Abstract
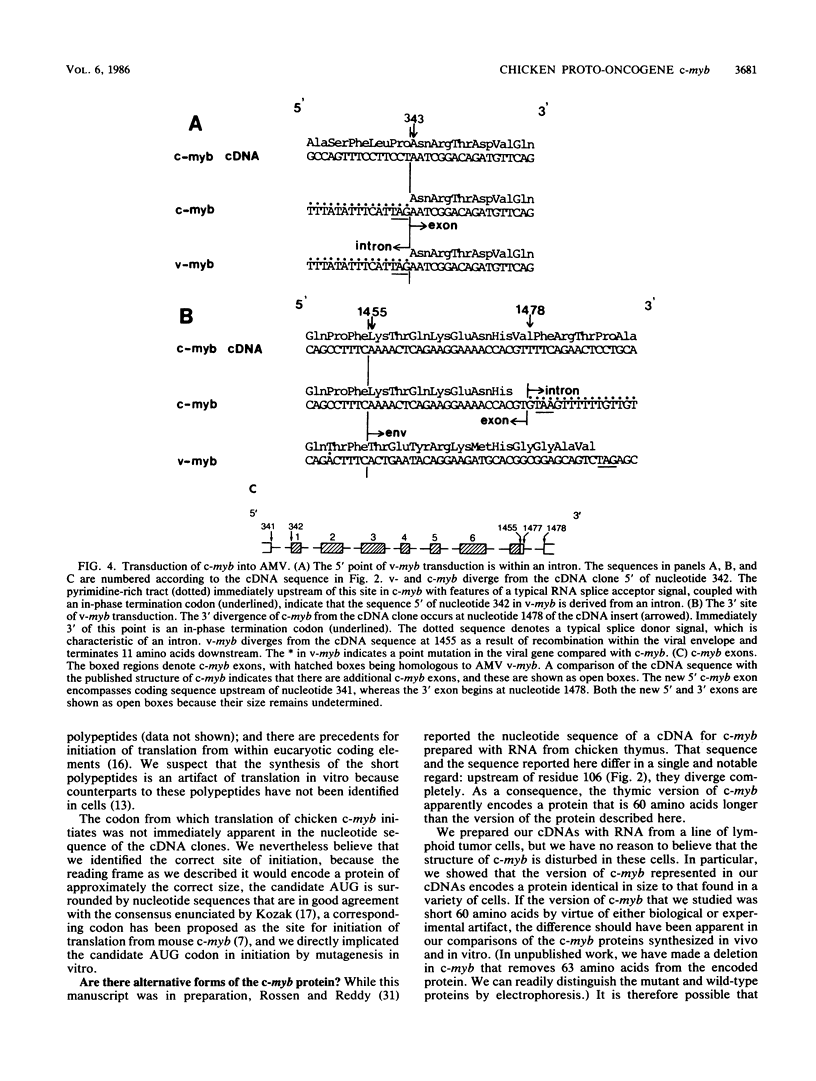
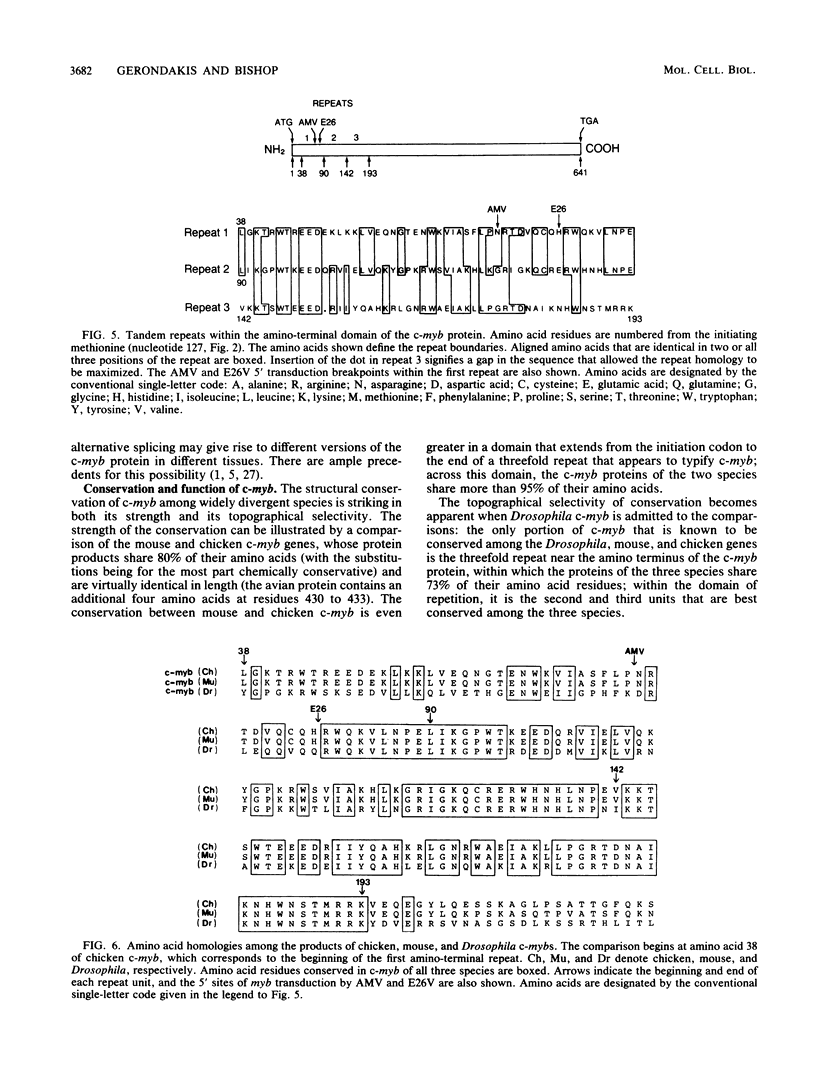
The retroviral oncogene v-myb arose by transduction of the chicken proto-oncogene c-myb. We isolated and sequenced cDNA that represents the entire coding domain of chicken c-myb. By transcribing the cDNA into mRNA in vitro and then translating the RNA, we were able to document the integrity of the cDNA and to identify the codon responsible for initiation of translation from c-myb. Two different alleles of v-myb are extant, one in the genome of avian myeloblastosis virus (AMV) and the other in the genome of erythroblastosis virus 26 (E26V). The proteins encoded by the AMV and E26V alleles of v-myb differ from the product of c-myb in three ways: at their amino termini, they lack 71 and 80 amino acids respectively; at their carboxy termini, they are deficient in 199 and 278 residues; and 11 substitutions of amino acids are scattered throughout the product of AMV allele, whereas the product of the E26V allele contains only a single substitution. The structural origins of tumorigenicity by v-myb and the biological functions of c-myb remain enigmatic. The findings and molecular clones described here should now permit a systematic exploration of these enigmas.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Neriah Y., Bernards A., Paskind M., Daley G. Q., Baltimore D. Alternative 5' exons in c-abl mRNA. Cell. 1986 Feb 28;44(4):577–586. doi: 10.1016/0092-8674(86)90267-9. [DOI] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. Cellular oncogenes and retroviruses. Annu Rev Biochem. 1983;52:301–354. doi: 10.1146/annurev.bi.52.070183.001505. [DOI] [PubMed] [Google Scholar]
- Early P., Rogers J., Davis M., Calame K., Bond M., Wall R., Hood L. Two mRNAs can be produced from a single immunoglobulin mu gene by alternative RNA processing pathways. Cell. 1980 Jun;20(2):313–319. doi: 10.1016/0092-8674(80)90617-0. [DOI] [PubMed] [Google Scholar]
- Evan G. I., Lewis G. K., Bishop J. M. Isolation of monoclonal antibodies specific for products of avian oncogene myb. Mol Cell Biol. 1984 Dec;4(12):2843–2850. doi: 10.1128/mcb.4.12.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonda T. J., Gough N. M., Dunn A. R., de Blaquiere J. Nucleotide sequence of cDNA clones of the murine myb proto-oncogene. EMBO J. 1985 Aug;4(8):2003–2008. doi: 10.1002/j.1460-2075.1985.tb03884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonda T. J., Sheiness D. K., Bishop J. M. Transcripts from the cellular homologs of retroviral oncogenes: distribution among chicken tissues. Mol Cell Biol. 1982 Jun;2(6):617–624. doi: 10.1128/mcb.2.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. C., Hay N., Bishop J. M. The role of RNA molecules in transduction of the proto-oncogene c-fps. Cell. 1986 Mar 28;44(6):935–940. doi: 10.1016/0092-8674(86)90016-4. [DOI] [PubMed] [Google Scholar]
