Abstract
Background:
Short and long sleep duration are associated with increased mortality and worse global cognitive function, but is unclear if these relations persist after accounting for the risk of sleep disordered breathing (SDB). The aim of our study is determine the association between short and long sleep duration with worse global cognitive function in a racially/ethnically diverse elderly cohort.
Methods:
We examined sleep hours and global cognitive function cross-sectionally within the population-based Northern Manhattan Study cohort. We conducted nonparametric and logistic regression to examine associations between continuous, short (< 6 h) and long (≥ 9 h) sleep hours with performance on the Mini Mental State Examination (MMSE).
Results:
There were 927 stroke-free participants with data on self-reported sleep hours and MMSE scores (mean age 75 ± 9 years, 61% women, 68% Hispanics). The median (interquartile range) MMSE was 28 (10-30). Sleep hours (centered at 7 h) was associated with worse MMSE (β = -0.01; SE [0.004], p = 0.0113) adjusting for demographics, vascular risk factors, medications, and risk for SDB. Reporting long sleep (≥ 9 h) compared to 6 to 8 h of sleep (reference) was significantly and inversely associated with MMSE (adjusted β = -0.06; SE [0.03], p = 0.012), while reporting short sleep was not significantly associated with MMSE performance. Long sleep duration was also associated with low MMSE score when dichotomized (adjusted OR: 2.4, 95% CI: 1.1-5.0).
Conclusion:
In this cross-sectional analysis among an elderly community cohort, long sleep duration was associated with worse MMSE performance.
Citation:
Ramos AR; Dong C; Elkind MSV; Boden-Albala B; Sacco RL; Rundek T; Wright CB. Association between Sleep Duration and the Mini-Mental Score: The Northern Manhattan Study. J Clin Sleep Med 2013;9(7):669-673.
Keywords: Sleep duration, cognition, short sleep, long sleep, cognitive impairment, mini mental score
Cognitive impairment and dementia are disabling conditions expected to rise in prevalence with the rapidly aging population.1,2 The identification of modifiable risk factors for cognitive impairment can provide important prevention strategies with significant public health implications. The impact of inadequate sleep on cognition can be profound. Besides producing sleepiness, which has detrimental effects on mood, job performance, and accident risk, poor sleep is associated with adverse health outcomes.3,4 This is particularly relevant to the aging population as sleep-wake patterns and sleep quality may change throughout the lifetime, with 50% of the elderly reporting sleep disturbances, and up to one-third reporting either short sleep or long sleep duration.5,6 Abnormal sleep duration may impair attention/vigilance and cause executive dysfunction,6,7 but it is unclear if these relationships persist after accounting for the risk of sleep disordered breathing (SDB). SDB is highly prevalent in the elderly, seen in up to 62% of those older than 65 years of age and is associated with poor cognitive function.8–11 Determining the relationship between sleep duration and cognition could lead to novel strategies to improve health as sleep duration is potentially modifiable.12
The aim of this analysis is to evaluate the association between self-reported sleep hours and short and long sleep duration with worse global cognitive function in an elderly racially/ ethnically diverse population-based cohort.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Cognitive impairment and dementia are expected to rise with the aging population. There is limited data from elderly race/ethnically diverse cohorts with evaluations of sleep duration and cognitive function. Determining the relation between sleep duration and cognitive function could lead to novel strategies to improve health.
Study Impact: Long sleep duration was associated with worse mini-mental score, a measure of global cognitive function, after adjusting for demographic, vascular risk factors and depressive symptoms. The results of this study suggest that long sleep duration may be an independent predictor of worse cognitive function in the elderly.
