Abstract
Objectives:
Little is known about the rate of obstructive sleep apnea (OSA) in patients with end stage lung disease (ESLD). Given the potential deleterious effect of OSA in these patients, we assessed the case-rate and severity of OSA and described associated patient characteristics.
Methods:
Retrospective survey of 60 patients with ESLD referred for lung transplantation evaluation. Demographic, polysomnographic, spirometric, and medication utilization data were extracted and analyzed.
Results:
As demographic and polysomnographic data did not differ between obstructive and restrictive patients, we present analysis of pooled data. Demographics/physiology: median age was 58.5 years, 52% males, mean BMI 32.3 kg/m2, 52% obstructive. Sleep variables (all medians): total sleep time (TST) 312 min, sleep efficiency 77%, minimal oxygen saturation 84%, apnea hypopnea (AHI) 9.7, respiratory disturbance index (RDI) 12.7 events/h of sleep. Sixty-seven percent had RDI > 5; 21% had RDI between 15 and 30; and 21% had RDI > 30. Periodic limb movement index ≥ 15/h sleep was present in 21.7%. An independent positive correlation between DLCO% and RDI was noted (r = 0.41, p < 0.01). The minimal oxygen saturation was negatively correlated with the RDI (r = -0.34, p < 0.01). The use of ACE inhibitors was associated with moderate-to-severe OSA (odd ratio of 4.67, CI 1.45-15.03; p = 0.017).
Conclusions:
In patients with ESLD, organic sleep disorders are common. Greater severity of OSA was associated with the higher DLCO% and lower oxygen saturation.
Keywords:
End-stage lung disease, sleep apnea, obstructive lung disease, restrictive lung disease, diffusion capacity, lung transplantation, sleep disorders, oxygen saturation
Citation:
Romem A; Iacono A; McIlmoyle E; Patel KP; Reed RM; Verceles AC; Scharf SM. Obstructive sleep apnea in patients with end-stage lung disease. J Clin Sleep Med 2013;9(7):687-693.
Sleep disordered breathing (SDB) describes a group of disorders of respiratory pattern or ventilation during sleep. Obstructive sleep apnea (OSA) is the most common subtype.1 Prevalence estimates of OSA vary widely, depending upon definition used and population studied. In the general population, prevalence estimates range from 5% to 22%.2–4
Several reports have assessed the epidemiologic relationship between chronic obstructive pulmonary disease (COPD) and OSA.5–7 Most data suggest that the prevalence of OSA in patients with COPD is similar to that of the general population, but previously studied cohorts include very few subjects with advanced lung disease. Patients undergoing evaluation for lung transplantation constitute a cohort of well-characterized subjects with advanced lung disease. Few studies have looked at the case rate of OSA in patients with ESLD being evaluated for lung transplantation. In one study of 50 patients with ILD, there was a high prevalence of OSA (88%).9 Both end-stage lung disease (ESLD) and OSA have been associated with decreased health-related quality of life (HRQOL) and important comorbidities.10–12 If the rate of OSA in ESLD patients is substantial, some of the associated changes in HRQOL and comorbidities could be due to the presence of concomitant OSA. In view of the scarcity of data on the case rate of OSA in patients with ESLD and a possible association between OSA and multiple comorbidities as well as poor HRQOL, we performed a retrospective review of patients with ESLD referred to our lung transplant service for evaluation. We hypothesized that OSA is common in this patient group. We also compared the frequency of OSA between patients with COPD and those with restrictive lung disease due to interstitial lung disease (ILD).
BRIEF SUMMARY
Current Knowledge/Study Rationale: The concomitant presence of organic sleep disorders including sleep disordered breathing (SDB) and periodic limb movement disorder (PLMD) could impact the quality of life and prognosis of patients with end-stage lung disease. Currently there are few reports on the prevalence of SDB and PLMD in such patients being evaluated for lung transplantation.
Study Impact: This study demonstrates a high prevalence of SDB and PLMD in patients with end-stage lung disease whether obstructive or restrictive, in a lung transplant clinic. Patients being evaluated for lung transplant should be evaluated for organic sleep disorders.
METHODS
Study Design and Sample
In this study, we retrospectively reviewed the archived data of 60 subjects with ESLD referred for initial lung transplantation evaluation to the lung transplant clinic of the University of Maryland. As part of the clinic protocol, patients being evaluated for lung transplant underwent polysomnography (PSG) in the sleep disorders center of the University of Maryland, regardless of preexisting risk factors for OSA. Patients also underwent pulmonary function testing (PFT) as well as extensive clinical evaluation. Demographic, polysomnographic, spirometric characteristics, comorbidities, and medication utilization data were extracted from patient records. Patients with Epworth Sleepiness Scale (ESS) scores ≥ 10 were considered to have excessive daytime sleepiness.13 Comorbidities presented are those present in ≥ 20% of the cohort. The University of Maryland School of Medicine Institutional Review Board approved this protocol.
Measurements
Pulmonary Function Testing
Spirometry (FEV1, FVC; FEV1/FVC ratio), measurement of static lung volumes (total lung capacity [TLC] by body box plethysmography) and measurement of diffusion capacity of the lung for carbon monoxide (DLCO% predicted) by the single-breath technique were performed (Vmax22, SensorMedics, Yorba Linda, CA, USA), with the patient in the seated position according to approved standards.14
Subjects with a ventilatory defect (defined as FEV1 < 70% predicted) were included. Subjects were categorized into obstructive (FEV1/FVC ratio < 70%, TLC > 80% predicted) or restrictive (FEV1/FVC > 70% and TLC < 80% predicted) disease.
