Abstract
Purpose:
Long-term antiglaucomatous drug administration may cause irritation, dry eye, allergy, subconjunctival fibrosis, or increased risk of glaucoma surgery failure, potentially due to the preservative benzalkonium chloride (BAK), whose toxic, proinflammatory, and detergent effects have extensively been shown experimentally. We hypothesize that BAK also influences trabecular meshwork (TM) degeneration.
Methods:
Trabecular specimens were examined using immunohistology and reverse transcriptase–polymerase chain reaction. A trabecular cell line was stimulated by BAK and examined for apoptosis, oxidative stress, fractalkine and SDF-1 expression, and modulation of their receptors. An experimental model was developed with BAK subconjunctival injections to induce TM degeneration. Mass spectrometry (MS) imaging assessed BAK penetration after repeated instillations in rabbit eyes.
Results:
Trabecular specimens showed extremely low densities of trabecular cells and presence of cells expressing fractalkine and fractalkine receptor and their respective mRNAs. Benzalkonium in vitro induced apoptosis, oxidative stress, and fractalkine expression and inhibited the protective chemokine SDF-1 and Bcl2, also inducing a sustained intraocular pressure (IOP) increase, with dramatic apoptosis of trabecular cells and reduction of aqueous outflow. MS imaging showed that BAK could access the TM at measurable levels after repeated instillations.
Conclusion:
BAK enhances all characteristics of TM degeneration typical of glaucoma—trabecular apoptosis, oxidative stress, induction of inflammatory chemokines—and causes degeneration in acute experimental conditions, potentially mimicking long-term accumulation. BAK was also shown to access the TM after repeated instillations. These findings support the hypothesis that antiglaucoma medications, through toxicity of their preservative, may cause further long-term trabecular degeneration and therefore enhance outflow resistance, reducing the impact of IOP-lowering agents.
INTRODUCTION
RATIONALE
Primary open-angle glaucoma (POAG) is the second overall leading cause of blindness and the leading cause of irreversible blindness in the world. POAG affects approximately 70 million people and is predicted to account for over 11 million cases of blindness by 2020 despite great progress in medical and surgical therapeutic strategies. Its prevalence continues to increase as the population ages.1 POAG is an optic neuropathy commonly initiated by an elevation of intraocular pressure (IOP). Abnormally elevated IOP, the most critical risk factor for POAG, is attributed to an increase in trabecular meshwork (TM) outflow resistance to aqueous humor (AH), resulting from trabecular degeneration, a process that is still not clearly understood but is associated with TM cell apoptosis, changes in extracellular matrix, oxidative stress, and increased cytokines in AH.
The current therapeutic strategy against POAG is mainly based on reducing IOP. In clinical practice, progressive therapeutic inefficacy marked by insufficient control of IOP associated with progression of retinal ganglion cell degeneration is often observed, leading to the need to combine two or more treatments to temporarily halt disease progression.2 This need to use several drugs over the long term is a common observation during the course of POAG, with possible harmful side effects developing over time, especially on the ocular surface, a fragile structure that protects the eye from environmental challenges and is most exposed to pollutants and xenobiotics.
The need for sterility in multidose eyedrops requires that an antimicrobial preservative be included in these solutions, most frequently the quaternary ammonium benzalkonium chloride (BAK). Although BAK provides useful antimicrobial properties in ophthalmic preparations, long-term exposure to BAK-containing eyedrops has been shown in a large variety of reports and experiments to cause chronic ocular inflammation over the ocular surface, resulting in irritancy symptoms, treatment discontinuation, and iatrogenic dry eye disease.3 Interestingly, BAK applied to the ocular surface may also infiltrate the eye, possibly accumulating in deeper ocular structures, such as the TM, especially after repeated use of several preservative-containing eyedrops over decades, and potentially causing harmful effects to this structure.4–6 Based on the literature and the results accumulated on BAK effects on ocular surface tissues, we wished to explore the hypothesis that, in addition to its impact on the ocular surface, BAK contained in antiglaucoma treatments could play a role in trabecular dysfunction, resulting in further damage to the TM with a possible impact on AH outflow and consequently IOP, even though drugs are specifically aimed at decreasing IOP.
BENZALKONIUM CHLORIDE, THE MOST WIDELY USED PRESERVATIVE
The pharmacopoeia recommends that eyedrops contain a preservative to prevent or limit microbial proliferation after the bottle is opened, which could induce a risk of potentially severe eye infection as well as the alteration of the formulation. Preservatives used in ophthalmic preparations belong to a variety of chemical families, including mercury derivatives, alcohols, parabens, EDTA, and chlorhexidine, but quaternary ammonium compounds, due to their low allergenic effects and apparently good safety profiles, have progressively become the major preservatives in use today. Quaternary ammoniums are bipolar compounds, which are highly hydrosoluble and have surfactant properties. They act mainly via their detergent properties, which dissolve and destroy the bacterial walls and membranes. BAK is an alkylbenzyldimethylammonium chloride mixture composed of C12 and C14 chains, commonly used at concentrations ranging from 0.004% to 0.025%. A large number of clinical and experimental investigations using in vitro or animal models suggested cytotoxic effects of BAK on several components of the eye.
THE OCULAR SURFACE IN GLAUCOMATOUS PATIENTS
Several consistent observational studies have pointed out the higher prevalence of dry eye in glaucomatous patients treated over the long term compared to the expected frequency of dry eye in the general population.7–10 Ocular symptoms and signs are therefore observed at rates ranging from 15% to 50%, much higher than in nonglaucomatous patients.11 In addition, a number of studies have clearly related the rate of symptoms or signs to the presence of BAK7,8 and the number of BAK-containing medications,10 and switching from BAK-containing to unpreserved eyedrops significantly improved these patients.7,12 From the literature, it appears that a chronic inflammatory process underlies dry eye development in glaucomatous patients, as has been clearly demonstrated by various techniques. Histopathological techniques in conjunctival specimens taken at the time of glaucoma surgery clearly demonstrated that inflammation is abnormally present in the conjunctival epithelium and substantia propria. Inflammatory infiltrates and a decrease in goblet cell density were found to be associated with longer duration of treatment and a higher number of medications.13,14 These data were directly correlated to risk of surgical failure.15 Similarly, in patients receiving medium- and long-term therapy, Nuzzi and colleagues16 described an increased fibroblast density in deep conjunctival tissues and a more compact connective tissue, richer in collagen fibers arranged in whirls, with inflammatory elements and increased expression of inflammatory markers.
An immunohistological study was also conducted in conjunctival biopsies and TM specimens from patients undergoing glaucoma surgery after various durations and numbers of medical treatments. Interestingly, specimens from patients who were receiving treatments had significantly greater expression of fibroblastic and inflammatory markers in both the conjunctiva and the TM, compared with cases of primary surgery or short-term treatments.4 Various innovative immunochemistry and flow cytometry techniques were also developed in impression cytology specimens, allowing to demonstrate in a minimally invasive way the ocular surface abnormalities in treated glaucomatous patients, the relationship with the number of eyedrops (ie, the amounts of BAK instilled over the long term), and the level of ocular surface inflammation.17,18
Using immunocytological and flow cytometry methods, it was also possible to measure HLA-DR expression in impression cytology specimens. HLA-DR antigens are normally not expressed by epithelial cells, except in inflammatory processes. Therefore, HLA-DR has become a very reliable marker for demonstrating that epithelial cells may be involved in inflammatory ocular surface diseases and for quantifying the level of inflammation. In the first immunocytological study investigating glaucoma, high rates of HLA-DR were found in patients treated with BAK-containing eyedrops compared to normal eyes and a group receiving chlorhexidine-containing eyedrops, for the first time highlighting a possible role played by BAK in conjunctival changes of glaucomatous patients.19
The technique of flow cytometry was further developed in impression cytology and repeatedly demonstrated that glaucoma patients, even though clinically asymptomatic, exhibit significant overexpression of HLA-DR class II antigens, ICAM-1, interleukins IL-6, IL-8, and IL-10, as well as CCR4 or CCR5 in the epithelium. Preserved drugs and multiple treatments reliably showed higher levels of inflammatory markers or cytokines compared to patients receiving unpreserved eyedrops and normal control eyes in patients of the same age.20–23
IN VITRO EFFECTS OF BAK ON OCULAR SURFACE CELLS
Based on human findings, new in vitro cytotoxicity tests were developed using cytofluorimetric microtitration and flow cytometry. The techniques of MiFALC (microtitration fluorimetric assays on live cells) allow tests on live cells, with series of complementary tests evaluating cell viability, apoptosis, and oxidative stress, each assessed by at least two biological tests. The potential toxicity of many antiglaucoma drugs and their preservatives has been investigated in Chang-derived conjunctival cells, a human conjunctiva-derived cell line widely used for toxicological purposes, and more recently in another conjunctival cell line, IOBA-NHC, a cell line developed by Dr Diebold, Valladolid, Spain.24 Even at very low concentrations, BAK appeared to be cytotoxic for ocular cells and responsible for cell death, apoptosis, and free radical production. Conversely, most of the active compounds tested, namely, beta-blockers and prostaglandin analogs, induced no apoptotic mechanism or pro-oxidative stress, which confirmed the main, if not exclusive, toxicity of BAK.23,25–32 From all these in vitro studies, it appeared that BAK induces a significant, concentration-dependent decrease in cellular viability as well as chromatin condensation associated with an alteration of mitochondrial activity and a decrease in glutathione, suggesting an apoptotic phenomenon. The proapoptotic effects were seen at very low concentrations of BAK with a threshold of toxicity found at about 0.005% (ie, below the usual concentration used in most eyedrops). In addition, reactive oxygen species (ROS) production was found with preserved formulations at significantly higher levels than those observed with unpreserved drugs.29,30 All these findings were in line with data from the literature reported by different groups that used other techniques but came to the same conclusions.33–35
However, despite their convenience and reliability, in vitro experiments on immortalized cells do not fully reflect the conditions of mucosa in vivo, as isolated cells may be more sensitive than tissues, and a tissue may also involve various cell components in complex interactions, namely, epithelial, inflammatory, and mucous cells. Therefore, the search for more appropriate models than cell monolayers has led to complex three-dimensional (3D) epithelial systems that are useful for toxicological studies. The reconstructed 3D model of human corneal epithelial cells, supplied by SkinEthic Laboratories (Nice, France), was found to resemble the corneal epithelium of the human eye in morphology and thickness and was proposed as a useful toxicological model for the assessment of eye irritation potential of chemicals and cosmetic products.36,37
Using this model, consistent with what was previously observed in monolayer cell models, BAK confirmed a dose-dependent response with significant toxic effects at concentrations as low as 0.005%.38,39 The most superficial cell layer, namely, the cells most exposed to the toxic effect, showed high TUNEL positivity, quite consistent with apoptotic and cell death features observed in the monolayer model, whereas deeper cell layers, which received much lower doses of toxic compounds, showed the activation of caspase-3, consistent with the early stage of apoptosis and demonstrating the range of effects that BAK may cause in a multilayered epithelium. At the same time, BAK increased ICAM-1 expression in the corneal epithelium, an adhesion molecule related to inflammation and inflammatory cell recruitment. Indeed, overexpression of ICAM-1 by conjunctival cells has been observed in glaucomatous patients treated over the long term.23 BAK also induced a dose-dependent disruption of the epithelial tight junctions in the superficial cells. As a possible compensatory mechanism, BAK stimulated the expression of Ki67, a cell marker of proliferation, most likely to replace dead cells and increase epithelial turnover. These results illustrate the tissue-level response to a toxic environment, most likely on stimulatory and inflammatory modes, in contrast to the cell-level response, which mainly responds in a binary cell death mode.
EXPERIMENTAL DATA IN ANIMAL MODELS
The assessment of the acute eye irritation potential of chemicals, cosmetics, and pharmaceuticals is still based on the Draize rabbit test proposed in 1944, a method widely criticized by animal welfare advocates and whose relevance, validity, and precision have been challenged because of the variability and low predictiveness of the human response.40 The rabbit model showed weaknesses in assessing nonsevere irritants and low concentrations of toxic compounds. Histological models in rats were also further developed. To overcome the natural resistance of healthy rats and the high variability of ocular behavior in these animals, higher concentrations are often required for toxicological purposes. Concentrations of 0.25% and 0.5% caused epithelial denudation, major damage in the deep structures such as stromal inflammation and neovascularization, and loss of endothelial visibility and fibrosis, with incomplete corneal recovery even long after toxic substance removal.41 At lower concentrations, ocular lesions due to a short duration of treatment in healthy animals are mild and more difficult to identify. Nevertheless, histological analyses comparing BAK-containing and BAK-free compounds consistently favored BAK-free compounds.4
To improve the reliability of standard clinical assessment and postmortem histology, corneal in vivo confocal microscopy (IVCM) was developed; using the recently developed Rostock Cornea Module of the Heidelberg Retina Tomograph (HRT/RCM; Heidelberg Engineering, Heidelberg, Germany), this is a nontraumatic technique for investigating the ocular surface, which provides histological-like levels of resolution, close to 1 μm. Various applications have been developed in human diseases,42–44 and the device has been further adapted to animal use.41,45–48 A scoring system was proposed to assess mild toxic changes in the ocular surface, today available for routine purposes.49 However, as rat models are poorly reliable, and despite the improvement provided by IVCM, other attempts have been made using animal models closer to human eyes, especially rabbits.
Ichijima and colleagues50 developed an acute stress mode suitable for mimicking the effects of long-term use of low-toxicity compounds over a short period of time and used IVCM to assess the toxicological profiles of BAK and antiglaucomatous drugs, further confirming the corneal toxicity of even low concentrations of BAK. Combined with clinical assessment, impression cytology, and immunohistology, the new-generation IVCM recently confirmed the overall toxicity of BAK and BAK-containing eyedrops, in a dose-dependent manner. The absence of toxicity of preservative-free prostaglandins was also clearly demonstrated, and, interestingly, excipients based on cationic emulsions were found to decrease BAK toxicity.46,47 In addition, in these experiments, it was possible to identify in vivo in rabbits the conjunctiva-associated lymphoid tissue,48 and a major inflammatory infiltration was found after an acute challenge with BAK or BAK-containing eyedrops, a model that is known in toxicology to reproduce long-term administration. This finding demonstrated immunoinflammatory involvement not restricted to the conjunctiva, but extending toward deep structures and most likely the immune system distant from the instillation site, as was found in animal models of dry eye, another closely related ocular surface disease.51 Additionally, as in dry eye, another major finding in animals challenged with BAK was a dramatic decrease in goblet cells. Similarly, Kahook and Noecker52 found significantly lower densities of goblet cells in animals receiving BAK-containing latanoprost compared to preservative-free artificial tears. Such findings are clearly in line with human findings of reduced goblet cell densities in patients treated with BAK-containing eyedrops.23,53,54
POSSIBLE IMPACT OF BAK ON DEEP OCULAR STRUCTURES
From all the previously mentioned studies and surveys, in humans, in experimental models, and in vitro, broad consensus has been reached that BAK causes a variety of ocular surface changes, including dry eye and allergic and immunoinflammatory reactions, with overexpression and/or synthesis of class II antigens, adhesion molecules, chemokines, chemokine receptors, interleukins, or cell death markers and mediators. Additionally, the substantia propria of the conjunctiva has been shown to be infiltrated by inflammatory cells and fibroblasts.4,13–16 Other reports have hypothesized that BAK plays a role in cataract development55 and the higher incidence of angiographic cystoid macular edema after cataract surgery.56 Based on the findings that the preservative causes increased synthesis of proinflammatory mediators and intensified postoperative inflammation, the term “pseudophakic preservative maculopathy” was even proposed by Miyake et al.56
Little is known about BAK pharmacokinetics, but this compound was shown to accumulate in the conjunctiva, since only a single drop of BAK could cause measurable amounts in the conjunctiva up to 7 days after instillation.57 No data are available in the literature for the TM, but it is highly likely that BAK accumulates in deep tissues after prolonged administration. In addition, the impact of inflammatory and toxic reactions in the ocular surface following long-term treatment with BAK-containing eyedrops, and clearly extending to deep conjunctiva and conjunctiva-associated lymphoid tissues, remains to be elucidated. Therefore, the consequences of high rates of cell death and inflammatory signals on deeper but still very close structures, such as the TM, cannot be excluded, and this hypothesis deserves further attention, gathered herein in a comprehensive series of experiments and investigations.
