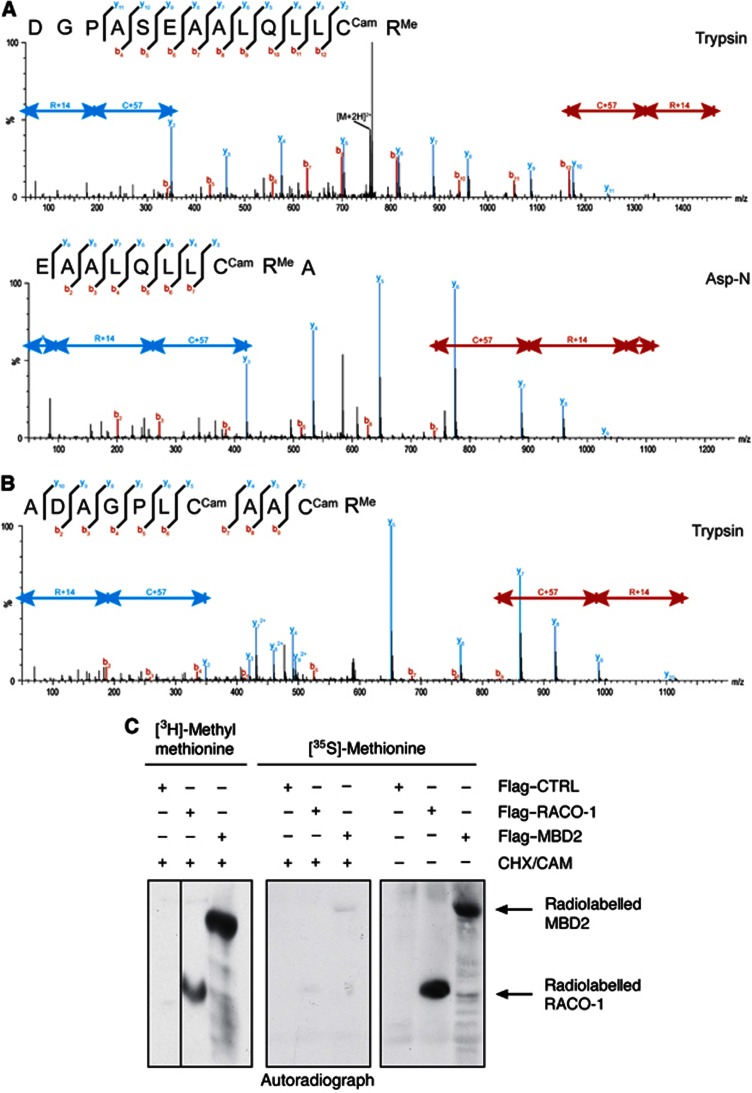
The complex inter- and intramolecular interaction of RACO-1 could represent a mechanism of functional regulation. To investigate this, we performed LC-MS/MS analysis to identify post-translational modifications occurring on RACO-1. We consistently detected the presence of methyl groups located on residues arginine 98 (R98) and arginine 109 (R109). These modifications could be detected using two different forms of enzymatic digestion (Trypsin or Asp-N; Figure 3A and B).
Figure 3.
RACO-1 is arginine methylated in vivo on residues R98 and R109. (A) Identification of arginine modifications on RACO-1 protein. The upper MS/MS spectrum shows the fragment ions of the 2+ charged peptide ion at m/z 757.876 generated from an ‘in gel’ tryptic digest of RACO-1. The lower spectrum indicates the fragment ions of the 2+ charged peptide ion at m/z 793.412 generated from an Asp-N proteolytic digestion of RACO-1. (B) Characterisation of arginine modifications on the RACO-1 protein. The MS/MS fragment ion spectrum of the 2+ charged peptide ion at m/z 588.267 is shown. The peptide was generated by the ‘in gel’ tryptic digestion of RACO-1. (C) RACO-1 is methylated in vivo. Cells were transfected with Flag-RACO-1 or Flag-MBD2 (control) and labelled with [3H]-methyl methionine (left panel) or [35S]-methionine (middle and right panels). Proteins were immunoprecipitated and incorporated methyl groups detected by SDS–PAGE followed by autoradiography. CHX/CAM treatment effectively blocked de novo protein synthesis as shown by the inability of [35S]-methionine to be incorporated into newly synthesised RACO-1 and MBD2 (compare middle and right panels).
Source data for this figure is available on the online supplementary information page.
To confirm that RACO-1 was methylated in vivo, we labelled cells with radioactive [3H]-methyl methionine. To exclude incorporation of radioactivity by protein synthesis, protein translation was blocked by addition of cyclohexamide (CHX). CHX treatment was efficient as it prevented incorporation of radioactively labelled 35S into RACO-1 and MBD2, a protein known to be arginine methylated (Tan and Nakielny, 2006) that was used as a positive control for this experiment (Figure 3C). The [3H]-methyl methionine in vivo serves as a precursor of SAM, which is the methyl donor for arginine methylation reactions. RACO-1 and MBD2 were then immunoprecipitated, resolved by SDS–PAGE and exposed to autoradiography film. The [3H]-methyl methionine was incorporated into bands corresponding to the molecular weights of RACO-1 and of MBD2 (Figure 3C). Hence, RACO-1 is methylated in vivo.
PRMT1 methylates RACO-1
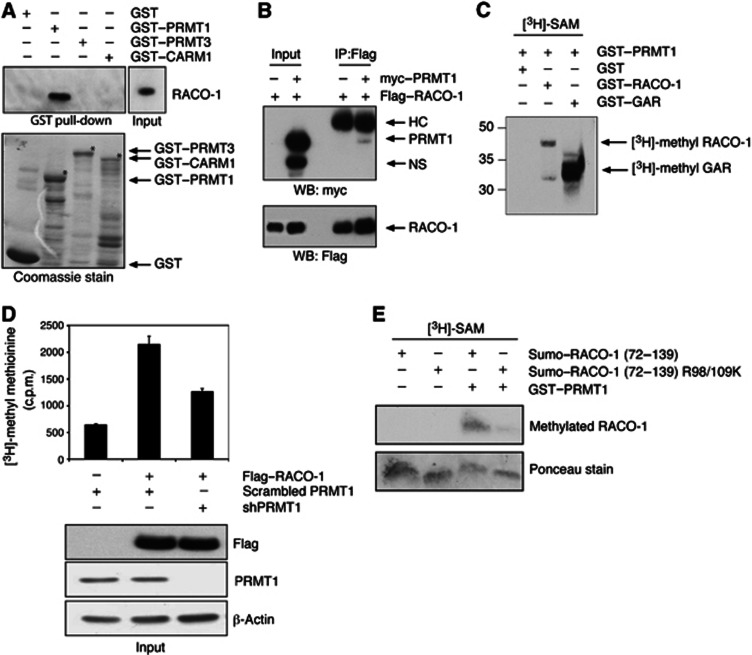
Mammalian arginine methyltransferases comprise a family of nine highly related proteins. To investigate which protein was responsible for methylating RACO-1, we performed pull-down experiments using recombinant GST–fusion proteins of PRMT1, PRMT3 and CARM1, three of the better-characterised arginine methyltransferases. Transfected RACO-1 interacted with GST–PRMT1 but not GST–PRMT3, GST–CARM1 or GST alone (Figure 4A) and RACO-1 and PRMT1 co-immunoprecipitated from cells (Figure 4B). To verify that RACO-1 is a novel substrate for PRMT1 and not solely a binding partner, we performed in vitro methylation assay using recombinant PRMT1 and RACO-1 in the presence of the methyl donor 3[H]-SAM. Recombinant RACO-1 was effectively methylated by recombinant PRMT1 (Figure 4C). We also attempted to generate methyl-specific antibodies directed towards asymmetric di-methyl-R98 and asymmetric di-methyl-R109 of RACO-1; however, neither a polyclonal nor a monoclonal antibody could be successfully produced (data not shown). Subsequently, to further demonstrate RACO-1 arginine methylation in vivo, we generated a stable cell line in which endogenous PRMT1 was silenced by expression of an shRNA construct (Figure 4D). We noted that when Flag–RACO-1 was IP-ed from [3H]-methyl methionine-labelled cells, a single radioactive band was immunoprecipitated (Figure 3C, left panel), implying that RACO-1 is the only protein methylated to a detectable level in the Flag IP. Therefore, we subsequently measured incorporated radioactivity into RACO-1 by scintillation counting. In vivo [3H]-methyl methionine labelling of this cell line after expression of Flag–RACO-1 demonstrated a substantial reduction in the methylation of RACO-1 compared to scrambled shPRMT1-expressing cells (Figure 4D).