Polysomnography
All PSGs included ≥ 6 h of overnight sleep in an American Academy of Sleep Medicine accredited sleep laboratory. The PSGs were performed according to commonly accepted clinical standards.15,16 The montage included encephalogram leads O1A2, O2A1, C1A2, C2A1, F1A2, F2A1; electromyogram leads for left eye, right eye, submentalis, and leg (left and right separately), electrocardiogram, and respiratory status measures by nasal airflow (nasal air pressure) and oronasal airflow (thermistor, used for backup), rib cage and abdominal respiratory effort (respiratory impedance plethysmographs), and pulse oximetry. Sleep scoring was done in 30-sec epochs according to the system of Rechtschaffen and Kales,17 as modified by the 2007 AASM scoring manual.18
Respiratory events were scored according to the 2007 AASM scoring manual.18 Obstructive apneas were scored where there was a decrease in nasal airflow to < 10% of baseline for ≥ 10 s with continued respiratory effort. Obstructive hypopneas were scored as a decrease in nasal airflow by 50% to 90% of baseline accompanied by oxygen desaturation > 4% for 10 s with continued respiratory effort. Respiratory event-related arousals (RERAs) were scored as a decrease in airflow by 30% to 90% accompanied by ≥ 3% decrease in oxygen saturation and/or a terminal arousal. Severity of SDB was quantified in two ways. First, we calculated the respiratory disturbance index (RDI), equal to the sum of apneas, hypopneas, and RERAs per hour of sleep. Second, we calculated the apnea-hypopnea index (AHI, equal to the sum of apneas and hypopneas per hour of sleep). For primary clinical purposes, the severity of OSA was defined as follows: “mild” = RDI 5-14.9, “moderate” RDI 15-29.9, and “severe” = RDI ≥ 30. Other PSG diagnoses were scored according to the AASM manual.18 Patients used oxygen during their PSG testing if ordered by their referring physician.
Data Analysis
Categorical variables are reported as counts and percentages, and were analyzed by using the Fisher exact test. For continuous variables, Gaussian distribution was evaluated by the Kolmogorov-Smirnov normality test. Normally distributed variables are presented as mean (standard deviation), and non-normally distributed variables are presented as median (interquartile range). Between-group comparisons employed non-paired t-tests for normally distributed variables and a Mann-Whitney rank sum test for skewed variables. The association between medication use and SDB was assessed with univariate logistic regression. Bivariate linear regression analyses were used to assess the association between clinical correlates with SDB. The following independent variables were tested: body mass index (BMI), DLCO%, and PSG measures of sleep architecture and indices of oxygen saturation. In addition, backward stepwise multivariate regression analyses were conducted while treating AHI/RDI as a dependent variable. Independent variables were chosen if the bivariate associations were significant. For all comparisons, a two-tailed p < 0.05 was considered significant. SigmaPlot 12.0 (Systat Software Inc., San Jose, CA) was used for all analyses and graph production.
RESULTS
Subject characteristics are summarized in Table 1. Sixty subjects meeting inclusion criteria were identified. Comparison based on their primary ventilatory defect (obstructive versus restrictive) showed similar demographic indices (age, gender distribution, BMI, ESS, and supplemental oxygen use) and major comorbid conditions (Tables 1, 2). The combined patient group was characterized by a median age of 58.5 years, BMI of 32.3, equally balanced gender distribution (52% male), and a median ESS of 9. Half the subjects were on home supplemental oxygen treatment, and 40% used supplemental oxygen during their overnight polysomnography.
Table 1.
Demographic, medication, and pulmonary function testing (PFT) characteristics according to underlying breathing abnormality
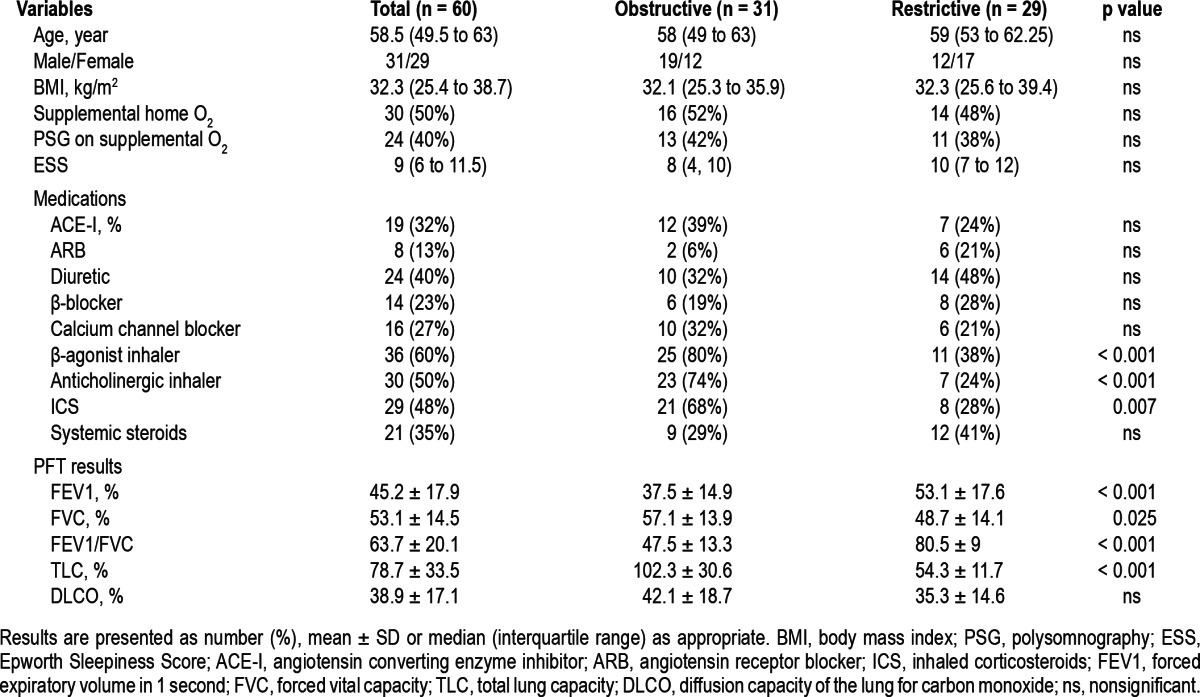
Table 2.