HYPOTHESES
We therefore speculate that BAK could accumulate in the TM and could cause trabecular dysfunction or degeneration by directly inducing chronic inflammation, inflammatory cytokine release, immune cell infiltration, and TM cell apoptosis, adding to the trabecular pathology characteristic of POAG. We may also raise the hypothesis that the previously demonstrated BAK-induced ocular surface toxicity could indirectly affect the very close structure comprising the TM, by diffusion of nearby inflammation and chemokine release. As a result, corneal and conjunctival epithelial cells could be considered not only as targets but also as stimulators of chronic inflammatory processes, further affecting the TM via roundabout pathways. Third, based on previous works on the TM,4–6 both ex vivo and in vitro, and glaucoma models, we assume that the TM is, in turn, a large source of inflammatory cytokines and, in particular, of chemokines. Therefore, the damage to TM would further cause release of chemoattractants, enhancing inflammatory infiltration and TM cell damage and degeneration.
At this time, these hypotheses cannot be confirmed in human experimental conditions, because almost all medications contain an IOP-lowering agent associated with the potentially toxic preservative; the result of this combination over the long term is very difficult to interpret and most likely depends on time, number of drugs administered, and individual responses, since glaucomatous patients are probably more sensitive with an already damaged TM. Additionally, drug-induced or drug-enhanced TM degeneration would likely take a long time to develop to significant levels, and it would be difficult and timely to conduct a prospective study comparing TM function over the long term with preserved and unpreserved drugs. This series of works therefore is aimed at collecting a range of experimental data, even though indirect and nonclinical, that would eventually support the hypothesis that BAK might have toxic side effects over the long term and would contribute to some extent to further aggravate glaucomatous TM degeneration. They could further be a basis for designing clinical studies investigating drug-induced TM degeneration in humans and compare preserved and unpreserved formulations in the long run.
METHODS
HUMAN TM SPECIMENS AND HUMAN GLAUCOMATOUS TM CELL LINE
A total of 50 human TM samples were obtained from 47 patients undergoing surgical nonpenetrating trabeculectomy. All patients presented with POAG without any other interfering ocular pathology or systemic disease. In all cases, surgery was required by glaucoma progression despite maximally tolerated medical treatment. All patients were receiving at least three different drugs, in fixed or unfixed combinations, totaling at least 3 drops a day of preservative-containing compounds. For ethical reasons, we could not obtain normal TMs or TM from untreated patients. Experiments were conducted in the Clinical Investigation Centre for Ocular Surface Pathology (INSERM 503, Quinze-Vingts National Ophthalmology Hospital, Paris, France) in accordance with the Declaration of Helsinki, Edinburgh (Scotland) amendment, 2000. Ethics committee approval was obtained (#10793, CPP Paris Ile de France V), and each patient gave informed consent for analyzing surgically obtained trabecular specimens.
After careful dissection of the TM under a deep scleral flap, the external trabeculum, composed of the inner wall of the Schlemm canal and corneoscleral trabeculum, as previously described,5 was gently removed and transferred onto a glass slide for immunohistological analyses (35 specimens) or immediately frozen in liquid nitrogen for reverse transcriptase–polymerase chain reaction (RT-PCR) (15 specimens).
Additionally, the human TM-derived cell line HTM3, an unrestricted gift from Alcon Laboratories (Fort Worth, Texas), was used for in vitro experiments. This immortalized cell line derived from a male 72-year-old patient with POAG whose IOP was controlled by topical beta-blocker at the time of death. The transformation characteristics and procedures have been reported previously.58 Trabecular cells were routinely cultured in standard humidified 5% CO2 atmosphere in serum-free Dulbecco’s Modified Eagle Medium (DMEM) (Gibco, Invitrogen, Carlsbad, California) supplemented with 4 mM l-glutamine, 10% fetal bovine serum, and 50 μg/mL gentamicin. For passages, monolayers were rinsed with phosphate-buffered saline (PBS), dislodged by trypsinization (0.25% trypsin, 0.02% EDTA), then cultured from an initial concentration of 80,000 cells/mL. All cells were used at passages 10 to 20.
TRABECULAR MESHWORK CELL STIMULATIONS AND TOXICOLOGICAL ASSAYS
After subconfluence was attained (culture surface covering 80%), cells were exposed to different stimulations. According to the experiments and their objectives, we used increasing concentrations of BAK (C-12 homolog 65.5 % w/w, C-14 homolog 34.6 % w/w; Sigma Aldrich, Fluka, Buchs, Switzerland), varying from 5×10−5% to 5×10−2%, the lower concentrations used for inflammatory assessments and higher concentrations (ie, those found in commercial eyedrops) for toxicological purposes. We also tested the induction of chemokines by tumor necrosis factor–α (TNF-α) (100 ng/mL, human recombinant TNF-α; R&D Systems, Abingdon, United Kingdom). For toxicological assessments, incubation times were 5 – 30 minutes, whereas proinflammatory challenges at low BAK were performed after 24 hours incubation. Regarding oxidative stress, commercial solutions of prostaglandin analogs, namely, latanoprost 0.005% (Xalatan; Pfizer, New York, New York), travoprost 0.004% (Travatan; Alcon, Fort Worth, Texas), and bimatoprost 0.03% (Lumigan; Allergan, Irvine, California), preserved with, respectively, BAK 0.02%, 0.015%, and 0.005%, the preservative-free tafluprost 0.0015% (Taflotan; Santen Oy, Tampere, Finland), and their corresponding BAK concentrations were tested. Additionally, as SDF-1 (CXCL12) is known in some systems to exert protective antiapoptotic properties,59 we analyzed the effects of BAK at concentrations ranging from 0.0001% to 0.01% on expressions of CXCL12 and its receptor CXCR4 (mouse IgG1 anti-human CXCL12 and mouse IgG2B anti-human CXCR4 monoclonal antibodies, both from R&D Systems), and those of CXCL12 on TM cell proliferation and apoptosis in a cellular model of toxicity induced by a pretreatment with BAK. Briefly, 80% confluent TM cells were incubated in PBS with 0.01% BAK for 15 minutes in order to induce approximately 50% apoptosis. Cells were washed with PBS, incubated in free medium only (control) or with CXCL12 (10 ng/mL human CXCL12 7.8 kDa, R&D Systems) for a 24-hour recovery period. Microplate cytofluorometry was performed on a Safire microplate reader (Tecan Instruments, Lyon, France) using apoptosis-related assays. All these in vitro experiments were performed in triplicate.
IMMUNOHISTOLOGICAL TECHNIQUES
Trabecular meshwork cells were grown on 22-mm glass cover slips, seeded and stimulated, then dried and fixed in 4% paraformaldehyde for 15 minutes. Nonspecific binding was blocked with normal goat serum in PBS and 0.1% Triton X100 for 60 minutes, washed three times in PBS with 1% bovine serum albumin (BSA), then incubated for 60 minutes at 4°C with the primary antibody. After three more washings, cells were counterstained with the secondary fluorescent antibody for 2 hours at room temperature. Specimens were finally washed three times in PBS, counterstained with propidium iodide or DAPI for 3 minutes (Sigma-Aldrich), and mounted in gel mounting medium. Isotype-matched monoclonal antibodies were used as controls for each sample and each primary antibody. Immunocytology was used for assessing cell morphology (Alexa Fluor 488 phalloidin staining; Molecular Probes, Eugene, Oregon), vimentin (Dako, Glostrup, Denmark), active caspase-3 (BD Pharmingen, Franklin Lakes, New Jersey), fractalkine CX3CL1 (R&D systems), or CX3CR1 (Imgenex, San Diego, California). A similar technique was used for immunohistology in surgically removed TM specimens. The protocol was the same as the one used for cultured cells, except for the incubation time, which was prolonged to 3 hours for each primary antibody, to allow better penetration in the TM. Immunolocalization of SDF-1/CXCL12, its receptor CXCR4, fractalkine, its receptor CX3CR1, and cell nuclei was performed in at least eight TM specimens each, as TM tissues could allow simultaneous examination of only one marker and TM cell nuclei. Vimentin staining was also performed to assess inflammatory cell infiltration. Samples were examined using a laser confocal microscope (E800, PCM2000; Nikon, Champigny-sur-Marne, France).
FLOW CYTOMETRY IN TM CELL CULTURES
Human trabecular meshwork (HTM) cells were plated in 6-well plates (Costar, Cambridge, Massachusetts) and grown to 80% confluence. After stimulations with BAK or TNF-α, cells were carefully detached, suspended in PBS containing 1% BSA, blocked with normal rabbit serum, and incubated with the primary antibodies—anti-fractalkine, anti-CX3CR1, anti-CXCR4, anti-CXCL12 (R&D Systems)—or expression of the anti-apoptotic protein Bcl2 (Dako) for 1 hour on ice. They were then washed three times with cold 1% BSA in PBS and incubated for 30 minutes with FITC-conjugated goat anti-mouse antibody. Negative controls were incubated with isotype-matched primary antibodies. Cells were fixed in 0.5% paraformaldehyde and analyzed on a FC 500 flow cytometer (Beckman Coulter, Miami, Florida). The results were reported as percentages of positive cells among the gated cells or as the relative “fold” change of the mean fluorescence intensity (MFI) compared to unstimulated controls.
COLD LIGHT CYTOFLUORIMETRY FOR BAK TOXICITY ASSESSMENTS
Cells were cultured under standard conditions and were seeded on plates to be at 80% to 85% confluence before BAK treatment. According to previously validated studies on conjunctival cells, we used a protocol based on 15 minutes of treatment with several dilutions of BAK or prostaglandin analogs followed by 24 hours of cell recovery.25–28 The BAK concentrations used were equivalent to or lower than the concentrations of commercially available eyedrops, namely, 0.005% to 0.02%. Viability tests using microtitration fluorescence assays in live cells were performed on the Safire microplate reader with the neutral red test, based on cell membrane integrity, and the alamar blue test, based on cellular enzyme activity. Apoptosis-related membrane permeability was evaluated by the same technique using the YO-PRO-1 assay (Molecular Probes, Eugene, Oregon), based on P2X7 membrane pore opening that leads to apoptosis in challenged cells, and Hoechst 33342/propidium iodide (Molecular Probes) revealing chromatin condensation during apoptosis. Oxidative stress was also assessed using the total ROS measurement (H2DCF-DA test, Molecular Probes) and superoxide anion (hydroethidine test, Molecular Probes), using protocols already described in previous studies in conjunctival cell lines.29,31
QUANTITATIVE RT-PCR
Total mRNA was isolated from cultured trabecular cells using the NucleoSpin RNA II extraction kit (Macherey-Nagel, Düren, Germany) and was then reverse-transcribed (TaqMan reverse transcription reagents; Applied Biosystems, Foster City, California). Levels of mRNA were assessed using RT 7300 (Applied Biosystems) and assays-on-demand primers for human CX3CL1, CX3CR1, and S18. Relative quantitation of target genes was calculated according to the comparative Ct method (ie, normalized to an endogenous control S18 gene and relative to a calibrator after calculating the efficiency coefficient). For the 15 surgical human trabecular tissues, mRNA was extracted using the NucleoSpin RNA XS kit (Macherey-Nagel), adapted to very small samples, then conditioned and determined following the same previously described procedure.60 The results are presented as the relative “fold” change compared to unstimulated control. A negative control was routinely used omitting mRNA from the RT reaction mixture. In the 15 postsurgery samples used for RT-PCR, 10 patients had received topical 0.1% indomethacin in the preoperative period, used for its anti-inflammatory properties, as this treatment had previously been shown to reduce inflammatory cells in TM specimens.5 Despite the relatively low number of specimens in each group, comparative measurements were made between treated and untreated patients.
ANIMAL MODEL OF BAK-INDUCED TM DEGENERATION
Animals and IOP Monitoring
Six male 8-week-old Long-Evans rats weighing 300 to 350 gm were used (Janvier, Le Genest St Isle, France). Animals were kept in pathogen-free conditions with food and water available ad libitum and housed in a 12-hour light/12-hour dark cycle. Ocular integrity was checked using the slit-lamp biomicroscope. On D0, a first subconjunctival injection of 0.01% BAK (100 μL) was given in the right eye, whereas the left eye received PBS only as control. A second injection was given 7 days later, following the same protocol. Animals were monitored daily for IOP using a handheld tonometer (TonoLab; Medtronics, Jacksonville, Florida) without sedation. All experiments were conducted in accordance with the Association for Research in Vision and Ophthalmology for the Use of Animals in Ophthalmic Research.
In Vivo Outflow Facility Measurement
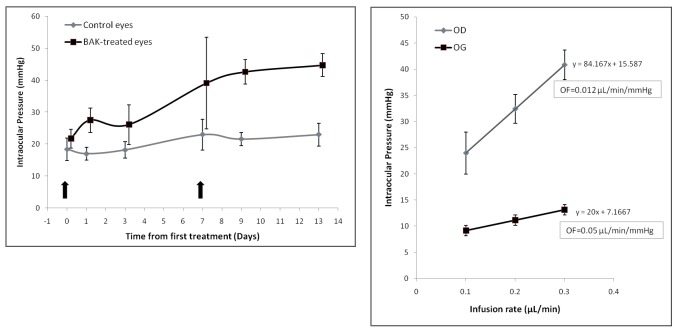
Six days after the second injection of BAK, trabecular outflow facility was measured in vivo under general anesthesia (intraperitoneal injection of ketamine, 75 mg/kg [Imalgene 500; Merial, Lyon, France] and xylazine, 10 mg/kg [Bayer, Puteaux, France]). Briefly, the eyes were anteriorly cannulated with a 30-gauge needle connected by tubing to a 1-mL syringe filled with PBS and loaded into a microdialysis infusion pump (World Precision Instruments, Sarasota, Florida). IOP was measured after a 10-minute stabilization period for three different infusion flow rates (0.1, 0.2, and 0.3 μL/min). Aqueous humor outflow facility (μL/min/mm Hg) was calculated as the reciprocal of the slope of the respective ocular pressure/flow rate curves.61
Ex Vivo TM Cell Apoptosis
At the end of the experiments, the animals were euthanized and the eyes were immediately conditioned for terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL). The eyes were fixed in 4% paraformaldehyde, embedded in an optimal cutting-temperature compound (OCT) (Tissue-Tek; Miles Inc, Bayer Diagnostic, Puteaux, France), and cut into 15-μm cryosections. A TUNEL assay (Roche Diagnostics, Meylan, France) was performed to detect apoptosis in rat ocular tissues following the manufacturer’s instructions. Specimens were mounted in aqueous mounting medium with DAPI to be further analyzed using light epifluorescence microscopy. TUNEL-labeled cells were observed and counted in five 0.01-mm² fields in each sample in order to compare the trabecular density of apoptotic cells between groups.
MASS SPECTROMETRY IMAGING OF BAK IN RABBIT EYES
Mass spectrometry (MS) imaging is the only analytical method capable of providing the spatial distribution of a wide range of molecules over the surface of a biological sample in a single run. It was used for assessing BAK distribution after instillation in animals. The eyes of four New Zealand albino rabbits were instilled twice a day with 2 drops of a BAK solution at 0.01% in saline, a commonly used concentration in eyedrops, for 1 month (two rabbits) or 5 months (two rabbits). One additional rabbit was instilled with preservative-free saline for 5 months and used as control. After sacrifice, the eyes were quickly enucleated and embedded in tragacanth gum and frozen at −80°C. Serial cryosections (14 μm thick) were performed at −30°C with a CM3050-S cryostat (Leica Microsystems SA, Nanterre, France) and alternately deposited on glass slides for immunohistological control and on silicon wafers (2-inch-diameter polished silicon wafers; ACM, Villiers-Saint-Frédéric, France) for MS imaging. Time-of-flight (ToF) secondary ion mass spectrometry (SIMS) (ToF-SIMS) or matrix-assisted laser desorption/ionization imaging (MALDI)-ToF was used in these experiments. To identify the ocular structures with BAK signal, the rabbit eyes were stained with hematoxylin-eosin. Most eyes were cut horizontally and some vertically to investigate possible differential distribution of BAK after instillation. To assess the specificity of the peaks obtained by MS imaging, mass spectra of the pure standard compounds of BAK, which correspond to a mixture of benzododecinium C12 (C21H38N+, m/z 304.32) and myristalkonium C14 (C23H42N+, m/z 332.36), were assessed separately. SIMS was performed using a commercial ToF-SIMS IV mass spectrometer (ION-TOF GmbH, Münster, Germany). For MALDI-ToF MS, after matrix deposition, the eye sections were analyzed in an Ultraflex III TOF/TOF instrument (Bruker Daltoniks GmbH, Bremen, Germany) and an AutoFlex Speed (Bruker Daltoniks GmbH). Optical images were recorded with an Olympus BX51 microscope (Olympus, Rungis, France) equipped with ×1.25 to ×50 lenses and a Color View I camera, monitored by CellB software (Soft Imaging System, GmbH, Münster, Germany).