METHODS
Study Population
The Northern Manhattan Study (NOMAS) enrolled 3,298 stroke-free participants randomly sampled from the Northern Manhattan population between 1993 and 2001 using the following criteria: (1) resident of Northern Manhattan ≥ 3 months; (2) from a household with a telephone; (3) age ≥ 40 years at the time of first in-person assessment; and (4) no history of stroke.13 For the purpose of this analysis, we included participants with self-reported sleep hours obtained during annual telephone follow-up evaluation in 2006 and Mini-Mental Examination (MMSE) scores within one year of reported sleep hours. From the parent cohort, a total of 2,266 subjects were available for follow up in 2006. Of the available sample, a total of 927 participants had reports of sleep hours and MMSE within one year of each assessment. The sample of 927 participants had a similar proportion of women (61%), but a greater proportion of Hispanics (68%) compared to the overall baseline cohort (53%). NOMAS was approved by the Columbia University Medical Center and University of Miami, Miller School of Medicine IRBs, and all participants provided written informed consent.
Cognitive Assessment
Cognitive status was assessed in person by bilingual (English or Spanish) trained research assistants using MMSE.14 The MMSE is a brief 30-point questionnaire test used to evaluate cognitive function. The MMSE measures various domains of cognitive functioning including memory, orientation to place and time, naming, reading, visuospatial orientation/construction ability, writing, and the ability to follow a 3-stage command. It has good sensitivity (71% to 92%) and specificity (56% to 96%) to screen for cognitive impairment and dementia.14
We used the total score of the MMSE as the outcome. Lower educational levels (≤ 8th grade) can adversely affect the MMSE scores. We defined low MMSE scores as a dichotomous outcome by adjusting for age and educational level based on established MMSE cutoffs. A cutoff of MMSE < 24 was used for those with > 8 years education and MMSE < 20 for those with ≤ 8 years of education.15,16
Sleep Hours
We collected self-reported sleep duration as an estimate of hours of nightly sleep in the four weeks prior to the annual telephone follow-up interview in 2006, using the following question: “During the past 4 weeks, how many hours, on average, did you sleep each night?” Respondents reported in 30-min increments of each hour.17 The responses ranged from 3 to 12 h of sleep with a median of 7 hours.
High Risk for Sleep Disordered Breathing
High risk for SDB was estimated by constructing the Berlin questionnaire,18 based on reports of frequent snoring and daytime sleepiness along with objective information on hypertension and obesity in our sample. Sleep symptoms were derived from a sleep questionnaire during follow-up examination in 2004-2005.17 The questionnaires were administered in English or Spanish. Habitual snoring was defined as self-report of snoring > 3 times per week, based on prior definitions of habitual snoring.19 The Epworth Sleepiness Scale was used and adapted for relevance to characteristics of people living in northern Manhattan.20 Daytime sleepiness was categorized as sum score ≥ 10 based on the established definition for daytime sleepiness from the Epworth Sleepiness Scale.21 The presence of 2 of the 3 following items was used to classify participants into high risk for SDB: (1) frequent snoring (snoring > 3 times per week), (2) daytime sleepiness (sum score ≥ 10), and (3) presence of hyper-tension or obesity (BMI > 30 kg/m2).
Risk Factor Assessments
Data were collected through interviews by trained bilingual research assistants using standardized data collection instruments described elsewhere.13 Race and ethnicity were defined by self-identification based on questions modeled after the US census. Race/ethnicity were categorized into mutually exclusive groups as non-Hispanic Black, non-Hispanic White, and Hispanic. Depressive symptoms were evaluated with the Center for Epidemiological Studies Depression scale (CES-D). The CESD is a 20-item scale documenting 4 factors: depressive affect, somatic complaints, positive affect, and interpersonal relations. Scores on the CES-D range from 0 to 60, with higher scores indicating more symptoms of depression.22 Depressive symptoms were categorized as present if the sum of the scores was ≥ 16 or if the participant was taking an antidepressant medication.22 Hypertension was defined as a systolic blood pressure > 140 mm Hg or a diastolic blood pressure > 90 mm Hg or a patient's self-report of a history of hypertension or use of antihypertensive medications. Diabetes mellitus was defined as fasting blood glucose ≥ 126 mg/dL or the patient's self-report of diabetes or use of insulin or hypoglycemic medications. Cardiac disease included history of angina, MI, coronary artery disease, atrial fibrillation, congestive heart failure, or valvular heart disease.