Major comorbid conditions grouped by ventilator defect and RDI
No difference was noted in DLCO% predicted between the obstructive and restrictive subgroups. Both groups included mostly patients with severely diminished pulmonary function as indicated by a low FEV1 < 50% predicted and/or DLCO% < 40% predicted.19
The use of antihypertensive medications and systemic steroid use was equally distributed between both obstructive and restrictive patient groups (Table 1). Inhaled medication use was significantly more common in the obstructive group.
PSG data are summarized in Table 3. None of the variables differed between the obstructive and restrictive groups. Hence, for further analysis, we have pooled the data for the entire cohort. Median sleep efficiency was reduced, with half the patients demonstrating sleep efficiency < 77.3%. Slow wave and REM sleep were also severely reduced. Sleep onset latency was slightly increased. The median AHI (9.7) and RDI (12.7) were in the mild range. Forty of 60 subjects (67%) had OSA, as defined by an RDI > 5/h (Figure 1). Fourteen (23%) had mild OSA and 26 (43%) had moderate-to-severe OSA (RDI > 15/h). Periodic leg movement index (PLMI) was elevated (> 15/h) in 13 patients (21.7%), with no differences between obstructive and restrictive patients. Time with oxygen saturation below 90% (T90%) was 17.7% ± 22.6% of total sleep time, with a median minimal saturation of 84%.
Table 3.
Sleep architecture characteristics according to underlying breathing abnormality
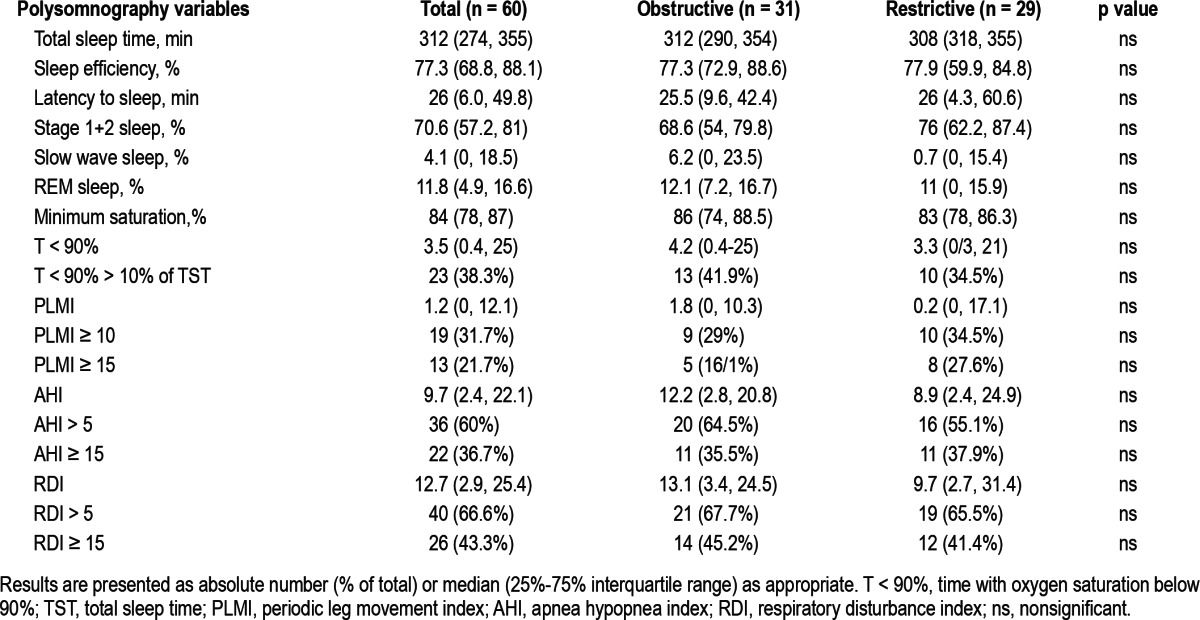
Figure 1. Prevalence of obstructive sleep apnea according to severity.
