Abstract
Study Objectives:
The aim was to determine the feasibility of using an unattended 2-channel device to screen for obstructive sleep apnea in a population of high-risk patients using a targeted, case-finding strategy. The case finding was based on the presence of risk factors not symptoms in the studied population.
Methods:
The study took place from June 2007 to May 2008 in rural and metropolitan Queensland and New South Wales. Family doctors were asked to identify patients with any of the following: BMI > 30, type 2 diabetes, treated hypertension, ischemic heart disease. Participants applied the ApneaLink+O2 at home for a single night. The device recorded nasal flow and pulse oximetry. Data were analyzed by proprietary software, then checked and reported by either of two sleep physicians.
Results:
1,157 patients were recruited; mean age 53 ± 14.6, M/F% = 62/38, mean BMI = 31.8, obesity = 35%, diabetes = 16%, hypertension = 39%, IHD = 5%, Mean Epworth Sleepiness Scale score (ESS) = 8.3. The prevalence of unrecognized OSA was very high: 71% had an AHI > 5/h, 33% had an AHI > 15/h, and 16% had an AHI > 30/h. The ApneaLink+O2 device yielded technically adequate studies in 93% of cases.
Conclusion:
The study shows that a “real world” simple low cost case finding and management program, based on unattended home monitoring for OSA, can work well in a population with risk factors and comorbidities associated with OSA, independent of the presence of symptoms. The prevalence of unrecognized OSA was very high.
Citation:
Burgess KR; Havryk A; Newton S; Tsai WH; Whitelaw WA. Targeted case finding for OSA within the primary care setting. J Clin Sleep Med 2013;9(7):681-686.
Keywords: Obstructive sleep apnea, ApneaLink, unattended sleep study
Obstructive sleep apnea (OSA) is a modern epidemic of great health and economic importance. Young et al.1 found in a population aged 30-60, that 9% of women and 24% of men had OSA, defined as apnea-hypopnea index (AHI) > 5/h, while 2% of women and 4% of men had AHI > 5/h, in association with excessive sleepiness (obstructive sleep apnea syndrome [OSAS]).
Untreated OSA is an important cause of impaired alertness and daytime sleepiness. Patients with OSA have been found to consume more health care resources than those without, take more sick leave, and have more work disability.2,3 Large numbers of cases of OSA remain undiagnosed and untreated, in large part because of limited resources for case finding and diagnosis.4 Male gender, increasing age, and obesity are known risk factors for OSA.5 OSA is an independent risk factor for vascular disease,6 stroke,7,8 and probably hypertension,9,10 and contributes to glucose intolerance11–13 and difficult-to-control atrial fibrillation.14 Patients with such risk factors are more likely to have OSA. They are also more likely to benefit from treatment for OSA that may not only improve energy and alertness but also to help control associated diseases. Currently diagnosis usually results from testing, which is traditionally triggered by patient symptomatology. If investigation is not triggered unless there are symptoms, many cases in these high-risk groups may go undiagnosed.
It is important to find and treat patients with OSA. Screening of the whole population would have a relatively low yield. We wished to assess the feasibility and the yield of a program to screen high-risk patients for unrecognized OSA, regardless of whether they had typical symptoms, hoping to improve the health of many of them and to reduce the health care costs associated with untreated OSA. To limit costs, we used an unattended portable monitor at home that has been shown to give AHI values similar to those from attended laboratory polysomnography.
BRIEF SUMMARY
Current Knowledge/Study Rationale: It is important to find and treat patients with OSA, but screening of whole populations would have a relatively low yield. We wished to assess the feasibility and the yield of a targeted case finding program to screen high risk patients for unrecognized OSA regardless of whether they had typical symptoms.
Study Impact: The study shows that a simple low cost case finding and management program, based on unattended home monitoring for OSA, focused on patients with obesity, hypertension and diabetes can work well in a population with risk factors and comorbidities associated with OSA. The prevalence of unrecognized OSA in this population was high, so these data support the testing for OSA in high risk groups whether they have the traditional symptoms of OSA or not.
The aim of the study was to determine the prevalence of unrecognized OSA in a population of high risk patients, in the practices of family physicians, using a targeted case finding strategy.