Figure 4.
Arginine methylation on RACO-1 is catalysed by PRMT1 methyltransferase. (A) Recombinant PRMT1 interacts with RACO-1. RACO-1-expressing cell lysates were incubated in pull-down assays with recombinant GST-tagged PRMT1, PRMT3 or CARM1 (indicated by asterisks on Coomassie stain). (B) RACO-1 and PRMT1 interact in vivo. Cells coexpressing Flag–RACO-1 and myc–PRMT1 were immunoprecipitated with α-Flag antibody and associated myc-tagged PRMT1 detected by immunoblot. The top arrow represents the position of immunoglobulin heavy chain (HC) and the bottom arrow represents a nonspecific band (NS). (C) PRMT1 directly methylates RACO-1 in vitro. Recombinant RACO-1 and PRMT1 were incubated with the methyl donor [3H]-SAM and incorporated methyl groups detected by SDS–PAGE and autoradiography. GST alone and GST–GAR serve as controls. (D) Methylation of RACO-1 in the presence of PRMT1 silencing. HEK293T cell lines with stable knockdown of PRMT1 or control scrambled silencing construct (scrambled PRMT1) were generated and in vivo labelled with [3H]-methyl methionine. After immunoprecipitation of RACO-1, incorporated [3H]-methyl groups were detected by scintillation counting. Western blot underneath demonstrates the levels of RACO-1 and knockdown of PRMT1. (E) PRMT1 methylates RACO-1 at R98 and R109. Wild-type recombinant SUMO-tagged RACO-1 fragment corresponding to residues 72–139 (SUMO–RACO-1 (72–139)), or RACO-1 (72–139) expressing R98K and R109K mutations (SUMO–RACO-1 (72–139) R98/R109K) were incubated with recombinant PRMT1 and in vitro methylation assays carried out in the presence of [3H]-SAM. Ponceau stain depicts equal levels of RACO-1 recombinant protein.
Source data for this figure is available on the online supplementary information page.
As further negative controls, we observed that immunoprecipitated PRMT5 failed to promote the incorporation of [3H]-methyl groups into recombinant RACO-1 (Supplementary Figure 1A). Moreover, in vivo methylation assay in PRMT5-silenced cell lines did not prevent RACO-1 methylation (Supplementary Figure 1B).
Finally, to further validate R98 and R109 as targets for PRMT1-mediated methylation, we compared in vitro methylation of a recombinant wild-type RACO-1 fragment corresponding to residues 72–139 to a mutant RACO-1 form in which the arginine residues R98 and R109 were substituted to lysine (R98/109K), thereby maintaining the positive charge. Mutation of both arginine R98 and R109 reduced methylation of RACO-1 to background levels (Figure 4E). Taken together, these results demonstrate that the arginines at positions 98 and 109 are the predominant methylated residues within RACO-1, and that PRMT1 is the methyltransferase that binds to and methylates RACO-1.
Arginine methylation stabilises RACO-1
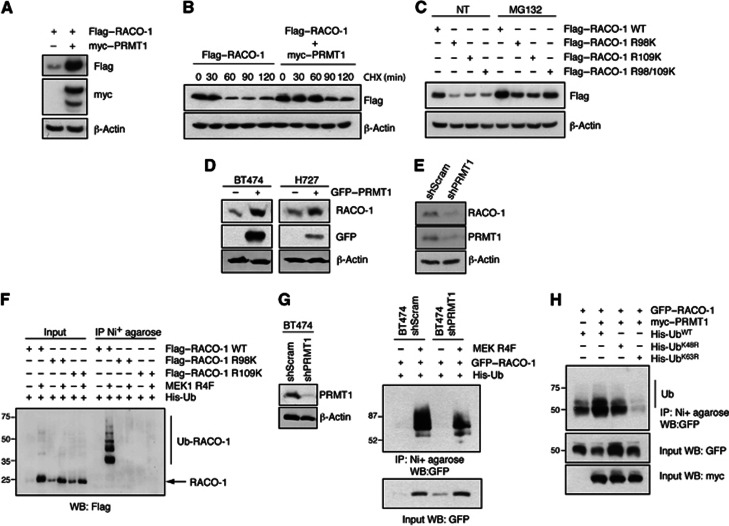
RACO-1 is a highly labile protein and RACO-1 stability is controlled by the competition of degradative K48- and nondegradative K63-linked ubiquitylation. (Davies et al, 2010). Arginine methylation appeared to counteract RACO-1 degradation, as overexpression of myc–PRMT1 led to a considerable increase in RACO-1 protein levels (Figure 5A) and half-life as determined by CHX chase (Figure 5B). Moreover, mutation of the methylation acceptor arginine residues R98 and R109 to lysines decreased RACO-1 protein levels, an effect that could be partially reverted by proteasome inhibition (Figure 5C). However, mutations of R98 and R109 did not change the subcellular localisation of RACO-1 (Supplementary Figure S2). PRMT1 overexpression increased endogenous RACO-1 protein levels in both the breast cancer cell line BT474 and the lung cancer cell line H727 (Figure 5D), while silencing of PRMT1 reduced RACO-1 levels (Figure 5E).
Figure 5.
Methylation of RACO-1 is required for protein stabilisation. (A) Overexpression of PRMT1 stabilises RACO-1 expression levels and (B) increases protein half-life as determined by cycloheximide (CHX) chase. (C) Mutation of R98 or R109 to lysine reduces protein expression and this can be rescued by proteasome inhibitor treatment (MG132). (D) Overexpression of PRMT1 increases endogenous RACO-1 expression in BT474 and H727 cells, while (E) silencing of PRMT1 decreases endogenous RACO-1 levels in H727 cells. (F) Ubiquitylation of RACO-1 by MEK1-R4F requires R98 and R109. Cells were transfected as indicated and ubiquitylated RACO-1 resolved by Ni2+-NTA affinity purification and immunoblotting with Flag antibodies. (G) Silencing of PRMT1 reduces MEK1-R4F induced RACO-1 ubiquitylation. BT474 stable cell lines expressing scrambled or shPRMT1 silencing constructs were generated (left panel) and then transfected with GFP–RACO-1 and MEK1-R4F. Ubiquitylated RACO-1 was resolved by Ni2+-NTA affinity purification and immunoblotting with GFP antibodies (right panel). (H) Overexpression of PRMT1 induces K63-linked ubiquitylation. HEK293T cells were transfected as indicated and ubiquitylated RACO-1 resolved by Ni2+-NTA affinity purification and immunoblotting with GFP antibodies.
