Abstract
Here we describe a novel method utilizing double stable isotope ultra performance liquid chromatography-tandem mass spectrometry to measure tissue contents and activity of phenylethanolamine N-methyltransferase (PNMT), the enzyme responsible for synthesis of the stress hormone, epinephrine. The method is based on measurement of deuterium-labeled epinephrine produced from reaction of norepinephrine with deuterium-labeled S-adenosyl-L-methionine as the methyl donor. In addition to enzyme activity the method allows for determination of tissue contents of PNMT using human recombinant enzyme for calibration. The calibration curve for epinephrine was linear over the range of 0.1 to 5000 pM, with 0.5 pM epinephrine representing the lower limit of quantification. The calibration curve relating PNMT to production of deuterium-labeled epinephrine was also linear from 0.01 ng to 100 ng PNMT. Intra- and inter-assay coefficients of variation were respectively 12.8% (n=10) and 10.9% to 13.6% (n=10). We established utility of the method by showing induction of the enzyme by dexamethasone in mouse pheochromocytoma cells and strong relationships to PNMT gene expression and tissue epinephrine levels in human pheochromocytomas. Development of this assay provides new possibilities for investigations focusing on regulation of PNMT, the crucial final enzyme responsible for synthesis of epinephrine, the primary fight-or-flight stress hormone.
Keywords: UPLC-MS/MS, PNMT, pheochromocytoma
Introduction
Epinephrine (EPI) is the major effector hormone of the sympathoadrenal limb of the stress system vital to the fight-or-flight response [1]. Important in maintaining physiological homeostasis, EPI also contributes to the etiology of numerous stress-related pathologies, including cardiovascular and neuropsychiatric disorders, immune dysfunction and even cancer [2]. These influences are produced via diverse pathways and mechanisms, including downstream beta2-adrenoceptor-mediated damage to DNA [3].
Because of its role as a neuroendocrine regulator involved in numerous stress-related disorders, considerable effort continues to focus on understanding the control of EPI production by phenylethanolamine N-methyltransferase (PNMT; EC 2.1.1.28), the final crucial enzyme in the catecholamine biosynthetic pathway [4]. This reaction involves the transfer of a methyl group from S-adenosyl-L-methionine (SAM) to the amino group of norepinephrine (NE) to produce EPI and S-adenosyl-L-homocysteine (SAH) [5].
In addition to polymerase chain reaction-based measurements of PNMT gene expression there are several methods for measuring PNMT at the protein level, but all have limitations. Western blot analysis is at best semi quantitative. Although several enzyme assays have been described, those that use production of unlabeled EPI are compromised by presence of endogenous catecholamines in tissue preparations, either requiring labor-intensive sample preparation, use of radioactive precursors or assessment of the conversion of SAM to SAH by relatively insensitive detection methods [5 – 14].
With recognition of the above short-comings in currently described assay techniques for quantitative assessments of PNMT enzyme activity, we sought to develop an accurate, rapid and sensitive method for determination of not only PNMT enzyme activity, but also tissue enzyme contents, applicable to both tissue and cell line specimens. To avoid use of radioactive materials and overcome problems associated with presence of endogenous catecholamines this method employs ultra performance liquid chromatography tandem mass spectrometry (UPLC-MS/MS) for measurements of deuterium-labeled EPI by isotope-dilution using a second stable-isotopically labeled EPI internal standard (Fig. 1).
Fig. 1.
Assay principle and chemical structures.
