Summary
Background
Tamoxifen and raloxifene reduce the risk of breast cancer in women at elevated risk of disease, but the duration of the effect is unknown. We assessed the effectiveness of selective oestrogen receptor modulators (SERMs) on breast cancer incidence.
Methods
We did a meta-analysis with individual participant data from nine prevention trials comparing four selective oestrogen receptor modulators (SERMs; tamoxifen, raloxifene, arzoxifene, and lasofoxifene) with placebo, or in one study with tamoxifen. Our primary endpoint was incidence of all breast cancer (including ductal carcinoma in situ) during a 10 year follow-up period. Analysis was by intention to treat.
Results
We analysed data for 83 399 women with 306 617 women-years of follow-up. Median follow-up was 65 months (IQR 54–93). Overall, we noted a 38% reduction (hazard ratio [HR] 0·62, 95% CI 0·56–0·69) in breast cancer incidence, and 42 women would need to be treated to prevent one breast cancer event in the first 10 years of follow-up. The reduction was larger in the first 5 years of follow-up than in years 5–10 (42%, HR 0·58, 0·51–0·66; p<0·0001 vs 25%, 0·75, 0·61–0·93; p=0·007), but we noted no heterogeneity between time periods. Thromboembolic events were significantly increased with all SERMs (odds ratio 1·73, 95% CI 1·47–2·05; p<0·0001). We recorded a significant reduction of 34% in vertebral fractures (0·66, 0·59–0·73), but only a small effect for non-vertebral fractures (0·93, 0·87–0·99).
Interpretation
For all SERMs, incidence of invasive oestrogen (ER)-positive breast cancer was reduced both during treatment and for at least 5 years after completion. Similar to other preventive interventions, careful consideration of risks and benefits is needed to identify women who are most likely to benefit from these drugs.
Funding
Cancer Research UK.
Introduction
Large reductions in contralateral tumours shown in adjuvant trials with tamoxifen suggest that this drug could prevent breast cancer.1,2 Studies of other selective oestrogen receptor modulators (SERMs) in trials designed to prevent fractures in women with osteoporosis have also suggested a preventive effect on breast cancer. Although SERMs have different chemical structures, which can affect their specific activities, they all work by binding to the oestrogen receptor and inhibiting the stimulus for cell division. A comprehensive review of the mechanisms of action of SERMs has been published.3 An earlier meta-analysis4 summarised the early follow-up results of the tamoxifen and raloxifene prevention trials. The results showed that tamoxifen significantly reduced the risk of oestrogen receptor (ER)-positive breast cancer by 48%, but no effect was noted for ER-negative tumours. Here, we update previous meta-analyses, with additional data for short-term follow-up of lasofoxifene and arzoxifene, to assess the effect of SERMs on breast cancer incidence.
Methods
Study selection
We searched PubMed with the keywords breast cancer, prevention, selective oestrogen receptor modulator (or SERM), and chemoprevention. Table 1 provides details of the included breast cancer prevention trials. We identified nine randomised trials that compared SERMs with placebo or another drug in women without breast cancer, and had at least 2 years of follow-up. Four trials5,7,9,11 assessed 20 mg per day tamoxifen versus placebo for at least 5 years in healthy women who were mostly at increased risk of breast cancer. Two trials13,14 investigated raloxifene versus placebo in postmenopausal women who had either osteoporosis, or had risk factors for or established coronary heart disease.15 A third trial16 compared raloxifene to tamoxifen in women at increased risk of developing breast cancer. One trial18 compared lasofoxifene at two different doses with placebo in postmenopausal women with osteoporosis. Finally, one trial20 compared arzoxifene with placebo in postmenopausal women with osteoporosis. The trials are summarised in table 1.
Table 1.
Details of breast cancer prevention trials
| N | Recruitment period | Treatment groups and daily dose | Treatment duration (years) | Entry criteria | Present status | Median follow-up (months) | |
|---|---|---|---|---|---|---|---|
| Marsden5,6 | 2471 | 1986–96 | Placebo (1233) Tamoxifen 20 mg (1238) |
5–8 | High risk, family history | Blinded, further follow-up | 171·6 (153·9–184·0) |
| IBIS-I7,8 | 7109 | 1992–2001 | Placebo (3566) Tamoxifen 20 mg (3573) |
5 | Greater than two times relative risk | Blinded, further follow-up | 96 (80·1–117·1) |
| NSABP-P-19,10 | 13 205 | 1992–97 | Placebo (6707) Tamoxifen 20 mg (6681) |
5 | >1·6% 5 year risk | Unblinded, no follow-up | 57·6 (35·4–64·9) |
| Italian11,12 | 5408 | 1992–97 | Placebo (2708) Tamoxifen 20 mg (2700) |
5 | Normal risk, women with hysterectomy | Unblinded, further follow-up | 139·6 (122·0–146·1) |
| MORE13/CORE14* | 7705/6511 | 1994–98/ 1998–2002 | Placebo (2576) Raloxifene 60 mg (2557)/Placebo (2576) Raloxifene 120 mg (2572) | 4/8 | Normal risk, postmenopausal women with osteoporosis | Unblinded, no follow-up | 71·3 (47·1–95·4) |
| RUTH15 | 10 101 | 1998–2000 | Placebo (5057) Raloxifene 60 mg (5044) |
5 | Normal risk, postmenopausal women with established or risk of CHD | Unblinded, no follow-up | 66·7 (60·1–72·3) |
| STAR16,17 | 19 490 | 1999–2004 | Raloxifene 60 mg (9875) Tamoxifen 20 mg (9872) |
5 | >1·6% 5 year risk, postmenopausal women | Unblinded, no follow-up | 81 (60·8–96.6) |
| PEARL18,19 | 8856 | 2001–07 | Placebo (2852) Lasofoxifene 0·50 mg (2852) Lasofoxifene 0·25 mg (2852) |
5 | Normal risk, postmenopausal women with osteoporosis | Blinded, no follow-up | 59·6 (58·8–60·1) |
| GENERATIONS20,21 | 9354 | 2004–09 | Placebo (4678) Arzoxifene 20 mg (4676) |
4 | Normal risk, postmenopausal with low BMD or osteoporosis | Unblinded, no follow-up | 54·3 (28·3–56·1) |
Data in parenthesis are number of randomised participants. CHD=coronary heart disease. BMD=bone mineral density.