STATISTICAL ANALYSES
All data in the text and in bar graphs are reported as means (SEM) and represent at least three independent experiments. Statistical comparisons were performed using analysis of variance (ANOVA) with Statview 4.0 for Windows (Abacus Concepts, Berkeley, California). The significance threshold was chosen at P<.05%. For quantitative RT-PCR, increases in mRNA levels were considered significant when superior to twofold change relative to control.
RESULTS
TRABECULAR MESHWORK SPECIMENS ASSOCIATE CELL DEATH AND INFLAMMATION
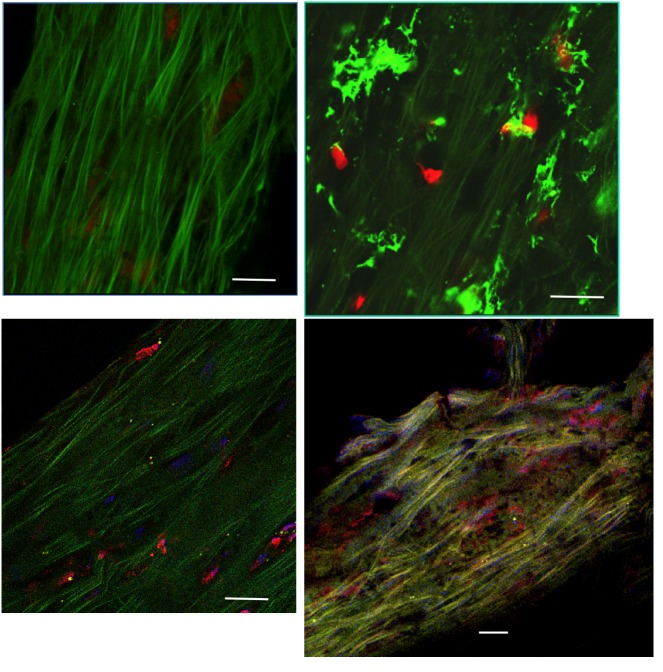
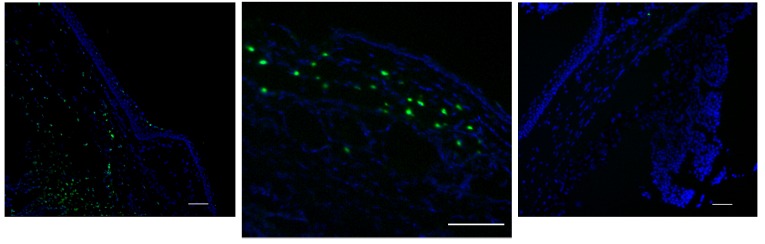
Consistent with previous studies on TM specimens,5 confocal microscopy examinations showed typical features consisting of a strip of TM, approximately 300 μm wide, 30 to 35 μm thick, 3 to 5 mm long, with a dense extracellular matrix composed of numerous elastic fibers, appearing with a bright green autofluorescence and very low density of cells scattered throughout the TM (Figure 1, top left). Based on the literature and previous investigations on TMs, cell density was dramatically decreased compared to patterns already described in normal eyes or glaucoma of other origins, namely, traumatic, angle-closure, or uveitic.5 The relative proportion of resident TM cells and infiltrating inflammatory cells could not be determined precisely, even though in some specimens a typical dendritiform pattern was observed (Figure 1, top right). Staining for fractalkine or its receptor CX3CR1 appeared in isolated cells throughout the TM, with no specific pattern or characteristics allowing precise identification of the cells bearing those markers (Figure 1, bottom left and right). As both markers could not be assessed on the same specimens, we could not determine whether TM cells expressed the chemokine and its receptor at the same time, as was found in HTM cells in vitro. Nonetheless, the staining in ex vivo specimens was very similar to the staining found in cultured trabecular cells. Not all cells were stained, indicating at least two contingents of cells, depending on how they expressed the fractalkine complex.
FIGURE 1.
Immunohistological assessment of human trabecular meshwork (TM) specimens examined by confocal microscopy. Top left, A few TM cells, visible through their red nuclei, scattered throughout a dense green autofluorescent matrix. Top right, Dendritiform inflammatory cells infiltrating the TM (vimentin green staining). Bottom left, Trabecular cells expressing fractalkine (red immunostaining). Bottom right, Trabecular cells expressing CX3CR1 (red immunostaining). (Red nuclear staining by propidium iodide (top), blue staining by DAPI (bottom). Bars = 50 μm.
Fractalkine mRNA was significantly detected in 10 of 15 TMs (data not shown). Six of these 10 specimens had received topical indomethacin before surgery, whereas among the 5 TMs showing undetectable fractalkine mRNA expression, 4 had received indomethacin preoperatively. Fractalkine was statistically less expressed when an anti-inflammatory agent was used preoperatively (P=.01). Although few cells showed CX3CR1 immunohistologically, no significant RNA synthesis was found in the group of TMs studied.
BAK STIMULATES CELL DEATH IN TRABECULAR CELLS
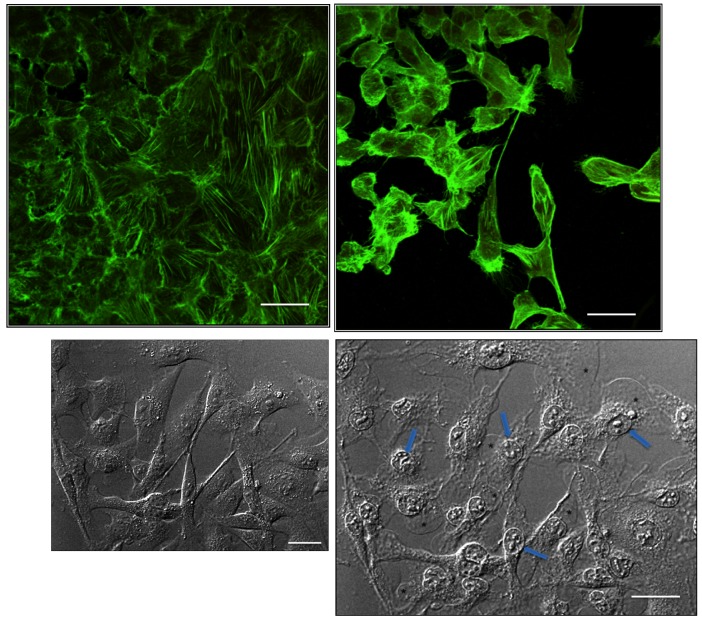
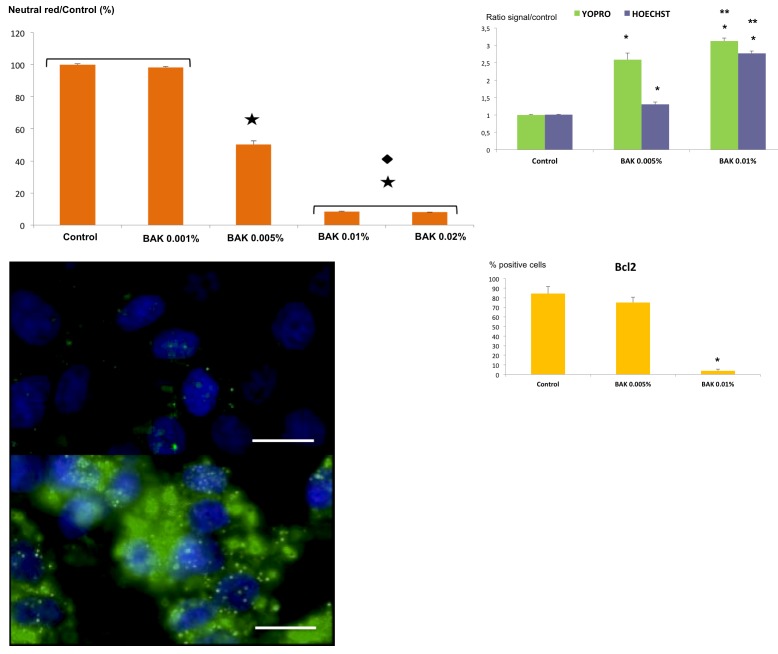
The morphological analysis of trabecular cell lines showed that administration of BAK reduced TM cell density and cell size and caused major morphological changes. TM cells lost their specific elongated form, becoming round or detaching from the culture plates, as BAK concentration increased (Figure 2). Very soon after contact with BAK, TM cells underwent features typical of apoptosis with fragmentation of nuclei and leakage of cytoplasmic material (Figure 2). The neutral red test (Figure 3, top left) showed a cell viability decrease of about 50% with BAK 0.005%, giving a BAK LC (lethal concentration) 50 on trabecular cells of 0.005%. This decrease of viability was even greater with the two higher concentrations (0.01% and 0.02%), reaching less than 10% viability. The lowest concentration (0.001%) showed no difference with the control. BAK 0.005% and 0.01% were used for the other tests. We observed the same BAK toxicity profile using the other alamar blue viability test (not shown).
FIGURE 2.
Cytological assessment of proapoptotic effects of benzalkonium (BAK) in trabecular meshwork (TM) cells. Top, Phalloidin staining shows actin cytoskeleton arrangement in HTM3 cells submitted to 0.01% BAK for 15 minutes. Control cells are at left. At right, BAK-stimulated cells show major shrinkage and apoptotic features. Bottom, Phase contrast examination of TM cells submitted to BAK 0.01%. Normal state at baseline at left. At right, after 5 minutes of contact, retraction and fragmentation of nuclei can be observed (arrows) with leakage of cytoplasmic material in response to membrane disruption (stars). Bars = 50 μm.
FIGURE 3.
Cell viability and expression of apoptosis-related markers. Top left, Evaluation of cellular viability of benzalkonium (BAK)-treated HTM3 cells using the neutral red test. Results are expressed as the percentage of positive cells reported to the control; ★<.001 vs control. ♦P<.001 vs BAK 0.005% (ANOVA). (Bar = Standare Error.) Top right, Evaluation of apoptosis in BAK-treated HTM3 cells using YO-PRO-1 and Hoechst 33342 tests. Results are expressed as the ratio of signal over the control; *P<.001 vs control; **P<.01 vs BAK 0.005% (ANOVA). (Bar = Standare Error.) Bottom left, Immunofluorescence staining of control (top) or BAK-treated (bottom) HTM3 cells, showing intracellular activated caspase-3 (in green) and nuclei stained with DAPI (in blue). (Bars = 50 μm.) Bottom right, Flow cytometry measurement of the anti-apoptosis molecule Bcl2 in control or BAK-stimulated HTM3 cells. Results are expressed as the percentage of positive cells; *P<.001 vs control and BAK 0.005% (ANOVA). Bar = SE.
Likewise, the apoptosis-related YO-PRO-1 and Hoechst 33342 tests (Figure 3, top right) showed a significant concentration-dependent increase in apoptosis between control, BAK 0.005%, and BAK 0.01%. At the concentration of 0.01%, BAK induced significantly higher apoptotic levels than did BAK 0.005%. Activated caspase 3 (Figure 3, bottom left) was found to be highly expressed on cells treated with BAK 0.005% in comparison with untreated cells, confirming the induction of effective apoptosis by BAK treatment even at very low concentrations, before cell viability was impaired. Additionally, the protective anti-apoptotic intracellular protein Bcl2 was not changed under BAK 0.005% but significantly decreased, from 80% to 5%, with BAK 0.01%, showing a loss of adaptive response to the toxic challenge (Figure 3, bottom right).
BAK AND BAK-CONTAINING PROSTAGLANDIN ANALOGS INDUCE OXIDATIVE STRESS IN TRABECULAR CELLS
Prostaglandin analogs were tested using cold light fluorimetry for oxidative stress as well as BAK at the concentrations corresponding to those found in commercially available prostaglandin analogs, ranging from 0.005% to 0.02%. At the concentrations causing significant cell loss, significant dose-dependent increases in total ROS production (H2DCF-DA) and anion superoxide (hydroethidine) were found in HTM3 cells (Figure 4), consistent with previous findings in conjunctival cells, at similar concentrations of BAK.27,62 Prostaglandin analogs showed similar oxidative stress stimulation, except for travoprost solution, which showed a lower hydroethidine level than that induced by BAK alone at the concentration found in the commercial solution. This possible slight antioxidative effect of the travoprost molecule had already been suggested from previous studies conducted in a conjunctival cell line.62 The nonpreserved solution of tafluprost and bimatoprost preserved with low concentration of BAK showed nonsignificantly different levels compared to the control. These results therefore demonstrate that BAK, alone or in combination, but not the prostaglandin analogs, cause significant oxidative stress in trabecular cells in vitro.
FIGURE 4.
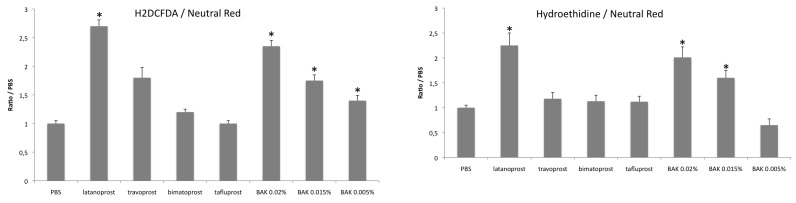
Cold light cytofluorimetry assessment of oxidative stress in trabecular meshwork (TM) cells submitted to benzalkonium (BAK). Reactive oxygen species (ROS) production was assessed in HTM3 cells after stimulation by BAK or commercial solutions of prostaglandin analogs: latanoprost, travoprost, and bimatoprost, preserved with BAK 0.02%, 0.015%, and 0.005%, respectively, and preservative-free tafluprost. Results are reported as the ratio of the signal over that of the neutral red test as a marker of viable cells. Left, Total ROS production with the H2DCF-DA probe. Right, Superoxide anion using the hydroethidine test. *P<.001 compared to the control (ANOVA). Bars = SE.
BAK STIMULATES INFLAMMATORY MEDIATORS IN TRABECULAR CELLS
In the HTM3 cell line, immunostaining showed slight basal fractalkine (Figure 5) and CX3CR1 (Figure 6) expression in unstimulated cells. Immunofluorescence staining intensity was strongly enhanced by TNF-α and, to a lesser extent, by BAK stimulations. However, at the same time, cells exposed to higher concentrations of BAK clearly lost their elongated shape and took on features of apoptotic cells.
FIGURE 5.
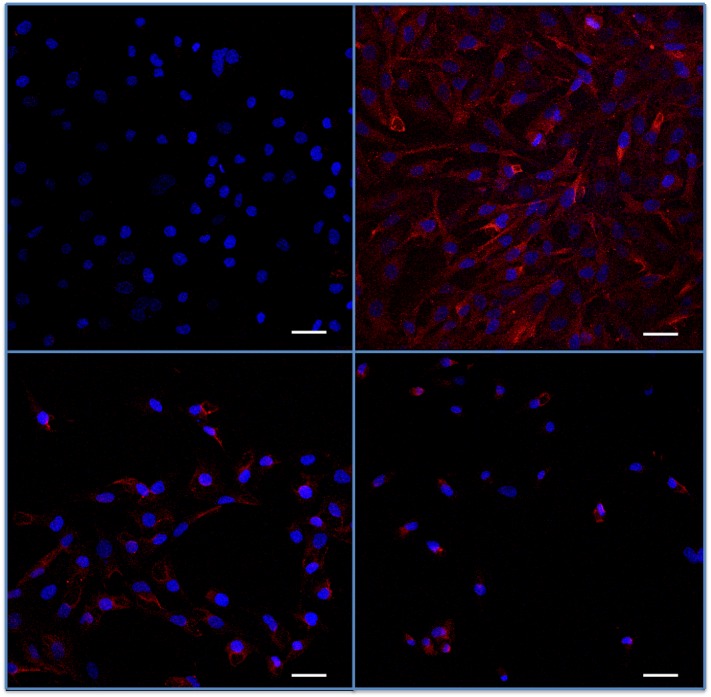
Assessment of fractalkine in trabecular meshwork cells submitted to benzalkonium (BAK) or TNF-α. Indirect immunostaining for fractalkine (in red) in trabecular cells in vitro (nuclei are counterstained in blue by DAPI). Top left, Low, almost negative fluorescence intensity in basal condition. Top right, Strong expression following TNF-α. Bottom left, BAK 5×10−5% stimulation for 24 hours. Bottom right, 5×104% stimulation for 24 hours. After BAK stimulations, cells lost their elongated form and cell density was reduced. Bars = 50 μm.