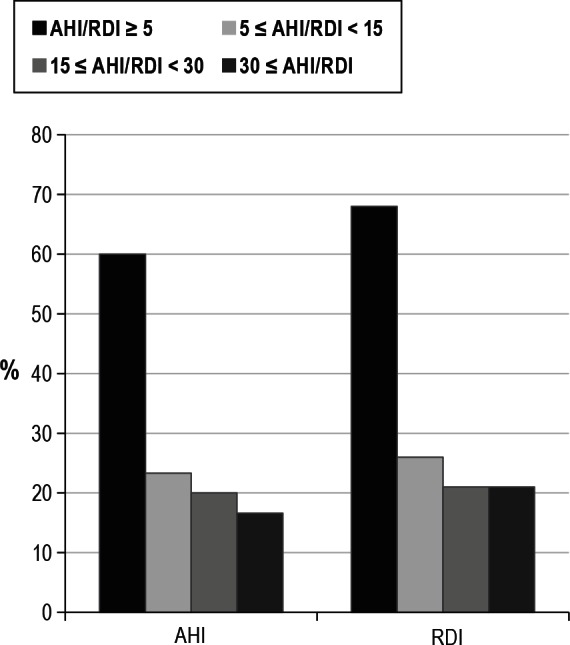
OSA as defined by an AHI or RDI ≥ 5 events/h was prevalent among 60% and 68% of the subjects (n = 60) respectively.
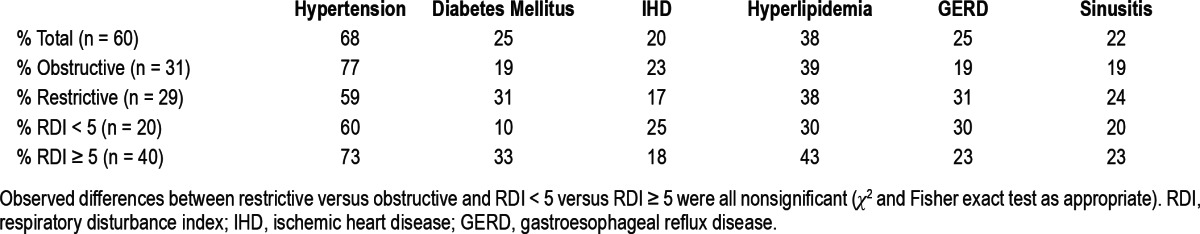
Table 4 summarizes the observed correlations to RDI and AHI. RDI and AHI correlated positively to both predicted FEV1% and predicted DLCO% values (p < 0.05 and p < 0.01, respectively). RDI and AHI were inversely correlated with the minimal oxygen saturation value (p < 0.01) and the percentage of time spent in slow wave sleep (p < 0.01). Subgroup analysis of subjects who had PSG done with and without supplemental oxygen showed persistence of these associations. Neither the Epworth score nor any of the other spirometric values correlated with the RDI or AHI. None of the major comorbid conditions was associated with an RDI ≥ 5/h (Table 2). No association could be found between the PLMI and the AHI, RDI, or ESS.
Table 4.
Univariate coefficients of correlation between sleep disordered breathing indices and demographic, spiro-metric, and polysomnographic variables
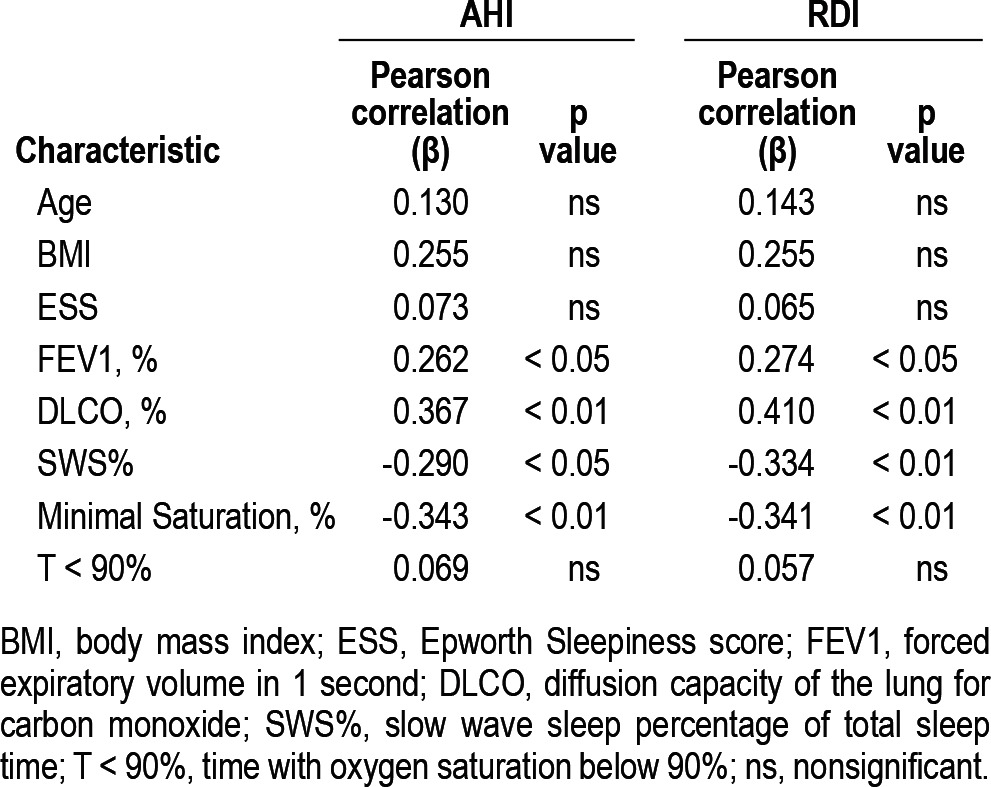
Multivariable modeling included a backward stepwise regression using significant predictors from Table 4 was performed. Only DLCO% and minimum O2 saturation were found to be significant predictors (p = 0.013 and p = 0.023, respectively) of RDI (Figures 2 and 3, respectively) and AHI (p = 0.034 and p = 0.023, respectively). A multi-linear regression model incorporating the above predictors generated the following equations:
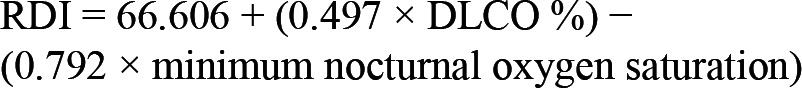 |
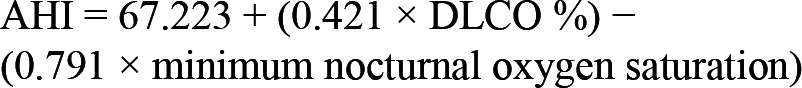 |
Figure 2. Bivariate correlation between RDI and CO diffusion capacity (DCO).