The CORE trial was done in a subset of women originally enrolled in the MORE trial.
Statistical analysis
We obtained individual participant data directly from the trial investigators. Comparisons were on an intention-to-treat basis. We assessed fixed-effects and random-effects models. Our primary endpoint was incidence of all breast cancer (including ductal carcinoma in situ) during 10 years of follow-up. Secondary endpoints were incidence in years 0–5 and years 5–10, and all invasive ER-positive or ER-negative cancers, and ductal carcinoma in situ. Other predefined secondary endpoints were incidence of other cancers, venous thromboembolic events, cardiovascular events, fractures, cataract, and all-cause mortality.
For the fixed-effects models, we computed log hazard ratios (HRs) and their variance separately for each trial and then used the inverse variance-weighted method to calculate a fixed-effect estimate of the overall log HR and its variance. For indirect comparisons between raloxifene and placebo in the National Surgical Adjuvant Breast and Bowel Project (NSABP) Study of Tamoxifen and Raloxifene (STAR) trial, we calculated the log HR for the intervention between raloxifene and placebo by subtracting the ratio for the direct comparison between raloxifene and tamoxifen by that for the direct comparison between tamoxifen and placebo for the other trials. We computed corresponding standard errors in a similar way. We included the STAR trial only for the raloxifene effects, and results for the overall effect do not include data from that trial.
We explored random-effects models, which account for variability between trials, and assessed trial heterogeneity with Q statistics and I2 estimates.22 Data are plotted as the proportion of women with the event as a function of follow-up time with Kaplan-Meier methods.23 To compare outcomes between tamoxifen and raloxifene we computed the ratio of HRs for comparisons of each drug with placebo, then added the direct comparison from the STAR trial as a separate stratum to obtain a summary hazard ratio. For analysis we used STATA (version 11.2) with the meta command. Results are presented as HRs with 95% CIs and two-sided p-values.
Role of the funding source
Neither Cancer Research UK nor the funding sources for the individual studies had a role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author and IS had full access to all the data in the study, and all authors had access to analyses. All authors read and approved the final decision to submit for publication.
Results
We included nine trials with 83 399 participants and 306 617 women-years of follow-up (table 1). Median follow-up was 65 months (IQR 54–93). Figure 1 shows Kaplan-Meier curves for all breast cancers and invasive ER-positive breast cancer for all trials except the STAR trial. Annual rates of breast cancer incidence varied substantially between trials (table 2), probably because of different entry criteria. The overall reduction in all breast cancer (including ductal carcinoma in situ) was 38% (p<0·0001; table 2), with an estimated 10 year cumulative incidence of 6·3% in the control groups and 4·2% in the SERM groups. We noted the reduction in both years 0–5 of follow-up (42%, p<0·0001) and years 5–10 (25%, p=0·007; table 2 and figure 2). Despite the smaller effect in years 5–10, there was no evidence of heterogeneity between trials (p=0·3). Random-effects models produced similar HRs to those for the fixed-effects models, but larger 95% CIs (table 2).
Figure 1.
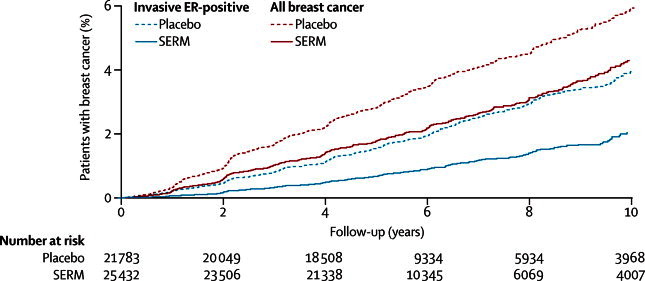
Cumulative incidence for all breast cancer (including ductal carcinoma in situ) and all ER-positive invasive cancers in years 0–10 according to treatment allocation
SERM=selective oestrogen receptor modulator. ER=oestrogen receptor.
Table 2.