Source data for this figure is available on the online supplementary information page.
We have previously shown that K48-linked autoubiquitylation is counteracted by Ras/MEK1-induced K63-linked ubiquitylation (Davies et al, 2010). Given that arginine methylation can affect the deposition of other post-translational modifications on the same protein (Hyllus et al, 2007; Yamagata et al, 2008), we thus investigated the impact of arginine methylation on K63-linked ubiquitylation of RACO-1. Mutation of either R98 or R109 completely suppressed ubiquitylation of RACO-1 induced by constitutively active MEK1 (MEK1 R4F) (Figure 5F), while silencing of PRMT1 reduced MEK1 R4F-induced ubiquitylation (Figure 5G). Moreover, experiments using ubiquitin mutants, which are unable to form specific chains (UbK48R or UbK63R), showed that PRMT1 promoted the formation of K63-linked ubiquitin chains on RACO-1 (Figure 5H). Hence, PRMT1-mediated methylation of RACO-1 at R98 and R109 is a prerequisite for K63-linked ubiquitylation and RACO-1 protein stabilisation.
Arginine methylation mediates RACO-1 dimer formation
The minimal N-terminal region of RACO-1 required for interaction with the RACO-1 C-terminus mapped to amino acids 72–139 and thus includes the methylated arginine residues at positions 98 and 109. We therefore investigated whether arginine methylation plays a role in RACO-1 intramolecular interactions.
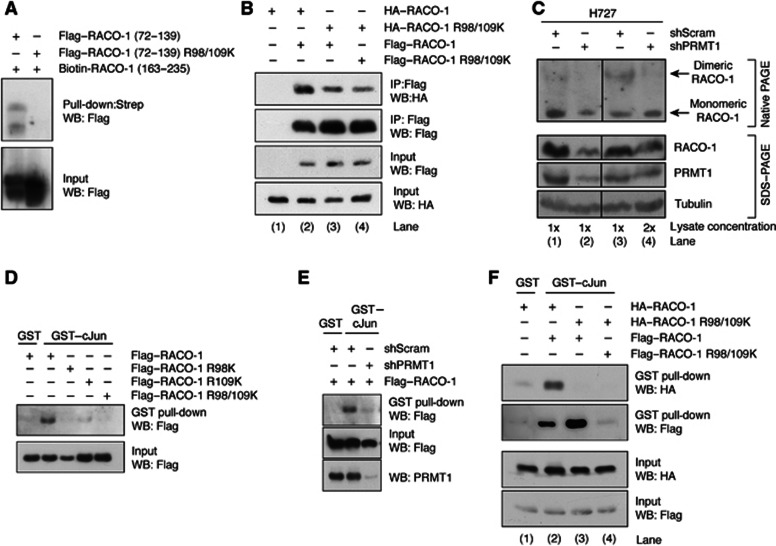
A biotinylated peptide corresponding to residues 163–235 of RACO-1 interacted with Flag–RACO-1 (72–139). In contrast, mutation of R98 and R109 to lysine within this fragment (Flag–RACO-1 (72–139 R98/109K)) prevented interaction (Figure 6A). Thus, arginine methylation regulates RACO-1 intramolecular binding.
Figure 6.
Methylation of RACO-1 by PRMT1 on R98 and R109 is required for dimerisation and c-Jun interaction. (A) Intramolecular interaction requires methylation on arginine 98 and arginine 109. Lysates from Flag–RACO-1 (72–139) or Flag–RACO-1 (72–139) R98/109K-expressing cells were incubated with a biotinylated RACO-1 peptide corresponding to residues 163–235. RACO-1-associated proteins were resolved by SDS–PAGE and immunoblotted with α-Flag antibody. (B) Mutation of methyl arginine residues on one RACO-1 monomer is sufficient to disrupt dimer formation. Cells were transfected as in the Figure, Flag–RACO-1 immunoprecipitated and associated HA–RACO-1 detected by immunoblotting. (C) Silencing of PRMT1 suppresses endogenous RACO-1 dimer formation. Stable H727 cell lines expressing scrambled or PRMT1 silencing constructs were generated, and endogenous RACO-1 dimers detected by nondenaturing PAGE (top panel). However, denaturing SDS–PAGE analysis using equivalent lysates demonstrates that silencing of PRMT1 reduces RACO-1 protein levels (bottom panel, compare lanes 1 and 2). Subsequently, to normalise RACO-1 expression levels, the right-hand panel depicts results when twice the concentration of lysate is used (compare lanes 3 and 4). Hence, RACO-1 and PRMT1 expression levels are equivalent in lanes 3 and 4, but the appearance of dimeric RACO-1 is reduced. (D) Mutation of one methyl arginine residue on RACO-1 is sufficient to disrupt c-Jun binding. Cells were transfected with various RACO-1 methyl mutants and cell lysates incubated with GST–c-Jun. Associated Flag–RACO-1 was detected by immunoblotting. (E) Silencing of PRMT1 prevents RACO-1 from binding to c-Jun. (F) Methylation-induced dimer formation is required for binding of RACO-1 to c-Jun. Lysates derived from cells transfected as depicted in the Figure were incubated with GST–c-Jun and associated RACO-1 detected by immunoblotting for HA or Flag tag.
Source data for this figure is available on the online supplementary information page.
Next, we investigated whether RACO-1 methylation would impact on dimer formation. Disruption of methylation on only one monomer was sufficient to greatly diminish dimerisation (Figure 6B, compare lanes 2 and 3), suggesting that arginine methylation is required in cis for dimer formation, that is, concurrent methylation of both molecules is required for efficient RACO-1 dimerisation. To further support this, we found that in vivo PRMT1-mediated methylation of RACO-1 is required for dimer formation. Nondenaturing gel analysis of endogenous RACO-1 showed that silencing of PRMT1 greatly reduced the abundance of dimeric RACO-1, and increased the prevalence of monomeric RACO-1. This was particularly apparent when lysate concentrations were adjusted to take into account that silencing of PRMT1 reduces RACO-1 protein levels (Figure 6C, compare lanes 2 and 4). Thus, RACO-1 methylation promotes a conformational change that increases RACO-1 protein stability and makes RACO-1 competent for dimerisation.