Abbreviations: NE, Norepinephrine; D3-SAM, deuterium-labeled S-adenosyl-L-methionine; D3-EPI, deuterium-labeled epinephrine
Materials and methods
Chemicals and reagents
Materials included (±)-epinephrine-2, 5, 6, α, β, β-d6 (d6-EPI, CDN isotopes, Augsburg, Germany), (±)-epinephrine-α-d1, β-d2, •HCl (d3-EPI, Medical Isotopes, Pelham, USA), L-(-)-norepinephrine (Sigma, St. Louis, USA), S-adenosyl-L-methionine-d3 (S-methyl-d3) tetra (p-toluenesulfonate) salt (d3-SAM, C/D/N Isotopes, Augsburg, Germany), human recombinant phenylethanolamine-N-methyltransferase (PNMT, Biotrend, Cologne, Germany), cell disruption buffer (Life Technologies, Darmstadt, Germany), Quant-iT™ Protein Assay kit (Life technologies, Darmstadt, Germany), dexamethasone (Sigma, St. Louis, USA), formic acid (Sigma, St. Louis, USA), acetonitrile (UPLC/MS, Biosolve, Valkenswaard, The Netherlands), tris-(hydroxymethyl)-aminomethane (Tris, Roth, Karlsruhe, Germany), alumina (MP Biomedicals, Eschwege, Germany) and hydrochloric acid (HCl, Merck, Darmstadt, Germany). Water was purified with a Milli-Q reagent water system (Millipore, Billerica, MA, USA).
UPLC-MS/MS
UPLC-MS/MS analysis was performed on an API QTRAP 5500 (AB Sciex) coupled to a Waters Acquity ultra performance liquid chromatography (UPLC) system. This system includes a binary solvent manager, which controls the passage of mobile phases through analytical columns, a sample manager, which controls the injection of prepared samples onto analytical columns, and a column manager, which enables automated switching between multiple analytical columns depending on the application. For the presently described application analyte enrichment and chromatographic separation was facilitated using a Waters Acquity UPLC® HSS T3 column (1.8μm, 2.1 mm x 100 mm) with a flow of 0.53 mL/min using a gradient of mobile phases A (0.2 % formic acid in water) and B (0.2 % formic acid in acetonitrile). The gradient profile consisted of an initial isocratic step of 2% mobile phase B for 0.5 min followed by increases to 40% B at 1.15 min and 98% B at 1.5 min until 1.8 min, at which time the column was re-equilibrated with 2% of mobile phase A. The total run time was 2.5 min. Column temperature was maintained at 25°C and sample manager temperature at 5 °C.
The QTRAP mass spectrometer was operated in the positive ion electrospray (+ESI) mode with multi reaction monitoring used for detection according to the transitions for quantification and qualification defined in Table 1. Optimization of compound dependent source parameters (declustering and entrance potentials) and fragmentation parameters (collision energy and cell exit potential) was performed by injection of single standard substances using the instrument integrated syringe pump. Therefore, standards were diluted in water/acetonitrile (4:1 by volume) acidified with 0.2 % formic acid. Ionization source (ESI) parameters were further optimized by automatic flow injection analyses provided by the Analyst software package (Vers 1.51, AB Sciex) with curtain gas (30 psi), ESI voltage (5500V), temperature (600°C), gas 1 (70 psi) and gas 2 (60 psi).
Table 1.
Multiple Reaction Monitoring transitions and respective fragmentation parameters
| Compound | Q1 | Q3 | DP [V] | EP [V] | CE [V] | CXP [V] |
|---|---|---|---|---|---|---|
| d3-EPIα | 169.0 | 77.1a, 79.0, 135.0, 138.0 | 131 | 10 | 51/37/21/21 | 12 |
| d6-EPIα | 172.0 | 112.0a, 139 | 131 | 10 | 29/23 | 12 |
fragments used as quantifier ions
Abbreviations: Q1, Quadrupole 1; Q3, Quadrupole 3; DP, Declustering Potential; EP, Entrance Potential; CE, Collision Energy; CXP, Cell Exit Potential
Estimation of tissue PNMT enzyme activity
Tissue PNMT enzyme activity was expressed in pmol d3-EPI per minute. The reaction mixture for measurement of PNMT enzyme activity consisted of the following components to a total volume of 1000 μL in 1.6 mL polypropylene eppendorf tubes: 920 μL of 0.4 M Tris/HCl buffer (pH 8.5); 20 μL of 2.5 mM d3-SAM; 20 μL of 2.5 mM NE; and 40 μL of tissue preparation (see below for details). The blank reaction mixture contained no enzyme. This mixture was incubated for 20 min at 37°C in a water bath. The reaction was stopped by addition of 10 μL of glacial acetic acid and then mixed for 20 min on a Vortex-Genie 2T (VWR, Darmstadt, Germany) with 0.25mM d6-EPI (in 20 μL) as internal standard, 400 μL of 1M Tris/HCl (pH 8.6 with EDTA) and 5 mg alumina (stored at 100°C before use). After brief centrifugation the supernatant was aspirated and the alumina washed once with Milli-Q water. Following aspiration of the water wash, 50 μL of a solution consisting of water/acetonitrile (98:2, V/V) and 2% formic acid was added to the alumina and vortexed for 45 sec in order to elute the catecholamines. After centrifugation, the supernatant was transferred into sample vials ready for injection onto the autosampler.