We obtained self-reported medication use at baseline. We created a dichotomous variable (yes vs. no) based on the use of the following medications: antidepressant, antiepileptic, pain, and antipsychotic, that could affect sleep duration and or cognitive function.17
Statistical Analysis
Results are presented as mean ± standard deviation or median (interquartile range) for continuous variables according to the variable distribution, and proportion for categorical variables. The χ2 test was used to compare proportions, while ANOVA, or the Kruskal-Wallis test if data were not normally distributed, was used to compare mean or median for continuous variables. We examined self-reported sleep hours in categories of short sleep (< 6 h) and long sleep (≥ 9 h), with 6 to 8.9 h of nightly sleep as the reference.23 We performed nonparametric regression to test for the association between sleep hours centered at 7 h of sleep continuously and comparing categories of < 6 h and ≥ 9 h versus the reference with the non-normally distributed MMSE using SAS procedure COUNTREG. Sequential models were done to evaluate the unadjusted association between sleep hours (centered at 7 h) and MMSE. We then adjusted for demographic factors: age, sex, education, race/ethnicity, and insurance status (Model 2); alcohol consumption, hypertension, diabetes, depression, medications, and risk for SDB (Model 3). Logistic regression was performed with the categories for low MMSE score as the outcome. As a sensitivity analysis, we also evaluated the relation between sleep hours and memory performance on the MMSE, given that verbal 3-word recall on the MMSE has been reported as an acceptable estimate of episodic memory in epidemiologic studies.24 Among participants able to register 3 initial words, impaired verbal recall was defined by a score of 0 or 1 obtained on the subsequent 3-word recall task of the MMSE.24 Additionally, we evaluated the interactions between sleep hours and the covariates. All analyses were performed using SAS software version 9.3 (SAS Institute Inc, Cary, NC).
RESULTS