PRMT1-mediated RACO-1 arginine methylation is required for c-Jun interaction and AP-1 target gene expression
Since arginine methylation is required for RACO-1 stabilisation and dimerisation, and dimerisation is a prerequisite for c-Jun binding (Figure 1F), we tested whether RACO-1 methylation might be essential for c-Jun binding. Indeed, mutation of both methylated RACO-1 arginine residues R98 and R109, and also mutation of each single arginine, was sufficient to substantially reduce RACO-1 binding to c-Jun (Figure 6D). Moreover, silencing of PRMT1 markedly reduced RACO-1 interaction with c-Jun, confirming the central role of PRMT1 in the regulation of RACO-1 function (Figure 6E). Strikingly, when wild-type and methylation-defective R98/R109K mutant RACO-1 proteins were co-overexpressed, c-Jun only interacted with wild-type RACO-1 (Figure 6F). This demonstrates that c-Jun can only interact with dimeric arginine methylated RACO-1, and that both molecules of the RACO-1 dimer need to be methylated, reminiscent of the requirement for arginine methylation in cis for RACO-1 dimer formation (Figure 6B and C).
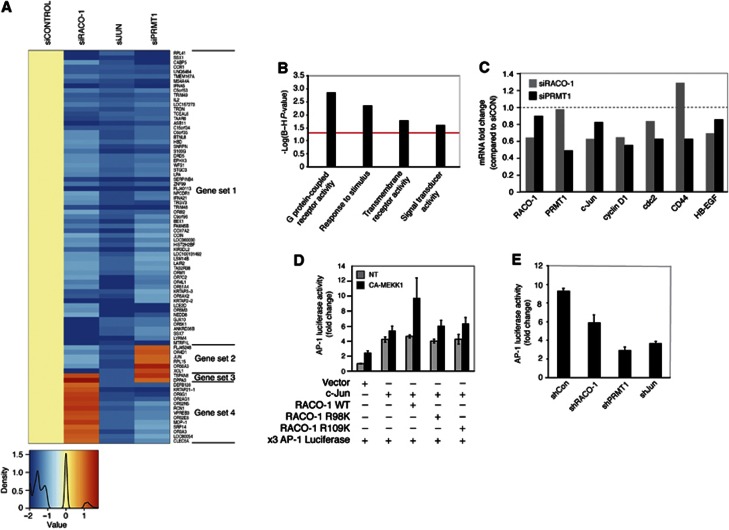
In order to investigate the biological importance of RACO-1 arginine methylation, we performed microarray analysis on HCT116 cells transfected with PRMT1-silencing oligos (siPRMT1) and compared gene expression of PRMT1-depleted HCT116 cells with the effects of c-Jun (siJun) and RACO-1 knockdown (siRACO-1) (Figure 7A). We have observed previously that of the 88 genes displaying >1.5-fold reduction in expression by siJun, 72 are also downregulated by RACO-1 knockdown, albeit to variable extents (Davies et al, 2010). We subjected the 72 genes that were downregulated upon knockdown of c-Jun or RACO-1 to gene ontological (GO) analysis. Of these 72 genes, 48 have known molecular function and 45 genes are involved in known biological processes. The GO analysis revealed that genes involved in G protein-coupled receptor activity, response to stimulus, transmembrane receptor activity and signal transducer activity were enriched, suggesting a function of c-Jun/RACO-1 target genes in cellular signal transduction (Figure 7B). Strikingly, the vast majority of genes downregulated by more than 1.5-fold after c-Jun knockdown were also found to be downregulated in response to PRMT1 silencing, and the expression of 75% of all c-Jun target genes was dependent on both RACO-1 and PRMT1 function (Figure 7A). Accordingly, expression of many known c-Jun/AP-1 target genes, including cyclin D1, cdc2, HB-EGF and c-jun itself, was decreased after silencing of RACO-1 or PRMT1 (Figure 7C). To further analyse the role of PRMT-1 and arginine methylation in gene activation by c-Jun and RACO-1, we performed an AP-1 luciferase reporter assay. We have previously shown that RACO-1 cooperates with c-Jun to induce AP-1 reporter gene activation in the presence of constitutively active MEKK1 (CA-MEKK1) (Davies et al, 2010). Mutation of each arginine residue greatly reduced RACO-1-mediated c-Jun/AP-1 activation (Figure 7D), in agreement with the finding that each individual residue is required for c-Jun binding (Figure 6D). Likewise, silencing of RACO-1, c-Jun or PRMT1 all led to a substantial reduction in AP-1-mediated gene transactivation from a minimal c-Jun promoter, validating the findings that c-Jun, RACO-1 and PRMT1 cooperate in AP-1-mediated gene expression (Figure 7E). Taken together, these results imply that arginine methylation of RACO-1 by PRMT1 is required for efficient expression of c-Jun target genes and identify PRMT1 as an important physiological regulator of c-Jun/AP-1 function.
Figure 7.
RACO-1, PRMT1 and c-Jun cooperate in AP-1 gene expression. (A) Microarray heatmap of genes downregulated by si-Jun more than 1.5-fold compared to control, and the corresponding changes in gene expression after silencing of RACO-1 or PRMT1. Four data sets can be distinguished. Gene set 1=common genes downregulated in response to silencing c-Jun, RACO-1 or PRMT1; gene set 2=downregulated genes independent of PRMT1; gene set 3=c-Jun exclusive genes; and gene set 4=downregulated genes independent of RACO-1. Colour key and density plot underneath is represented as a log scale. (B) Gene ontology analysis of gene sets 1 and 2. Biological pathways significantly enriched for genes downregulated by si-Jun and si-RACO-1. Red line indicates threshold of P-value=0.05 (Benjamini and Hochberg corrected). (C) Silencing of RACO-1 or PRMT1 results in a concomitant reduction in classic AP-1 target genes. Quantitative RT–PCR analysis of cJun, cyclinD1, cdc2, CD44 and HB-EGF in HCT116 cells after silencing RACO-1 or PRMT1. The results were normalised to actin and expressed as fold change, compared to control siRNA in HCT116 cells. (D) The methyl targeted residues R98 and R109 are required for RACO-1-mediated AP-1 coactivation. Luciferase reporter assay of HCT116 cells co-transfected with a 2 × AP-1 luciferase reporter construct, c-Jun, wild-type RACO-1, RACO-1 R98K or RACO-1 R109K. Activation of the MEK/ERK signalling pathways was achieved by coexpression of constitutively active MEKK1 (CA-MEKK1). (E) Silencing RACO-1 or PRMT1 inhibits AP-1 luciferase activity. HCT116 cells were co-transfected with a 2 × AP-1 Firefly luciferase reporter construct along with shRACO-1, shPRMT1 or shJun constructs. Activation of MAP kinase signalling pathways was achieved by coexpressing CA-MEKK1. Ubiquitin-Renilla was transfected as an internal control. Results are expressed as ratio of Firefly luciferase/Renilla luciferase.
Discussion
We have described RACO-1 as a growth factor-regulated coactivator of c-Jun (Davies et al, 2010). Here we demonstrate an additional level of control of RACO-1 that involves methylation on two arginine residues by PRMT1. This is therefore the first report showing that arginine methylation regulates the AP-1 transcriptional response.