METHODS
The study used data which was collected from June 2007 to May 2008 at sites in rural and metropolitan Queensland, (Bundaberg and Brisbane) and 4 regions across the state of New South Wales (the Northern, Eastern and North Western Suburbs of Sydney and the rural Southern Highlands). It was done with the cooperation of general practitioners located across these regions and Healthy Sleep Solutions (HSS), a private company that specializes in facilitating home diagnostic studies and provision of CPAP therapy on behalf of Specialist Sleep Physicians. The geographic catchment area was approximately equally divided between urban and rural areas, (47% and 53% respectively), with a total population of approximately 560,000. The de-identified data were provided by HSS. Patients gave consent to HSS for the data collection. The data analysis was approved by an institutional review board (IRB); the Northern Sydney Central Coast Human Research Ethics Committee [1101-032M(QA)]. The results of the testing and reporting physician recommendations were conveyed to the patients by the referring family physicians. Subsequent treatment decisions and implementation were then the result of doctor and patient decisions, which were beyond the control of the authors.
Recruitment
Doctors were approached for participation in the study initially by mail and then by office visits from a specially trained clinical coordinator representing Healthy Sleep Solutions or a cooperating representative of a pharmaceutical company. General practitioners were asked to identify from among their patients those with a body mass index (BMI) > 30, type 2 diabetes, treated hypertension, ischemic heart disease, or with the traditional risk factors of snoring, sleepiness, and witnessed apneas.5 Prior to the study, the consenting subjects were interviewed by one of the HSS clinical coordinators to record clinical data, including height, weight, blood pressure, and details of their history and medications. Subjects also completed an Epworth Sleepiness Scale.15
Hypertension was considered present according to self-report or use of antihypertensive medication. Type 2 diabetes was defined by an abnormal fasting plasma glucose, or use of oral hypoglycemic medications. Ischemic heart disease was defined by self-report, or use of cardiac medications.
Each patient was then instructed in the use of the ApneaLink+O2, took a device home, and applied it for a single night of recording. Data were extracted and analyzed by proprietary software (ResMed) and were then reviewed and reported by 2 respiratory physicians experienced in sleep medicine, and approved by the Health Insurance Commission to report laboratory polysomnograms.
ApneaLink+O2
ApneaLink+O2 is a proprietary monitor based on analysis of nasal pressure and oxygen saturation signals. An earlier version, employing only a nasal pressure signal, has been tested against simultaneously recorded laboratory polysomnography in a study of 59 subjects, and found to have sensitivity 0.91 and specificity 0.84 for OSA,16 comparable to other similar monitors that have been validated against polysomnography.17,18 Very recently, the 2-channel device used in this study has been validated in a study of 143 subjects against home polysomnography (PSG) in a Chinese population.19 Those authors found very high sensitivity and specificity for the diagnosis of OSA compared to in home PSG; the areas under the reader operator curves (ROC) were both 0.933. The new version continues to base its calculation of AHI on the nasal pressure tracing, but a second channel recording oxygen saturation allows it to count the number of dips per hour > 4%, which was used as a substitute for AHI if the nasal pressure probe was lost, and has the potential to reduce the study failure rate. (This approach is supported by the similar high sensitivities and specificities of both the oximetry and nasal flow signals [∼85%]19). The ApneaLink+O2 displays tracings of nasal pressure, oxygen saturation, and pulse rate. The pulse oximeter was a Nonin Xpod 3012 with a Nonin 7000A finger probe and a sampling rate of 1 Hz. Flow was sampled at 100 Hz. Tracings were reviewed initially at 5 cm/min, but expanded as necessary. The device provides statistics, including an apnea-hypopnea index derived from the flow tracing plus minimum saturation, the number per hour of desaturations of 4%, oxygen desaturation index (ODI), and mean pulse rate.
To be considered technically “good,” a study must have lasted ≥ 4 h and must have recorded data from both flow and oxygen saturation channels ≥ 90% of the time. A second category was considered “acceptable,” which meant that either the oximetry or the flow signal was missing, or of poor quality for > 10% of the recording, but the study could still be interpreted with confidence on data from the remaining channel.
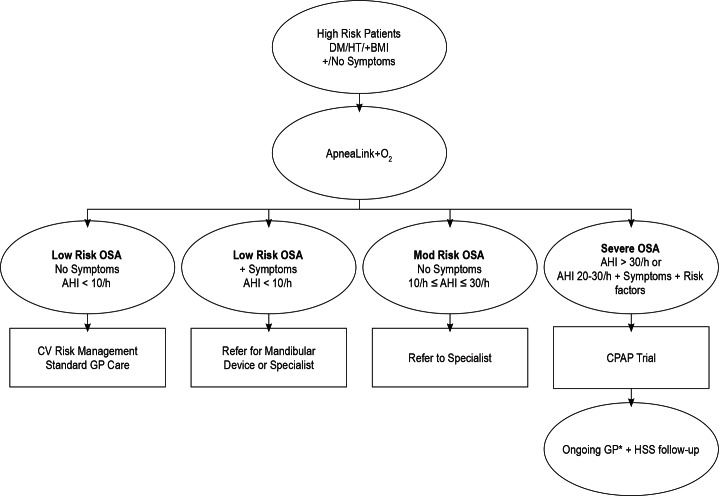
One of two accredited sleep physicians reviewed each tracing, assessed their quality, noted the Epworth score, (a score ≥ 11 being considered excessive sleepiness), medications, medical history, and daytime oxygen saturation and issued a report and recommendation based on an algorithm (Figure 1). AHI was used as the primary criterion of severity, but on the few occasions when the oxygen desaturation index was higher than the AHI because of a problem with the flow signal for part of the recording, the higher number was used. If the initial test was inadequate, a repeat test was requested.