Breast cancer incidence in the chemoprevention trials
| Overall* | Annual rates per 1000† | HR (95% CI) | ER-positive invasive | HR (95% CI) | ER-negative invasive | HR (95% CI) | DCIS | HR (95% CI) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Tamoxifen trials | ||||||||||
| Marsden | 96 vs 114 | 6·4 | 0·87 (0·63-1·21) | 51 vs 83 | 0·66 (0·44-0·99) | 25 vs 17 | 1·66 (0·81-3·40) | 14 vs 9 | 1·40 (0·44-4·40) | |
| IBIS I | 143 vs 198 | 6·7 | 0·72 (0·58-0·90) | 88 vs 131 | 0·69 (0·52-0·90) | 36 vs 38 | 0·97 (0·62-1·54) | 16 vs 27 | 0·52 (0·27-0·99) | |
| NSABP-P-1 | 130 vs 248 | 6·1 | 0·52 (0·42-0·64) | 44 vs 134 | 0·33 (0·23-0·46) | 39 vs 31 | 1·26 (0·78-2·02) | 38 vs 70 | 0·54 (0·36-0·80) | |
| Italian | 62 vs 74 | 4·2 | 0·83 (0·58-1·19) | 36 vs 48 | 0·73 (0·45-1·17) | 16 vs 17 | 0·87 (0·43-1·79) | 9 vs 6 | 1·80 (0·60-5·38) | |
| Total (0–10 years) | 431 vs 634 | .. | 0·67 (0·59-0·76) | 219 vs 396 | 0·56 (0·47-0·67) | 116 vs 103 | 1·13 (0·86-1·49) | 77 vs 112 | 0·72 (0·57-0·92) | |
| Total (0–5 years) | 256 vs 409 | .. | 0·62 (0·53-0·73) | 121 vs 235 | 0·51 (0·41-0·64) | 78 vs 76 | 1·03 (0·75-1·41) | 47 vs 83 | 0·56 (0·39-0·81) | |
| Total (5–10 years) | 175 vs 225 | .. | 0·78 (0·62-0·97) | 98 vs 161 | 0·63 (0·47-0·83) | 38 vs 27 | 1·55 (0·88-2·72) | 30 vs 29 | 0·87 (0·49-1·57) | |
| Raloxifene trials | ||||||||||
| MORE/CORE | 57 vs 65 | 4·2 | 0·42 (0·29-0·60) | 22 vs 44 | 0·24 (0·15-0·40) | 15 vs 7 | 1·06 (0·43-2·59) | 13 vs 7 | 0·91 (0·36-2·28) | |
| RUTH | 52 vs 76 | 4·2 | 0·67 (0·47-0·96) | 25 vs 55 | 0·45 (0·28-0·72) | 13 vs 9 | 1·44 (0·61-3·63) | 11 vs 5 | 2·17 (0·75-6·25) | |
| STAR‡ (tamoxifen vs raloxifene) | 358 vs 447 | 5·9 | 0·81 (0·70-0·93) | 182 vs 221 | 0·83 (0·69-1·02) | 60 vs 70 | 0·79 (0·56-1·11) | 111 vs 137 | 0·82 (0·64-1·05) | |
| Total (0–10 years) | 467 vs 588 | .. | 0·66 (0·55-0·80) | 229 vs 320 | 0·44 (0·34-0·58) | 88 vs 93 | 1·37 (0·96-1·95) | 135 vs 149 | 1·07 (0·68-1·68) | |
| Total (0–5 years) | 327 vs 421 | .. | 0·63 (0·51-0·79) | 168 vs 224 | 0·40 (0·29-0·56) | 62 vs 71 | 1·27 (0·83-1·95) | 86 vs 108 | 1·08 (0·60-1·96) | |
| Total (5–10 years) | 140 vs 167 | .. | 0·84 (0·51-1·27) | 61 vs 96 | 0·72 (0·49-1·06) | 26 vs 22 | 1·70 (0·84-3·47) | 49 vs 41 | 0·88 (0·45-1·74) | |
| PEARL | ||||||||||
| 0·25 mg | 20 vs 24 | 2·0 | 0·82 (0·45-1·49) | 9 vs 18 | 0·49 (0·22-1·10) | 7 vs 2 | 2·83 (0·57-14·02) | 4 vs 4 | 0·99 (0·25-3·99) | |
| 0·5 mg | 5 vs 24 | 2·0 | 0·21 (0·08-0·55) | 3 vs 18 | 0·17 (0·05-0·56) | 0 vs 2 | .. | 3 vs 4 | 0·50 (0·09-2·73) | |
| GENERATIONS | 22 vs 53 | 3·2 | 0·42 (0·25-0·68) | 9 vs 30 | 0·30 (0·14-0·63) | 10 vs 10 | 1·01 (0·42-2·41) | 3 vs 10 | 0·30 (0·08-1·09) | |
| All trials (fixed effect; random effect)§ | ||||||||||
| Total (0–10 years) | 587 vs 852 | 4·7 | 0·62 (0·56-0·69); 0·61 (0·49-0·75) | 287 vs 543 | 0·49 (0·42-0·57); 0·44 (0·33-0·60) | 160 vs 131 | 1·14 (0·90-1·45) 1·14 (0·90-1·45) | 110 vs 138 | 0·69 (0·53-0·90); 0·79 (0·53-1·19) | |
| Total (0–5 years) | 376 vs 594 | 4·6 | 0·58 (0·51-0·66); 0·58 (0·47-0·73) | 174 vs 360 | 0·45 (0·38-0·54); 0·42 (0·29-0·61) | 111 vs 90 | 1·05 (0·80-1·39); 1·05 (0·80-1·39) | 73 vs 107 | 0·66 (0·48-0·90); 0·73 (0·47-1·14) | |
| Total (5–10 years) | 211 vs 258 | 4·9 | 0·75 (0·61-0·93); 0·75 (0·59-0·94) | 113 vs 183 | 0·58 (0·45-0·76); 0·58 (0·45-0·76) | 49 vs 32 | 1·66 (0·98-2·81) 1·66 (0·98-2·81) | 37 vs 31 | 0·94 (0·53-1·66); 0·94 (0·53-1·66) | |
HR=hazard ratio. ER=oestrogen receptor. DCIS=ductal carcinoma in situ. Data are for selective oestrogen receptor modulator versus vs placebo, unless otherwise indicated
All cancers (including DCIS) and those with unknown receptor status.