PRMT1 regulates transcription by methylating both histone and nonhistone substrates. PRMT1 stimulates transcription by methylating histone H4 arginine 3 (H4R3), which is read by the scaffolding protein TDRD3 that facilitates gene transcription (Yang et al, 2010). PRMT1 is the main arginine methyltransferase in mammalian cells (Tang et al, 2000), but only a small number of nonhistone substrates involved in transcriptional regulation are known. PRMT1 stimulates transcription by methylating coactivator proteins such as PGC-1α and NIP45 and the nuclear receptor corepressor RIP140 (Mowen et al, 2004; Teyssier et al, 2005; Mostaqul Huq et al, 2006). The identification of RACO-1 thus extends the rather short list of PRMT1 substrates involved in transcription. PRMT1 recognises substrates with glycine/argine-rich (GAR) motifs (especially RGG repeats). However, many PRMT1 substrates, including RACO-1, lack this consensus sequence, and it has been suggested that PRMT1 recognises additional binding sites on substrates distal to the methylated residue (Osborne et al, 2007; Wooderchak et al, 2008). This means that the number of PRMT1 substrates may be underestimated by conventional in silico analysis, suggesting that the importance of arginine methylation is yet to be fully appreciated.
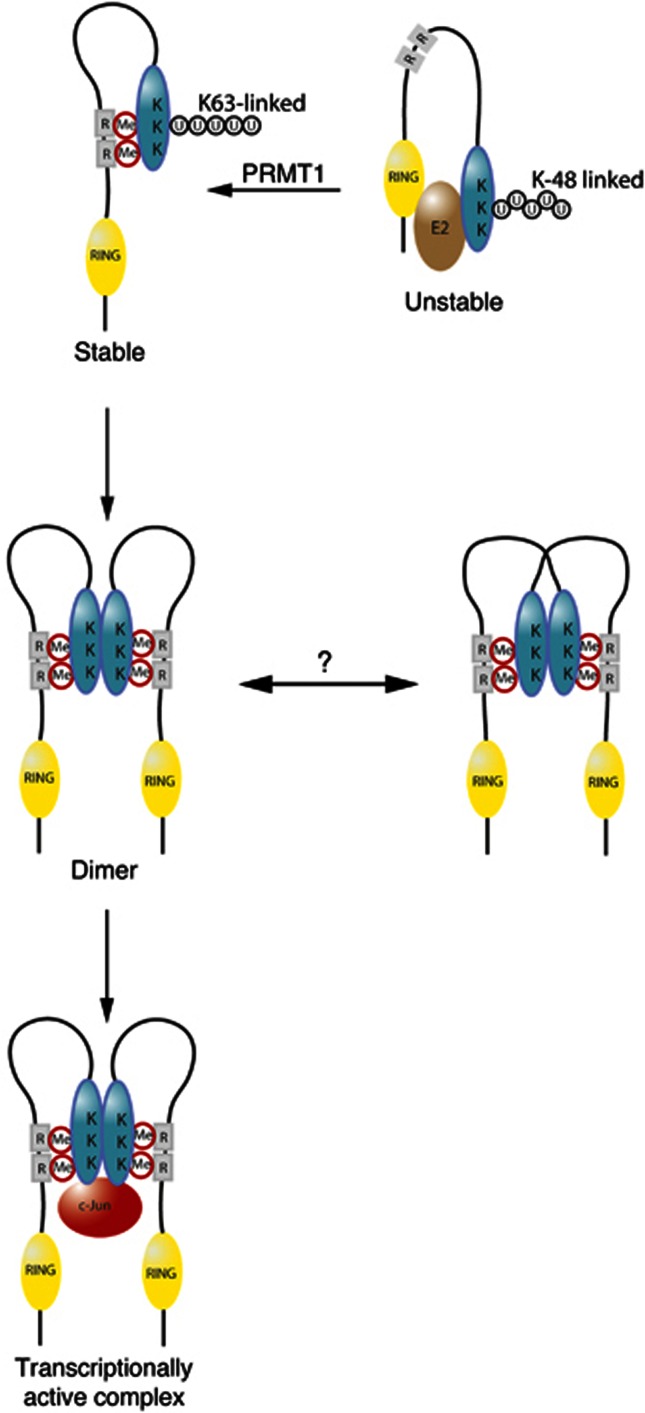
PRMT1-mediated methylation of arginines 98 and 109 controls several aspects of RACO-1 function. RACO-1 is a highly unstable protein due to autoubiquitylation. It is noteworthy that the three most C-terminal lysines (K195, K223 and K224) within RACO-1 are the preferential targets of autoubiquitylation (Davies et al, 2010). Thus, the N-terminal RING domain and the RACO-1 C-terminus, while separated in primary amino acid sequence, may be in proximity in the tertiary structure to allow degradative autoubiquitylation (Figure 8). We hypothesise that methylation of arginines 98 and 109 would enable the C-terminal region to bind to the methylated domain, thus inducing a conformational change that displaces the RING domain from the lysines targeted by autoubiquitylation, thereby facilitating K63-linked ubiquitylation. This stable conformer of RACO-1 would then be competent for dimerisation, and this conformation would also be capable of c-Jun binding. It is worth noting that we cannot discriminate whether the interaction between the RACO-1 C-terminus region and the methylated domain occurs in cis or in trans. However, we favour the hypothesis that this interaction occurs in cis, since the local concentration of the interacting domains is vastly higher for an intramolecular interaction and, secondly, methylation of each dimer partner protein is required for efficient dimer formation and c-Jun binding (Figure 6B and F). Thus, we propose that a conformational change in RACO-1 is the key mechanism that underlies the regulation of RACO-1 by arginine methylation.
Figure 8.
Schematic representation illustrating a potential mechanism for effects of PRMT1-mediated arginine methylation on RACO-1 protein stability and c-Jun binding. Demethylated RACO-1 (top right) is unstable due to autoubiquitylation targeting 3 lysine residues within the C-terminal domain of the protein. Methylation of arginine 98 and arginine 109 induces a conformational change enabling an intramolecular interaction between the N-terminal and C-terminal portions of the protein. Autoubiquitylation is subsequently inhibited and K63-linked ubiquitylation induced, leading to protein stabilisation. Once stabilised by methylation, RACO-1 forms a dimeric complex via the C-terminal dimerisation domain of each monomer (blue oval shape). The dimeric configuration of RACO-1 could exist in two forms; either the binding of two monomers in N- to C-terminal cis configuration or via the binding of two monomers in N- to C-terminal trans configuration. Dimeric RACO-1 can subsequently interact with c-Jun and drive AP-1 gene activation. K, lysine residues; R, arginine methylation (Me) sites (98 and 109); U, ubiquitin.
Arginine methylation has been most extensively studied in the context of histone tail modification and the epigenetic regulation of gene expression. PRMT1-mediated methylation of RACO-1 is the first time that arginine methylation has been shown to be important for AP-1 activation. PRMT1 appears to be a major regulator of AP-1-mediated transcription, since a large number of genes whose expression is dependent on c-Jun and RACO-1 are also dependent on PRMT1 (Figure 7A). This suggests that PRMT1 inactivation may substantially impair AP-1 function. In support of this, mouse embryonic fibroblasts lacking PRMT1 exhibit growth arrest and accumulate in G2/M phase of the cell cycle (Yu et al, 2009), which are phenotypes also observed in c-Jun-deficient MEFs (Johnson et al, 1993; Schreiber et al, 1999; Wada et al, 2004). c-Jun has important functions in intestinal tumour formation (Nateri et al, 2005; Sancho et al, 2009), and RACO-1 overexpression cooperated with oncogenic Ras and Apc in intestinal tumourigenesis (Davies et al, 2010). Expression of PRMT1 gene variant v1 was shown to be elevated in colorectal tumours and high expression of PRMT1v1 correlated positively with disease progression and aggressiveness (Mathioudaki et al, 2008). Moreover, patients strongly expressing the PRMT1v1 have a higher probability of relapse and lower survival compared with patients with low expression. Therefore, it may be worthwhile to investigate a potential function of PRMT1 in intestinal cancer and AP-1 regulation, especially since pharmacological PRMT1 inhibitors are being developed (Infantino et al, 2010; Dowden et al, 2011).
