Abstract
Purpose:
In conjunctival melanoma, tumor thickness and nonlimbal location are associated with poor prognosis. However, other established high-risk features for cutaneous melanoma, including ulceration, mitotic figures, epithelioid cell type, and lymphovascular invasion, have not previously been studied extensively for their prognostic value in conjunctival melanoma. We examined the hypothesis that these features also predict regional nodal metastasis and death in conjunctival melanoma.
Methods:
The medical records of 44 of 46 consecutive conjunctival melanoma patients treated between June 2003 and December 2009 were retrospectively reviewed; tumor tissue was not available for the two excluded patients. Demographic and clinicopathologic features, including tumor location, tumor thickness, ulceration, mitotic rate, histology, lymphovascular invasion, and microsatellitosis, were reviewed. Outcome measures included regional nodal metastasis, distant metastasis, and death.
Results:
Twenty-six women and 18 men had a median age of 62 years. Regional nodal metastasis occurred in 7 patients (16%) and distant metastasis in 9 (20%). Median follow-up was 40 months. At last follow-up, 10 patients (23%) had died of disease. Tumor thickness >2.0 mm, ulceration, and mitotic figure >1/mm2 predicted regional nodal metastasis and death from disease. In addition to these three histologic features, vascular invasion, epithelioid cell type, and microsatellitosis significantly predicted death from disease. Tumor location (bulbar vs nonbulbar) was not correlated with regional nodal metastasis or death.
Conclusions:
In conjunctival melanoma, as in cutaneous melanoma, thicker tumor, ulceration, and higher mitotic rate are correlated with regional nodal metastasis. In addition, lymphovascular invasion, epithelioid cell type, and microsatellitosis are correlated with melanoma-related death.
INTRODUCTION
Conjunctival melanoma is an uncommon malignancy with the potential for significant morbidity and mortality.1 A study of 150 patients with conjunctival melanoma found a 5-year mortality rate of 8%.2 Recent studies on conjunctival melanoma have reported a 10-year estimated risk of regional nodal metastasis of 11%3 and a 10-year overall metastasis rate of 19%.4 Tumor thickness and a nonlimbal location have been associated with poor prognosis in patients with conjunctival melanoma according to several previous reports.3–6 In addition to tumor thickness, several other histologic features, including ulceration, mitotic figures, epithelioid cell type, and vascular invasion, are known to be high-risk features for cutaneous melanoma.7–12 These histologic features have not been studied extensively for their prognostic value in conjunctival melanoma.
The purpose of this study was to evaluate whether any of these other histologic features that are known high-risk histologic features for cutaneous melanoma are also relevant for risk of regional lymph node metastasis and death associated with conjunctival melanoma.
MATERIALS AND METHODS
After obtaining approval from the Institutional Review Board of The University of Texas MD Anderson Cancer Center (protocol DR07-0795), we performed a retrospective chart review including all consecutive patients with a diagnosis of conjunctival melanoma who were treated by the principal investigator or were enrolled in a prospective trial of sentinel lymph node biopsy for conjunctival melanoma at our institution between June 2003 and December 2009. The study was in compliance with the Health Insurance Portability and Accountability Act and adhered to the tenets of the Declaration of Helsinki. The Institutional Review Board waived the requirement for informed consent because of the retrospective nature of the study.
We identified 46 consecutive patients with conjunctival melanoma who met the criteria for chart review; 44 of those patients were included. Tissue from the conjunctival tumor was not available for review for the other 2 patients; they were excluded from the study.
Baseline clinical variables assessed included age, gender, race/ethnicity, and location of tumor (bulbar, palpebral, caruncular, or a combination of these locations). For purposes of statistical analysis, any tumor that was either isolated to a nonbulbar anatomic location or had a component that was nonbulbar was counted as “nonbulbar.” Viewed a different way, only tumors that were limited to the bulbar conjunctiva were counted as “bulbar”; all other tumors were counted as “nonbulbar” even if they had a bulbar component.