FIGURE 6.
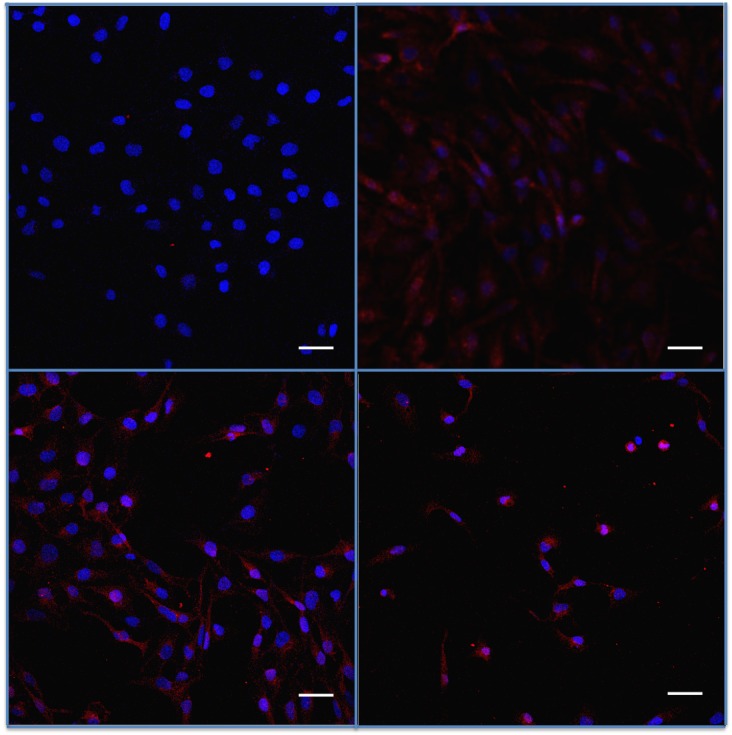
Assessment of fractalkine receptor CX3CR1 in trabecular meshwork cells submitted to benzalkonium (BAK) or TNF-α. Indirect immunostaining for CX3CR1 (in red) in trabecular cells (nuclei in blue). Top left, Negative fluorescence intensity in basal conditions. Top right, Increased expression after TNF-α. Bottom left, BAK 5×10−5%. Bottom right, BAK 5×10−4% stimulations for 24 hours. Bars = 50 μm.
Using flow cytometry in the basal condition, fractalkine expression was found at low levels in TM3 cells and significantly increased after BAK and TNF-α stimulations, although the higher concentration of BAK, possibly through the toxic patterns morphologically observed, did not reach statistical significance (Figure 7, left). The maximum intensity fluorescence peak was found 4 hours after stimulation, whereas fractalkine expression was nonsignificantly different at other times (15 minutes, 1 hour, and 12 hours; data not shown). Expression of CX3CR1 was also found to be higher after stimulation by BAK and TNF-α than in untreated cells but later than for fractalkine (Figure 7, right), as the maximum peak of intensity was found 12 hours after stimulation.
FIGURE 7.
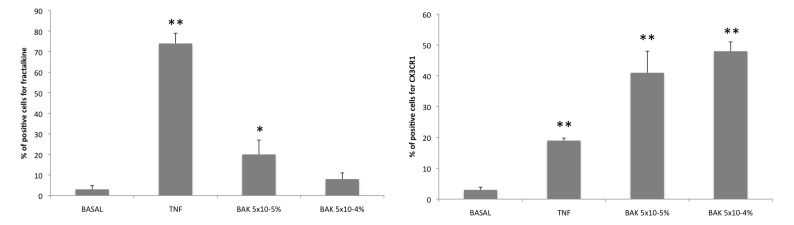
Fractalkine and CXC3CR1 expression in trabecular cells assessed by flow cytometry. Left, Fractalkine expression was low in the basal condition. It was enhanced after TNF-α and benzalkonium (BAK) 5×10−5% stimulations and was increased but not significantly for BAK 5×10−4% stimulation. *P<.05; **P<.001 (ANOVA). Right, CX3CR1 expression rate in flow cytometry was also low in basal condition. It was increased after all BAK stimulations in HTM3 cells. **P<.001 (ANOVA). Bars = SE.
Quantitative RT-PCR demonstrated an overexpression of fractalkine mRNA by HTM3 cells after TNF-α and BAK stimulations (Figure 8, left). The maximum peak of expression appeared very early, 1 hour after stimulation. The very low concentration of 5×10−5% BAK induced a significant elevation of the rate of fractalkine mRNA in HTM3 cells, whereas it was not significantly found elevated with BAK 5×10−4%, possibly due to a higher rate of toxicity, impairing the capacities of RNA synthesis or causing technical problems using this very sensitive detection technique. Similar results were found for CX3CR1 (Figure 8, right), as CX3CR1 expression was significantly elevated by BAK 5×10−5% and TNF-α stimulations in TM3 cells. Otherwise, BAK 5×10−4% stimulation induced a significant elevation of CX3CR1 mRNA level compared to the basal condition.
FIGURE 8.
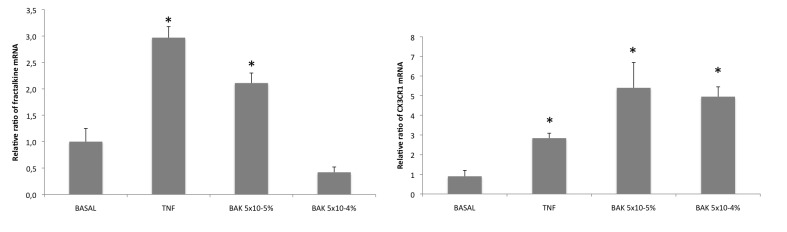
Fractalkine and CXCR3 mRNA levels in trabecular cells assessed by quantitative RT-PCR. Left, Increased fractalkine mRNA level after TNF-α stimulation and after benzalkonium (BAK) 5×10−5% stimulation compared to basal condition. No significant difference for BAK 5×10−4%. * P<.05 (ANOVA). Right, statistically significant increase of CX3CR1 mRNA expression after TNF-α and BAK stimulations in TM3 cells. *P<.05 (ANOVA). Bars = SE.
BAK DECREASES THE PROTECTIVE CHEMOKINE SDF-1
As expected from CXCL12 effects in other systems,59 cell apoptosis was mildly but significantly decreased in BAK-exposed TM cells recovering with CXCL12 (10 ng/mL) over 24 hours compared to BAK-exposed TM cells recovering in free medium only (P<.05) (Figure 9, left). Conversely, BAK induced a decrease in TM cell expression of CXCL12 in a dose-dependent manner as assessed by flow cytometry. Interestingly, a rapid 15-minute exposure to extremely low concentrations of BAK (0.0001% and higher) significantly reduced CXCL12 expression in TM cells, with a dose dependency (P<.001) (Figure 9, middle). In parallel, 0.0001% and 0.001% BAK stimulations significantly increased the membrane expression of CXCR4 in TM cells (P<.001) (Figure 9, right). A higher concentration of BAK (0.01%) did not influence CXCR4 membrane expression, most likely through a toxic mechanism or an interaction with membrane-bound receptors. These two latter results could be interpreted as an attempt of TM cells challenged with low BAK concentrations to compensate for the loss of protective CXCL12 by increasing its receptor, whereas at slightly higher concentrations, BAK induced a loss of both the chemokine and its receptor, eventually leading to the loss of its protective effects and further apoptosis and cell death.
FIGURE 9.
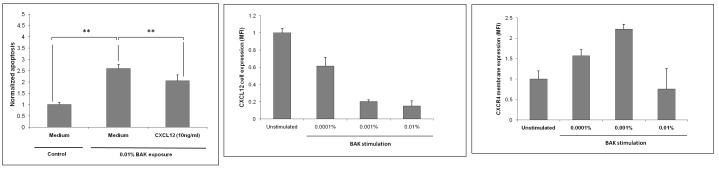
Interactions between benzalkonium (BAK) and CXCL12. Left, Anti-apoptotic effect of CXCL12 on trabecular meshwork cells exposed to a toxic dose of BAK. Apoptosis was measured using the Hoechst test in cold light cytofluorimetry. Apoptosis was normalized after reporting Hoechst over the neutral red signal; **P<.05 (ANOVA). Middle, Effects of BAK on CXCL12 expression in TM cells assessed by flow cytometry. P <.001 for all BAK stimulations compared to the control (ANOVA). Right, Effect of BAK on CXCR4 expression assessed by flow cytometry. P<.001 for 0.0001% and 0.001% BAK stimulations (ANOVA); not significant for BAK 0.01%. MFI, mean fluorescence intensity. Bars = Standard Error.
BAK ADMINISTRATION IN ANIMAL MODELS
The model of subconjunctival injections of BAK was used to demonstrate the concept that BAK could cause in vivo TM cell death and induce toxic trabecular damage resulting in a sustained rise in IOP. Therefore, to induce significant effects with a rapid onset, the model had to use relatively high concentrations. The first subconjunctival injection of BAK at 0.01% had no effect for 6 days. Interestingly, the IOP significantly rose at D7 in BAK-injected eyes compared to vehicle-injected eyes. After a second injection, the IOP remained significantly elevated in BAK-injected eyes until the end of the experiments (Figure 10, left). Additionally, outflow facility was significantly reduced in eyes treated with BAK compared to controls (P<.01), further confirming that BAK-induced elevated IOP resulted from a decrease in AH outflow facility (Figure 10, right). Indeed, histologically, the density of apoptotic cells in the TM and iris root was dramatically increased in BAK-injected eyes as assessed by TUNEL labeling compared to controls (Figure 11). No major histological changes were found in the anterior segment, thus suggesting a local toxic effect in the TM impairing the aqueous outflow, without significant toxic effects to the cornea or iris or visible anterior segment inflammation. Although subconjunctival injections were performed in the upper quadrant of conjunctiva, BAK disseminated along the entire subconjunctival space and no difference in localization was noticed in trabecular damage induced by BAK.
FIGURE 10.
Effects of benzalkonium (BAK) in experimental models on intraocular pressure and aqueous outflow. Left, Effects of two consecutive subconjunctival injections (arrows) of 0.01% BAK on intraocular pressure in rats. Right, Blockage of outflow (OF) facility in BAK-treated eyes (OD) compared to control eyes (OG). Bars = Standard Error.
FIGURE 11.
Effects of benzalkonium (BAK) in experimental models on trabecular cell apoptosis. Left, Immunostaining using the TUNEL technique for apoptotic cells in the trabecular meshwork (TM) and iris root after BAK injection. Staining of apoptotic cells appears in green in the nuclei. Numerous cells are stained in the iridocorneal angle structures. Nuclei are counterstained in blue by DAPI. The cornea, limbus, and conjunctiva are visible on the right, the iris root and ciliary body at bottom left, and TM in between. Middle, Higher magnification of the TM containing many green-stained apoptotic cells. Right, Control eye subconjunctivally injected only with saline: no green staining of apoptotic cells in the anterior segment (ciliary processes are clearly visible at bottom right, cornea on left, and iris in between). Bars = 100 μm.
BAK PENETRATION AFTER INSTILLATIONS IN THE RABBIT EYE
Mass spectrometry ToF-SIMS ion images were acquired in the limbal and iridocorneal angle areas. In all BAK-treated eyes, both BAK compounds, BAK C12 (C21H38N+, m/z 304.32) and BAK C14 (C23H42N+, m/z 332.36), were found in ocular structures. As expected, the control eyes did not show any signal with either ion. In all eyes, the ion image corresponding to C12 exhibited a large distribution in the peripheral cornea, limbus, and conjunctiva. Similar results were obtained with BAK C14. This technique remains semiquantitative and could not measurably count the signals, but color codes are associated with the signal strength, reflecting molecule density. Hence, the signals increased over time with higher levels in eyes treated for 5 months, which showed strong accumulation of the two forms of BAK in the tissues of the front of the eye (Figure 12). At the iridocorneal angle, the signals remained low in the eyes treated for 1 month, at the limit of the method’s sensitivity (data not shown), but increased in the eyes treated for 5 months, which showed clearly visible signals of both BAK C12 and C14 in the trabecular area, more in the nasal part of the eye than in the temporal area, and more in the inferior angle than in the superior angle, suggesting an accumulation with regard to the lacrimal lake and inferior fornix, where drugs are considered to be in contact with the eye longer after instillation. The iris did not show visible signals of BAK in any eye, and as suggested by the superimposition of histological images and MS signals, it seems that BAK mainly diffused to the trabecular area from the limbus and conjunctiva, where the signals were extremely high and appeared to progressively decrease from the surface to the deep structures. However, in two eyes the BAK signal was found at the level of the anterior capsule of the lens (Figure 12, right), but not in the iris, suggesting a direct route through AH.
FIGURE 12.
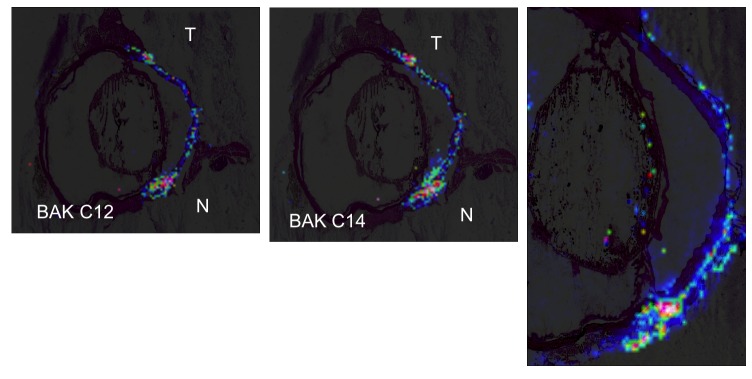
Mass spectrometry imaging of benzalkonium (BAK) distribution after instillation in the rabbit eye. Left, Distribution of BAK C12 after instillation of 0.01% BAK for 5 months. Higher signal levels tend toward red color, lower toward blue. The highest concentrations are found along the ocular surface, with diffusion in the limbal area toward deeper structures, especially in the nasal part of the eye. Spatial resolution of MALDI-ToF MS imaging is 150 μm. Middle, Similar distribution of BAK C14. Right, BAK C14 MS imaging in another rabbit eye showing the same distribution as well as the signal along the anterior capsule of the lens. T, temporal; N, nasal.