- Katzen A. L., Kornberg T. B., Bishop J. M. Isolation of the proto-oncogene c-myb from D. melanogaster. Cell. 1985 Jun;41(2):449–456. doi: 10.1016/s0092-8674(85)80018-0. [DOI] [PubMed] [Google Scholar]
- Klempnauer K. H., Bishop J. M. Transduction of c-myb into avian myeloblastosis virus: locating points of recombination within the cellular gene. J Virol. 1983 Dec;48(3):565–572. doi: 10.1128/jvi.48.3.565-572.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klempnauer K. H., Gonda T. J., Bishop J. M. Nucleotide sequence of the retroviral leukemia gene v-myb and its cellular progenitor c-myb: the architecture of a transduced oncogene. Cell. 1982 Dec;31(2 Pt 1):453–463. doi: 10.1016/0092-8674(82)90138-6. [DOI] [PubMed] [Google Scholar]
- Klempnauer K. H., Ramsay G., Bishop J. M., Moscovici M. G., Moscovici C., McGrath J. P., Levinson A. D. The product of the retroviral transforming gene v-myb is a truncated version of the protein encoded by the cellular oncogene c-myb. Cell. 1983 Jun;33(2):345–355. doi: 10.1016/0092-8674(83)90416-6. [DOI] [PubMed] [Google Scholar]
- Klempnauer K. H., Sippel A. E. Subnuclear localization of proteins encoded by the oncogene v-myb and its cellular homolog c-myb. Mol Cell Biol. 1986 Jan;6(1):62–69. doi: 10.1128/mcb.6.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klempnauer K. H., Symonds G., Evan G. I., Bishop J. M. Subcellular localization of proteins encoded by oncogenes of avian myeloblastosis virus and avian leukemia virus E26 and by chicken c-myb gene. Cell. 1984 Jun;37(2):537–547. doi: 10.1016/0092-8674(84)90384-2. [DOI] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Mechanism of mRNA recognition by eukaryotic ribosomes during initiation of protein synthesis. Curr Top Microbiol Immunol. 1981;93:81–123. doi: 10.1007/978-3-642-68123-3_5. [DOI] [PubMed] [Google Scholar]
- Krieg P. A., Melton D. A. Functional messenger RNAs are produced by SP6 in vitro transcription of cloned cDNAs. Nucleic Acids Res. 1984 Sep 25;12(18):7057–7070. doi: 10.1093/nar/12.18.7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leprince D., Gegonne A., Coll J., de Taisne C., Schneeberger A., Lagrou C., Stehelin D. A putative second cell-derived oncogene of the avian leukaemia retrovirus E26. Nature. 1983 Nov 24;306(5941):395–397. doi: 10.1038/306395a0. [DOI] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moelling K., Pfaff E., Beug H., Beimling P., Bunte T., Schaller H. E., Graf T. DNA-binding activity is associated with purified myb proteins from AMV and E26 viruses and is temperature-sensitive for E26 ts mutants. Cell. 1985 Apr;40(4):983–990. doi: 10.1016/0092-8674(85)90358-7. [DOI] [PubMed] [Google Scholar]