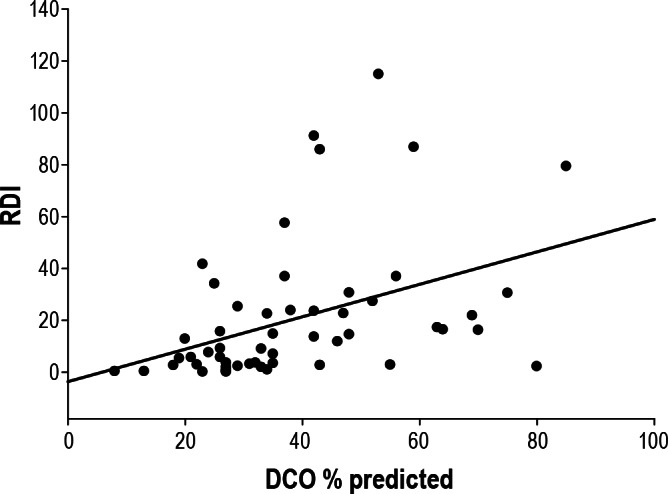
For linear regression: RDI = -3.653 + (0.626 × DCO%); r = 0.410; p = 0.002.
Figure 3. Bivariate correlation between RDI and minimal O2 saturation during polysomnography.
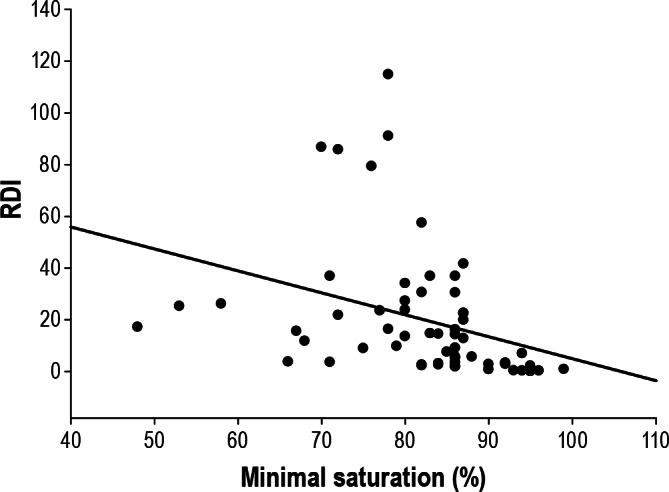
For linear regression: RDI = 89.934 − (0.850 × minsat); r = -0.341; p = 0.008.
The use of ACE inhibitors was strongly associated with the occurrence of moderate-to-severe OSA (odd ratio of 4.67 CI 1.45-15.03; p = 0.017). This association was not evident for any other antihypertensive medications, including angiotensin receptor blockers. Only patients with hypertension received ACE inhibitors. Also, the association between ACE inhibitors and the occurrence of moderate-to-severe OSA was independent of comorbid conditions and BMI.
DISCUSSION
In this study of patients with ESLD, we demonstrated a high rate of sleep fragmentation, OSA, and PLM disorder. Factors such as BMI and ESS failed to predict OSA in patients with ESLD. Conversely, higher DLCO% and lower saturation during sleep correlated to higher RDI and AHI. In the ensuing discussion we consider these findings in the light of the currently available literature.
Few studies on sleep disorders have been directed at patients with ESLD. The majority (67%) of the patients had obstructive sleep apnea (OSA) as defined by an RDI ≥ 5 events per hour, with 23 of 40 (57.5%) subjects diagnosed with moderate-to severe OSA (RDI ≥ 15/h). No difference in the case rate of OSA was noted between the “obstructive” group and the “restrictive” group, despite the inherent differences in the underlying respiratory mechanical abnormalities. These facts underscore the complexity and yet poorly understood etiology of OSA among subjects with lung diseases.