Materials and methods
Cell lines and antibodies
Human embryonic kidney (HEK) 293T, HCT116 human colon adenocarcinoma cells, BT474 breast carcinoma cells and H727 lung cancer cells were maintained in DMEM supplemented with 10% FCS. β-Actin, Flag, RACO-1 and HA (rabbit) antibodies were obtained from Sigma, GFP (rabbit) from Abcam (Cambridge, UK), myc (monoclonal clone 9E10) was generated in-house and PRMT1 (rabbit) was obtained from Cell Signalling.
cDNA constructs and cell transfection
All mutagenic/fragment constructs generated were confirmed by sequence analysis and primer sequences for all constructs are available on request. Myc–PRMT1 and GST–GAR were a gift from Uta-Maria Bauer (University of Marburg). GFP–PRMT1 was a gift from Mark Bedford (MD Anderson Cancer Center, The University of Texas), GST–PRMT1, GST–PRMT3 and GST–CARM1 were kind gifts from Steven Clarke (UCLA). HEK293T cells were transfected with Lipofectamine Plus (Invitrogen) and HCT116 with Lipofectamine 2000 (Invitrogen). siRNA oligos were transfected using DharmaFECT 1 (Dharmacon).
RNAi constructs and generation of cell lines with stable knockdown of PRMT1 and RACO-1
Annealed hairpin oligo siRNA sequences directed towards human PRMT1 (sense strand 5′-AGATTACTACTTTGACTCC-3′; scrambled PRMT1: sense strand 5′-GCATATATTTCCCCATAGT-3′) were cloned into pRetroSuper (puro) (OligoEngine). For stable knockdown cell lines, HEK293T, BT474 and H727 cells were transfected and individual clones isolated by puromycin selection (2 μg/ml). Generation of RACO-1 stable knockdown cell lines was performed by lentiviral infection as described previously (Davies et al, 2010). Hairpin sequences (depicted in uppercase characters) were: shRACO-1 (5′-tgTGATGGACCGTAGGAAGAAttcaagagaTTCTTCCTACGGTCCATCAttttttc-3′) and shLUC (5′-tgCGTACGCGGAATACTTCGAttcaagagaTCGAAGTATTCCGCGTACGttttttc-3′). Nontargeting shRNA and shJun constructs were obtained from Sigma.
Reporter assay and microarray analysis
HCT116 cells were seeded in 24-well plates and transfected with 250 ng 2 × AP-1 luciferase reporter construct (Fontana et al, 2012), 50 ng ubiquitin-Renilla, 425 ng of respective shRNA constructs and 50 ng CA-MEKK1. After transfection, cells were maintained in serum-free medium for 24 h before cell lysis. The Dual Luciferase reporter assay (Promega) was used according to the manufacturer’s instructions. For microarray analysis, HCT116 cells were transiently transfected by DharmaFECT 1 (Dharmacon) with silencing oligos directed towards RACO-1 (Sigma), c-Jun (Sigma) and PRMT1 (Dharmacon). At 48 h after transfection, cells were harvested for RNA extraction using the RNeasy kit (Qiagen) and subjected to DNase treatment (Ambion) according to the manufacturer’s instructions. Microarray analysis was performed by the Molecular Biology Core Facility, Paterson Institute for Cancer Research, Manchester, UK. Biological pathway enrichment analysis of the genes was carried out using DAVID (Database for Annotation, Visualization and Integrated Discovery v6.7)(Huang da et al, 2009a).
qRT–PCR on cell lines
RNA was extracted from cells using the Qiagen RNeasy mini kit, DNase treated (Ambion) and cDNA synthesised using Superscript III (Invitrogen) according to the manufacturer’s instructions. QPCR was performed using a ABIPrism 7900HT Sequence Detection System with SYBR Green incorporation. QPCR primers were:
Human c-Jun forward:. 5′-ccaaaggatagtgcgatgttt-3′ and reverse: 5′-ctgtccctctccactgcaac-3′; human CyclinD1 forward: 5′-agctcctgtgctgcgaagtggaaac-3′ and reverse: 5′-agtgttcaatgaaatcgtgcggggt-3′; human cdc2 forward: 5′-tggatctgaagaaatacttggattcta-3′ and reverse: 5′-caatcccctgtaggatttgg-3′; human CD44 forward: 5′-tgccgctttgcaggtgat-3′ and reverse: 5′ ggcctccgtccgagaga-3′; human RACO-1 forward: 5′-tggaaatcatgagaaaggacttg-3′ and reverse: 5′ acggtccatcacgtgtcc-3′; human PRMT1 forward: 5′-accttggctaatgggatgag-3′ and reverse: 5′-gggcttctcactgctttcc-3′; human Actin forward: 5′-tggatcagcaagcaggagtat 3′ and reverse: 5′-gcatttgcggtggacgat-3′. All primer pairs generated a single product as determined by dissociation curve analysis.
GST fusion protein expression
cJun (1–285), lacking the leucine zipper motif, was cloned by PCR into pGEX-6P1. DNA was transformed into BL21 (DE3) bacteria and a single colony expanded. Cultures were grown to an optical density of 0.4 and protein expression was induced by the addition of 400 μM IPTG for 4 h at 37 °C. Pelleted bacteria were lysed by sonication in Buffer B (20 mM Tris, pH 7.5, 150 mM NaCl, 0.5% Triton X-100 and 10 mM DTT). Fusion proteins were isolated by affinity purification using Glutathione Sepharose 4B beads (Amersham) and washed four times with Buffer B.
Isolation of cytoplasmic and nuclear extracts and Ni 2+ NTA-agarose purification
Cytoplasmic and nuclear extracts were isolated as described previously (Davies et al, 2010). For co-immunoprecipitations, volumes of nuclear and cytoplasmic lysates were made up to 1 ml with Buffer A (20 mM Tris–Cl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 0.2% NP-40, protease inhibitor cocktail (Sigma), 100 mM PMSF, 1 mM Na3VO4, 50 mM NaF and 1 mM β glycerophosphate) before immunoprecipitation. For 4 h before lysis, 25 μM of the proteasome inhibitor MG132 (Calbiochem) was added to cells. For in vivo ubiquitylation assays, His-Ubi was affinity purified with Ni2+-NTA-Agarose beads as described previously (Campanero and Flemington, 1997).