Estimation of tissue PNMT content
The calibration curve relating production of d3-EPI to the amount of PNMT was constructed using purified human recombinant PNMT. Twenty μL of 2.5 mM d3-SAM and 20 μL of 2.5 mM NE were used as substrates. The incubation was carried out at 37°C for 20 min. Amounts of PNMT protein used in the assay ranged from 0.01 to 100 ng and included a blank without enzyme. The content of tissue PNMT was calculated from the calibration curve as a percent of total tissue protein. Total protein was quantified using the Quant-iT™ Protein Assay kit. This method is not compromized by the presence of high levels of catecholamines, which strongly interfere with the more common protein assays based on chemical reactions of aromatic amino acid residues.
Assay Validation
Assay linearity was established for d3-EPI by injection of 10 μl injections of concentrations ranging from 0.1 to 5000 pM. Lower limits of quantification were estimated as the minimal concentration of d3-EPI measureable at an accuracy within ±20%.
Matrix effects related to ion suppression were assessed for alumina extracted pheochromocytoma tumors and mouse pheochromocytoma cells (MPC). The internal standard was injected continuously post column to eluates of the alumina extracted sample matrices, which is a common procedure in mass spectrometry for assessing the ion suppression.
Absolute recovery of the analyte, d3-EPI, through the extraction procedure were estimated by dividing the peak areas of spiked extracted (pre-spike) samples by the peak areas of extracted samples spiked with the same amount of d3-EPI after extraction (post-spike). Relative recoveries of the d3-EPI analyte to the d6-EPI internal standard were estimated from the ratios of these analytes in pre- and post-spike samples.
Assay precision was estimated from intra- and inter-assay coefficients of variation for respective series of 10 identical quality control (QC) samples taken through enzyme incubations, extractions and UPLC-MS/MS measurements in a single run and in multiple runs. Two different types of QC samples were used for estimation of intra- and inter-assay coefficients of variation: 1. A tumor tissue sample preparation from which multiple aliquots of extracts were stored at −80°C until required; and 2. a solution of recombinant PNMT (10 ng/40 μl) in cell disruption buffer.
Accuracy of PNMT measurements was determined from calculation of the mass amount of PNMT enzyme estimated in each of 10 measurements of the recombinant PNMT QC sample. Measured mass amounts relative to true amounts were calculated for each sample and measures of accuracy provided as a mean percent recovery with 95% confidence intervals.
Cell lines and pheochromocytoma specimens
To validate the method and establish its utility we examined the activity and amounts of PNMT enzyme in cell lines and in human pheochromocytoma tumor specimens. For the former we used a mouse pheochromocytoma (MPC) cell line established to express PNMT and developed from pheochromocytomas in mice with a heterozygous knockout of the NF1 gene [15]. MPC cell lines were a gift kindly provided by Prof. A.S. Tischler (Department of Pathology, Tufts University School of Medicine, Boston, MA, USA). Human pheochromocytoma specimens were obtained from 79 patients undergoing surgical resection of tumors under intramural review board approved protocols at Dresden or the National Institutes of Health, Bethesda, MD. USA.