In control group.
STAR data not included for overall effect.
STAR data not excluded for overall effect.
Figure 2.
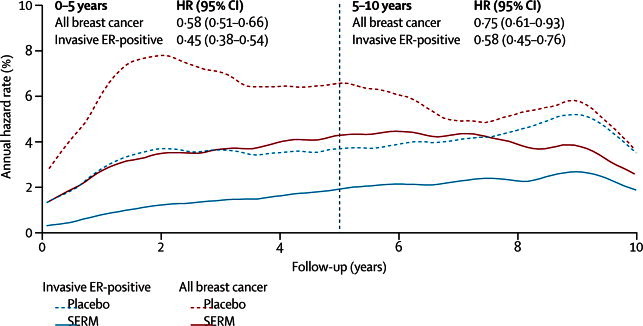
Annual hazard rate for all breast cancers (including ductal carcinoma in situ) and invasive ER-positive breast cancer in years 0–10 with fixed-effects models23
ER=oestrogen receptor. SERM=selective oestrogen receptor modulator.
Overall, the frequency of invasive ER-positive cancer was reduced from 4·0% to 2·1% (p<0·0001; table 2). This reduction was apparent in years 0–5 (p<0·0001) and in years 5–10 (p<0·0001; table 2 and figure 2). The number needed to treat to prevent one diagnosis of breast cancer in the first 10 years was 42; when restricted to invasive ER-positive breast cancer the number was 53. Although all trials showed a reduction in breast cancer incidence, we noted substantial heterogeneity between trials in the size of the effect for all breast cancers and invasive ER-positive cancers (figure 3), and for invasive cancers. We noted a non-significant increase in invasive ER-negative breast cancers (p=0·3; table 2). The incidence of ductal carcinoma in situ was significantly reduced overall by 31% (p=0·006; table 2). We noted a 38% reduction in incidence in the tamoxifen trials, but no effect for raloxifene; however, significant heterogeneity was shown between trials. Little information was available for the effect of lasofoxifene and arzoxifene on ductal carcinoma in situ.
Figure 3.
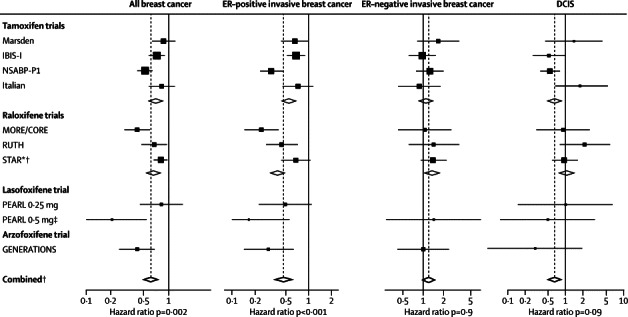
All breast cancers, invasive breast cancer, and DCIS in years 0–10
ER=oestrogen receptor. DCIS=ductal carcinoma in situ. *Adjusted by overall tamoxifen effect to give raloxifene versus placebo comparisons. † STAR data not included in comparisons. ‡Data for ER-invasive cancer are pooled.
For tamoxifen trials, we noted a significant reduction of 33% (p<0·0001) in all breast cancers compared with placebo (table 2 and figure 3). This reduction was mainly due to a large effect on ER-positive invasive breast cancer, for which we noted a reduction of 44% (p<0·0001; table 2) and a significant reduction in DCIS (p=0·009; table 2), but a non-significant increase in ER-negative tumours was recorded (p=0·4; table 2). Significant heterogeneity was shown between trials for all breast cancers (p=0·02) and invasive ER-positive breast cancers (p=0·03). For raloxifene trials, we noted a significant reduction in incidence of all breast cancer (p<0·0001; table 2) due to a reduction in invasive ER-positive breast cancers, with a non-significant increase in the incidence of invasive ER-negative breast cancers and no effect on DCIS (table 2 and figure 3). When we compared raloxifene with tamoxifen, the only significant difference in effect size was a greater effect for tamoxifen in DCIS (HR 0·78, 95% CI 0·61–0·99; p=0·04).