The mean age was 75 ± 9 years, with 61% women, 68% Hispanics, and 41% with less than a high school education. Table 1 presents the characteristics of the overall sample and across categories of sleep duration. Self-reports < 6 h were seen in 24% of the sample, and ≥ 9 h were reported by 9% (n = 87). Participants reporting ≥ 9 h of sleep were older, had greater frequencies of hypertension, diabetes, and lower MMSE scores than the reference (p < 0.0001). There was no statistical difference in the frequencies of sex, education, race-ethnicity, Medicaid or no insurance status, BMI, depression, cardiac disease, or risk of SDB among the groups.
Table 1.
Demographic and vascular risk factors and cognitive scores across categories of sleep hours
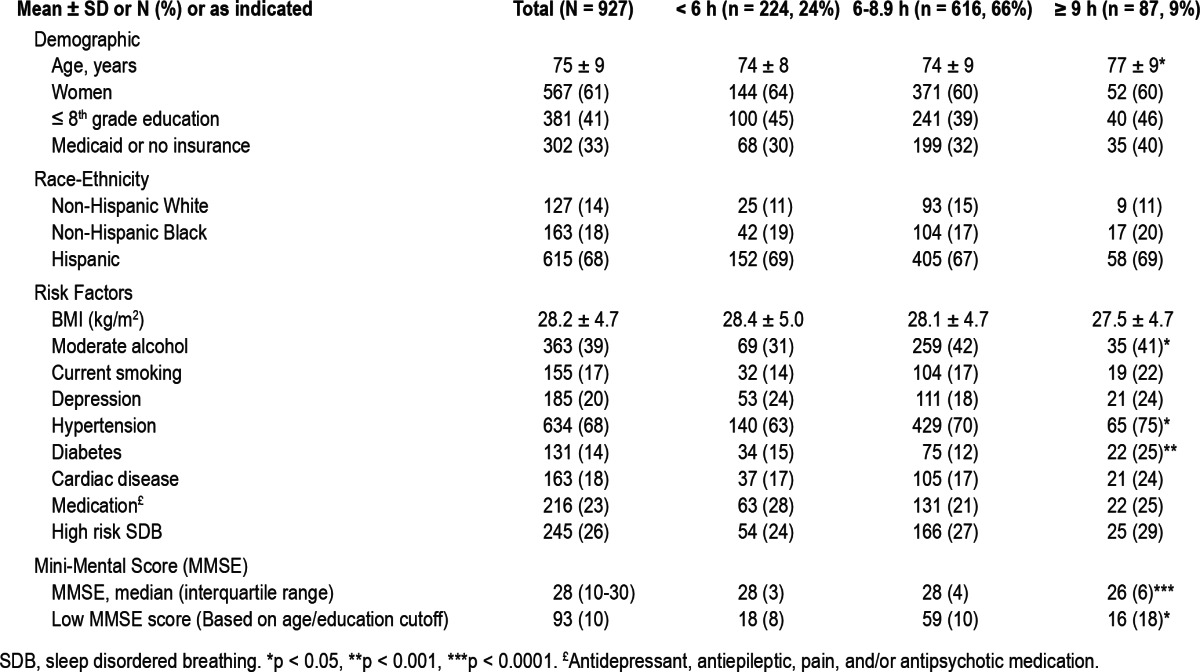
The covariates that were associated with the lower MMSE scores were increased age (β = -0.004; p < 0.0001), ≤ 8th grade education (β = -0.14; p < 0.0001), having Medicaid or no insurance (β = -0.14; p < 0.0001), Hispanic race/ethnicity (β = -0.09, p < 0.0001) compared to non-Hispanic white, depression (β = -0.05, p = 0.0029), diabetes (β = -0.05, p = 0.012), hyper-tension (β = -0.05, p = 0.0007), and medications (β = -0.08, p < 0.0001). Male sex (β = 0.04; p = 0.0065) and moderate alcohol consumption alcohol (β = 0.04, p = 0.009) were positively associated with the MMSE. The BMI (β = 0.0005, p = 0.74), non-Hispanic black (β = 0.006, p = 0.71) compared to non-Hispanic white race/ethnicity, current smoking (β = 0.001, p = 0.94), cardiac disease (β = 0.006, p = 0.71), and risk for SDB (β = -0.02, p = 0.11) were not associated to the MMSE.
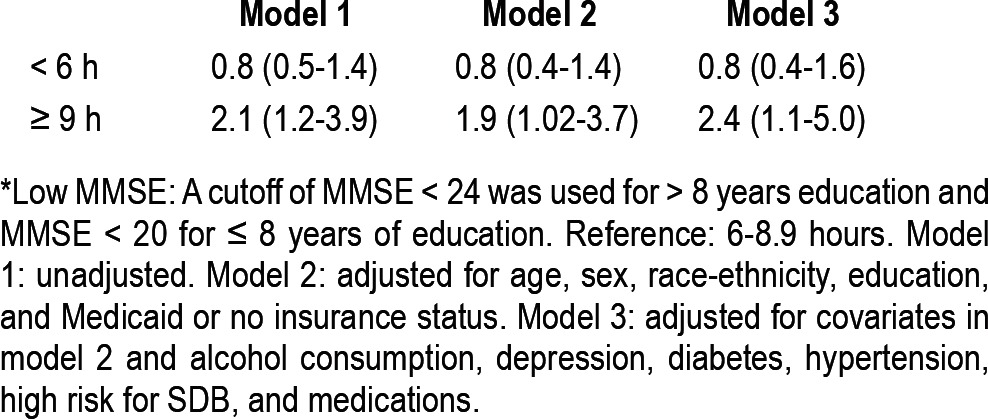
Self-reported sleep hours (continuous) was associated with worse MMSE scores in sequential models (Table 2). In addition, categorical analysis showed that self-reports of ≥ 9 h (long sleep duration), compared to 6-8.9 h of sleep were associated with worse MMSE scores in fully adjusted models (Table 2). When evaluating cognitive scores as a binary outcome, we found that long sleep duration (≥ 9 h) was associated with increased odds of low MMSE scores (Table 3). There was no association between sleep hours and delayed verbal memory and no interactions between sleep hours and demographic, vascular risk factors, medications, and risk for SDB.
Table 2.