Cell Culture
MPC cells were plated in collagen coated flasks and maintained in RPMI 1640 medium containing 5% fetal calf serum, 10% horse serum, at 37°C in a water-saturated atmosphere of 95% air and 5% CO2. Dexamethasone treatment was initiated by replacing the growth medium with fresh medium containing 1 μM dexamethasone 24 hours after seeding the cultures.
Tissue sample preparation
MPC cells were washed with PBS and immediately resuspended in ice-cold cell disruption buffer for lysis. At least 300 μL cell disruption buffer was used for ≥106 cells. Human tissue specimens, including pheochromocytoma tumor tissues, were homogenized in 100–600 μL (6–8 volumes per tissue mass) ice-cold cell disruption buffer with a SilentCrusher M (Heidolph, Schwabach, Germany). Homogenates from cells or tissues were centrifuged at 10,000 g for 5 min and supernatants were used without further purification.
Quantitative real-time RT-PCR
Total RNA was extracted and purified from cultured cells or homogenized tissues with a mirVana Paris kit (Life Technologies, Darmstadt, Germany). Isolated RNA was converted to cDNA using the GeneAmp® RNA PCR kit (Applied Biosystems, Foster City, CA, USA). Real-time RT-PCR was performed using Rotor-Gene Q instrument and Rotor-Gene SYBR Green RT-PCR Kit (Qiagen, Hilden, Germany). Primers for PNMT utilized the following base sequences: 5′-GTG CGT AGT GGC TAC AAG GT - 3′ and 5′-TTC AAA GAA CAG GGA ATC CA - 3′. β-Actin and 18S rRNA were used as housekeeping genes to calculate the expression level of PNMT.
Results and discussion
Here we describe a simple, sensitive and novel non-radioactive-based method to quantify not only PNMT activity, but also the mass and tissue contents of active enzyme. The assay uses stable isotope deuterium-labeled SAM as a methyl donor and the measurement of the deuterated product, d3-EPI, by liquid chromatography-tandem mass spectrometry with d6-EPI as internal standard to correct for recovery by isotope dilution. The double stable isotopic nature of the assay provides advantages over previously described techniques by ensuring a high level of precision and accuracy without compromise by influences of endogenous EPI or complications related to use of radioactivity.
Assay validation
The detector response showed linearity (r=0.993) for d3-EPI over a concentration range of 0.1 to 5000 pM EPI, broad enough to quantify PNMT in any biological sample without necessity for repeat measurement of diluted sample extracts. The lower limit of quantification was 0.5 pM d3-EPI. Injection of alumina extracted pheochromocytoma tumor and MPC cell samples during continuous post-column injection of internal standard revealed negligible matrix effects. Absolute recovery of the analyte, d3-EPI, was estimated at 71.2%. Recoveries of the analyte, d3-EPI, relative to the d6-EPI internal standard were 99.8%. Illustrating the high precision of the method, the intra- and inter-assay coefficients of variation, for the 10 ng recombinant PNMT QC sample, were respectively 12.8% (n=10) and 10.9% (n=10). The inter-assay coefficient of variation for the tumor sample QC preparation was 13.6% (n=10). Accuracy of the method for correct determination of measured amounts of PNMT enzyme was determined at 90.4% with 95% confidence intervals of 82.3% to 98.5%.
Optimization of enzymatic reaction conditions
The natural stereoisomer, L-(-)-NE, was used as the substrate since PNMT exhibits a high degree of both stereochemical and structural specificity [16]. Similarly L-SAM was used as a methyl donor since D-SAM is nearly inactive towards PNMT [17]. Substrate concentrations of 50 μM for both NE and d3-SAM were chosen based on their Kms [6]. Using these substrate concentrations PNMT enzyme was saturated with substrate and the rate of reaction was maximized. Under these conditions the enzymatically formed d3-EPI showed a linear increase in concentrations with incubation time up to 40 minutes (Fig. 2a). A standard incubation time of 20 min was selected to ensure linearity with sufficient time to guarantee easily measurable amounts of d3-EPI.
Fig. 2.