Figure 1. OSA management algorithm.
*If GP is not keen to follow up then patient witll be directed to specialists. Incorporating guidelines produced by the Thoracic Society of Australia & New Zealand and Australasian Sleep Association. OSA, obstructive sleep apnea; DM, diabetes mellitus; HT, hypertension; AHI, apnea hypopnea index; CV, cardiovascular disease; GP, general practitioner (family doctor); CPAP, continuous positive airway pressure; HSS, Health Sleep Solutions Pty Ltd.
Recommendations were based on a modification of published algorithm.20 Because of previous uncertainty as to the accuracy of the ApneaLink device when AHI is between 10 and 30, and because of the recognized lack of certainty about benefits of treatment in that range, we recommended that these patients be referred to a respiratory/sleep physician for assessment, (probably including polysomnography), and management. We recommended that all patients with AHI > 30/h undergo a trial of treatment with continuous positive airway pressure (CPAP), because this degree of OSA is considered likely to be detrimental to a patient's health, even if no symptoms are reported. In addition, however, we recommended trials of treatment with CPAP for patients with an AHI > 20/h who had comorbidities that can be worsened by the presence of untreated OSA, e.g. type 2 diabetes, hypertension, and ischemic heart disease. These constituted 40% of patients for whom CPAP was recommended. Referral to a sleep physician was recommended for patients who had excessive daytime sleepiness and AHI < 10/h. If snoring was an important complaint and AHI < 10/h, a mandibular advancement device was recommended.
Statistics
Patients were described using mean and 95% confidence intervals. A χ2 test for linear trend was used to determine if the prevalence of comorbidity had a dose dependent relationship with OSA severity. Patient characteristics were treated as continuous variables, but where appropriate, (e.g., Sleepy [ESS > 10], and Obese [BMI > 30]), were also analyzed as categorical variables. Comorbidities were treated as categorical variables. The prevalence of OSA was identified in selected populations. Predictors of OSA (at varying levels of severity) were identified using multiple logistic regression.
RESULTS
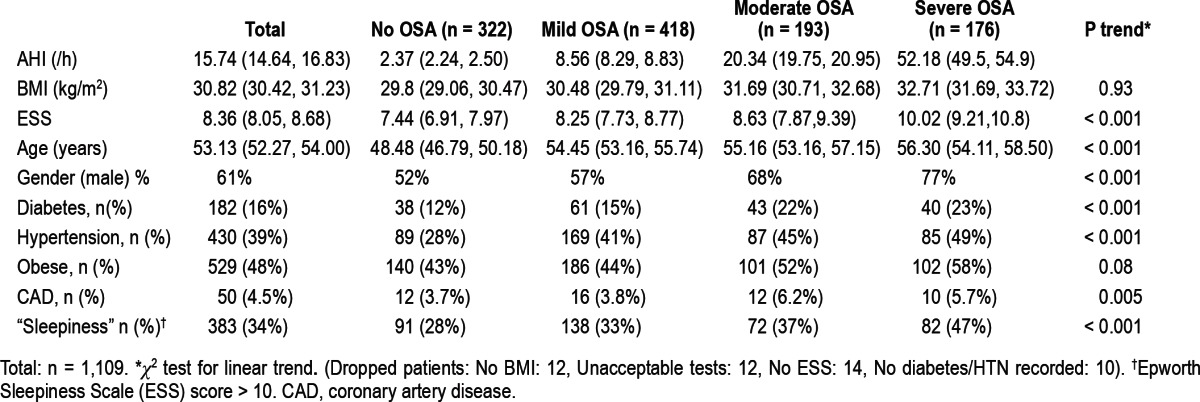
Of patients who agreed and underwent the initial clinical assessment, 95% attended for overnight testing and 92% completed testing. A total of 1,157 patients were referred to the study, had an initial workup, and underwent a night of monitoring. Characteristics of the population are listed in Table 1.