The PEARL and GENERATIONS trials had follow-up results for only years 0–5. All breast cancers (p<0·0001) and ER-positive cancers (p<0·0001; table 2) were significantly reduced with 0·5 mg per day of lasofoxifene compared with placebo, whereas only a small effect was noted for women receiving 0·25 mg per day (table 2 and figure 3). We noted a non-significant increase in incidence for invasive ER-negative breast cancer (HR 1·43, 95% CI 0·43–1·66) and a non-significant decrease for ductal carcinoma in situ (0·76, 0·26–2·21; p=0·6) when both treatment groups were combined. Arzoxifene reduced all breast cancer occurrence by 58% (p=0·001; table 2). Invasive ER-positive breast cancers were reduced by 70% (p=0·002), whereas no effect was noted for invasive ER-negative breast cancers (p=0·9; table 2 and figure 3). Incidence of ductal carcinoma in situ was reduced, but not substantially so (p=0·07; table 2).
No trial was designed to look at mortality as an endpoint, and no effect of any SERM was reported for all causes of death (table 2). Data for cause-specific mortality was not available for most of the non-tamoxifen trials. No effect on breast cancer death was reported in the tamoxifen trials on the basis of a total of 59 deaths (table 2).
Table 3 and figure 4 present major events for each trial. Overall, women receiving a SERM had a higher rate of endometrial cancer than did those given placebo (p=0·007; table 3) but the increase was confined to the first 5 years of follow-up (HR 1·64, 1·14–2·36; p=0·007) and was not apparent during years 5–10, the period after treatment (0·85, 0·38–1·89; p=0·7). The effect seemed to be limited to the tamoxifen trials (2·18, 1·39–3·42; p=0·001) and no increase was shown in the raloxifene trials (1·09, 0·74–1·62; p=0·7). Too few endometrial cancers were reported with lasofoxifene to make a meaningful interpretation, but an increase of 2·3 times was noted with arzoxifene (2·26, 0·70–7·32; p=0·2).
Table 3.
Major non-breast cancer events in the prevention trials
| Endometrial cancer | All other cancer* | Any death | Breast cancer death | Venous thrombolic events† | Cardiovascular events‡ | All fractures | Non-vertebral fractures | Vertebral fractures | Cataracts | |
|---|---|---|---|---|---|---|---|---|---|---|
| Marsden | 12 vs 5 | 55 vs 60 | 54 vs 54 | 12 vs 9 | .. | .. | .. | .. | .. | .. |
| IBIS-I | 19 vs 11 | 110 vs 113 | 65 vs 55 | 10 vs 12 | 65 vs 43 | 40 vs 38 | 229 vs 252 | 221 vs 244 | 8 vs 8 | 76 vs 70 |
| NSABP-P-1 | 36 vs 15 | 101 vs 103 | 59 vs 71 | 4 vs 6 | 55 vs 29 | 90 vs 82 | 502 vs 539 | 480 vs 509 | 22 vs 30 | 578 vs 513 |
| Italian | .. | 106 vs 91 | 36 vs 38 | 2 vs 2 | 11 vs 10 | 14 vs 10 | .. | .. | .. | .. |
| MORE/CORE | 6 vs 8 | 112 vs 132 | 81 vs 84 | .. | 47 vs 25 | 82 vs 78 | 353 vs 450 | 214 vs 225 | 139 vs 225 | 275 vs 280 |
| RUTH | 21 vs 17 | 204 vs 203 | 548 vs 585 | 2 vs 0 | 106 vs 73 | 487 vs 481 | 529 vs 591 | 470 vs. 499 | 59 vs 92 | 570 vs 561 |
| STAR§ (raloxifene vs tamoxifen) | 37 vs 65 | 354 vs 323 | 202 vs 236 | 4 vs 11 | 154 vs 202 | 233 vs 220 | 1272 vs 1364 | 1195 vs 1299 | 65 vs 77 | 603 vs 739 |
| PEARL (0·5 mg vs 0·25 mg vs placebo) | 2 vs 2 vs 3 |
25 vs 20 vs 22 |
92 vs 73 vs 65 |
.. | 48 vs 37 vs 18 |
47 vs 54 vs 76 |
359 vs 422 vs 508 |
203 vs 233 vs 246 |
156 vs 189 vs 262 |
320 vs 317 vs 330 |
| GENERATIONS | 9 vs 4 | 74 vs 75 | 103 vs 98 | .. | 43 vs 17 | 71 vs 64 | 426 vs 508 | 316 vs 327 | 110 vs 181 | 382 vs 400 |
| All events | 105 vs 63 | 787 vs 799 | 1038 vs 1050 | 30 vs 29 | 375 vs 215 | 831 vs 829 | 2398 vs 2848 | 1904 vs 2050 | 494 vs 798 | 2201 vs 2154 |
| HR or OR (95% CI) | HR 1·56 (1·13–2·14) | HR 0·98 (0·89–1·08) | HR 0·98 (0·90–1·06) | HR 1·03 (0·55–1·92) | OR 1·73 (1·47–2·05) | OR 0·99 (0·91–1·09) | OR 0·85 (0·80–0·89) | OR 0·93 (0·87–0·99) | OR 0·66 (0·59–0·73) | OR 1·01 (0·95–1·06) |
Data are number of patients for selective oestrogen receptor modulator versus vs placebo, unless otherwise indicated. HR=hazard ratio. OR=odds ratio
Excluding endometrial cancer.
Including deep vein thrombosis, pulmonary embolism, retinal thrombosis; excluding superficial thrombosis.
Including myocardial infarction, cerebrovascular accident, and transient ischaemic accident.
STAR data not included for overall effect.
Figure 4.