(a) Rate of deuterium-labeled epinephrine (d3-EPI) formation using pure PNMT as a function of incubation time. Incubation was carried out at 37°C over 0 to 60 min. (b) Rate of deuterium-labeled epinephrine (d3-EPI) formation using purified PNMT as a function of enzyme protein concentration. The incubation was carried out at 37°C for 20 min. Amounts of PNMT protein used in the assay ranged from 0.01 to 100 ng.
Using commercially available purified PNMT we established that production of d3-EPI showed a linear relationship with 0.01 to 100 ng amounts of the purified enzyme added to the reaction mixture (Fig. 2b). Thus, in addition to measurements of enzyme activity, construction of calibration curves relating amount of PNMT to production of d3-EPI allows calculation of absolute amounts of the active PNMT enzyme, which we expressed as a percent of total protein.
PNMT measurements in cell line and tumor specimens
Since PNMT expression can be induced by dexamethasone, the ‘canonical’ PNMT inducer [15], we examined expression of the enzyme after incubation of MPC cells with the steroid. The activity of PNMT and the cellular contents of EPI in MPC cells were found to increase respectively by 75- and 50-fold after incubation with dexamethasone (Fig. 3). These findings are in agreement with the report by Powers et al. [15], who reported 75-fold increases in PNMT mRNA levels after dexamethasone in the MPC 10/9CRC1 cell line and 800-fold increases in the MPC 862L cell line.
Fig. 3.
Effects of dexamethasone (Dex, 1 μM; 24 h) on the expression of PNMT protein and EPI in MPC cell.
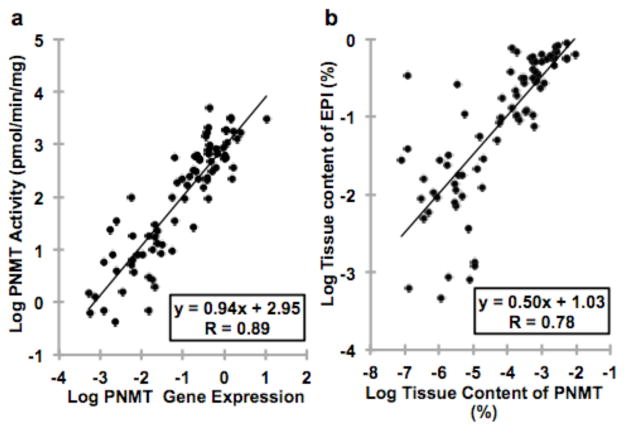
For studies in human pheochromocytomas we simultaneously isolated the protein and RNA from tumor specimens using the mirVana Paris kit (Life Technologies, Darmstadt, Germany). This simultaneous extraction enabled direct comparisons of PNMT gene expression and enzyme activity in the same portion of tumor tissue. Consequently we established a strong positive relationship (r=0.89, P<0.0001) between PNMT gene expression and enzyme activity covering a range of over 4 orders of magnitude (Fig. 4a).
Fig. 4.
(a) Correlation between the PNMT mRNA and PNMT enzyme activity (n=79). (b) Correlation between tissue content of PNMT and EPI content (as a percentage of sum of tissue NE and EPI).
Using separately obtained measurements of tumor tissue NE and EPI concentrations in other specimens from the same tumor [18], we further compared tumor tissue contents of PNMT with those of EPI (Fig. 4b). Again we established a strong positive relationship (r=0.78, P<0.0001) between tumor tissue contents of PNMT and EPI.
Our data and findings in pheochromocytoma cell lines and tumor specimens not only validate the method but also establish its utility for exploring phenotypic features of these highly heterogeneous tumors, which on the one hand depend on the underlying germ-line mutation and on the other determine diverse variations in hormonal secretory patterns and clinical presentation [18]. As the major fight-or-flight stress hormone, the clinical implications of excess circulating concentrations of EPI reach far beyond catecholamine-producing tumors to many other disorders involving stress-related etiologies. For example, the catecholamine-based etiology of myocardial dysfunction in patients with pheochromocytoma is understood to be similar to that more commonly found in stress cardiomyopathy, otherwise known as Takosubo or apical ballooning syndrome [19]. The precise, accurate and simple measurements of PNMT afforded by the presently described method provide a means to explore how variations in this enzyme may influence or contribute to this and numerous other stress-related disorders.