Association between sleep hours and Mini-Mental Score Examination
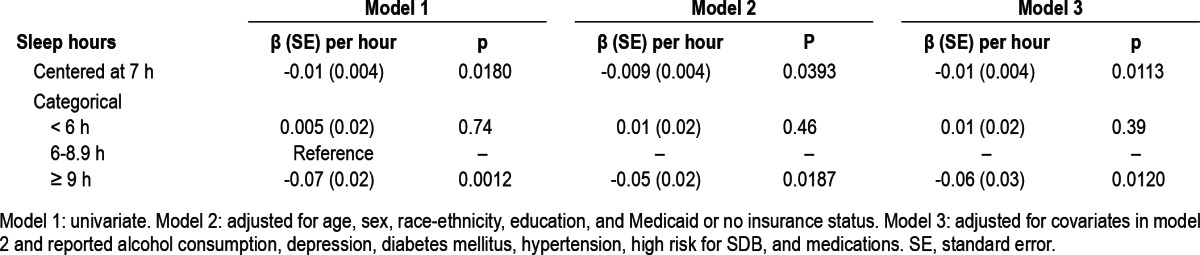
Table 3.
Odds ratio and 95% confidence interval among categories of sleep duration and low Mini-Mental score (MMSE)*
DISCUSSION
In this cross-sectional study, we found that self-reported long sleep duration was associated with worse global cognitive function in the elderly, racially/ethnically diverse NOMAS sample, after adjusting for vascular risk factors, depression, and risk for SDB.
Self-reported long sleep duration is linked to greater mortality, and an increased risk of stroke and cardiovascular disease.3,23,25,26 While very few NOMAS participants had dementia and our results most likely reflect subtle cognitive differences, our findings are in agreement with prospective data from an elderly (≥ 65 years) population-based cohort showing a positive association between long sleep duration (≥ 9 h) and incident dementia.6 A cross-sectional analysis of the Osteoporotic Fractures in Men Study (MrOS) also demonstrated an association between long sleep (≥ 8 h) by actigraphy and worse global cognitive scores.27 Population-based studies have reported associations between long sleep duration and worse cognitive performance by measures of global cognition (MMSE),24,28 as well as verbal fluency, delayed recall,29 and psychomotor speed.30
Most studies on sleep hours and cognitive function have examined homogenous populations, with a paucity of data from racially/ethnically diverse communities. Studies comparing self-reported sleep duration in Hispanics and non-Hispanic blacks have provided inconsistent results, suggesting that habitual sleep duration is possibly dependent on factors other than race-ethnicity.9,31,32 We previously described greater long sleep duration in Hispanics compared to non-Hispanic whites17 and observed an inverse relation between Hispanic race/ethnicity and MMSE scores. In NOMAS, a greater proportion of Hispanics have less than eight years of formal education as well as Medicaid or no insurance, both surrogate markers of lower SES. Lower SES is associated with a number of comorbidities that could result in long sleep duration.25,26,33
Self-reports of long sleep duration have been associated with older age, low socioeconomic status (SES), diabetes, and vascular disease,25 but we observed an association between long sleep duration and MMSE after controlling for these factors. Additionally, long sleep duration was associated with worse MMSE score after controlling for depressive symptoms, medications (e.g., antidepressants, antiepileptics) and risk for SDB, factors that may worsen cognitive function.11,34–36 Our findings suggest that in the elderly, long sleep duration (≥ 9 h) could be an independent predictor of worse cognitive function.
It is suggested that the relation between long sleep and adverse health outcomes could be confounded by SDB.30,37 In our study, there was no difference in risk for SDB among the sleep duration groups, and high risk for SDB did not modify the relation between self-reported long sleep and worse MMSE scores. Our findings are in accordance with an analysis of the Osteoporotic Fractures in Men study38 that characterized differences in demographic, sleep, and vascular risk factors among elderly participants (mean age 76.4 years) with long sleep duration compared to average sleepers. In this study there were no differences in the apnea-hypopnea index between long sleep compared to 7-8 hours of sleep.38 In addition, self-reported long sleep duration was positively associated with increased time in bed and sleep time by actigraphy that was not explained by sleep disorders, such as SDB or vascular risk factors.