Table 1.
Analysis of results by severity of OSA
Comments about technical adequacy were provided by the reporting physicians for 1,098, or 95% of the 1,157 studies performed (Table 1). Of those, 79% were technically good and an additional 13% were acceptable. Only 7% were technically unsatisfactory, for whom repeat studies were requested. Review of the studies for which the physicians had not made a comment at the time, shows that they were similar with respect to distribution of AHI. All but 7 of the patients eventually had a technically satisfactory test. The most common reason for technical inadequacy was complete or partial absence of data, from both the flow channel and the oximetry channel. The second most common reason was a study of short duration, (defined as < 4 h). In most cases, short studies were not due to equipment failures, but the patient's decision to abandon the test, or remove the oximeter probe, or the nasal cannulae, because of discomfort or difficulty sleeping.
Prevalence of Unrecognized OSA
Prevalence of OSA at different levels of severity is shown in Table 1. Mean AHI for the population was 15.8 ± 14.6 and mean lowest oxygen saturation was 83% ± 7%. Table 1 also shows the percentage prevalences within the whole group and various subgroups, (by severity of AHI) of key clinical features: diabetes, hypertension, obesity, coronary artery disease, and sleepiness (Epworth score > 10). As OSA severity increased, there were highly significant increases in prevalence of diabetes (p < 0.001), hypertension (p < 0.001), coronary artery disease (p = 0.005), and sleepiness (p < 0.001), but not obesity (p = 0.08). Some subjects were not included in the subgroup analysis because of missing data as shown below Table 1 (Dropped patients: No BMI: 12, Unacceptable tests: 12, No ESS: 14, No diabetes/HTN recorded: 10).
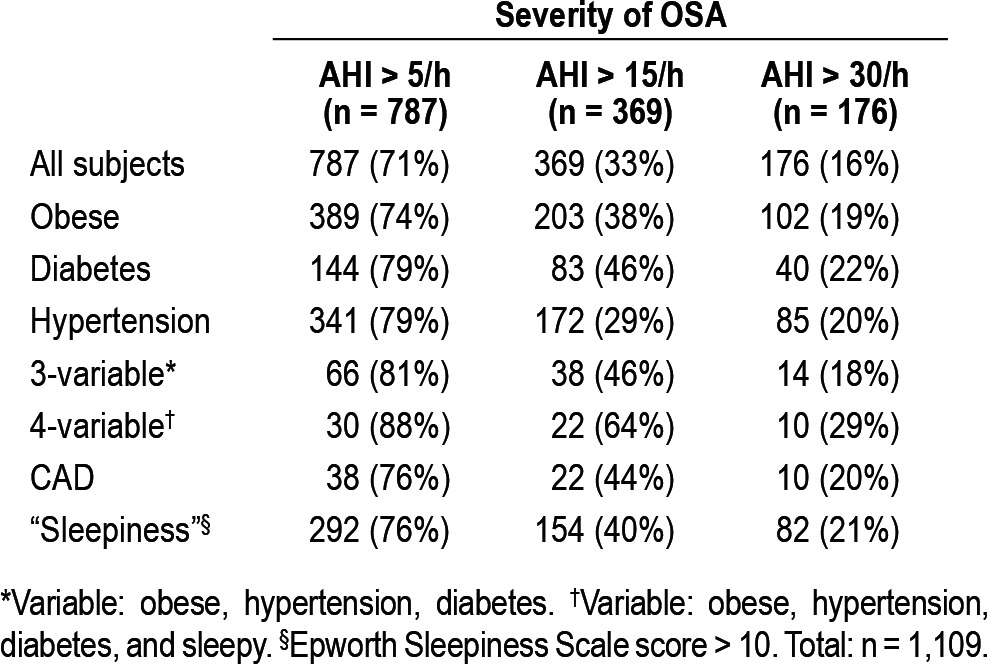
Table 2 shows the analysis of results by selected risk factors for OSA. “Sleepiness” indicates an Epworth Sleepiness score > 10. Note that the patients in the columns marked “AHI > 15/h” and “AHI > 30/h” have already been included in the column “AHI > 5/h”.
Table 2.