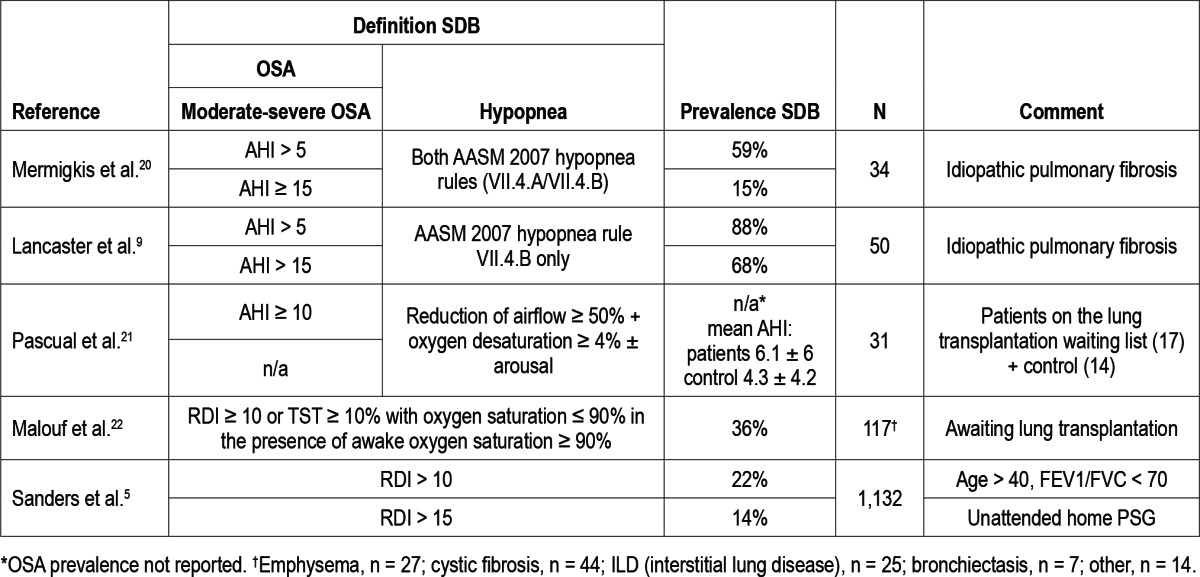
We note that the median BMI among our patients was high. Patients with higher BMIs are known to have an increased incidence of OSA.23 Since we did not have a control group matched for BMI, we cannot say that the rate of OSA was higher than would be expected on the basis of BMI alone. The effect of age on polysomnographic respiratory abnormalities in healthy individuals was previously reported by Pavlova et al.,24 who showed a positive correlation between age and RDI with an average RDI of 12.8/h in the 50-65 year age range. Thus, it is possible that part of the high prevalence of sleep disordered breathing reported here is related to the age of the patients we evaluated. Prior reports of lung disease patients including COPD, idiopathic pulmonary fibrosis (IPF), and patients on the waiting list for lung transplantation reported different frequencies of OSA. Some of these are reviewed in Table 5. Depending on the definition of sleep disordered breathing and differences in population base, rates of 14% to 88% have been reported in patients with ESLD. The reasons for differences between reported prevalence estimates between studies are likely attributable to differences in patient selection, techniques, scoring criteria, definitions, and severity of lung disease.
Table 5.
Previously reported prevalences of OSA in lung disease patients (polysomnographic recordings)
The potential to predict the occurrence of OSA in patients with advanced lung disease would facilitate more effective and efficient screening of this growing patient population. In our study, associations were found between OSA (as indicated by higher AHI/RDI scores) and multiple physiological parameters, including higher DLCO%, lower oxygen saturations during sleep, and percent of total sleep time spent in slow wave sleep. Though all of the above parameters have been found to correlate with OSA in previous studies, only DLCO% and minimal oxygen saturation remained independent predictors in the multivariate analyses. High rates of nocturnal oxygen de-saturation have been described extensively in both COPD and ILD patients,25,26 being further deranged among those with superimposed OSA. The concept of predicting OSA by overnight pulse oximetry to monitor severity of nocturnal oxygen desatu-ration was thoroughly reviewed by Netzer et al.27 Those authors concluded that overnight pulse oximetry is a useful tool for the screening for OSA, although they did not specifically consider patients with ESLD. Our study is consistent with the notion that OSA and ESLD have additive negative effects on oxygenation, and that indices of oxygenation could be useful for prescreening patients with OSA being considered for lung transplant. Interestingly, the association between minimum saturation and RDI was also present in those patients receiving oxygen at night.
The reasons for the direct correlation between DLCO% and OSA are not clear. This could be explained by a number of factors. Keens et al.28 have shown that adding external resistance during inspiration leads to an increase in DLCO in normal subjects. Therefore, increased inspiratory airflow resistance, typical of OSA patients,29 could have resulted in improved DLCO. It is possible that increased inspiratory effort associated with increased upper airways resistance leads to increased pulmonary capillary volume, both related to increases in venous return and increased afterloading of the left ventricle,30 hence leading to improved DLCO with worse OSA. Elevated DLCO has been reported in adults with increased BMI,31 although these findings are not universal.32 It is also possible that in ESLD capillary destruction (lower DLCO), results in a greater degree of intermittent hypoxemia, which in turn could lead to increased respiratory drive (peripheral chemoreceptors), including the abductors of the upper airway—thus providing some protection from OSA. Hence, with worsening DLCO the severity of OSA might have been mitigated, as we observed (Figure 2).
Excessive sleepiness as assessed by the ESS did not correlate with severity or the occurrence of OSA. The reasons for the dissociation between excessive sleepiness and severity of OSA are not clear. Others have also demonstrated that the ESS is a poor predictor of the severity of sleep disorders and that the ESS may not reflect sleepiness measured objectively.33 It is possible that sympathetic hyperstimulation, or anxiety could induce a “hyperalert” state, thereby diminishing the extent of perceived sleepiness. In their study of sleep quality in patients with COPD, Scharf et al.34 found that in spite of a high incidence of self-reported poor sleep quality and insufficient sleep, few patients had excessive sleepiness as measured by ESS. These authors speculated that these patients also had a “hyperalert” state.