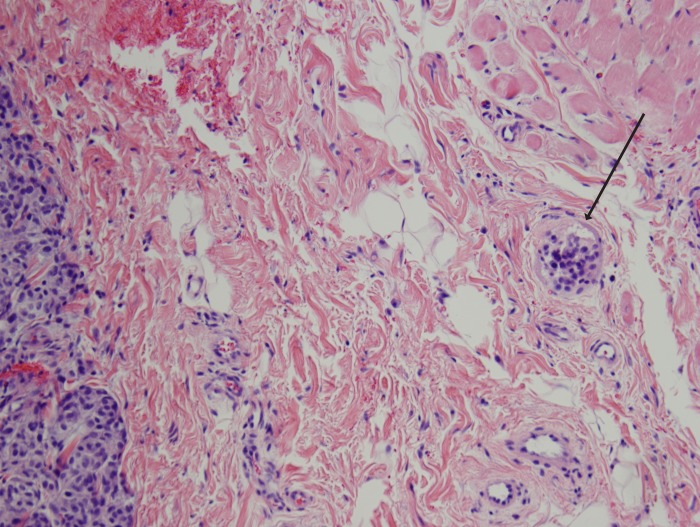
Histologic features for each tumor were also reviewed, including tumor thickness (Figure 1), presence of ulceration (Figure 2), mitotic rate (Figure 3), presence of tumor-infiltrating lymphocytes, perineural invasion, vascular invasion (Figure 4), predominant histology (epithelioid vs spindle), vertical growth phase, and microscopic satellitosis (microsatellitosis) (Figure 5).
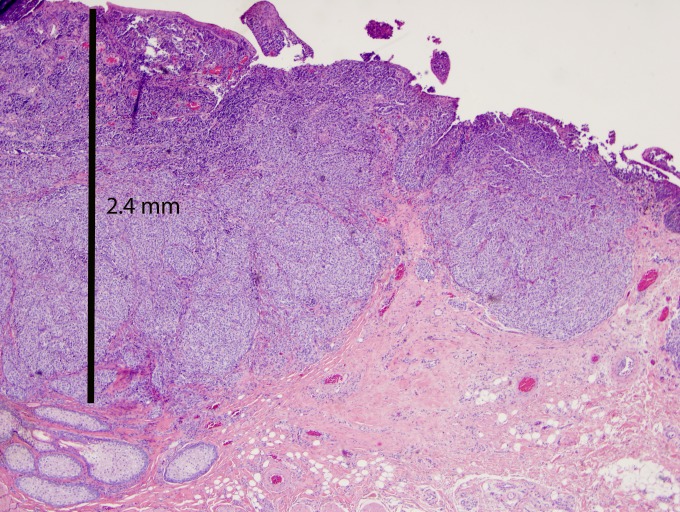
FIGURE 1.
Conjunctival melanoma tumor thickness is measured from the epithelial surface to the deepest tumor cell. This lesion measures 2.4 mm in thickness (hematoxylin-eosin stain, original magnification ×2).
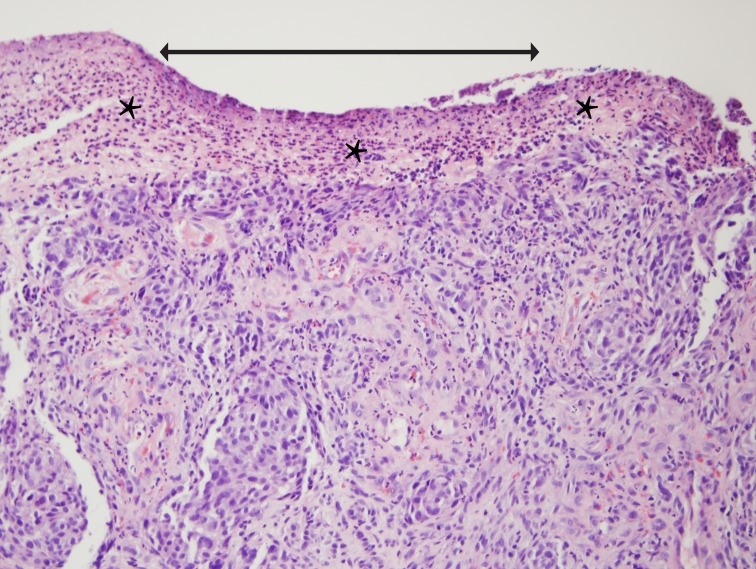
FIGURE 2.
Ulceration on the surface of conjunctival melanoma. Arrow marks the extent of ulcer; asterisks mark a band of fibrin and inflammatory cells that replaces the epithelium (hematoxylin-eosin stain, original magnification ×20).