DISCUSSION
TRABECULAR MESHWORK DEGENERATION IN POAG
Trabecular meshwork degeneration has largely been demonstrated as the main cause of aqueous outflow resistance in POAG and consequently elevated IOP.63 The main glaucoma-related trabecular modifications resemble age-related TM degeneration and involve accumulation of trabecular extracellular matrix together with a decrease in TM cellularity.64–66 The role played by trabecular cells in trabecular function is essential because of their phagocytosis activity and their ability to produce extracellular matrix components.67,68 Trabecular cell depletion has therefore been involved in structural trabecular alterations, such as trabecular thickening, trabecular fusion, and accumulation of extracellular material in the endothelial meshwork.64 The trabecular cell loss that occurs in glaucoma, however, is not fully understood. It is known to develop through apoptotic mechanisms and was found as a characteristic of POAG, as cell death in the TM appears greater in POAG patients than in primary angle-closure glaucoma.69 Several mechanisms have been proposed for explaining TM cell loss in POAG, but one underlying condition widely investigated is an increase in oxidative stress resulting in TM senescence, TM cell loss, and functional alterations of TM cells.70–72 Accumulation of end products of lipid peroxidation in TM, AH, and the Schlemm canal and significantly higher levels of DNA oxidation products have been observed in the TM of glaucoma patients compared with age-matched and sex-matched controls.73–75 Chronic oxidative stress can contribute to reduced outflow by inhibiting the intracellular proteasome system that works to degrade intracellular and intercellular debris. After exposure to oxidative stress (40% oxygen) for 10 days, HTM cells lose proteasome activity and undergo increased cell death.75,76 Likewise, a significant down-regulation of miR-29b was found in HTM cell lines, in association with an increase in the expression of several extracellular matrix genes known to be regulated by miR-29b.77 Indeed, TM oxidative stress is a relevant mechanism in glaucoma, as correlations were found between ROS-mediated damage to the TM and increased AH outflow resistance78,79 and elevated IOP.77,80 Recently, an increase in mitochondrial DNA deletion as a result of oxidative stress was found, with a genetic susceptibility to oxidative stress due to a polymorphism of the GSTM1 genes encoding for antioxidant defenses.81
Among possible candidates for inducing oxidative stress in the TM is transforming growth factor-β (TGF-β), a member of a family of dimeric polypeptide growth factors. The three isoforms of TGF-β—TGF-β1, TGF-β2, and TGF-β3—are each encoded by a distinct gene. TGF-β2 is the major cytokine associated with glaucoma, repeatedly found elevated in the AH of POAG patients compared to normal AH or other causes of glaucoma.82–84 TGF-β has immunosuppressant properties that play a role in the phenomenon of anterior chamber–associated immune deviation but is also a well-known growth factor promoting fibrosis through increased extracellular matrix synthesis and deposition.84 Additionally, TGF-β has been shown to increase lipid peroxidation and ROS production in TM cells,85 reinforcing the cascade of events occurring in POAG, which include, in an order that remains to be determined, TGF-β dysregulation and overexpression, TM oxidative stress, TM cell loss, matrix accumulation, and further reduction in AH outflow.
In addition to increased oxidative stress–mediated senescence of TM, another mechanism may deserve further attention, namely, the role of low-grade inflammatory reactions and inflammatory cell infiltration. In previous studies investigating TM specimens from POAG patients, inflammatory cells were found together with a dramatic decrease in TM cells.4,5 As in the present work assessing fractalkine mRNA, the use of anti-inflammatory agents prior to surgery was associated with a lower inflammatory cell density,5 and, even more interesting, this study demonstrated a relationship between the number of medications received by the patients and TM inflammatory cell density and fibroblast presence in TM tissues.4 Therefore, we could not rule out that the long-term administration of antiglaucoma treatments, especially in multitherapy, might contribute to the apoptosis of TM cells and therefore raised the hypothesis that preservatives might play a role in TM cell loss and chronic inflammatory changes within the glaucomatous TM.
CLINICAL PERSPECTIVES AND HYPOTHESES
From a clinical perspective, this would indeed raise the new and ominous hypothesis that the BAK present in antiglaucomatous treatments could stimulate trabecular senescence, resulting in further damage to the TM with a possible impact on outflow and consequently contributing to increasing IOP, even though drugs are specifically aimed at decreasing it. This hypothesis cannot be confirmed in the clinical setting because access to TM tissues in control groups is impossible and because TM degeneration cannot be followed up over time. For obvious ethical reasons, patients could not be prospectively treated with BAK-based eyedrops, namely, without IOP-lowering medications, and followed up over time for possible IOP changes or reduced outflow facility. Moreover, it is most likely that if it exists, BAK toxicity to TM may be more prominent in already damaged TM, as in glaucoma, and in patients treated with multiple medications for long periods of time. Therefore, the respective effects of the IOP decrease stemming from the action of the active compound and of a low-grade TM degeneration through oxidative stress, as well as toxic or inflammatory phenomena, resulting in further reduction of the AH outflow, cannot be accurately measured. It can be hypothesized, however, that the result of this combination of the positive IOP-lowering and negative toxic effects over the long term would appear as a progressive loss of efficacy of antiglaucoma eyedrops, as eventually often occurs in glaucoma care.
Indeed, the progression in the number of medications required for controlling IOP, with high switch or combination rates, is a very common feature in glaucoma. One possibility would be a loss of efficacy of the active compound over time, a mechanism called tachyphylaxis, which has already been described with beta-blockers and may occur in up to 30% of patients,86 but has rarely been addressed with newer drugs, given that loss of efficacy over time is multifactorial and is often observed after long periods of time. This concept has moved to that of persistence, which also seems highly variable and influenced by several factors, such as efficacy, tolerance, or failure to refill the prescribed drug. As a few representative examples, in a 24-month follow-up in 191 patients, persistence with monotherapy was found at variable, significantly different levels of 81.6% with latanoprost vs only 22.9% for bimatoprost, 65.4% for travoprost, and 60.5% for timolol.87 Treatment failure, defined as a need for additional therapy or switching to a new drug, seems to be even worse in more severe patients already on dual therapy: Denis and colleagues88 found that failure at 1 year was experienced by 58.8% of patients receiving carbonic anhydrase inhibitors plus prostaglandin analogs and 66.0% of patients receiving alpha2 agonists plus prostaglandin analogs. Therefore, although retrospective findings in a database may sometimes be difficult to interpret, considering these relatively low success rates, which actually seem lower in real life than expected from clinical trials in standardized settings, several mechanisms could be suggested.
In addition to tachyphylaxis, one logical cause could be the natural history of IOP, which could spontaneously increase at the same time drugs are aimed at decreasing it, the result appearing as a need for additional medications. However, although these two hypotheses may account for significant proportions of cases, questions are raised by the results of the Ocular Hypertension Treatment Study (OHTS).2 This prospective, multicenter, randomized study had two arms: 817 individuals received topical ocular hypotensive medication and 819 were assigned to observation. The treatment goal was to reduce IOP by 20% or more and to reach an IOP of 24 mm Hg or less. Treatment was changed and/or added until both goals were reached or the participant was receiving maximum-tolerated topical medical therapy. Patients were followed up every 6 months. At 60 months, in the untreated group, the median IOP showed a slight decrease of 4% ± 11.6%, and in the treated group, the mean reduction in IOP was 22.5% ± 9.9%. During the follow-up, the IOP decrease curves in both groups remained parallel, but to maintain the same level of IOP reduction in the treated group, it was necessary to give increasingly strong treatment, because at the end of the 5-year observation period, 40% of the patients received two drugs and 9% received three or more. The fact that IOP remained roughly stable in the observation group, with similar stability found over a 6-year period in the Early Manifest Glaucoma Trial in the untreated group, does not support the hypothesis that the natural history of IOP increase and TM degeneration would explain the need for increasing IOP-lowering medications.89
Additionally, in the OHTS, an increased rate of cataract extraction and cataract/filtration surgery was observed in patients receiving medication (7.6%) compared with the untreated control group (5.6%), with an odds ratio of 1.56.90 After 8 more years of observation in the OHTS (ie, 13 years after randomization), no difference in cataract or cataract surgery was observed in the two groups. Indeed, the original observation group had a median of 5.5 years with treatment, which may suggest that their rate of cataract reached that of the group treated from the beginning.91 The Blue Mountains Eye Study also showed that antiglaucoma therapy seems to increase the risk of cataract formation (odds ratio, 1.90).92 These observations raise the hypothesis that antiglaucoma treatment may increase the lens opacity rate and/or accelerate cataract formation.55 No evidence of a specific role for one or another compound could be found, which again argues for a possible interaction with the common toxic compound found in eyedrops, namely, the preservative. BAK is a small molecule that interacts with lipid membranes and disrupts cell junctions38,39; it is highly susceptible to accumulating in the eyes of patients treated with several drugs over the long term.57 It may therefore infiltrate or stimulate inflammatory and/or toxic reactions in deeper structures than those previously identified, namely, the ocular surface and lacrimal film.3 Indeed, Goto and colleagues93 investigated the effects of latanoprost, timolol, and BAK in a human lens epithelial cell line and found that BAK strongly stimulated the expression by lens epithelial cells of soluble mediators involved in inflammatory and/or apoptotic processes (ie, prostaglandin E2, IL-1α, and IL-6).
Taking these observations together, a possible role for BAK-induced oxidative stress and inflammatory changes at the TM or lens levels could be hypothesized. BAK is a major toxic compound, influencing the ocular surface components in the long run, stimulating inflammatory reactions, and causing oxidative stress as part of its toxic mechanisms.3,19–23,27,29,62 Therefore, the possibility that BAK could stimulate hidden, low-grade toxic and/or inflammatory changes in deep ocular structures, especially in the TM, can no longer be excluded. The lack of clinical tools for measuring such effects in patients emphasizes the value of experimental, in vitro or animal, models for further investigating these hypotheses.
BAK INDUCES OXIDATIVE STRESS AND CELL DEATH IN TRABECULAR MESHWORK CELLS
Yu and colleagues85 also raised this hypothesis: they found that BAK stimulated oxidative stress, cell death, and fibronectin mRNA in an in vitro model of trabecular cells, findings similar to those observed in the TM degeneration observed in POAG. Likewise, Samples and colleagues94 demonstrated in vitro that BAK caused a significant inhibition of the growth of TM cells at extremely low concentrations. Another in vitro study was conducted on drug-induced apoptosis in TM cells, evaluating the effects of BAK-containing or BAK-free antiglaucoma medications on cell cytoskeleton and apoptotic marker expression in two cultured human TM cell lines. In a 1/100 dilution (ie, at an extremely low concentration), unpreserved beta-blockers had no apoptotic effect, preserved beta-blockers and latanoprost significantly increased expression of only Apo2.7, an early apoptosis marker, whereas BAK significantly increased all the apoptotic markers investigated. When tested in a 1/10 dilution, all drugs except unpreserved timolol triggered a 2- to 3.5-fold increase in apoptotic patterns, whereas BAK caused a dramatic 95% cell reduction upon apoptotic mechanisms.95 In the current series of investigations, we confirmed that TM cells are sensitive to BAK, undergoing degenerative processes when submitted to BAK for a very short period of time. An oxidative stress mechanism induced by BAK was also confirmed, similar to Yu and colleagues’ findings and our own previous experiments in conjunctival cells.27,62 Very recently, two studies similarly showed the toxic effects of BAK on TM cells even at low concentrations.96,97
Additionally, we found that BAK inhibits the secretion of stromal cell–derived factor-1 (SDF-1), a chemokine that we also found to have antiapoptotic properties toward trabecular cells. SDF-1, also termed CXCL12, belongs to the CXC subfamily of chemokines and is known to bind mainly to a G-protein-coupled receptor, CXCR4. Aside from its involvement in the immune system, the CXCL12 axis is also deeply involved in axonal development, neurotransmission, chemotaxis of cancer cells, and extracellular matrix adhesion of hematopoietic cells in bone marrow or damaged tissues. We herein report a constitutive autocrine function of CXCL12 in TM cells via its classic receptor CXCR4, as already described in the brain. Some investigators59 previously reported a protective role of CXCL12 in a primary neuronal cell line. In TM cells, we demonstrated that a physiological concentration of CXCL12 could at least partly protect TM cells against BAK-induced apoptosis, confirming the protective potential of the chemokine in this ocular system. Conversely, even at extremely low concentrations, BAK induced a dramatic decrease in CXCL12 expression by TM cells, along with dysregulation of the membrane expression of CXCR4, namely, an overexpression at low BAK concentrations, likely as a compensatory mechanism, and a dropout at a higher concentration when toxicity overcomes adaptive responses, leading to a total loss of both the protective chemokine and its receptor. Hence, we reasoned that BAK deregulates the protective autocrine function of CXCL12 in TM cells by inhibiting its expression.
BAK INDUCES PROINFLAMMATORY EFFECTS IN THE TRABECULAR MESHWORK
Chemokines, a group of cytokines that attract and activate leukocytes into inflamed tissues, have been associated with the pathogenesis of various inflammatory diseases. Some cytokines or their receptors are enhanced in glaucoma at the ocular surface level by the long-term use of preservatives, as we found in impression cytology IL-6, IL-8, CCR4, and CCR5 overexpression by conjunctival epithelial cells.20–22 Basically, TM cells synthesize and secrete inflammatory mediators, among them the chemotactic cytokines IL-8, CXCL6, and MCP-1, even in the absence of any stimulation. The secretion of these chemokines was found augmented by treatment with the pro-inflammatory cytokines TNF-α and IL-1β.98 The TM may thus play a natural role in the regulation of inflammatory reactions in the anterior chamber by attracting inflammatory cells abnormally entering the anterior chamber with potentially harmful effects. However, this work points out that TM cells also may have proinflammatory potential and that TM cells also may attract inflammatory cells when submitted to a local stress (eg, toxic, degenerative, oxidative, or inflammatory) and become the target of inflammatory reactions bearing destructive potential.
Among the large family of inflammatory chemokines, fractalkine (CX3CL1) is the sole member of the CX3C family. It is a transmembrane glycoprotein expressed by epithelial cells, endothelial cells, dendritic cells, neurons, and astrocytes.99 In the eye, it has been described in the iris, ciliary body, and choroid,100 and in some ocular pathologies, such as in age-related macular degeneration, fractalkine has been investigated and suggested to play a key role.101 CX3CL1 is involved in chemotaxis by inducing adhesion and retention of monocytes and T cells into inflammatory tissues. These actions are mediated through its sole receptor, CX3CR1, which is mainly expressed by inflammatory cells. TNF-α, a major proinflammatory and cell death–inducing cytokine, stimulates fractalkine synthesis in various cellular types.102 To our knowledge, our experiments have demonstrated for the first time the presence of fractalkine and its receptor CX3CR1 in a human trabecular cell line, basically and after stimulation, as well as in glaucomatous trabecular tissue. Both the chemokine and its receptor were overexpressed upon stimulation by TNF-α and BAK in trabecular cells, as assessed by various complementary techniques, namely, immunostaining and molecular biology in TM and TM cell cultures, and flow cytometry in TM cells.
TNF-α has long been known to be associated with the progression of glaucomatous neuropathy. It has been found in glial cells of the optic nerve head, and further studies demonstrated its role in loss of oligodendrocytes and retinal ganglion cells.103 TNF-α is also increased in optic nerve glia in association with ROS production, demonstrating a clear interaction between oxidative stress and inflammatory cytokines, especially TNF-α.104 Recent reports demonstrated that TNF-α may be found in the AH of glaucomatous patients more frequently than in nonglaucomatous patients105 and that elevated TNF-α levels in AH are associated with a poor prognosis of filtering surgery.106 Additionally, an experimental study by Husain and colleagues107 suggested that latanoprost could stimulate TNF-α secretion by the ciliary body. TNF-α, as well as many other inflammatory cytokines, was also demonstrated at significantly higher levels in the tears of patients treated for glaucoma compared to control eyes.108 All these findings thus strongly support that TNF-α could play an important role in the TM of glaucomatous patients in relation to the disease itself or, to some extent still impossible to evaluate, antiglaucomatous treatments or drug-induced inflammatory reactions.
Glaucomatous trabecular cells, submitted to TNF-α stimulation, may continually synthesize fractalkine and express CX3CR1. TNF-α–related overexpression of fractalkine and fractalkine receptor could therefore be involved in the trabecular changes related to glaucoma and possibly influenced by further long-term BAK administration, even if submitted to low but sustained amounts. Epstein and colleagues109 demonstrated that BAK induced significant amounts of TNF-α by corneal and conjunctival epithelial cells. We could thus speculate that protracted trabecular exposure to low concentrations of BAK, possibly along with stimulation of TNF-α, itself related to both the disease and BAK stimulation, could cause overexpression of fractalkine and CX3CR1, further stimulating the inflammatory challenge within the TM.
Indeed, preoperative use of topical indomethacin significantly reduced fractalkine mRNA levels in TMs of glaucomatous patients, suggesting that this anti-inflammatory medication could have an effect on fractalkine synthesis. In a previous study, Hamard and colleagues5 had shown that patients who had anti-inflammatory treatment before surgery presented decreased inflammatory cell infiltration in the TM compared to those who had not been pretreated. One could hypothesize that anti-inflammatory treatment reduces fractalkine synthesis by the trabeculum, decreasing the fractalkine-related chemotaxis of inflammatory cells into the TM. These points, such as the respective roles of glaucoma and antiglaucoma medications, as well as those of TNF and fractalkine synthesis in the cascade leading to TM degeneration, should be further investigated.