In vivo [ 3 H]-methyl methionine and [ 35 S]-methionine labelling
Transfected cells were placed in complete DMEM (methionine free) media and incubated with cycloheximide (100 μg/ml) and chloramphenicol (40 μg/ml) for 1 h to inhibit de novo protein synthesis. L-[Methyl-3H]-methionine (specific activity 70–85 Ci (2.59–3.145 TBq)/mmol, PerkinElmer) or EasyTag L-[35S]-methionine (specific activity 1000 Ci (37.0 TBq)/mmol, PerkinElmer) was then added to cultures for a further 4 h. Cells were harvested and lysed in Buffer A and Flag–RACO-1 immunoprecipitated overnight with α-Flag–agarose beads (Sigma). After four washes with Buffer A, immunoprecipitates were denatured, resolved by SDS–PAGE and proteins transferred to nitrocellulose membrane. To verify equal expression of transfected plasmids, one-tenth of the immunoprecipitation was retained for standard SDS–PAGE/western blotting analysis. To enhance the tritium signal, membranes were treated with EN3HANCE (PerkinElmer), and exposed to autoradiography film for at least 1 month at −80°C. For some experiments, after immunoprecipitation and washes with Buffer A, beads were resuspended in 100 μl Buffer A and added directly to 4 ml scintillation fluid and counted using a tritium programme on a scintillation counter.
In vitro methylation assay
Purified recombinant GST–PRMT1 was incubated with 10 μg His–SUMO–RACO-1 and 2 μl S-[methyl-3H]–adenosyl-L-methionine (3[H]-SAM) (specific activity: 55–85 Ci/mmol, PerkinElmer) in a total volume of 55 μl supplemented with 100 mM sodium phosphate (pH 7.5) buffer. Reactions were incubated at 37°C for 1 h and denatured protein resolved by SDS–PAGE. Protein was transferred onto PVDF membrane, the tritium signal was enhanced by treating membranes with EN3HANCE (PerkinElmer) and was exposed to autoradiography film for at least 1 month at −80 °C.
Mass spectrometry
pIRES2-HA-RACO-1 was transfected into 293T cells (4 × 10 cm dishes per condition) and 24 h later treated with the proteasome inhibitor MG132 (25 μM, Calbiochem) for 4 h before lysis with Buffer A. After sonication and clarification, cell lysates were incubated with HA–agarose beads (Sigma) for 2 h and then washed 4 × with Buffer A. Immunoprecipitations were resolved on a 4–12% Bis/Tris gradient gel (NuPage, Invitrogen) and stained without fixing using a methanol-free colloidal coomassie blue stain (ProtoBlue Safe, National Diagnostics). Polyacrylamide gel slices (1–2 mm) containing purified RACO-1 were prepared for mass spectrometric analysis using the Janus liquid handling system (PerkinElmer, UK). Briefly, the excised protein gel pieces were placed in a well of a 96-well microtitre plate and destained with 50% v/v acetonitrile and 50 mM ammonium bicarbonate, reduced with 10 mM DTT, and alkylated with 55 mM iodoacetamide. After alkylation, proteins were digested with either 6 ng/μl Trypsin (Promega, UK) or 6 ng/μl endoproteinase Asp-N (Roche Diagnostics, Germany) overnight at 37°C. The resulting peptides were extracted in 1% v/v formic acid, 2% v/v acetonitrile. The digest was analysed by nano-scale capillary LC-MS/MS using a nanoAcquity UPLC (Waters, UK) to deliver a flow of 300 nl/min. A C18 Symmetry 5 μm, 180 μm × 20 mm μ-Precolumn (Waters) trapped the peptides prior to separation on a C18 BEH130 1.7 μm, 75 μm × 100 mm analytical UPLC column (Waters). Peptides were eluted with a gradient of acetonitrile. The analytical column outlet was interfaced with a Triversa nanomate microfluidic chip for mass spectrometric analysis (Advion, UK). Mass spectrometric information was obtained using an orthogonal-acceleration quadrupole-time-of-flight mass spectrometer (SYNAPT HDMS, Waters). Data-dependent analysis was carried out, where automatic MS/MS was acquired on the eight most intense, multiply-charged precursor ions in the m/z range 400–1500. MS/MS data were acquired over the m/z range 50–1995. LC/MS/MS data were then searched against a protein database (UniProt KB, release 15.5 or an in-house sequence database) using the Mascot search engine programme (Matrix Science, UK)(Perkins et al, 1999). The experiment was repeated eight times.
In vitro chemical crosslinking assay
HEK293T cells were transfected for 6 h using Lipofectamine PLUS reagent with Flag-RACO-1. After 24 h, cells were lysed in Buffer A and immunoprecipitated for 2 h using α-Flag-agarose beads (Sigma). After three washes with Buffer A and two washes with 100 mM sodium phosphate buffer (pH 7.5), associated proteins were eluted with two rounds of Flag peptide elution (Sigma, 0.2 mg/ml, 10 min shaking at 25°C). Of the pooled eluate, 50% was left untreated while the remainder was chemically crosslinked with glutaraldehyde (0.025%, RT from 30 min). Reactions were terminated with 100 mM Tris, pH 7.5, resolved by SDS–PAGE and immunoblotted with α-Flag antibody. In some experiments, after chemical crosslinking, reactions were further subdivided and incubated with GST or GST-c-Jun for 2 h at 4°C with rotation. Pull-downs were washed with Buffer A and resolved by SDS–PAGE and immunoblotted with anti-Flag antibody.
Biotin pull-down assays
Biotin-labelled RACO-1 peptides were synthesised in-house and immobilised onto streptavidin-coated DynaBeads (Dynal) as follows: biotinylated peptide (1 mg) was incubated with Dynabeads M280 streptavidin in buffer containing 50 mM Tris–Cl, pH 7.5, 1 M NaCl and 0.5 mM EDTA at 4°C overnight. Peptide-bound beads were washed and resuspended in Buffer A. HEK293T cells overexpressing RACO-1 wt or mutants were lysed in Buffer A and then incubated with the Dynabead bound peptide for 2 h at 4°C. The beads were washed thrice with Buffer A and analysed by western blot.
Nondenaturing PAGE analysis
H727 cells, with stably integrated control shRNA or shRNA against RACO-1 or PRMT1, were lysed in Buffer A. Cell extracts were dissolved in gel loading buffer (375 mM Tris–Cl, pH 8.8, 10% Glycerol and 0.01% Bromophenol Blue) and loaded onto 8% continuous nondenaturing PAGE (375 mM Tris–Cl, pH 8.8). Gel was run using electrophoresis buffer (25 mM Tris and 192 mM Glycine, pH 8.3) at 4°C. Analysed samples were immunoblotted using anti-RACO-1 antibody.
Supplementary Material
Acknowledgments
Support was given from the Bioinformatics and Biostatistics and the Equipment Park resources at the London Research Institute (CRUK). Mass spectrometry was performed by the Protein Analysis and Proteomics Group, Clare Hall, the London Research Institute (CRUK). We thank U-M Bauer, M Bedford and S Clarke for reagents, and P Chakravarty, Bioinformatics Department, London Research Institute (CRUK), for help in gene ontology analysis. We also thank H Walden and B Thompson for critical reading of the manuscript and the Mammalian Genetics Lab for input and discussions. The London Research Institute is funded by Cancer Research UK. This work was supported by grants from AICR (12-1049) and MRC (G0901677).
Author contributions: CCD and AC performed the experiments, compiled data, generated figures and co-wrote the manuscript, MED generated shRACO1 stable cell lines, MS performed MS analysis and AB directed the project and wrote the manuscript.
Footnotes
The authors declare that they have no conflict of interest.