Intriguingly, we found a significant and substantial positive association between moderate-severe OSA and the use of an angiotensin-converting enzyme inhibitor (odds ratio 4.67). This association did not exist for other antihypertensive medication categories. While possibly due to chance, these findings are in agreement with those of Cicolin et al.35 who, in a small case series, showed that withdrawal of ACE inhibitors can lead to a significant decrease in AHI. These findings led the authors to suggest that through inhibition of degradation of bradykinin, ACE inhibitors induced vasodilatation and plasma extravasation in the upper airway in turn increasing the propensity for OSA.
We failed to find that the presence of any particular RDI cutoff was predictive of the occurrence of self-reported hypertension. The association between OSA and hypertension is well established.36 In our cohort, it is possible that confounding factors such as obesity and the presence of lung disease may have weakened the association between hypertension and OSA, so that it was not detected in our small cohort.
Finally, the finding of the high prevalence of sleep disordered breathing in patients with end-stage lung disease might appear surprising. The association between lung disease and OSA could be related to the release of proinflammatory cytokines due to the underlying lung inflammation with subsequent edema and swelling of the upper airway, which would increase the likelihood for OSA. Alternatively, during OSA there is release of cytokines and oxidant stress, which could have accelerated the progression of the underlying lung.37
Our study has several limitations. It is limited by its relatively small size and its retrospective nature. Furthermore, clinical necessity mandated the use of supplemental oxygen during the overnight sleep study in a large portion of our study population, potentially masking hypoxic episodes and leading to underestimation of the true prevalence of nocturnal oxygen desaturation—a critical determinant of hypopnea definition.
In summary, we observed a high case rate of OSA among a group of patients with advanced lung disease, irrespective of underlying etiology or subjective symptoms of excessive sleepiness. Diagnosis of OSA could well lead to improved quality of life, better perioperative management, and possibly improved course of the underlying disease.38 Additional studies will be necessary to further understand the true prevalence of OSA in advanced lung disease and its consequences, as well as the effects of treatment of OSA. There is a large disease burden borne by patients with ESLD. Addressing a common and underdiagnosed comorbidity such as OSA would appear to be an important component of their evaluation. Our study suggests that OSA is common in patients with ESLD, and therefore these patients might benefit from OSA screening with overnight polysomnography.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The study was performed at the University of Maryland Medical Center.
REFERENCES
- 1.American Academy of Sleep Medicine Task Force. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 2.Young T, Palta M, Dempsey J, et al. The occurrence of sleep disordered breathing in middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 3.Gottlieb DJ, Whitney CW, Bonekat WH, et al. Relation of sleepiness to respiratory disturbance index: the sleep heart health study. Arch Intern Med. 2002;162:893–900. doi: 10.1164/ajrccm.159.2.9804051. [DOI] [PubMed] [Google Scholar]
- 4.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea. Am J Respir Crit Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 5.Sanders MH, Newman AB, Haggerty CL, et al. Sleep and sleep-disordered breathing in adults with predominantly mild obstructive airway disease. Am J Respir Crit Care Med. 2003;167:7–14. doi: 10.1164/rccm.2203046. [DOI] [PubMed] [Google Scholar]
- 6.Bednarek M, Plywaczewski R, Jonczak L, Zielinski J. There is no relationship between chronic obstructive pulmonary disease and obstructive sleep apnea syndrome: a population study. Respiration. 2005;72:142–9. doi: 10.1159/000084044. [DOI] [PubMed] [Google Scholar]
- 7.Chaouat A, Weitzenblum E, Krieger J, et al. Association of chronic obstructive pulmonary disease and sleep apnea syndrome. Am J Respir Crit Care Med. 1995;151:82–6. doi: 10.1164/ajrccm.151.1.7812577. [DOI] [PubMed] [Google Scholar]
- 8.American Thoracic Society; European Respiratory Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. Am J Respir Crit Care Med. 2000;161:646–64. doi: 10.1164/ajrccm.161.2.ats3-00. [DOI] [PubMed] [Google Scholar]
- 9.Lancaster LH, Mason WR, Parnell JA, et al. Obstructive sleep apnea is common in idiopathic pulmonary fibrosis. Chest. 2009;3:772–8. doi: 10.1378/chest.08-2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaplan RM, Ries AL. Health-related quality of life in emphysema. Proc Am Thorac Soc. 2008;5:561–6. doi: 10.1513/pats.200706-080ET. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swigris JJ, Kuschner WG, Jacobs SS, Wilson SR, Gould MK. Health-related quality of life in patients with idiopathic pulmonary fibrosis: a systematic review. Thorax. 2005;60:588–94. doi: 10.1136/thx.2004.035220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moyer CA, Sonnad SS, Garetz SL, Helman JI, Chervin RD. Quality of life in obstructive sleep apnea: a systematic review of the literature. Sleep Med. 2001;2:477–91. doi: 10.1016/s1389-9457(01)00072-7. [DOI] [PubMed] [Google Scholar]