ANIMAL MODEL CONFIRMATORY MECHANISMS
Furthermore, additional proof of the widespread, potentially harmful effects of BAK was obtained by an experimental model consisting of the subconjunctival injection of 0.01% BAK, the concentration of most antiglaucomatous eyedrops, lower than that found in some compounds. Of course, in this model the subconjunctival injection caused a concentration of the toxic substance in the subepithelial space. A high level of inflammation was observed initially but decreased, whereas IOP progressively increased after several days, then remained stable, especially when a second injection was made. Determining whether or not this raises a new glaucoma model and whether lower doses would be effective in increasing IOP calls for further studies. Nonetheless, this model proves that BAK may eventually block the AH outflow through an apoptotic and/or inflammatory reaction, leading to increased IOP that remains stable for at least 14 days. It was associated with increased TM cell apoptosis, demonstrating a BAK-induced TM impairment with the consequences of TM cell damage to the outflow and therefore IOP. As a model it cannot fully mimic what occurs in a chronic human disease, but it strongly supports our hypotheses that cell death and inflammation interact in AH outflow reduction through trabecular degeneration and that BAK may play a direct role in enhancing such effects.
RELEVANCE OF THE TESTED CONCENTRATIONS
We lack information on the possible penetration of BAK in deep ocular structures. Tear concentrations have often been considered a marker of BAK penetration, with low tear concentrations probably falsely considered reassuring. Due to its chemical properties, BAK is a molecule with a high potential for accumulation in deep structures. To our knowledge, only one study has reported BAK pharmacokinetic data after instillation. BAK is retained in tissues and can be found 168 hours after a single drop of 0.01% BAK in rabbits. The half-life of BAK in the corneal and conjunctival epithelium was found to be 20 hours, with a half-life in deeper conjunctival structures of 11 hours.57 This is important to consider because tear washout may considerably reduce tear concentration of toxic substances, including BAK,110 whereas deeper structures may be impregnated, especially after repeated use of several preservative-containing eyedrops over the long term. No data are available from the literature for the TM, but it is most likely that BAK accumulates in deep tissues after prolonged administration, and the AH route is probably not the only one to cause contact of BAK or BAK-induced toxic components, such as inflammatory cytokines or ROS, with trabecular tissues. Benzalkonium chloride could thus also reach the TM directly through conjunctival and scleral pathways, so that long-term instillation may lead to increased retention of BAK with a potential toxicity to trabecular structures, particularly following repeated instillation over time of BAK-containing eyedrops. Hence, our MS imaging work confirmed that instillations of BAK in the rabbit eye for several months may make it possible to identify this compound in the iridocorneal angle region, probably diffusing from the surface where it was shown to accumulate. MS imaging techniques (ie, ToF-SIMS and MALDI-ToF) are new, sensitive, and extremely sophisticated methods for assessing presence of and analyzing compounds in tissues.111,112 Although semiquantitative, MS imaging allowed us to confirm that BAK accumulates in deep structures with time, as higher signals were found after 5 months than after only 1 month. Furthermore, in some eyes, BAK was also found in the lens capsule, thus accrediting the hypothesis that BAK may also influence cataract formation in some patients. This is quite consistent with recent work by Garrett and colleagues,113 who identified by MS imaging the presence of BAK intraocularly, in two flat-mounted human eye tissues from one donor, but not in an age-matched rhesus monkey tissue prepared in a similar manner, which was interpreted as the result of premortem treatments administered to the patient.
Even considering that only low amounts of preservative may be in contact with the TM, the proapoptotic and proinflammatory effects of BAK have been previously reported on trabecular cells at extremely low concentrations, such as 2×10−5% and 2×10−6% for 7 days, found to be sufficient to inhibit human cell proliferation, induce morphological and functional changes, and alter the TM function.94 Our findings also showed proinflammatory effects at very low concentrations of BAK, with fractalkine synthesis and release at subtoxic levels, which make the in vitro effects found on cellular models relevant.
In addition to direct accumulation of BAK in the TM, research should also address the possible indirect effects, resulting from proinflammatory cytokine release, locally or from neighboring areas of the anterior segment, still impossible to discriminate from a direct toxic effect. In humans, in experimental models, and in vitro, conjunctival epithelial cells have largely been shown to be involved, as targets and/or stimulators, in major inflammatory changes such as overexpression and/or synthesis of class II antigens, adhesion molecules, chemokines, chemokine receptors, interleukins, or cell death markers and mediators.3 Additionally, the substantia propria of the conjunctiva of patients receiving long-term treatment is infiltrated by inflammatory cells and fibroblasts,4,13–16 and at least in the rabbit eye, where it can be assessed in vivo, the conjunctiva-associated lymphoid tissue follicles are stimulated by repeated instillations of BAK-containing eyedrops.51 Therefore, the impact of high rates of cell death and inflammatory signals on deeper but still very close structures, such as the TM, cannot be ruled out.
Furthermore, possible cataract issues and direct or indirect toxicity to the lens have already been discussed, suggesting that BAK either penetrates the eye over time or induces inflammatory reactions that would also be toxic for intraocular structures. Antiglaucoma eyedrops have also been reported to cause cystoid macular edema following cataract surgery, most likely through an effect of BAK. Miyake and colleagues56 demonstrated that BAK caused disruption of the blood-aqueous barrier in postoperative pseudophakia, increasing aqueous flare and incidence of angiographic cystoid macular edema in BAK-receiving eyes, likely through an increased synthesis of proinflammatory mediators and intensified postoperative inflammation. The investigators proposed the concept of pseudophakic preservative maculopathy. Consistently, a very recent prospective study found significantly higher flare levels in the anterior chamber of eyes receiving BAK-containing timolol compared to preservative-free timolol, thus demonstrating a clear effect of BAK on the blood-aqueous barrier.114
Therefore, even though no data are available today on BAK penetration in human deep ocular structures when eyedrops accumulate over time, and although it remains difficult to discriminate between the direct effects of BAK and the indirect consequences of chronic inflammatory mediator release and oxidative stress, clues are accumulating that BAK may also influence the TM in addition to the ocular surface. Further pharmacokinetic studies will be necessary with appropriate methods, such as MS imaging in human tissues, to establish how BAK comes in contact with the TM, but any indirect inflammatory effect or oxidative stress diffusing from the surface or resulting from extremely low BAK local amounts will not be addressed, only based on BAK pharmacokinetic evaluations.
CONCLUSIONS
The hypothesis that antiglaucomatous medications administered over the long term may have a positive therapeutic effect by decreasing IOP and may also, at least in some cases, adversely affect not only the ocular surface but also deeper ocular tissues, including the TM, and therefore induce further TM degeneration, is somewhat troublesome in that it may contradict all current knowledge and beliefs in the therapeutic effects of topical drugs. Short-term randomized clinical trials, of course, can only detect positive therapeutic effects on lowering IOP. There is no doubt regarding the value of decreasing IOP and the largely positive efficacy of currently available eyedrops, especially prostaglandin analogs. However, almost all drugs are associated with preservatives that account for observed side effects that cannot be ruled out. The most spectacular are allergic reactions, but there is growing evidence that in the long run, glaucomatous patients develop other side effects, such as dry eye, chronic irritation, fibrotic reactions after filtering surgery, or hidden chronic inflammation related to release of inflammatory cytokines and expression of inflammatory mediators.3 Possible infiltration of BAK into deeper structures, as we found using MS imaging, or diffusion of inflammatory reactions from the surface to the TM, are hypotheses that cannot be excluded or confirmed, based solely on clinical trials. Actually, nearly 50% of patients receive more than one drug, and approximately the same proportion suffer from dry eye,7–10 a disease that involves chronic inflammatory reactions distant from the surface.51 A clear relationship between BAK amounts and disruption of the tear film measured by a tear film osmolarimeter was recently demonstrated.115 Furthermore, it is quite frequent that one drug is not sufficient after a more or less prolonged period of time, and IOP control very often requires additional treatments, as if IOP were relentlessly increasing over time. One of the possible mechanisms for the apparent progressive loss of efficacy of topical IOP-lowering drugs could therefore be the undermining toxicity of the neglected part of the eyedrop, namely, the preservative that may exert significant toxic, pro-oxidative, and/or proinflammatory effects on the TM. Nevertheless, the proportion of the spontaneous increase in IOP, loss of drug efficacy, and drug-induced TM alterations cannot be measured, and all patients certainly do not respond in the same way.
It is most likely that other factors may also influence individual responses, such as genetic susceptibility of the TM to oxidative stress, age, race, inflammatory changes over the ocular surface, abnormalities of the surface epithelia, allowing higher or easier penetration of toxic compounds, the number of medications, the duration of treatments, or the inability of the TM to compensate for toxic or inflammatory stress. As we demonstrated in vitro with the SDF-1 (CXCL12) pathway, to some extent TM cells are capable of resisting, by inducing compensatory mechanisms such as inducing more receptors when the ligand is lacking, but their resistance may be overcome when the stress increases, even at very low BAK concentrations. Individual susceptibility and other confounding factors may therefore influence both BAK penetration and patient responses. Nonetheless, the concept of preservative-induced trabeculopathy may be proposed as a hypothesis accounting for some cases of glaucoma progression despite apparently appropriate treatments and medical care.
The future and definitive answers to these important and confounding questions will probably be given by the development of preservative-free compounds. In the glaucoma domain, preservative-free beta-blockers already exist and have shown similar efficacy compared to their preserved counterpart.116–118 Preservative-free tafluprost and BAK-free travoprost have recently been developed, also showing IOP-lowering equivalence with potentially better tolerance.12,119 However, these compounds have not been evaluated over the long term, given that the clinical trials were designed to show equivalence in monotherapy settings. It is still not possible to evaluate the hypothesis of a better preservation of the TM, clinically translated as a lower failure rate over time, which may require years and will obviously be submitted to individual variations. Nonetheless, as the tolerance issues have now become a concern supported by the authorities, physicians, and a growing number of companies that develop BAK-free compounds, patients will be able to avoid potentially harmful drugs. The most sensitive among them will benefit from ocular surface improvement and comfort. Clinical trials and longitudinal observational studies will confirm whether improvement will affect only the surface of the eye or also overall glaucoma care.
Acknowledgments
Funding/Support: This series of extended studies was conducted during the years 2009–2011, at the Vision Institute, University Pierre et Marie Curie (UPMC) and Quinze-Vingts National Ophthalmology Hospital, Paris, France, with the administrative and financial support of INSERM (Institut National de la Santé et la Recherche Médicale), UMRS968, and UPMC. Mass spectrometry imaging was supported by the Agence Nationale pour la Recherche (grant ANR-09-PIRI-0012 MASDA-EYE).
Financial Disclosures: Dr Baudouin is a consultant for or benefited from unrestricted research grants from several companies: Allergan, Alcon, Merck, Novagali Pharma, Pfizer, Thea, and Santen. However, none of the studies presented in this thesis was supported by any industrial grant.
Author Contributions: Design and conduct of the study (C.B.); Collection, management, analysis, interpretation of data (C.B., A.D., N.D., G.H., A.G.); Manuscript Preparation, review, and approval (C.B.).
Other Acknowledgments: Dr Baudouin wishes to thank the members of his research group (Team 8 “Chemokines and Glaucoma,” Vision Institute, University Pierre et Marie Curie, Paris, France) who have also participated in this work at various supportive levels: William Rostène, PhD, DRCE, for his advice in research on chemokines; Sophie Bauer, PharmD, who worked on RT-PCR; Elise Warcoin, PharmD, who investigated TM cell apoptosis; Chloe Clouzeau, PharmD, PhD student, who worked on oxidative stress; Antoine Labbé, MD, PhD, Rachid Tahiri, MD, and Pascale Hamard, MD, PhD, for their help in TM specimen collection and clinical assessments; Luisa Riancho for her help in cold light fluorimetry measurements; and Françoise Brignole-Baudouin, MD, PhD, who coordinated flow cytometry works and participated to Mass Spectrometry Imaging, with Hong Liang, MD,PhD.
Finally, he wishes to thank the consortium constituted for exploring BAK penetration using mass spectrometry imaging, which is the result of a fruitful collaboration between several French universities and research centers: Alain Brunelle, David Touboul, and Vincent Guérineau (Centre de recherche de Gif, Institut de Chimie des Substances Naturelles, CNRS, Gif-sur-Yvette, France), Jean-Pierre Both (Laboratoire d’Intégration des Systèmes et des Technologies, CEA-LIST, Gif-sur-Yvette, France), Maxence Wisztorski, Isabelle Fournier, and Michel Salzet (Laboratoire de Neuroimmunologie des Annélides, EA 4550, Université de Lille, Villeneuve d’Ascq, France), Jonathan Stauber (ImaBiotech), Olivier Laprévote (Chimie Toxicologie Analytique et Cellulaire, EA 4463, Faculté des Sciences Pharmaceutiques et Biologiques, Université Paris Descartes, Paris, France), Françoise Brignole-Baudouin and Hong Liang (Vision institute and Faculté des Sciences Pharmaceutiques et Biologiques, Université Paris Descartes, Paris, France).