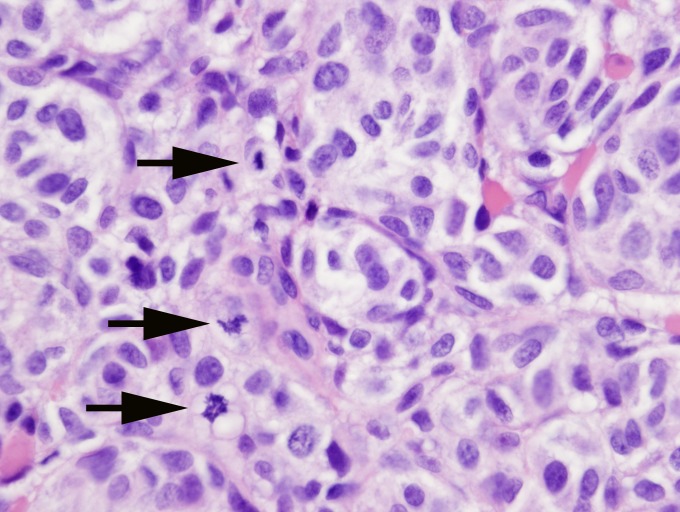
FIGURE 3.
Conjunctival melanoma section with a high mitotic rate. This high-power field has three mitotic figures (arrows) (hematoxylin-eosin stain, original magnification ×40).
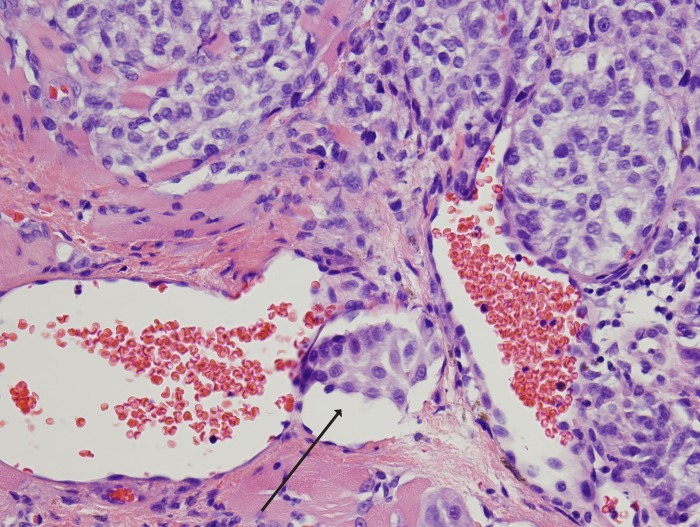
FIGURE 4.
Conjunctival melanoma with vascular invasion. Note the cluster of tumor cells located in a vascular space with red blood cells (arrow) (hematoxylin-eosin stain, original magnification ×20).
FIGURE 5.
Conjunctival melanoma with microsatellitosis (arrow) (hematoxylin-eosin stain, original magnification ×10).
For each tumor, the entire surgical specimen was submitted. A minimum of five high-power fields were examined in all cases. For patients with recurrent lesions at presentation at our institution, prior specimens were also reviewed.
Histologic ulceration is defined as absence of epithelium in the area overlying the melanoma and not caused by trauma (eg, previous surgery).
For calculation of mitotic rate, using the recommendation of the College of American Pathologists (http://www.cap.org/apps/docs/committees/cancer/cancer_protocols/2011/SkinMelanoma_11protocol.pdf), the pathologist looks for the “hot spot” in which mitotic figures are found and then counts at least 1 mm2 of tumor.
Microsatellitosis or microscopic satellitosis refers to the presence of discrete tumor nests that are separated from the main body of the tumor by normal stroma and not by fibrosis, scar, regression, or similar findings.
The primary outcome measures included regional lymph node metastasis, overall survival, disease-free survival, and length of follow-up time in months.
STATISTICAL METHODS
Two-tailed Fisher exact tests were done to examine correlations between variables and end points. Relative risks were calculated using logistic regression. Effects of survival were measured by making Kaplan-Meier plots; the differences between the plots were tested using the log-rank test. Cox proportional hazards test was used to establish proportional hazard ratios and to test the multivariate model for disease-specific survival.