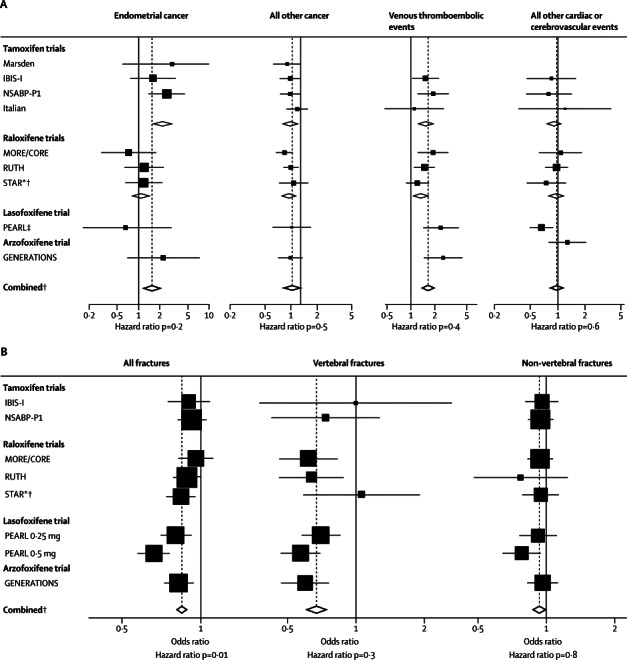
Forest plots for adverse events
(A) Endometrial cancer, other cancer, venous thromboembolic events, and cardiac or stroke events. (B) All fractures, vertebral fractures and non-vertebral fractures. *Adjusted by overall tamoxifen effect to give raloxifene versus placebo comparisons. † STAR data not included in comparisons. ‡Data are pooled.
1586 cancers other than breast or endometrial cancer were reported. These cancers were evenly distributed between the treatment groups (p=0·8; table 3) and no heterogeneity between trials was noted (p=0·8). A non-significant reduction was noted for ovarian cancer (OR 0·84, 95% CI 0·60–1·19; p=0·3) and there was no effect on colorectal cancer (1·04, 0·85–1·27; p=0·7). Venous thromboembolic events were significantly increased overall (p<0·0001; table 3 and figure 4). We noted similar ORs in the tamoxifen and raloxifene trials (1·60, 1·21–2·12; p=0·001 vs 1·45, 1·18–1·76; p<0·0001; figure 4), but the rate was higher for arzoxifene (2·55, 1·45–4·47; p=0·001) and lasofoxifene (ORpooled 2·38, 1·43–3·97; p=0·001), and no significant heterogeneity was noted between trials. Overall, no effect of SERMs was noted for myocardial infarction, stroke, or transient ischaemic attacks, and there was no evidence for heterogeneity, except for a significant reduction in strokes for lasofoxifene (OR 0·67, 0·48–0·92; p=0·01; figure 4).
All fractures were significantly reduced by SERMs (p<0·001; table 3 and figure 4). This reduction was mainly driven by a decrease in the PEARL trial (0·73, 0·66–0·81; p<0·0001), but decreases were also noted in the raloxifene and GENERATIONS trials (figure 4). By contrast, no effect was seen with tamoxifen (0·92, 0·83–1·02). We noted a greater effect when we restricted findings to those for vertebral fractures; however, such fractures were rare in the tamoxifen and STAR trials, and not recorded in the Italian trial, and only well documented in the osteoporosis trials in which follow-up spinal radiographs were done (MORE and CORE, PEARL, and GENERATIONS trials). When restricted to these trials, we noted a 41% reduction in vertebral fractures (0·59, 0·52–0·67; p<0·0001). We recorded a small effect for non-vertebral fractures overall (table 3), which seemed to be greatly affected by the 0·5 mg dose of lasofoxifene (OR 0·81, 0·67–0·98; figure 4); however, no heterogeneity was shown (p=0·8). Overall cataracts were evenly distributed between treatment groups (table 3), but a small increase was observed with tamoxifen (1·10, 1·01–1·21; p=0·04).
Discussion
This report is the only comprehensive analysis of all the SERM prevention trials, and use of individual participant data enabled us to undertake various analyses that were not done in the published reports. We provide here a substantial update of our previous report,4 which was limited to results for short term follow-up and only assessed tamoxifen and raloxifene. Our findings clearly show that SERMs significantly reduce the risk of all breast cancer in high-risk and average-risk women who do not have the disease, which is due to a reduction in ER-positive invasive breast cancer. No effect was noted for ER-negative breast cancers, for which new approaches are still needed. All SERMs except raloxifene had an effect on ductal carcinoma in situ.
Benefits were noted during the active treatment period, but also after treatment was completed. The reduction in ER-positive invasive tumours in years 5–10 of follow-up was largely restricted to the tamoxifen and raloxifene trials. Long-term follow-up is needed to establish the full duration of protection for these drugs and to identify whether any carryover effect will be shown for lasofoxifene and arzoxifene. Whether the non-significant increase in ER-negative invasive tumours is a chance finding or biologically relevant is unclear. For example, some of these tumours could have arisen as ER-positive cancers in the absence of a SERM, and treatment might have delayed their emergence, but they developed endocrine resistance, eventually escaped control, and emerged as ER-negative tumours. No evidence that SERMs had an effect on breast-cancer-specific or overall mortality was noted. In view of the continuing effect on breast cancer incidence in years 5–10, further follow-up will be needed to establish whether there is a reduction in deaths from breast cancer. All drugs increased venous thromboembolic events, but only tamoxifen showed a clear increase in endometrial cancers. No other type of cancer seemed to be affected by SERM use. Despite a 10–20% reduction in LDL cholesterol with SERMs, no reduction in cardiovascular disease was noted.