Our findings could be explained by sleep fragmentation. Fragmented sleep has been linked to long sleep duration.4 Fragmented sleep measured by actigraphy was associated with worse global cognitive function, independent of sleep duration, in a cross-sectional analysis of the Rush Memory and Aging Project.39 Sleep fragmentation is directly related to time in bed,25 and perhaps long sleep duration could be a surrogate or a compensatory response to fragmented sleep in those with worse cognitive function.29 Sleep-wake disturbances could also exacerbate cognitive dysfunction and cause further sleep disturbances, such as advancement of circadian phase with subsequent prolongation of sleep duration.24
In our study, self-reported short sleep duration was not associated with MMSE. Short sleep duration has been associated with worse global cognitive function, memory impairment, and psychomotor speed,7,30,40,41 but stronger associations have been described for long sleep duration. Our findings are in accordance with population based studies where short sleep duration was not associated, either by self-report28 or actigraphy,27 with MMSE. Short sleep duration can cause deficits in attention and vigilance through excessive sleepiness,42 but the mechanisms by which long sleep duration could affect cognitive function are not fully understood.
Several limitations should be noted. The current study is cross-sectional and does not allow assessment of causality between self-reported sleep hours and cognition. Sleep duration was obtained by subjective reports from a sleep questionnaire. In particular, it could be that those with cognitive impairment tend to report longer sleep duration. Also, we were not able to capture night to night variability of sleep duration, daytime napping, or objective measures of sleep. However, other observational studies of sleep duration and adverse health outcomes are similarly based on subjective reports from sleep questionnaires. Self-reports of long sleep duration might represent a greater sleep time or just more time in bed, which cannot be determined from the current data. There might be unmeasured confounders (e.g., autoimmune disorders) that could cause fatigue and sleepiness and in part explain the results of our study. In spite of these limitations there are several strengths to our study. We evaluated a relatively large, racially/ethnically diverse, community-based cohort with a high burden of vascular risk factors, risk of SDB, depression, and systematically applied measures of cognition with the MMSE.
In conclusion, we found a cross-sectional association between self-reported long sleep duration, and greater odds of worse cognitive scores that were not explained by high risk of SDB. Prospective studies in racially/ethnically diverse samples are needed to confirm our findings and determine if long sleep duration is in the causal pathway and a harbinger of cognitive decline.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This work was supported by grants from the National Institute of Neurological Disorders and Stroke: Supplement to R37 NS029993 (to Dr. Ramos); R37 NS029993 (to Drs. Sacco, Elkind, Rundek, Wright, Boden-Albala); K24 NS 062737 (to Dr. Rundek); and Evelyn F. McKnight Brain Institute (to Drs. Wright, Sacco, Rundek). All authors had access to the data and contributed substantially to the design, acquisition, and analysis of data, and writing of manuscript. The Northern Manhattan study cohort is followed at Columbia University. The administrative support and statistical analysis was performed at the University of Miami-Miller School of Medicine. The authors are thankful to the study participants for their collaboration and to all staff of the Northern Manhattan Study for their efforts to this study, and in particular Edison Sabala and Janet DeRosa.