Analysis of results by selected risk factors for OSA
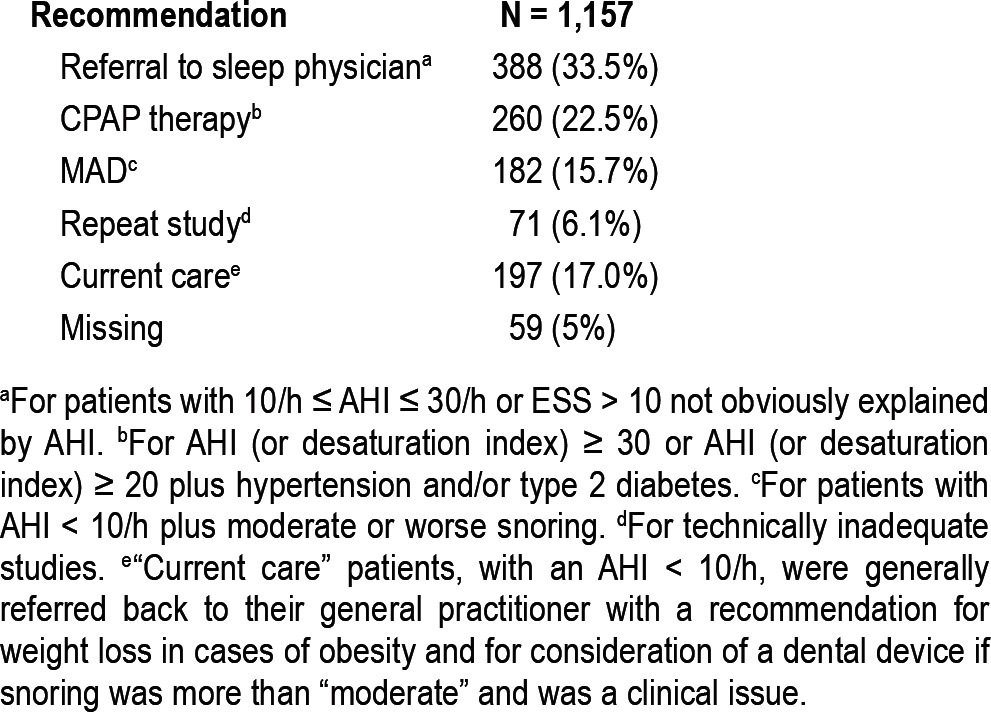
Management recommendations are summarized in Table 3. For the purposes of this study we assumed that the device would reliably detect OSA when present.19 The study was not intended to validate the device against home or laboratory PSG, because that had been previously done by others,16,19 The study was intended to test the feasibility of using a targeted case finding strategy with the device in a primary care setting.
Table 3.
Management recommendations
DISCUSSION
Prevalence of OSA
The prevalence of unrecognized OSA was very high: 71% had an AHI > 5/h, 33% had an AHI > 15/h; and 16% had an AHI > 30/h. Table 2 shows the prevalence of OSA, divided into the 3 usual grades of severity by AHI (AHI > 5/h, > 15/h, > 30/h) for the whole study population and for subgroups of subjects defined by comorbidity or risk factors, (obesity, diabetes, hypertension, coronary artery disease and sleepiness). The prevalence of OSA remained high even when cases were identified by a single risk factor.
Table 2 shows that for a similar population among people with all 3 factors of obesity, hypertension, and diabetes, 81% could be expected to have some OSA (AHI > 5/h), 46% to have moderate or severe OSA (AHI > 15/h), and 18% to have severe OSA. If the same analysis is performed using 4 variables (adding in sleepiness), the prevalence increases to 88% for some OSA, 64% for moderate or severe OSA, and 29% for severe OSA. However, due to the lower number of patients with multiple risk factors, there was no advantage in using any specific risk factor or multiple risk factors in combination over case selection based on a single risk factor alone.
In keeping with the previous literature, we found, incidentally, that the prevalence of OSA was higher than for the rest of the population, in patients with diabetes who are sleepy (ESS > 10), but not in patients with diabetes who were not sleepy.21 Also, as expected, there was a dose-dependent relationship between OSA severity and hypertension or coronary artery disease.
The implications for the management of patients with a high risk of having OSA, such as the population that we have chosen to study, are very significant in terms of both health outcome and economic effects.
Hillman's data3 showed that untreated OSA patients consume more health related dollars than those who are treated or do not have OSA, and Sivertsen et al. have shown increased work disability.22 It can be expected, therefore, that a plan to identify and treat cases early, would reduce health care costs and thus be of interest to both the community at large and to funding organizations.