Conclusions
In summary, we outline a method for measuring tissue content and enzyme activity of PNMT using a double stable isotope-based UPLC-MS/MS method with measurements of enzymatically formed d3-EPI from NE and d3-SAM. Calibration with a commercial source of purified PNMT enables additional precise measurement of the mass of active enzyme. High sensitivity, low variation and simple sample preparation facilitates accurate measurements of PNMT activity. As demonstrated, the method provides new possibilities for investigations of PNMT in a variety of experimental preparations from cell lines to human tissues. For the latter application we further demonstrated utility of the method for examination of pheochromocytoma tumor specimens in which high catecholamine tissue contents precludes all but isotope-based methods of quantification. Development of this assay provides new possibilities for investigations focusing on regulation of the PNMT, the crucial final enzyme responsible for synthesis of EPI, the primary fight-or-flight stress hormone.
Acknowledgments
We gratefully acknowledge Dr. A. Tischler for providing the MPC cell line and Carmen Berndt for her expert technical assistance. This work was supported by the Deutsche Forschungsgemeinschaft (DFG) (DFG EI 855/1-1, 252/0 Microenvironment of the adrenal).
Contributor Information
Nan Qin, Institute of Clinical Chemistry and Laboratory Medicine, University Hospital Dresden, Fetscherstr 74, 01307 Dresden, Germany.
Mirko Peitzsch, Institute of Clinical Chemistry and Laboratory Medicine, University Hospital Dresden, Fetscherstr 74, 01307 Dresden, Germany.
Mario Menschikowski, Institute of Clinical Chemistry and Laboratory Medicine, University Hospital Dresden, Fetscherstr 74, 01307 Dresden, Germany.
Gabriele Siegert, Institute of Clinical Chemistry and Laboratory Medicine, University Hospital Dresden, Fetscherstr 74, 01307 Dresden, Germany.
Karel Pacak, Program in Reproducitive and Adult Endocrinology, National Institutes of Health, Bethesda MD, Maryland 20892 USA.
Graeme Eisenhofer, Institute of Clinical Chemistry and Laboratory Medicine and Department of Medicine III, University Hospital Dresden, Fetscherstr 74, 01307 Dresden, Germany.
References
- 1.Kvetnansky R, Sabban EL, Palkovits M. Catecholaminergic systems in stress: structural and molecular genetic approaches. Physiol Rev. 2009;89:535–606. doi: 10.1152/physrev.00042.2006. [DOI] [PubMed] [Google Scholar]
- 2.Wong DL, Tai TC, Wong-Faull DC, et al. Epinephrine: A Short- and Long-Term Regulator of Stress and Development of Illness : A Potential New Role for Epinephrine in Stress. Cell Mol Neurobiol. 2011 doi: 10.1007/s10571-011-9768-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hara MR, Kovacs JJ, Whalen EJ, et al. A stress response pathway regulates DNA damage through beta2-adrenoreceptors and beta-arrestin–1. Nature. 2011;477:349–353. doi: 10.1038/nature10368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong DL, Siddall BJ, Ebert SN, et al. Phenylethanolamine N-methyltransferase gene expression: synergistic activation by Egr-1, AP-2 and the glucocorticoid receptor. Brain Res Mol Brain Res. 1998;61:154–161. doi: 10.1016/s0169-328x(98)00225-3. [DOI] [PubMed] [Google Scholar]
- 5.Axelrod J. Purification and properties of phenylethanolamine-N-methyl transferase. J Biol Chem. 1962;237:1657–1660. [PubMed] [Google Scholar]