References
- Bedford MT, Clarke SG (2009) Protein arginine methylation in mammals: who, what, and why. Mol Cell 33: 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanero MR, Flemington EK (1997) Regulation of E2F through ubiquitin-proteasome-dependent degradation: stabilization by the pRB tumor suppressor protein. Proc Natl Acad Sci USA 94: 2221–2226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies CC, Chakraborty A, Cipriani F, Haigh K, Haigh JJ, Behrens A (2010) Identification of a co-activator that links growth factor signalling to c-Jun/AP-1 activation. Nat Cell Biol 12: 963–972 [DOI] [PubMed] [Google Scholar]
- Davis RJ (2000) Signal transduction by the JNK group of MAP kinases. Cell 103: 239–252 [DOI] [PubMed] [Google Scholar]
- Dowden J, Pike RA, Parry RV, Hong W, Muhsen UA, Ward SG (2011) Small molecule inhibitors that discriminate between protein arginine N-methyltransferases PRMT1 and CARM1. Org Biomol Chem 9: 7814–7821 [DOI] [PubMed] [Google Scholar]
- Eferl R, Sibilia M, Hilberg F, Fuchsbichler A, Kufferath I, Guertl B, Zenz R, Wagner EF, Zatloukal K (1999) Functions of c-Jun in liver and heart development. J Cell Biol 145: 1049–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eferl R, Wagner EF (2003) AP-1: a double-edged sword in tumorigenesis. Nat Rev Cancer 3: 859–868 [DOI] [PubMed] [Google Scholar]
- Fontana X, Hristova M, Da Costa C, Patodia S, Thei L, Makwana M, Spencer-Dene B, Latouche M, Mirsky R, Jessen KR, Klein R, Raivich G, Behrens A (2012) c-Jun in Schwann cells promotes axonal regeneration and motoneuron survival via paracrine signaling. J Cell Biol 198: 127–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang da W, Sherman BT, Lempicki RA (2009a) Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res 37: 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang da W, Sherman BT, Lempicki RA (2009b) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4: 44–57 [DOI] [PubMed] [Google Scholar]
- Hyllus D, Stein C, Schnabel K, Schiltz E, Imhof A, Dou Y, Hsieh J, Bauer UM (2007) PRMT6-mediated methylation of R2 in histone H3 antagonizes H3 K4 trimethylation. Genes Dev 21: 3369–3380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infantino S, Benz B, Waldmann T, Jung M, Schneider R, Reth M (2010) Arginine methylation of the B cell antigen receptor promotes differentiation. J Exp Med 207: 711–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R, Spiegelman B, Hanahan D, Wisdom R (1996) Cellular transformation and malignancy induced by ras require c-jun. Mol Cell Biol 16: 4504–4511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RS, van Lingen B, Papaioannou VE, Spiegelmann BM (1993) A null mutation at the c-jun locus causes embryonic lethality and retarded cell growth in culture. Genes Dev 7: 1309–1317 [DOI] [PubMed] [Google Scholar]
- Mathioudaki K, Papadokostopoulou A, Scorilas A, Xynopoulos D, Agnanti N, Talieri M (2008) The PRMT1 gene expression pattern in colon cancer. Br J Cancer 99: 2094–2099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechta-Grigoriou F, Gerald D, Yaniv M (2001) The mammalian Jun proteins: redundancy and specificity. Oncogene 20: 2378–2389 [DOI] [PubMed] [Google Scholar]
- Mostaqul Huq MD, Gupta P, Tsai NP, White R, Parker MG, Wei LN (2006) Suppression of receptor interacting protein 140 repressive activity by protein arginine methylation. EMBO J 25: 5094–5104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowen KA, Schurter BT, Fathman JW, David M, Glimcher LH (2004) Arginine methylation of NIP45 modulates cytokine gene expression in effector T lymphocytes. Mol Cell 15: 559–571 [DOI] [PubMed] [Google Scholar]
- Nateri AS, Spencer-Dene B, Behrens A (2005) Interaction of phosphorylated c-Jun with TCF4 regulates intestinal cancer development. Nature 437: 281–285 [DOI] [PubMed] [Google Scholar]
- Osborne TC, Obianyo O, Zhang X, Cheng X, Thompson PR (2007) Protein arginine methyltransferase 1: positively charged residues in substrate peptides distal to the site of methylation are important for substrate binding and catalysis. Biochemistry 46: 13370–13381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlak MR, Scherer CA, Chen J, Roshon MJ, Ruley HE (2000) Arginine N-methyltransferase 1 is required for early postimplantation mouse development, but cells deficient in the enzyme are viable. Mol Cell Biol 20: 4859–4869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins DN, Pappin DJ, Creasy DM, Cottrell JS (1999) Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis 20: 3551–3567 [DOI] [PubMed] [Google Scholar]
- Sancho R, Nateri AS, de Vinuesa AG, Aguilera C, Nye E, Spencer-Dene B, Behrens A (2009) JNK signalling modulates intestinal homeostasis and tumourigenesis in mice. EMBO J 28: 1843–1854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber M, Kolbus A, Piu F, Szabowski A, Mohle-Steinlein U, Tian J, Karin M, Angel P, Wagner EF (1999) Control of cell cycle progression by c-Jun is p53 dependent. Genes Dev 13: 607–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaulian E, Karin M (2001) AP-1 in cell proliferation and survival. Oncogene 20: 2390–2400 [DOI] [PubMed] [Google Scholar]
- Tan CP, Nakielny S (2006) Control of the DNA methylation system component MBD2 by protein arginine methylation. Mol Cell Biol 26: 7224–7235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Frankel A, Cook RJ, Kim S, Paik WK, Williams KR, Clarke S, Herschman HR (2000) PRMT1 is the predominant type I protein arginine methyltransferase in mammalian cells. J Biol Chem 275: 7723–7730 [DOI] [PubMed] [Google Scholar]
- Teyssier C, Ma H, Emter R, Kralli A, Stallcup MR (2005) Activation of nuclear receptor coactivator PGC-1alpha by arginine methylation. Genes Dev 19: 1466–1473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada T, Joza N, Cheng HY, Sasaki T, Kozieradzki I, Bachmaier K, Katada T, Schreiber M, Wagner EF, Nishina H, Penninger JM (2004) MKK7 couples stress signalling to G2/M cell-cycle progression and cellular senescence. Nat Cell Biol 6: 215–226 [DOI] [PubMed] [Google Scholar]
- Wooderchak WL, Zang T, Zhou ZS, Acuna M, Tahara SM, Hevel JM (2008) Substrate profiling of PRMT1 reveals amino acid sequences that extend beyond the “RGG” paradigm. Biochemistry 47: 9456–9466 [DOI] [PubMed] [Google Scholar]
- Yamagata K, Daitoku H, Takahashi Y, Namiki K, Hisatake K, Kako K, Mukai H, Kasuya Y, Fukamizu A (2008) Arginine methylation of FOXO transcription factors inhibits their phosphorylation by Akt. Mol Cell 32: 221–231 [DOI] [PubMed] [Google Scholar]
- Yang Y, Lu Y, Espejo A, Wu J, Xu W, Liang S, Bedford MT (2010) TDRD3 is an effector molecule for arginine-methylated histone marks. Mol Cell 40: 1016–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z, Chen T, Hebert J, Li E, Richard S (2009) A mouse PRMT1 null allele defines an essential role for arginine methylation in genome maintenance and cell proliferation. Mol Cell Biol 29: 2982–2996 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.