- Moscovici C. Leukemic transformation with avian myeloblastosis virus: present status. Curr Top Microbiol Immunol. 1975;71:79–101. doi: 10.1007/978-3-642-66193-8_2. [DOI] [PubMed] [Google Scholar]
- Moscovici M. G., Jurdic P., Samarut J., Gazzolo L., Mura C. V., Moscovici C. Characterization of the hemopoietic target cells for the avian leukemia virus E26. Virology. 1983 Aug;129(1):65–78. doi: 10.1016/0042-6822(83)90396-3. [DOI] [PubMed] [Google Scholar]
- Nabeshima Y., Fujii-Kuriyama Y., Muramatsu M., Ogata K. Alternative transcription and two modes of splicing results in two myosin light chains from one gene. Nature. 1984 Mar 22;308(5957):333–338. doi: 10.1038/308333a0. [DOI] [PubMed] [Google Scholar]
- Nunn M. F., Seeburg P. H., Moscovici C., Duesberg P. H. Tripartite structure of the avian erythroblastosis virus E26 transforming gene. Nature. 1983 Nov 24;306(5941):391–395. doi: 10.1038/306391a0. [DOI] [PubMed] [Google Scholar]
- Papas T. S., Lautenberger J. A. Sequence curiosity in v-myc oncogene. Nature. 1985 Nov 21;318(6043):237–237. doi: 10.1038/318237a0. [DOI] [PubMed] [Google Scholar]
- Prywes R., Hoag J., Rosenberg N., Baltimore D. Protein stabilization explains the gag requirement for transformation of lymphoid cells by Abelson murine leukemia virus. J Virol. 1985 Apr;54(1):123–132. doi: 10.1128/jvi.54.1.123-132.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosson D., Reddy E. P. Nucleotide sequence of chicken c-myb complementary DNA and implications for myb oncogene activation. Nature. 1986 Feb 13;319(6054):604–606. doi: 10.1038/319604a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Shaw J., Hayman M. J., Enrietto P. J. Analysis of a deleted MC29 provirus: gag sequences are not required for fibroblast transformation. J Virol. 1985 Dec;56(3):943–950. doi: 10.1128/jvi.56.3.943-950.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen-Ong G. L., Morse H. C., 3rd, Potter M., Mushinski J. F. Two modes of c-myb activation in virus-induced mouse myeloid tumors. Mol Cell Biol. 1986 Feb;6(2):380–392. doi: 10.1128/mcb.6.2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda Y., Ohlendorf D. H., Anderson W. F., Matthews B. W. DNA-binding proteins. Science. 1983 Sep 9;221(4615):1020–1026. doi: 10.1126/science.6308768. [DOI] [PubMed] [Google Scholar]
- Thompson C. B., Challoner P. B., Neiman P. E., Groudine M. Expression of the c-myb proto-oncogene during cellular proliferation. 1986 Jan 30-Feb 5Nature. 319(6052):374–380. doi: 10.1038/319374a0. [DOI] [PubMed] [Google Scholar]