REFERENCES
- 1.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(6):701–713. doi: 10.1001/archopht.120.6.701. [DOI] [PubMed] [Google Scholar]
- 3.Baudouin C, Labbé A, Liang H, Pauly A, Brignole-Baudouin F. Preservatives in eyedrops: the good, the bad and the ugly. Prog Retin Eye Res. 2010;29(4):312–334. doi: 10.1016/j.preteyeres.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Baudouin C, Pisella PJ, Fillacier K, et al. Ocular surface inflammatory changes induced by topical antiglaucoma drugs: human and animal studies. Ophthalmology. 1999;106(3):556–563. doi: 10.1016/S0161-6420(99)90116-1. [DOI] [PubMed] [Google Scholar]
- 5.Hamard P, Valtot F, Sourdille P, Bourles-Dagonet F, Baudouin C. Confocal microscopic examination of trabecular meshwork removed during ab externo trabeculectomy. Br J Ophthalmol. 2002;86(9):1046–1052. doi: 10.1136/bjo.86.9.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamard P, Blondin C, Debbasch C, Warnet JM, Baudouin C, Brignole F. In vitro effects of preserved and unpreserved antiglaucoma drugs on apoptotic marker expression by human trabecular cells. Graefes Arch Clin Exp Ophthalmol. 2003;241(12):1037–1043. doi: 10.1007/s00417-003-0777-7. [DOI] [PubMed] [Google Scholar]
- 7.Pisella PJ, Pouliquen P, Baudouin C. Prevalence of ocular symptoms and signs with preserved and preservative free glaucoma medication. Br J Ophthalmol. 2002;86(4):418–423. doi: 10.1136/bjo.86.4.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jaenen N, Baudouin C, Pouliquen P, Manni G, Figueiredo A, Zeyen T. Ocular symptoms and signs with preserved and preservative-free glaucoma medications. Eur J Ophthalmol. 2007;17(3):341–349. doi: 10.1177/112067210701700311. [DOI] [PubMed] [Google Scholar]
- 9.Erb C, Gast U, Schremmer D. German register for glaucoma patients with dry eye. I. Basic outcome with respect to dry eye. Graefes Arch Clin Exp Ophthalmol. 2008;246(11):1593–1601. doi: 10.1007/s00417-008-0881-9. [DOI] [PubMed] [Google Scholar]
- 10.Leung EW, Medeiros FA, Weinreb RN. Prevalence of ocular surface disease in glaucoma patients. J Glaucoma. 2008;17(5):350–355. doi: 10.1097/IJG.0b013e31815c5f4f. [DOI] [PubMed] [Google Scholar]
- 11.Dry Eye WorkShop The epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007;5(2):93–107. doi: 10.1016/s1542-0124(12)70082-4. [DOI] [PubMed] [Google Scholar]
- 12.Uusitalo H, Chen E, Pfeiffer N, et al. Switching from a preserved to a preservative-free prostaglandin preparation in topical glaucoma medication. Acta Ophthalmol. 2010;88(3):329–336. doi: 10.1111/j.1755-3768.2010.01907.x. [DOI] [PubMed] [Google Scholar]
- 13.Sherwood MB, Grierson I, Millar L, Hitchings RA. Long-term morphologic effects of antiglaucoma drugs on the conjunctiva and Tenon’s capsule in glaucomatous patients. Ophthalmology. 1989;96(3):327–335. doi: 10.1016/s0161-6420(89)32888-0. [DOI] [PubMed] [Google Scholar]
- 14.Broadway DC, Grierson I, O’Brien C, Hitchings RA. Adverse effects of topical antiglaucoma medication. I. The conjunctival cell profile. Arch Ophthalmol. 1994;112(11):1437–1445. doi: 10.1001/archopht.1994.01090230051020. [DOI] [PubMed] [Google Scholar]
- 15.Broadway DC, Grierson I, O’Brien C, Hitchings RA. Adverse effects of topical antiglaucoma medication. II. The outcome of filtration surgery. Arch Ophthalmol. 1994;112(11):1446–1454. doi: 10.1001/archopht.1994.01090230060021. [DOI] [PubMed] [Google Scholar]
- 16.Nuzzi R, Vercelli A, Finazzo C, Cracco C. Conjunctiva and subconjunctival tissue in primary open-angle glaucoma after long-term topical treatment: an immunohistochemical and ultrastructural study. Graefes Arch Clin Exp Ophthalmol. 1995;233(3):154–162. doi: 10.1007/BF00166608. [DOI] [PubMed] [Google Scholar]
- 17.Baudouin C, Brignole F, Becquet F, Pisella PJ, Goguel A. Flow cytometry in impression cytology specimens. A new method for evaluation of conjunctival inflammation. Invest Ophthalmol Vis Sci. 1997;38(7):1458–1464. [PubMed] [Google Scholar]
- 18.Brignole-Baudouin F, Ott AC, Warnet JM, Baudouin C. Flow cytometry in conjunctival impression cytology: a new tool for exploring ocular surface pathologies. Exp Eye Res. 2004;78(3):473–481. doi: 10.1016/j.exer.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 19.Baudouin C, Garcher C, Haouat N, Bron A, Gastaud P. Expression of inflammatory membrane markers by conjunctival cells in chronically treated patients with glaucoma. Ophthalmology. 1994;101(3):454–460. doi: 10.1016/s0161-6420(94)31322-4. [DOI] [PubMed] [Google Scholar]
- 20.Baudouin C, Hamard P, Liang H, Creuzot-Garcher C, Bensoussan L, Brignole F. Conjunctival epithelial cell expression of interleukins and inflammatory markers in glaucoma patients treated over the long term. Ophthalmology. 2004;111(12):2186–2192. doi: 10.1016/j.ophtha.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 21.Baudouin C, Liang H, Bremond-Gignac D, et al. CCR4 and CCR5 expression in conjunctival specimens as differential markers of T(H)1/T(H)2 in ocular surface disorders. J Allergy Clin Immunol. 2005;116(3):614–619. doi: 10.1016/j.jaci.2005.05.033. [DOI] [PubMed] [Google Scholar]
- 22.Baudouin C, Liang H, Hamard P, et al. The ocular surface of glaucoma patients treated over the long term expresses inflammatory markers related to both T-helper 1 and T-helper 2 pathways. Ophthalmology. 2008;115(1):109–115. doi: 10.1016/j.ophtha.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 23.Pisella PJ, Debbasch C, Hamard P, et al. Conjunctival proinflammatory and proapoptotic effects of latanoprost and preserved and unpreserved timolol: an ex vivo and in vitro study. Invest Ophthalmol Vis Sci. 2004;45(5):1360–1368. doi: 10.1167/iovs.03-1067. [DOI] [PubMed] [Google Scholar]
- 24.Diebold Y, Calonge M, Enriquez de Salamanca A, et al. Characterization of a spontaneously immortalized cell line (IOBANHC) from normal human conjunctiva. Invest Ophthalmol Vis Sci. 2003;44(10):4263–4274. doi: 10.1167/iovs.03-0560. [DOI] [PubMed] [Google Scholar]
- 25.De Saint Jean M, Brignole F, Bringuier AF, Bauchet A, Feldmann G, Baudouin C. Effects of benzalkonium chloride on growth and survival of Chang conjunctival cells. Invest Ophthalmol Vis Sci. 1999;40(3):619–630. [PubMed] [Google Scholar]
- 26.De Saint Jean M, Debbasch C, Brignole F, Rat P, Warnet JM, Baudouin C. Toxicity of preserved and unpreserved antiglaucoma topical drugs in an in vitro model of conjunctival cells. Curr Eye Res. 2000;20(2):85–94. doi: 10.1076/0271-3683(200002)20:2;1-d;ft085. [DOI] [PubMed] [Google Scholar]
- 27.Debbasch C, Brignole F, Pisella PJ, Warnet JM, Rat P, Baudouin C. Quaternary ammoniums and other preservatives’ contribution in oxidative stress and apoptosis on Chang conjunctival cells. Invest Ophthalmol Vis Sci. 2001;42(3):642–652. [PubMed] [Google Scholar]
- 28.Debbasch C, De La Salle SB, Brignole F, Rat P, Warnet JM, Baudouin C. Cytoprotective effects of hyaluronic acid and Carbomer 934P in ocular surface epithelial cells. Invest Ophthalmol Vis Sci. 2002;43(11):3409–3415. [PubMed] [Google Scholar]
- 29.Debbasch C, Pisella PJ, De Saint Jean M, Rat P, Warnet JM, Baudouin C. Mitochondrial activity and glutathione injury in apoptosis induced by unpreserved and preserved beta-blockers on Chang conjunctival cells. Invest Ophthalmol Vis Sci. 2001;42(11):2525–2533. [PubMed] [Google Scholar]
- 30.Guenoun JM, Baudouin C, Rat P, Pauly A, Warnet JM, Brignole-Baudouin F. In vitro study of inflammatory potential and toxicity profile of latanoprost, travoprost, and bimatoprost in conjunctiva-derived epithelial cells. Invest Ophthalmol Vis Sci. 2005;46(12):2444–2450. doi: 10.1167/iovs.04-1331. [DOI] [PubMed] [Google Scholar]
- 31.Brasnu E, Brignole-Baudouin F, Riancho L, Guenoun JM, Warnet JM, Baudouin C. In vitro effects of preservative-free tafluprost and preserved latanoprost, travoprost, and bimatoprost in a conjunctival epithelial cell line. Curr Eye Res. 2008;33(4):303–312. doi: 10.1080/02713680801971857. [DOI] [PubMed] [Google Scholar]
- 32.Brasnu E, Brignole-Baudouin F, Riancho L, Warnet JM, Baudouin C. Comparative study on the cytotoxic effects of benzalkonium chloride on the Wong-Kilbourne derivative of Chang conjunctival and IOBA-NHC cell lines. Mol Vis. 2008 Mar 4;14:394–402. [PMC free article] [PubMed] [Google Scholar]
- 33.Patarca R, Rosenzwei JA, Zuniga AA, Fletcher MA. Benzalkonium salts: effects on G protein-mediated processes and surface membranes. Crit Rev Oncog. 2000;11(3–4):255–305. [PubMed] [Google Scholar]
- 34.Buron N, Micheau O, Cathelin S, Lafontaine PO, Creuzot-Garcher C, Solary E. Differential mechanisms of conjunctival cell death induction by ultraviolet irradiation and benzalkonium chloride. Invest Ophthalmol Vis Sci. 2006;47(10):4221–4230. doi: 10.1167/iovs.05-1460. [DOI] [PubMed] [Google Scholar]
- 35.Deutschle T, Porkert U, Reiter R, Keck T, Riechelmann H. In vitro genotoxicity and cytotoxicity of benzalkonium chloride. Toxicol In Vitro. 2006;20(8):1472–1477. doi: 10.1016/j.tiv.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 36.Nguyen DH, Beuerman RW, De Wever B, Rosdy M. Three-dimensional construct of the human corneal epithelium for in vitro toxicology. In: Salem H, Katz S, editors. Alternative Toxicological Methods. Boca Raton, FL: CRC Press; 2003. pp. 147–159. [Google Scholar]
- 37.Doucet O, Lanvin M, Thillou C, et al. Reconstituted human corneal epithelium: a new alternative to the Draize eye test for the assessment of the eye irritation potential of chemicals and cosmetic products. Toxicol In Vitro. 2006;20(4):499–512. doi: 10.1016/j.tiv.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 38.Pauly A, Meloni M, Brignole-Baudouin F, Warnet JM, Baudouin C. Multiple endpoint analysis of the 3D-reconstituted corneal epithelium after treatment with benzalkonium chloride: early detection of toxic damage. Invest Ophthalmol Vis Sci. 2009;50(4):1644–1652. doi: 10.1167/iovs.08-2992. [DOI] [PubMed] [Google Scholar]
- 39.Meloni M, Pauly A, Servi BD, Varlet BL, Baudouin C. Occludin gene expression as an early in vitro sign for mild eye irritation assessment. Toxicol In Vitro. 2010;24(1):276–285. doi: 10.1016/j.tiv.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 40.Curren RD, Harbell JW. Ocular safety: a silent (in vitro) success story. Altern Lab Anim. 2002;30(Suppl 2):69–74. doi: 10.1177/026119290203002S10. [DOI] [PubMed] [Google Scholar]
- 41.Pauly A, Brignole-Baudouin F, Labbe A, Liang H, Warnet JM, Baudouin C. New tools for the evaluation of toxic ocular surface changes in the rat. Invest Ophthalmol Vis Sci. 2007;48(12):5473–5483. doi: 10.1167/iovs.06-0728. [DOI] [PubMed] [Google Scholar]
- 42.Labbe A, Dupas B, Hamard P, Baudouin C. In vivo confocal microscopy study of blebs after filtering surgery. Ophthalmology. 2005;112(11):1979–1986. doi: 10.1016/j.ophtha.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 43.Labbe A, De Nicola R, Dupas B, Auclin F, Baudouin C. Epithelial basement membrane dystrophy: evaluation with the HRT II Rostock cornea module. Ophthalmology. 2006;113(8):1301–1308. doi: 10.1016/j.ophtha.2006.03.050. [DOI] [PubMed] [Google Scholar]
- 44.Labbe A, Niaudet P, Loirat C, Charbit M, Guest G, Baudouin C. In vivo confocal microscopy and anterior segment optical coherence tomography analysis of the cornea in nephropathic cystinosis. Ophthalmology. 2009;116(5):870–876. doi: 10.1016/j.ophtha.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 45.Labbe A, Pauly A, Liang H, et al. Comparison of toxicological profiles of benzalkonium chloride and polyquaternium-1: an experimental study. J Ocul Pharmacol Ther. 2006;22(4):267–278. doi: 10.1089/jop.2006.22.267. [DOI] [PubMed] [Google Scholar]
- 46.Liang H, Baudouin C, Pauly A, Brignole-Baudouin F. Conjunctival and corneal reactions in rabbits following short- and repeated exposure to preservative-free tafluprost, commercially available latanoprost and 0.02% benzalkonium chloride. Br J Ophthalmol. 2008;92(9):1275–1282. doi: 10.1136/bjo.2008.138768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liang H, Brignole-Baudouin F, Rabinovich-Guilatt L, et al. Reduction of quaternary ammonium-induced ocular surface toxicity by emulsions: an in vivo study in rabbits. Mol Vis. 2008 Jan 31;14:204–216. [PMC free article] [PubMed] [Google Scholar]
- 48.Liang H, Baudouin C, Dupas B, Brignole-Baudouin F. Live conjunctiva-associated lymphoid associated tissue (CALT) analysis in rabbit using in vivo confocal microscopy (IVCM) under inflammatory stimuli. Invest Ophthalmol Vis Sci. 2010;51(2):1008–1015. doi: 10.1167/iovs.09-3509. [DOI] [PubMed] [Google Scholar]
- 49.Pauly A, Labbe A, Baudouin C, Liang H, Warnet JM, Brignole-Baudouin F. In vivo confocal microscopic grading system for standardized corneal evaluation: application to toxic-induced damage in rat. Curr Eye Res. 2008;33(10):826–838. doi: 10.1080/02713680802381460. [DOI] [PubMed] [Google Scholar]
- 50.Ichijima H, Petroll WM, Jester JV, Cavanagh HD. Confocal microscopic studies of living rabbit cornea treated with benzalkonium chloride. Cornea. 1992;11(3):221–225. [PubMed] [Google Scholar]
- 51.Liang H, Baudouin C, Labbe A, Riancho L, Brignole-Baudouin F. Conjunctiva-associated lymphoid tissue (CALT) reactions to antiglaucoma prostaglandins with or without BAK-preservative in rabbit acute toxicity study. PLoS One. 2012;7(3):e33913. doi: 10.1371/journal.pone.0033913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kahook MY, Noecker R. Quantitative analysis of conjunctival goblet cells after chronic application of topical drops. Adv Ther. 2008;25(8):743–751. doi: 10.1007/s12325-008-0078-y. [DOI] [PubMed] [Google Scholar]
- 53.Herreras JM, Pastor JC, Calonge M, Asensio VM. Ocular surface alteration after long-term treatment with an antiglaucomatous drug. Ophthalmology. 1992;99(7):1082–1088. doi: 10.1016/s0161-6420(92)31847-0. [DOI] [PubMed] [Google Scholar]
- 54.Rolando M, Brezzo V, Giordano G, Campagna P, Burlando S, Calabria G. The effect of different benzalkonium chloride concentrations on human normal ocular surface. In: Van Bijsterveld O, Lemp M, Spinelli D, editors. The Lacrimal System. Amsterdam, Berkely, Milano: Kugler and Ghedini Publications; 1991. pp. 87–91. [Google Scholar]
- 55.Brandt JD. Does benzalkonium chloride cause cataract? Arch Ophthalmol. 2003;121(6):892–893. doi: 10.1001/archopht.121.6.892. [DOI] [PubMed] [Google Scholar]
- 56.Miyake K, Ibaraki N, Goto Y, et al. ESCRS Binkhorst lecture 2002: pseudophakic preservative maculopathy. J Cataract Refract Surg. 2003;29(9):1800–1810. doi: 10.1016/s0886-3350(03)00560-1. [DOI] [PubMed] [Google Scholar]
- 57.Champeau E, Edelhauser H. Effect of ophthalmic preservatives on the ocular surface: conjunctival and corneal uptake and distribution of benzalkonium chloride and chlorhexidine digluconate. In: Holly F, editor. The Preocular Tear Film. Lubbock, Texas: Dry Eye Institute, Inc; 1986. pp. 292–302. [Google Scholar]
- 58.Pang IH, Shade DL, Clark AF, Steely HT, DeSantis L. Preliminary characterization of a transformed cell strain derived from human trabecular meshwork. Curr Eye Res. 1994;13(1):51–63. doi: 10.3109/02713689409042398. [DOI] [PubMed] [Google Scholar]