The most widely quoted prevalence data for OSA comes from the Wisconsin Cohort Study, which took place over two decades ago.1 Recent survey data from the National Sleep Foundation, suggests that the prevalence may be higher, most likely because of the rising prevalence of obesity. Using the Berlin Questionnaire in 1,506 respondents, Hiestand et al. in 2006 estimated a prevalence of OSA of up to 25%.23
In a population survey in Spain, Duran et al.24 found the overall prevalence of AHI > 10/h in men aged 30 to 70 to be 19%, in women 15%. Prevalence increased considerably with age, from 8% in the 30-40 decade to 32% in the 60-70 decade in men, and from 2% to 26% in women. That population had a mean BMI in men of 26.2 and in women of 25.1. Bixler at al25 conducted a telephone survey of 4,364 men and chose from them a stratified random sample, taking progressively higher percentages of subjects, according to how many of four risk factors for OSA (snoring, daytime sleepiness, obesity, and hypertension) they had. They found the prevalence of AHI > 15/h increased from 2% in those with no risk factors to 34% for those with 4 risk factors, (but including only 2 of our risk factors—obesity and hypertension). Their prevalence of AHI > 15/h increased from 3% in those aged 20-44, to 13% in those over 65, confirming aging as a separate risk factor for obstructive sleep apnea syndrome (OSAS).
Critique of the Methods
The prevalence found in a program like this would depend on criteria for selection. The nature of the recruiting process, where family physicians were invited to refer patients, made it impossible to define rigorously the criteria for selection. Although the primary criterion was intended to be the presence of type 2 diabetes, obesity, or hypertension, a small number of patients were selected by the family physicians primarily because of symptoms that suggested OSA. (The study itself raised awareness of sleep apnea by participating physicians). The rate of discovery of previously unsuspected cases will depend on awareness on the part of both patients and physicians and on local resources for assessment and treatment.
Portable monitors, similar to the ApneaLink+O2, used unattended at home, have been assessed by many authors over recent years.17,26–30 Most have been found to reliably identify patients with severe OSA and those with minimal OSA as determined by PSG. In general, agreement with PSG for classifying mild and moderate OSA (AHI 5-15/h range) is not as good. Although the very recent study by Gantner et al.19 found good correlation between ApneaLink+O2 and home PSG for both AHI and oxygen desaturation index (ODI) in the normal to mild OSA range, they found underestimation of severity by the ApneaLink+O2 in the severe range. Despite that, they reported a high level of diagnostic accuracy in the moderate to severe range of OSA.19 The clinical importance of finding an exact AHI, or of the differences in results between polysomnography and unattended portable monitors used at home, however, is questionable. Unattended portable monitors have been compared in randomized controlled trials with laboratory polysomnography, as decision making tools for the investigation and management of patients with OSA.31,32 These showed no difference in treatment outcome between patients tested by portable monitors at home and those tested by polysomnography in the laboratory.32
The statistics presented here assume that all cases of sleep apnea that were found were OSA, rather than central sleep apnea (CSA). The analysis software calculated a central sleep apnea index for each study based on a proprietary algorithm (Resmed Pty Ltd, Sydney, Australia). This was available to the reviewing physicians, but they placed more emphasis on their interpretation of the raw data tracings than the CSA index, when deciding whether events were central or obstructive. Although tracings from ApneaLink can give strong indications, such as round rather than flat topped inspiratory flow curves, lack of snoring, and constant cycle length, which favor a diagnosis of CSA, polysomnography is usually considered essential to verify that. The number of cases of CSA in the study population is therefore not known. It is likely very small, however, since only two cases were being treated for congestive heart failure, and only three were known to have cerebrovascular disease.
Although the technical inadequacy rate for laboratory polysomnography is lower than that for the portable monitor in this study, it has been reported as 3%.33 A rate of 7% for a portable monitor should therefore be very acceptable for most purposes, given the low unit cost of the test.
We did not evaluate the effect of targeted screening on outcome measures such as CPAP adherence or quality of life. The goal of the study was to determine the feasibility of a targeted case finding strategy in high-risk patients using simple criteria likely to be found in the primary care chart. We also wished to determine whether there was a sufficiently high prevalence of patients with unrecognized OSA to justify case finding in the first place. Given the results of this study, further research is necessary to determine if targeted case finding results in effective treatment of the identified cases.