REFERENCES
- 1.Korczyn AD. The underdiagnosis of the vascular contribution to dementia. J Neurol Sci. 2005;229–230:3–6. doi: 10.1016/j.jns.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 2.2010 Alzheimer's disease facts and figures. Alzheimers Dement. 2010;6:158–94. doi: 10.1016/j.jalz.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 3.Cappuccio FP, Cooper D, D'Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J. 2011;32:1484–92. doi: 10.1093/eurheartj/ehr007. [DOI] [PubMed] [Google Scholar]
- 4.Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002;59:131–6. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- 5.Mesas AE, Lopez-Garcia E, Leon-Munoz LM, Graciani A, Guallar-Castillon P, Rodriguez-Artalejo F. The association between habitual sleep duration and sleep quality in older adults according to health status. Age Ageing. 2011;40:318–23. doi: 10.1093/ageing/afr004. [DOI] [PubMed] [Google Scholar]
- 6.Benito-Leon J, Bermejo-Pareja F, Vega S, Louis ED. Total daily sleep duration and the risk of dementia: a prospective population-based study. Eur J Neurol. 2009;16:990–7. doi: 10.1111/j.1468-1331.2009.02618.x. [DOI] [PubMed] [Google Scholar]
- 7.Goel N, Rao H, Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2009;29:320–39. doi: 10.1055/s-0029-1237117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ancoli-Israel S, Kripke DF, Klauber MR, Mason WJ, Fell R, Kaplan O. Sleep-disordered breathing in community-dwelling elderly. Sleep. 1991;14:486–95. doi: 10.1093/sleep/14.6.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baldwin CM, Ervin AM, Mays MZ, et al. Sleep disturbances, quality of life, and ethnicity: the Sleep Heart Health Study. J Clin Sleep Med. 2010;6:176–83. [PMC free article] [PubMed] [Google Scholar]
- 10.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 11.Zimmerman ME, Aloia MS. Sleep-disordered breathing and cognition in older adults. Curr Neurol Neurosci Rep. 2012;12:537–46. doi: 10.1007/s11910-012-0298-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Youngstedt SD, Kripke DF. Long sleep and mortality: rationale for sleep restriction. Sleep Med Rev. 2004;8:159–74. doi: 10.1016/j.smrv.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 13.Sacco RL, Boden-Albala B, Gan R, et al. Stroke incidence among white, black, and Hispanic residents of an urban community: the Northern Manhattan Stroke Study. Am J Epidemiol. 1998;147:259–68. doi: 10.1093/oxfordjournals.aje.a009445. [DOI] [PubMed] [Google Scholar]
- 14.Boustani M, Peterson B, Hanson L, Harris R, Lohr KN. Screening for dementia in primary care: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2003;138:927–37. doi: 10.7326/0003-4819-138-11-200306030-00015. [DOI] [PubMed] [Google Scholar]
- 15.Tombaugh T, McDowell I, Kristjansson B, Hubley A. Mini-Mental State Examination (MMSE) and the Modified MMSE (3MS): A psychometric comparison and normative data. Psychol Assess. 1996;8:48–59. [Google Scholar]
- 16.Wright CB, Elkind MS, Rundek T, Boden-Albala B, Paik MC, Sacco RL. Alcohol intake, carotid plaque, and cognition: the Northern Manhattan Study. Stroke. 2006;37:1160–4. doi: 10.1161/01.STR.0000217439.73041.b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramos AR, Wohlgemuth WK, Dong C, et al. Race-ethnic differences of sleep symptoms in an elderly multi-ethnic cohort: the Northern Manhattan Study. Neuroepidemiology. 2011;37:210–5. doi: 10.1159/000334315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131:485–91. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 19.Young T, Shahar E, Nieto FJ, et al. Predictors of sleep-disordered breathing in community-dwelling adults: the Sleep Heart Health Study. Arch Intern Med. 2002;162:893–900. doi: 10.1001/archinte.162.8.893. [DOI] [PubMed] [Google Scholar]
- 20.Boden-Albala B, Roberts ET, Bazil C, et al. Daytime sleepiness and risk of stroke and vascular disease: Findings from the Northern Manhattan Study (NOMAS) Circ Cardiovas Qual Outcomes. 2012;5:500–7. doi: 10.1161/CIRCOUTCOMES.111.963801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 22.Salaycik KJ, Kelly-Hayes M, Beiser A, et al. Depressive symptoms and risk of stroke: the Framingham Study. Stroke. 2007;38:16–21. doi: 10.1161/01.STR.0000251695.39877.ca. [DOI] [PubMed] [Google Scholar]