The large amount of extended follow-up available for this analysis has provided a clear overview of the benefits and harms of these drugs. The higher (0·5 mg per day) dose of lasofoxifene is a promising candidate for prevention, because it not only had a large effect on breast cancer incidence but also showed benefits for stroke, cardiac events, and vertebral fractures, with no increase in endometrial cancer. Further studies on this compound should be a priority for prevention research. Furthermore, the direct comparison of tamoxifen with raloxifene in the STAR trial has shown that raloxifene is less effective than tamoxifen, but has fewer side-effects. Limitations of our analysis are that the GENERATIONS and PEARL trials, and one of the raloxifene trials, were done on average-risk women with osteoporosis. Longer follow-up is also desirable for the lasofoxifene and arzoxifene trials. Only tamoxifen has been assessed in premenopausal women, in whom it is the only drug with proven effectiveness.
New prevention trials with aromatase inhibitors are promising, but these drugs are only suitable for postmenopausal women. Several adjuvant studies have shown an effect in new contralateral tumours,24 and one trial in the preventive setting has shown a very large effect on short-term incidence for exemestane.25 Results from another prevention trial of anastrozole are awaited,24 and a comparison of aromatase inhibitors with SERMs might be needed when long-term data for aromatase inhibitors are available. The duration of the SERM benefit on breast cancer incidence is unknown, but this analysis confirms that benefits last for at least 5 years after treatment completion. Tamoxifen-specific adverse events (eg, thromboembolic events and endometrial cancer) are largely confined to the active treatment period and are few after treatment has ceased. However, similar to other preventive interventions, including oral contraceptives and prophylactic cardiovascular medicines, careful consideration of potential benefits and harms during the decision making process is needed to identify women most likely to benefit. Improved benefit–harm ratios are most likely to be achieved by enhanced targeting of women at high risk of ER-positive postmenopausal breast cancer. As such, use of mammographic breast density26 and panels of single nucleotide polymorphisms,27 each of which individually only identify a modest increase of risk, seem to be the most likely new risk factors.
Despite their effectiveness, SERMs have not been widely accepted as breast cancer preventive drugs by high-risk women and their primary care physicians, mainly because of concern about toxic effects and a perceived unfavourable balance between benefits and harms. Unfortunately, at the present time, none of these drugs are being actively marketed for breast cancer prevention, and approval by the US Food and Drug Administration or any other regulatory authority for this indication will probably not be sought for lasofoxifene or arzoxifene. Our longer term assessment shows that the benefit–harm balance is now more favourable than that calculated for short term follow-up, and, in view of this new evidence, assessment of these drugs, especially lasofoxifene, should be continued.
Acknowledgments
Acknowledgments
We thank many women who participated in these trials and all trialists who chose to share their data to make these analyses possible.
Contributors
JC, JPC, BHM, LF, ADC contributed to study design. IS did the statistical analyses. All authors contributed to data analysis and interpretation. JC and IS drafted the report and all authors approved the final manuscript.
Steering committee
JC (chair), IS, BB, JPC, SC, ADC, MD, JFF, LF, AZLC, JM, BHM, TP, IS, UV, VV, DLW
Conflicts of interest
JC has received a grant from AstraZeneca for chemoprevention trials. BHM is, and JM was, an employee and shareholder of Eli Lilly. IS, BB, JPC, VV, MD, TP, DLW, LF, SC, JFF, ADC, AZLC, UV declare that they have no conflicts of interest.