- 59.Khan MZ, Brandimarti R, Shimizu S, Nicolai J, Crowe E, Meucci O. The chemokine CXCL12 promotes survival of postmitotic neurons by regulating Rb protein. Cell Death Differ. 2008;15(10):1663–1672. doi: 10.1038/cdd.2008.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gabert J, Beillard E, van der Velden VH, et al. Standardization and quality control studies of “real-time” quantitative reverse transcriptase polymerase chain reaction of fusion gene transcripts for residual disease detection in leukemia—a Europe Against Cancer program. Leukemia. 2003;17(12):2318–2357. doi: 10.1038/sj.leu.2403135. [DOI] [PubMed] [Google Scholar]
- 61.Shepard AR, Millar JC, Pang IH, Jacobson N, Wang WH, Clark AF. Adenoviral gene transfer of active human transforming growth factor-{beta}2 elevates intraocular pressure and reduces outflow facility in rodent eyes. Invest Ophthalmol Vis Sci. 2010;51(4):2067–2076. doi: 10.1167/iovs.09-4567. [DOI] [PubMed] [Google Scholar]
- 62.Guenoun JM, Baudouin C, Rat P, Pauly A, Warnet JM, Brignole-Baudouin F. In vitro comparison of cytoprotective and antioxidative effects of latanoprost, travoprost, and bimatoprost on conjunctiva-derived epithelial cells. Invest Ophthalmol Vis Sci. 2005;46(12):4594–4599. doi: 10.1167/iovs.05-0776. [DOI] [PubMed] [Google Scholar]
- 63.Tektas OY, Lütjen-Drecoll E. Structural changes of the trabecular meshwork in different kinds of glaucoma. Exp Eye Res. 2009;88(4):769–775. doi: 10.1016/j.exer.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 64.Alvarado J, Murphy C, Juster R. Trabecular meshwork cellularity in primary open-angle glaucoma and non-glaucomatous normals. Ophthalmology. 1984;91(6):564–579. doi: 10.1016/s0161-6420(84)34248-8. [DOI] [PubMed] [Google Scholar]
- 65.Rohen JW, Lutjen-Drecoll E, Flugel C, Meyer M, Grierson I. Ultrastructure of the trabecular meshwork in untreated cases of primary open-angle glaucoma. Exp Eye Res. 1993;56(6):683–692. doi: 10.1006/exer.1993.1085. [DOI] [PubMed] [Google Scholar]
- 66.Grierson I, Howes RC. Age-related depletion of the cell population in the human trabecular meshwork. Eye. 1987;1(Pt2):204–210. doi: 10.1038/eye.1987.38. [DOI] [PubMed] [Google Scholar]
- 67.Sherwood ME, Richardson TM. Phagocytosis by trabecular meshwork cells: sequence of events in cats and monkeys. Exp Eye Res. 1988;46(4):881–895. doi: 10.1016/s0014-4835(88)80040-x. [DOI] [PubMed] [Google Scholar]
- 68.Hernandez MR, Weinstein BI, Schwartz J, Ritch R, Gordon GG, Southern AL. Human trabecular meshwork cells in culture: morphology and extracellular matrix components. Invest Ophthalmol Vis Sci. 1987;28(10):1655–1660. [PubMed] [Google Scholar]
- 69.Baleriola J, García-Feijoo J, Martínez-de-la-Casa JM, Fernández-Cruz A, de la Rosa EJ, Fernández-Durango R. Apoptosis in the trabecular meshwork of glaucomatous patients. Mol Vis. 2008 Aug 18;14:1513–1516. [PMC free article] [PubMed] [Google Scholar]
- 70.De La Paz MA, Epstein DL. Effect of age on superoxide dismutase activity of human trabecular meshwork. Invest Ophthalmol Vis Sci. 1996;37(9):1849–1853. [PubMed] [Google Scholar]
- 71.Sacca SC, Izzotti A, Rossi P, Traverso C. Glaucomatous outflow pathway and oxidative stress. Exp Eye Res. 2007;84(3):389–399. doi: 10.1016/j.exer.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 72.Fernández-Durango R, Fernández-Martínez A, García-Feijoo J, et al. Expression of nitrotyrosine and oxidative consequences in the trabecular meshwork of patients with primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2008;49(6):2506–2511. doi: 10.1167/iovs.07-1363. [DOI] [PubMed] [Google Scholar]
- 73.Babizhayev MA, Bunin A. Lipid peroxidation in open-angle glaucoma. Acta Ophthalmol (Copenh) 1989;67(4):371–377. doi: 10.1111/j.1755-3768.1989.tb01617.x. [DOI] [PubMed] [Google Scholar]
- 74.Zanon-Moreno V, Marco-Ventura P, Lleo-Perez A, et al. Oxidative stress in primary open-angle glaucoma. J Glaucoma. 2008;17(4):263–268. doi: 10.1097/IJG.0b013e31815c3a7f. [DOI] [PubMed] [Google Scholar]
- 75.Gasiorowskia JZ, Russell P. Biological properties of trabecular meshwork cells. Exp Eye Res. 2009;88(4):671–675. doi: 10.1016/j.exer.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Caballero M, Liton PB, Epstein DL, Gonzalez P. Proteasome inhibition by chronic oxidative stress in human trabecular meshwork cells. Biochem Biophys Res Commun. 2003;308(2):346–352. doi: 10.1016/s0006-291x(03)01385-8. [DOI] [PubMed] [Google Scholar]
- 77.Luna C, Li G, Qiu J, Epstein DL, Gonzalez P. Role of miR-29b on the regulation of the extracellular matrix in human trabecular meshwork cells under chronic oxidative stress. Mol Vis. 2009 Nov 28;15:2488–2497. [PMC free article] [PubMed] [Google Scholar]
- 78.Izzotti A, Bagnis A, Sacca SC. The role of oxidative stress in glaucoma. Mutat Res. 2006;612(2):105–114. doi: 10.1016/j.mrrev.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 79.Saccà SC, Izzotti A. Oxidative stress and glaucoma: injury in the anterior segment of the eye. Prog Brain Res. 2008;173:385–407. doi: 10.1016/S0079-6123(08)01127-8. [DOI] [PubMed] [Google Scholar]
- 80.Ohia SE, Opere CA, Leday AM. Pharmacological consequences of oxidative stress in ocular tissues. Mutat Res. 2005;579(1–2):22–36. doi: 10.1016/j.mrfmmm.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 81.Izzotti A, Saccà SC, Longobardi M, Cartiglia C. Mitochondrial damage in the trabecular meshwork of patients with glaucoma. Arch Ophthalmol. 2010;128(6):724–730. doi: 10.1001/archophthalmol.2010.87. [DOI] [PubMed] [Google Scholar]
- 82.Tripathi RC, Li J, Chan WFA, Tripathi BJ. Aqueous humor in glaucomatous eyes contains an increased level of TGF-β2. Exp Eye Res. 1994;59(6):723–727. doi: 10.1006/exer.1994.1158. [DOI] [PubMed] [Google Scholar]
- 83.Inatani M, Tanihara H, Katsuta H, Honjo M, Kido N, Honda Y. Transforming growth factor-β2 levels in aqueous humor of glaucomatous eyes. Graefes Arch Clin Exp Ophthalmol. 2001;39(2):109–113. doi: 10.1007/s004170000241. [DOI] [PubMed] [Google Scholar]
- 84.Fuchshofer R, Tamm ER. Modulation of extracellular matrix turnover in the trabecular meshwork. Exp Eye Res. 2009;88(4):683–688. doi: 10.1016/j.exer.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 85.Yu AL, Birke K, Moriniere J, Welge-Lüssen U. TGF-{beta}2 induces senescence-associated changes in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2010;51(11):5718–5723. doi: 10.1167/iovs.10-5679. [DOI] [PubMed] [Google Scholar]
- 86.Maclure GM. Chronic open angle glaucoma treated with Timolol. A four year study. Trans Ophthalmol Soc U K. 1983;103(Pt 1):78–83. [PubMed] [Google Scholar]
- 87.Arias A, Schargel K, Ussa F, Canut MI, Robles AY, Sánchez BM. Patient persistence with first-line antiglaucomatous monotherapy. Clin Ophthalmol. 2010 Apr 26;4:261–267. doi: 10.2147/opth.s7971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Denis P, Lafuma A, Berdeaux G. Costs and persistence of alpha-2 adrenergic agonists versus carbonic anhydrase inhibitors, both associated with prostaglandin analogues, for glaucoma as recorded by The United Kingdom General Practitioner Research Database. Clin Ophthalmol. 2008;2(2):321–329. doi: 10.2147/opth.s2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hyman L, Heijl A, Leske MC, Bengtsson B, Yang Z, Early Manifest Glaucoma Trial Group Natural history of intraocular pressure in the early manifest glaucoma trial: a 6-year follow-up. Arch Ophthalmol. 2010;128:601–607. doi: 10.1001/archophthalmol.2010.78. [DOI] [PubMed] [Google Scholar]
- 90.Herman DC, Gordon MO, Beiser JA, et al. Topical ocular hypotensive medication and lens opacification: evidence from the ocular hypertension treatment study. Am J Ophthalmol. 2006;142:800–810. doi: 10.1016/j.ajo.2006.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kass MA, Gordon MO, Gao F, et al. Delaying Treatment of Ocular Hypertension. The Ocular Hypertension Treatment Study. Arch Ophthalmol. 2010;128:276–287. doi: 10.1001/archophthalmol.2010.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chandrasekaran S, Cumming RG, Rochtchina E, Mitchell P. Associations between elevated intraocular pressure and glaucoma, use of glaucoma medications, and 5-year incident cataract: the Blue Mountains Eye Study. Ophthalmology. 2006;113:417–424. doi: 10.1016/j.ophtha.2005.10.050. [DOI] [PubMed] [Google Scholar]
- 93.Goto Y, Ibaraki N, Miyake K. Human lens epithelial cell damage and stimulation of their secretion of chemical mediators by benzalkonium chloride rather than latanoprost and timolol. Arch Ophthalmol. 2003;121:835–839. doi: 10.1001/archopht.121.6.835. [DOI] [PubMed] [Google Scholar]
- 94.Samples JR, Binder PS, Nayak S. The effect of epinephrine and benzalkonium chloride on cultured corneal endothelial and trabecular meshwork cells. Exp Eye Res. 1989;49(1):1–12. doi: 10.1016/0014-4835(89)90071-7. [DOI] [PubMed] [Google Scholar]
- 95.Hamard P, Blondin C, Debbasch C, Warnet JM, Baudouin C, Brignole F. In vitro effects of preserved and unpreserved antiglaucoma drugs on apoptotic marker expression by human trabecular cells. Graefes Arch Clin Exp Ophthalmol. 2003;241(12):1037–1043. doi: 10.1007/s00417-003-0777-7. [DOI] [PubMed] [Google Scholar]
- 96.Ammar DA, Kahook MY. Effects of benzalkonium chloride- or polyquad-preserved fixed combination glaucoma medications on human trabecular meshwork cells. Mol Vis. 2011 Jul 2;17:1806–1813. [PMC free article] [PubMed] [Google Scholar]
- 97.Ammar DA, Kahook MY. Effects of glaucoma medications and preservatives on cultured human trabecular meshwork and non-pigmented ciliary epithelial cell lines. Br J Ophthalmol. 2011;95(10):1466–1469. doi: 10.1136/bjophthalmol-2011-300012. [DOI] [PubMed] [Google Scholar]
- 98.Shifera AS, Trivedi S, Chau P, Bonnemaison LH, Iguchi R, Alvarado JA. Constitutive secretion of chemokines by cultured human trabecular meshwork cells. Exp Eye Res. 2010;91(1):42–47. doi: 10.1016/j.exer.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 99.Stievano L, Piovan E, Amadori A. C and CX3C chemokines: cell sources and physiopathological implications. Crit Rev Immunol. 2004;24(3):205–228. doi: 10.1615/critrevimmunol.v24.i3.40. [DOI] [PubMed] [Google Scholar]
- 100.Silverman MD, Zamora DO, Pan Y, et al. Constitutive and inflammatory mediator-regulated fractalkine expression in human ocular tissues and cultured cells. Invest Ophthalmol Vis Sci. 2003;44(4):1608–1615. doi: 10.1167/iovs.02-0233. [DOI] [PubMed] [Google Scholar]
- 101.Combadière C, Feumi C, Raoul W, et al. CX3CR1-dependent subretinal microglia cell accumulation is associated with cardinal features of age-related macular degeneration. J Clin Invest. 2007;117(10):2920–2928. doi: 10.1172/JCI31692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen YM, Lin SL, Chen CW, Chiang WC, Tsai TJ, Hsieh BS. Tumor necrosis factor-alpha stimulates fractalkine production by mesangial cells and regulates monocyte transmigration: down-regulation by cAMP. Kidney Int. 2003;63(2):474–486. doi: 10.1046/j.1523-1755.2003.00766.x. [DOI] [PubMed] [Google Scholar]
- 103.Nakazawa T, Nakazawa C, Matsubara A, et al. Tumor necrosis factor-alpha mediates oligodendrocyte death and delayed retinal ganglion cell loss in a mouse model of glaucoma. J Neurosci. 2006;26(49):12633–12641. doi: 10.1523/JNEUROSCI.2801-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tezel G, Yang X, Luo C, Peng Y, Sun SL, Sun D. Mechanisms of immune system activation in glaucoma: oxidative stress-stimulated antigen presentation by the retina and optic nerve head glia. Invest Ophthalmol Vis Sci. 2007;48(2):705–714. doi: 10.1167/iovs.06-0810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sawada H, Fukuchi T, Tanaka T, Abe H. Tumor necrosis factor-alpha concentrations in the aqueous humor of patients with glaucoma. Invest Ophthalmol Vis Sci. 2010;51(2):903–906. doi: 10.1167/iovs.09-4247. [DOI] [PubMed] [Google Scholar]
- 106.Cvenkel B, Kopitar AN, Ihan A. Inflammatory molecules in aqueous humour and on ocular surface and glaucoma surgery outcome. Mediators Inflamm. 2010;2010:939602. doi: 10.1155/2010/939602.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Husain S, Yates PW, Crosson CE. Latanoprost-induced changes in rat intraocular pressure: direct or indirect? J Ocul Pharmacol Ther. 2008;24(4):367–372. doi: 10.1089/jop.2008.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Malvitte L, Montange T, Vejux A, et al. Measurement of inflammatory cytokines by multicytokine assay in tears of patients with glaucoma topically treated with chronic drugs. Br J Ophthalmol. 2007;91(1):29–32. doi: 10.1136/bjo.2006.101485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Epstein SP, Chen D, Asbell PA. Evaluation of biomarkers of inflammation in response to benzalkonium chloride on corneal and conjunctival epithelial cells. J Ocul Pharmacol Ther. 2009;25(5):415–424. doi: 10.1089/jop.2008.0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Friedlaender MH, Breshears D, Amoozgar B, Sheardown H, Senchyna M. The dilution of benzalkonium chloride (BAK) in the tear film. Adv Ther. 2006;23(6):835–841. doi: 10.1007/BF02850204. [DOI] [PubMed] [Google Scholar]
- 111.Touboul D, Laprevote O, Brunelle A. Micrometric molecular histology of lipids by mass spectrometry imaging. Curr Opin Chem Biol. 2011;15(5):725–732. doi: 10.1016/j.cbpa.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 112.Benabdellah F, Seyer A, Quinton L, Touboul D, Brunelle A, Laprévote O. Mass spectrometry imaging of rat brain sections: nanomolar sensitivity with MALDI versus nanometer resolution by TOF-SIMS. Anal Bioanal Chem. 2010;396(1):151–162. doi: 10.1007/s00216-009-3031-2. [DOI] [PubMed] [Google Scholar]
- 113.Garrett TJ, Menger RF, Dawson WW, Yost RA. Lipid analysis of flat-mounted eye tissue by imaging mass spectrometry with identification of contaminants in preservation. Anal Bioanal Chem. 2011;401(1):103–113. doi: 10.1007/s00216-011-5044-x. [DOI] [PubMed] [Google Scholar]
- 114.Stevens AM, Kestelyn PA, De Bacquer D, Kestelyn PG. Benzalkonium chloride induces anterior chamber inflammation in previously untreated patients with ocular hypertension as measured by flare meter: a randomized clinical trial. Acta Ophthalmol. 2012;90(3):e221–224. doi: 10.1111/j.1755-3768.2011.02338.x. [DOI] [PubMed] [Google Scholar]
- 115.Labbé A, Terry O, Brasnu E, Van Went C, Baudouin C. Tear film osmolarity in patients treated for glaucoma or ocular hypertension. Cornea. 2012 Jun 15; doi: 10.1097/ICO.0b013e31823f8cb6. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 116.Denis P, Demailly P, Saraux H. Clinical evaluation of betaxolol in ophthalmic suspension with or without preservative agent in patients with glaucoma or ocular hypertension. J Fr Ophtalmol. 1993;16(5):297–303. [PubMed] [Google Scholar]
- 117.Baudouin C, de Lunardo C. Short-term comparative study of topical 2% carteolol with and without benzalkonium chloride in healthy volunteers. Br J Ophthalmol. 1998;82(1):39–42. doi: 10.1136/bjo.82.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gandolfi S, Paredes T, Goldberg I, et al. Comparison of a travoprost BAK-free formulation preserved with polyquaternium-1 with BAK-preserved travoprost in ocular hypertension or open-angle glaucoma. Eur J Ophthalmol. 2012;22(1):32–42. doi: 10.5301/ejo.5000001. [DOI] [PubMed] [Google Scholar]
- 119.Henry JC, Peace JH, Stewart JA, Stewart WC. Efficacy, safety, and improved tolerability of travoprost BAK-free ophthalmic solution compared with prior prostaglandin therapy. Clin Ophthalmol. 2008;2(3):613–621. doi: 10.2147/opth.s3881. [DOI] [PMC free article] [PubMed] [Google Scholar]