CONCLUSIONS
The study shows that a simple low cost case finding and management program, based on unattended home monitoring for OSA, focused on patients with obesity, hypertension, and diabetes, can work well in a population with risk factors and comorbidities associated with OSA. The prevalence of unrecognized OSA in this population was high, and many patients stand to benefit from the discovery and treatment of their disease. These data support the testing for OSA in high-risk groups, whether they have traditional symptoms of OSAS or not.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Burgess has received speaker honoraria from Glaxo Smith Kline. He is the director of Peninsula Health Care Pty Ltd. Dr. Burgess' Family Trust has shares in a private sleep laboratory and he does sleep consultations in one of the regions surveyed. Dr. Whitelaw serves as a consultant for R'ANA Respiratory Care Group. Mr. Newton is a director of Healthy Sleep Solutions Pty Ltd., a company that provides home diagnostic services and treatment for sleep apnea. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Ms. S. Coulson for her assistance in preparing this manuscript for publication and Ms. M. Bennett for assistance with data collection. Work for this study was performed at Peninsula Sleep Laboratory. Author contributions: Dr. Burgess was involved in study design, data collection and manuscript preparation. He is the Guarantor for the manuscript. Dr. Havryk was involved in data collection and manuscript preparation. Mr. Newton was involved in study design, data collection and analysis. Dr. Tsai was involved in data analysis and manuscript preparation. Dr. Whitelaw was involved in study design, data analysis and manuscript preparation.
REFERENCES
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Reuveni H, Greenberg-Dotan S, Simon-Tuval T, Oksenberg A, Tarasiuk A. Elevated healthcare utilisation in young adult males with obstructie sleep apnea. Eur Respir J. 2008;31:273–9. doi: 10.1183/09031936.00097907. [DOI] [PubMed] [Google Scholar]
- 3.Hillman D, Murphy A, Pezullo L. The economic cost of sleep disorders. Sleep. 2006;29:299–305. doi: 10.1093/sleep/29.3.299. [DOI] [PubMed] [Google Scholar]
- 4.Flemons W, Douglas N, Kuna S, Rodenstein D, Wheatley J. Access to diagnosis and treatment of patients with suspected sleep apnea. Am J Respir Crit Care Med. 2004;169:668. doi: 10.1164/rccm.200308-1124PP. [DOI] [PubMed] [Google Scholar]
- 5.Guilleminault C. Principles and practice of sleep medicine. Philadelphia: Saunders; 1989. [Google Scholar]
- 6.Wolk R, Kara T, Somers V. Sleep-disordered breathing and cardiovascular disease. Circulation. 2003;108:9–12. doi: 10.1161/01.CIR.0000072346.56728.E4. [DOI] [PubMed] [Google Scholar]
- 7.Artz M, Young T, Finn L, Skatrud J, Bradley T. Association of sleep-disordered breathing and the occurrence of stroke. Am J Respir Crit Care Med. 2005;172:1447–51. doi: 10.1164/rccm.200505-702OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yaggi H, Concato J, Kernan W, Lichtman J, Brass L, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–41. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 9.Peppard P, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 10.Logan A. Sleep-disordered breathing and hypertension. Am J Respir Crit Care Med. 2009;179:1082–3. doi: 10.1164/rccm.200811-1681ED. [DOI] [PubMed] [Google Scholar]
- 11.Ip M, Lam B, Ng M, Lam W, Tsang K, Lam K. Obstructive sleep apnea is independently associated with insulin resistance. Am J Respir Crit Care Med. 2002;165:670–6. doi: 10.1164/ajrccm.165.5.2103001. [DOI] [PubMed] [Google Scholar]
- 12.Punjabi N, Ahmed M, Polotsky V, Beamer B, O'Donnell C. Sleep-disordered breathing, glucose intolerance, and insulin resistance. Respir Physiol Neurobiol. 2003;136:167–78. doi: 10.1016/s1569-9048(03)00079-x. [DOI] [PubMed] [Google Scholar]
- 13.Resnick H, Redline S, Shahar E, et al. Diabetes and sleep disturbance: findings from the Sleep Heart Health Study. Diabetes Care. 2003;26:702–9. doi: 10.2337/diacare.26.3.702. [DOI] [PubMed] [Google Scholar]
- 14.Gami A, Pressman G, Caples S, et al. Association of atrial fibrillation and obstructive sleep apnea. Circulation. 2004;110:364–7. doi: 10.1161/01.CIR.0000136587.68725.8E. [DOI] [PubMed] [Google Scholar]