References
- 1.Cuzick J, Baum M. Tamoxifen and contralateral breast cancer. Lancet. 1985;2:282. doi: 10.1016/s0140-6736(85)90338-1. [DOI] [PubMed] [Google Scholar]
- 2.Early Breast Cancer Trialists' Collaborative Group Tamoxifen for early breast cancer: an overview of the randomised trials. Lancet. 1998;351:1451–1467. [PubMed] [Google Scholar]
- 3.Jordan VC. Chemoprevention of breast cancer with selective oestrogen-receptor modulators. Nat Rev Cancer. 2007;7:46–53. doi: 10.1038/nrc2048. [DOI] [PubMed] [Google Scholar]
- 4.Cuzick J, Powles T, Veronesi U. Overview of the main outcomes in breast-cancer prevention trials. Lancet. 2003;361:296–300. doi: 10.1016/S0140-6736(03)12342-2. [DOI] [PubMed] [Google Scholar]
- 5.Powles T, Eeles R, Ashley S. Interim analysis of the incidence of breast cancer in the Royal Marsden Hospital tamoxifen randomised chemoprevention trial. Lancet. 1998;352:98–101. doi: 10.1016/S0140-6736(98)85012-5. [DOI] [PubMed] [Google Scholar]
- 6.Powles TJ, Ashley S, Tidy A, Smith IE, Dowsett M. Twenty-year follow-up of the Royal Marsden randomized, double-blinded tamoxifen breast cancer prevention trial. J Natl Cancer Inst. 2007;99:283–290. doi: 10.1093/jnci/djk050. [DOI] [PubMed] [Google Scholar]
- 7.Cuzick J, Forbes J, Edwards R, for the IBIS investigators First results from the International Breast Cancer Intervention Study (IBIS-I): a randomised prevention trial. Lancet. 2002;360:817–824. doi: 10.1016/s0140-6736(02)09962-2. [DOI] [PubMed] [Google Scholar]
- 8.Cuzick J, Forbes JF, Sestak I. Long-term results of tamoxifen prophylaxis for breast cancer—96-month follow-up of the randomized IBIS-I trial. J Natl Cancer Inst. 2007;99:272–282. doi: 10.1093/jnci/djk049. [DOI] [PubMed] [Google Scholar]
- 9.Fisher B, Costantino JP, Wickerham DL. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 10.Fisher B, Costantino JP, Wickerham DL. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97:1652–1662. doi: 10.1093/jnci/dji372. [DOI] [PubMed] [Google Scholar]
- 11.Veronesi U, Maisonneuve P, Costa A. Prevention of breast cancer with tamoxifen: preliminary findings from the Italian randomised trial among hysterectomised women. Italian Tamoxifen Prevention Study. Lancet. 1998;352:93–97. doi: 10.1016/s0140-6736(98)85011-3. [DOI] [PubMed] [Google Scholar]
- 12.Veronesi U, Maisonneuve P, Rotmensz N. Tamoxifen for the prevention of breast cancer: late results of the Italian Randomized Tamoxifen Prevention Trial among women with hysterectomy. J Natl Cancer Inst. 2007;99:727–737. doi: 10.1093/jnci/djk154. [DOI] [PubMed] [Google Scholar]
- 13.Cauley JA, Norton L, Lippman ME. Continued breast cancer risk reduction in postmenopausal women treated with raloxifene: 4-year results from the MORE trial. Multiple outcomes of raloxifene evaluation. Breast Cancer Res Treat. 2001;65:125–134. doi: 10.1023/a:1006478317173. [DOI] [PubMed] [Google Scholar]
- 14.Martino S, Cauley JA, Barrett-Connor E. Continuing outcomes relevant to Evista: breast cancer incidence in postmenopausal osteoporotic women in a randomized trial of raloxifene. J Natl Cancer Inst. 2004;96:1751–1761. doi: 10.1093/jnci/djh319. [DOI] [PubMed] [Google Scholar]
- 15.Barrett-Connor E, Mosca L, Collins P. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006;355:125–137. doi: 10.1056/NEJMoa062462. [DOI] [PubMed] [Google Scholar]
- 16.Vogel VG, Costantino JP, Wickerham DL. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 Trial. JAMA. 2006;295:2727–2741. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- 17.Vogel VG, Costantino JP, Wickerham DL. Update of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 Trial: preventing breast cancer. Cancer Prev Res. 2010;3:696–706. doi: 10.1158/1940-6207.CAPR-10-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cummings SR, Ensrud K, Delmas PD. Lasofoxifene in postmenopausal women with osteoporosis. N Engl J Med. 2010;362:686–696. doi: 10.1056/NEJMoa0808692. [DOI] [PubMed] [Google Scholar]
- 19.LaCroix AZ, Powles T, Osborne CK. Breast cancer incidence in the randomized PEARL trial of lasofoxifene in postmenopausal osteoporotic women. J Natl Cancer Inst. 2010;102:1706–1715. doi: 10.1093/jnci/djq415. [DOI] [PubMed] [Google Scholar]
- 20.Cummings SR, McClung M, Reginster JY. Arzoxifene for prevention of fractures and invasive breast cancer in postmenopausal women. J Bone Miner Res. 2011;26:397–404. doi: 10.1002/jbmr.191. [DOI] [PubMed] [Google Scholar]
- 21.Powles TJ, Diem SJ, Fabian CJ. Breast cancer incidence in postmenopausal women with osteoporosis or low bone mass using arzoxifene. Breast Cancer Res Treat. 2012;134:299–306. doi: 10.1007/s10549-012-2041-5. [DOI] [PubMed] [Google Scholar]
- 22.Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:101–129. [Google Scholar]
- 23.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 24.Cuzick J. Aromatase inhibitors in prevention—data from the ATAC (arimidex, tamoxifen alone or in combination) trial and the design of IBIS-II (the second International Breast Cancer Intervention Study) Recent Results Cancer Res. 2003;163:96–103. doi: 10.1007/978-3-642-55647-0_9. [DOI] [PubMed] [Google Scholar]
- 25.Goss PE, Ingle JN, Ales-Martinez JE. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;364:2381–2391. doi: 10.1056/NEJMoa1103507. [DOI] [PubMed] [Google Scholar]
- 26.Boyd NF, Guo H, Martin LJ. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356:227–236. doi: 10.1056/NEJMoa062790. [DOI] [PubMed] [Google Scholar]
- 27.Evans DG, Warwick J, Astley SM. Assessing individual breast cancer risk within the U.K. National Health Service Breast Screening Program: a new paradigm for cancer prevention. Cancer Prev Res. 2012;5:943–951. doi: 10.1158/1940-6207.CAPR-11-0458. [DOI] [PubMed] [Google Scholar]