- 6.Beaudouin C, Haurat G, Fraisse L, et al. Assay of phenylethanolamine N-methyltransferase activity using high-performance liquid chromatography with ultraviolet absorbance detection. J Chromatogr. 1993;613:51–58. doi: 10.1016/0378-4347(93)80196-b. [DOI] [PubMed] [Google Scholar]
- 7.Borchardt RT, Vincek WC, Grunewald GL. A liquid chromatographic assay for phenylethanolamine N-methyltransferase. Anal Biochem. 1977;82:149–157. doi: 10.1016/0003-2697(77)90143-9. [DOI] [PubMed] [Google Scholar]
- 8.Burke WJ, Hanson DM, Chung HD. A highly sensitive assay for phenylethanolamine N-methyltransferase in human brain. Proc Soc Exp Biol Med. 1986;181:66–70. doi: 10.3181/00379727-181-42225. [DOI] [PubMed] [Google Scholar]
- 9.Connett RJ, Kirshner N. Purification and properties of bovine phenylethanolamine N-methyltransferase. J Biol Chem. 1970;245:329–334. [PubMed] [Google Scholar]
- 10.Kennedy B, Elayan H, Ziegler MG. Glucocorticoid induction of epinephrine synthesizing enzyme in rat skeletal muscle and insulin resistance. J Clin Invest. 1993;92:303–307. doi: 10.1172/JCI116567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee M, Nohta H, Ohkura Y, et al. Determination of phenylethanolamine N-methyltransferase by high-performance liquid chromatography with fluorescence detection. J Chromatogr. 1985;348:407–415. doi: 10.1016/s0021-9673(01)92479-3. [DOI] [PubMed] [Google Scholar]
- 12.Sole MJ, Hussain MN. A simple specific radioenzymatic assay for the simultaneous measurement of picogram quantities of norepinephrine, epinephrine, and dopamine and in plasma and tissues. Biochem Med. 1977;18:301–307. doi: 10.1016/0006-2944(77)90064-3. [DOI] [PubMed] [Google Scholar]
- 13.Trocewicz J, Kato N, Oka K, et al. Determination of phenylethanolamine N-methyltransferase activity in rat brain by high-performance liquid chromatography with fluorometric detection. J Chromatogr. 1982;233:328–333. doi: 10.1016/s0378-4347(00)81762-4. [DOI] [PubMed] [Google Scholar]
- 14.Trocewicz J, Oka K, Nagatsu T. Highly sensitive assay for phenylethanolamine N-methyltransferase activity in rat brain by high-performance liquid chromatography with electrochemical detection. J Chromatogr. 1982;227:407–413. doi: 10.1016/s0378-4347(00)80393-x. [DOI] [PubMed] [Google Scholar]
- 15.Evinger MJ, Powers JF, Tischler AS. Transcriptional silencing of glucocorticoid-inducible phenylethanolamine N-methyltransferase expression by sequential signaling events. Exp Cell Res. 2007;313:772–781. doi: 10.1016/j.yexcr.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 16.Patil PN, Lapidus JB. Stereoisomerism in adrenergic drugs. Ergeb Physiol. 1972;66:213–260. doi: 10.1007/3-540-05882-6_4. [DOI] [PubMed] [Google Scholar]
- 17.Borchardt RT, Shiong Y, Huber JA, et al. Potential inhibitors of S-adenosylmethionine-dependent methyltransferases. 6. Structural modifications of S-adenosylmethionine. J Med Chem. 1976;19:1104–1110. doi: 10.1021/jm00231a005. [DOI] [PubMed] [Google Scholar]
- 18.Eisenhofer G, Pacak K, Huynh TT, et al. Catecholamine metabolomic and secretory phenotypes in phaeochromocytoma. Endocr Relat Cancer. 2011;18:97–111. doi: 10.1677/ERC-10-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lyon AR, Rees PS, Prasad S, et al. Stress (Takotsubo) cardiomyopathy--a novel pathophysiological hypothesis to explain catecholamine-induced acute myocardial stunning. Nat Clin Pract Cardiovasc Med. 2008;5:22–29. doi: 10.1038/ncpcardio1066. [DOI] [PubMed] [Google Scholar]