- 15.Johns M. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 16.Erman M, Stewart D, Einhorn D, Gordon N, Casal E. Validation of the ApneaLink for the screening of sleep apnea: a novel and simple single-channel recording device. J Clin Sleep Med. 2007;3:387–92. [PMC free article] [PubMed] [Google Scholar]
- 17.Flemons W, Littner M, Rowley J, et al. Home diagnosis of sleep apnea: a systematic review of the literature. An evidence review cosponsored by the American Academy of Sleep Medicine, The American College of Chest Physicians, and the American Thoracic Society. Chest. 2003;124:1543–79. doi: 10.1378/chest.124.4.1543. [DOI] [PubMed] [Google Scholar]
- 18.Nakano H, Tanigawa T, Ohnishi Y, et al. Validation of a single-channel airflow monitor for screening of sleep-disordered breathing. Eur Respir J. 2008;32:1060–7. doi: 10.1183/09031936.00130907. [DOI] [PubMed] [Google Scholar]
- 19.Gantner D, Ge J-Y, Li L-H, et al. Diagnostic accuracy of a questionnaire and simple home monitoring device in detecting obstructive sleep apnea in a Chinese population at high cardiovascular risk. Respirology. 2010;15:952–60. doi: 10.1111/j.1440-1843.2010.01797.x. [DOI] [PubMed] [Google Scholar]
- 20.Flemons W. Clinical practice. Obstructive sleep apnea. N Engl J Med. 2002;347:498–504. doi: 10.1056/NEJMcp012849. [DOI] [PubMed] [Google Scholar]
- 21.Ronksley P, Hemmelgarn B, Heitman S, et al. Obstructive sleep apnea is associated with diabetes in sleepy subjects. Thorax. 2009;64:834–9. doi: 10.1136/thx.2009.115105. [DOI] [PubMed] [Google Scholar]
- 22.Sivertsen B, Overland S, Glozier N, Bjorvatn B, Maeland J, Mykletun A. The effect of OSAS on sick leave and work disability. Eur Respir J. 2008;32:1497–503. doi: 10.1183/09031936.00044908. [DOI] [PubMed] [Google Scholar]
- 23.Hiestand D, Britz P, Goldman M, Phillips B. Prevalence of symptoms and risk of sleep apnea in the US population: Results from the national sleep foundation in America 2005 poll. Chest. 2006;130:780–6. doi: 10.1378/chest.130.3.780. [DOI] [PubMed] [Google Scholar]
- 24.Duran J, Esnaola S, Rubio R, Iztueta A. Obstructive sleep apnea-hypopnea and related clinical features in a population-based sample of subjects aged 30 to 70 yr. Am J Respir Crit Care Med. 2001;163:685–9. doi: 10.1164/ajrccm.163.3.2005065. [DOI] [PubMed] [Google Scholar]
- 25.Bixler E, Vgontzas A, Ten H, Tyson K, Kales A. Effects of age on sleep apnea in men: I. Prevalence and severity. Am J Respir Crit Care Med. 1998;157:144–8. doi: 10.1164/ajrccm.157.1.9706079. [DOI] [PubMed] [Google Scholar]
- 26.Ayappa I, Norman R, Seelall V, Rapoport D. Validation of a self-applied unattended monitor for sleep disordered breathing. J Clin Sleep Med. 2008;4:26–37. [PMC free article] [PubMed] [Google Scholar]
- 27.Collop N, Anderson W, Boehlecke B, et al. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2007;3:737–47. [PMC free article] [PubMed] [Google Scholar]
- 28.Iber C, Redline S, Kaplan Gilpin A, et al. Polysomnography performed in the unattended home versus the attended laboratory setting - Sleep Heart Health Study methodology. Sleep. 2004;27:536–40. doi: 10.1093/sleep/27.3.536. [DOI] [PubMed] [Google Scholar]
- 29.Kirk V, Bohn S, Flemons W, Remmers J. Comparison of home oximetry monitoring with laboratory polysomnography in children. Chest. 2003;124:1702–8. doi: 10.1378/chest.124.5.1702. [DOI] [PubMed] [Google Scholar]
- 30.Zou D, Grote L, Peker Y, Lindblad U, Hedner J. Validation a portable monitoring device for sleep apnea diagnosis in a population based cohort using synchronized home polysomnography. Sleep. 2006;29:367–74. doi: 10.1093/sleep/29.3.367. [DOI] [PubMed] [Google Scholar]
- 31.Mulgrew A, Fox N, Ayas N, Ryan C. Diagnosis and initial management of obstructive sleep apnea without polysomnography: a randomized validation study. Ann Intern Med. 2007;146:157–66. doi: 10.7326/0003-4819-146-3-200702060-00004. [DOI] [PubMed] [Google Scholar]
- 32.Whitelaw W, Brant R, Flemons W. Clinical usefulness of home oximetry compared with polysomnography for assessment of sleep apnea. Am J Respir Crit Care Med. 2005;171:188–93. doi: 10.1164/rccm.200310-1360OC. [DOI] [PubMed] [Google Scholar]
- 33.Berry R, Hill G, Thompson L, McLaurin V. Portable monitoring and autotitration versus polysomnography for the diagnosis and treatment of sleep apnea. Sleep. 2008;31:1423–31. [PMC free article] [PubMed] [Google Scholar]