- 13.Patil SP, Schneider H, Schwartz AR, Smith PL. Adult obstructive sleep apnea: pathophysiology and diagnosis. Chest. 2007;132:325–37. doi: 10.1378/chest.07-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American Thoracic Society Standardization of spirometry, 1994 update. Am J Respir Crit Care Med. 1995;152:1107–36. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 15.Friedman M, Wilson MN, Pulver T, et al. Screening for obstructive sleep apnea/ hypopnea syndrome: subjective and objective factors. Otolaryngol Head Neck Surg. 2009;142:531–5. doi: 10.1016/j.otohns.2009.12.038. [DOI] [PubMed] [Google Scholar]
- 16.Olivarez SA, Maheshwari B, McCarthy M, et al. Prospective trial on obstructive sleep apnea in pregnancy and fetal heart rate monitoring. Am J Obstet Gynecol. 2010;202:552.e1–7. doi: 10.1016/j.ajog.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 17.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system of sleep stages in human subjects. Los Angeles: Brain Information Service/Brain Research Institute, University of California; 1968. [Google Scholar]
- 18.Iber C, Ancoli-Israel S, Chesson A, Quan SF. The AASM manual for scoring of sleep and associated events: rules, terminology and technical specifications, 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 19.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–68. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 20.Mermgkis C, Stagaki E, Tryfon S, et al. How common is sleep-disordered breathing in patients with idiopathic pulmonary fibrosis? Sleep Breath. 2010;14:387–90. doi: 10.1007/s11325-010-0336-5. [DOI] [PubMed] [Google Scholar]
- 21.Pascual N, Jurado B, Rubio JM, Santos F, Lama R, Cosano A. Respiratory disorders and quality of sleep in patients on the waiting list for lung transplantation. Transpl Proc. 2005;37:1537–9. doi: 10.1016/j.transproceed.2005.02.043. [DOI] [PubMed] [Google Scholar]
- 22.Malouf MA, Milross MA, Grunstein RR, et al. Sleep-disordered breathing before and after lung transplantation. J Heart Lung Transplant. 2008;27:540–6. doi: 10.1016/j.healun.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 23.Young T, Peppard PE, Taheri S. Excess weight and sleep-disordered breathing. J Appl Physiol. 2005;99:1592–9. doi: 10.1152/japplphysiol.00587.2005. [DOI] [PubMed] [Google Scholar]
- 24.Pavlova MK, Duffy JF, Shea SA. Polysomnographic respiratory abnormalities in asymptomatic individuals. Sleep. 2008;31:241–8. doi: 10.1093/sleep/31.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krachman S, Minai OA, Scharf SM. Sleep Abnormalities and treatment in emphysema. Proc Am Thorac Soc. 2008;5:536–42. doi: 10.1513/pats.200708-134ET. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agarwal S, Richardson B, Krishnan V, Schneider H, Collop NA, Danoff SK. Interstitial lung disease and sleep: What is known? Sleep Med. 2009;10:947–51. doi: 10.1016/j.sleep.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 27.Netzer N, Eliasson AH, Netzer C, Kristo DA. Overnight pulse oximetry for sleep-disordered breathing in adults: a review. Chest. 2001;120:625–33. doi: 10.1378/chest.120.2.625. [DOI] [PubMed] [Google Scholar]
- 28.Keens TG, Mansell A, Krastins IRB, et al. Evaluation of the single-breath diffusing capacity in asthma and cystic fibrosis. Chest. 1979;76:41–4. doi: 10.1378/chest.76.1.41. [DOI] [PubMed] [Google Scholar]
- 29.Stauffer JL, Zwillich CW, Cadieux RJ, et al. Pharyngeal size and resistance in obstructive sleep apnea. Am Rev Respir Dis. 1987;136:623–7. doi: 10.1164/ajrccm/136.3.623. [DOI] [PubMed] [Google Scholar]
- 30.Robotham J, Scharf SM. The effects of positive and negative pressure ventilation on cardiac performance. Clin Chest Med. 1983;4:161–87. [PubMed] [Google Scholar]
- 31.Reisin E, Messerli FG, Ventura HO. Renal hemodynamic studies in obesity hypertension. J Hypertens. 1987;5:397–400. [PubMed] [Google Scholar]
- 32.Collard P, Wilputte JY, Aubert G, Rodenstein DO, Frans A. The single-breath diffusing capacity for carbon monoxide in obstructive sleep apnea and obesity. Chest. 1996;110:1189–93. doi: 10.1378/chest.110.5.1189. [DOI] [PubMed] [Google Scholar]
- 33.Fong SY, Ho CK, Wing YK. Comparing MSLT and ESS in the measurement of excessive daytime sleepiness in obstructive sleep apnoea syndrome. J Psycho-som Res. 2005;58:55–60. doi: 10.1016/j.jpsychores.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 34.Scharf SM, Maimon N, Simon-Tuval T, Bernhard-Scharf BJ, Reuveni H, Tarasiuk A. Sleep quality predicts quality of life in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2010;22(6):1–12. doi: 10.2147/COPD.S15666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cicolon A, Mangiardi L, Mutani R, Bucca C. Angiotensin-converting enzyme inhibitors and obstructive sleep apnea. Mayo Clin Proc. 2006;81:53–5. doi: 10.4065/81.1.53. [DOI] [PubMed] [Google Scholar]
- 36.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 37.Williams A, Scharf SM. Obstructive sleep apnea, cardiovascular disease, and inflammation - is NF-kB the key? Sleep Breath. 2007;11:69–76. doi: 10.1007/s11325-007-0106-1. [DOI] [PubMed] [Google Scholar]
- 38.Marin JM, Soriano JB, Carrizo SJ, Boldova A, Celli BR. Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea-the overlap syndrome. Am J Respir Crit Care Med. 2010;182:325–31. doi: 10.1164/rccm.200912-1869OC. [DOI] [PubMed] [Google Scholar]