- 23.Gallicchio L, Kalesan B. Sleep duration and mortality: a systematic review and meta-analysis. J Sleep Res. 2009;18:148–58. doi: 10.1111/j.1365-2869.2008.00732.x. [DOI] [PubMed] [Google Scholar]
- 24.Potvin O, Lorrain D, Forget H, et al. Sleep quality and 1-year incident cognitive impairment in community-dwelling older adults. Sleep. 2012;35:491–9. doi: 10.5665/sleep.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grandner MA, Drummond SP. Who are the long sleepers? Towards an understanding of the mortality relationship. Sleep Med Rev. 2007;11:341–60. doi: 10.1016/j.smrv.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel SR, Malhotra A, Gottlieb DJ, White DP, Hu FB. Correlates of long sleep duration. Sleep. 2006;29:881–9. doi: 10.1093/sleep/29.7.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blackwell T, Yaffe K, Ancoli-Israel S, et al. Association of sleep characteristics and cognition in older community-dwelling men: the MrOS sleep study. Sleep. 2011;34:1347–56. doi: 10.5665/SLEEP.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Faubel R, Lopez-Garcia E, Guallar-Castillon P, Graciani A, Banegas JR, Rodriguez-Artalejo F. Usual sleep duration and cognitive function in older adults in Spain. J Sleep Res. 2009;18:427–35. doi: 10.1111/j.1365-2869.2009.00759.x. [DOI] [PubMed] [Google Scholar]
- 29.Kronholm E, Sallinen M, Suutama T, Sulkava R, Era P, Partonen T. Self-reported sleep duration and cognitive functioning in the general population. J Sleep Res. 2009;18:436–46. doi: 10.1111/j.1365-2869.2009.00765.x. [DOI] [PubMed] [Google Scholar]
- 30.Kronholm E, Sallinen M, Era P, Suutama T, Sulkava R, Partonen T. Psychomotor slowness is associated with self-reported sleep duration among the general population. J Sleep Res. 2011;20:288–97. doi: 10.1111/j.1365-2869.2010.00899.x. [DOI] [PubMed] [Google Scholar]
- 31.Hale L, Do DP. Racial differences in self-reports of sleep duration in a population-based study. Sleep. 2007;30:1096–103. doi: 10.1093/sleep/30.9.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loredo JS, Soler X, Bardwell W, Ancoli-Israel S, Dimsdale JE, Palinkas LA. Sleep health in U.S. Hispanic population. Sleep. 2010;33:962–7. doi: 10.1093/sleep/33.7.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel SR. Social and demographic factors related to sleep duration. Sleep. 2007;30:1077–8. doi: 10.1093/sleep/30.9.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Byers AL, Yaffe K. Depression and risk of developing dementia. Nat Rev Neurol. 2011;7:323–31. doi: 10.1038/nrneurol.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mezick EJ, Hall M, Matthews KA. Are sleep and depression independent or overlapping risk factors for cardiometabolic disease? Sleep Med Rev. 2011;15:51–63. doi: 10.1016/j.smrv.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yaffe K, Laffan AM, Harrison SL, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011;306:613–9. doi: 10.1001/jama.2011.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lavie P. Self-reported sleep duration--what does it mean? J Sleep Res. 2009;18:385–6. doi: 10.1111/j.1365-2869.2009.00813.x. [DOI] [PubMed] [Google Scholar]
- 38.Patel SR, Blackwell T, Ancoli-Israel S, Stone KL. Sleep characteristics of self-reported long sleepers. Sleep. 2012;35:641–8. doi: 10.5665/sleep.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lim AS, Yu L, Costa MD, et al. Increased fragmentation of rest-activity patterns is associated with a characteristic pattern of cognitive impairment in older individuals. Sleep. 2012;35:633–40. doi: 10.5665/sleep.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferrie JE, Shipley MJ, Akbaraly TN, Marmot MG, Kivimaki M, Singh-Manoux A. Change in sleep duration and cognitive function: findings from the Whitehall II Study. Sleep. 2011;34:565–73. doi: 10.1093/sleep/34.5.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu L, Jiang CQ, Lam TH, et al. Short or long sleep duration is associated with memory impairment in older Chinese: the Guangzhou Biobank Cohort Study. Sleep. 2011;34:575–80. doi: 10.1093/sleep/34.5.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ohayon MM, Vecchierini MF. Daytime sleepiness and cognitive impairment in the elderly population. Arch Intern Med. 2002;162:201–8. doi: 10.1001/archinte.162.2.201. [DOI] [PubMed] [Google Scholar]