RESULTS
There were 26 women and 18 men. The median age was 62 years (range, 24–85). Thirty-nine patients were white, 2 Hispanic, 2 black, and 1 Asian. Twenty patients (45%) had melanomas limited to the bulbar conjunctiva, 6 (14%) had melanomas limited to the palpebral conjunctiva, 1 (2%) had a caruncular melanoma, and 17 (39%) had melanomas involving a combination of the above locations. The median length of follow-up time from the date of presentation at MD Anderson to the date of last contact was 40 months (range, 12 to 119 months).
During the study period, 7 patients (16%) developed regional lymph node metastasis, and 9 patients (20%) had documented distant metastasis. Three of the patients who had distant metastasis did not have preceding or simultaneous regional lymph node metastasis. At the most recent follow-up, 10 patients (23%) had died of disease. The 3-year disease-free survival rate was 74.7% (Figure 6).
FIGURE 6.
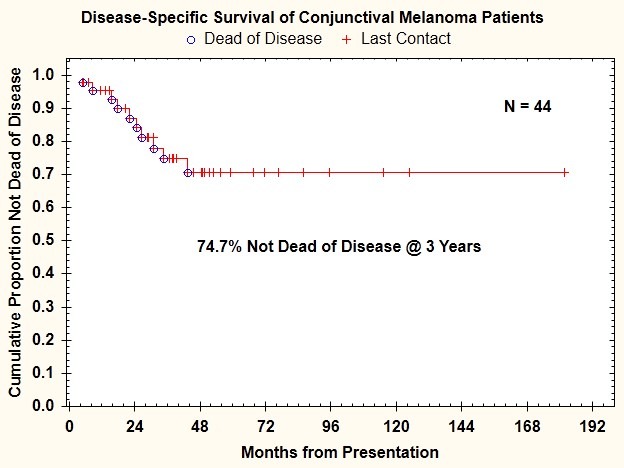
Disease-specific survival for 44 patients with conjunctival melanoma treated between 2003 and 2009.
RELATIONSHIP BETWEEN TUMOR LOCATION AND METASTASIS AND DISEASE-SPECIFIC SURVIVAL
Tumor location (bulbar vs nonbulbar) was not significantly correlated with lymph node metastasis (P=1.000); lymph node or distant metastasis (P=.150); or death from disease (P=.089) (Figure 7). Tumor limited to the bulbar conjunctiva was associated with a 15% (3 of 20 patients) risk of regional nodal metastasis. Tumor not limited to the bulbar conjunctiva was associated with a 16.67% (4 of 24 patients) risk of nodal metastasis. This difference was not statistically significant.
FIGURE 7.
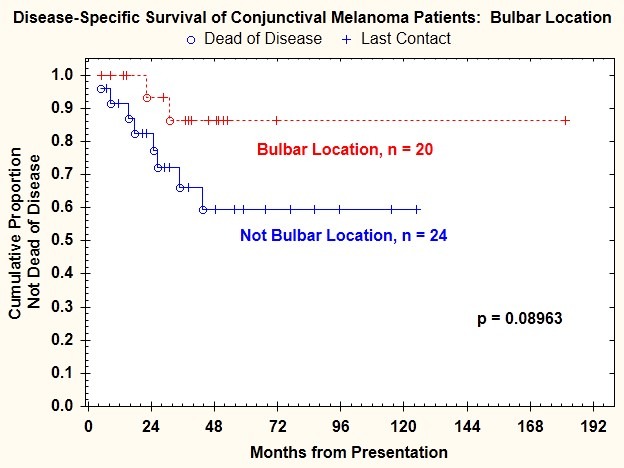
Disease-specific survival for patients with conjunctival melanoma as a function of tumor location.
HISTOLOGIC FEATURES CORRELATED WITH METASTASES AND DISEASE-SPECIFIC SURVIVAL
Tumor thickness was measurable in 37 patients and not determinable in 7 patients because of tangential cutting of the surgical specimen. Tumor thickness ranged from 0.12 mm to 50 mm (median, 1.8 mm). Seventeen of 44 patients (39%) had tumors >2 mm thick; of these, 6 developed regional nodal metastasis, 8 developed distant metastasis, 5 had both lymph node and distant metastasis, and 9 (53%) died. Tumor thickness >2 mm was significantly correlated with regional lymph node metastasis (P=.033), regional lymph node or distant metastasis (P=.005), and death from disease (P=.004) (Figure 8).
FIGURE 8.
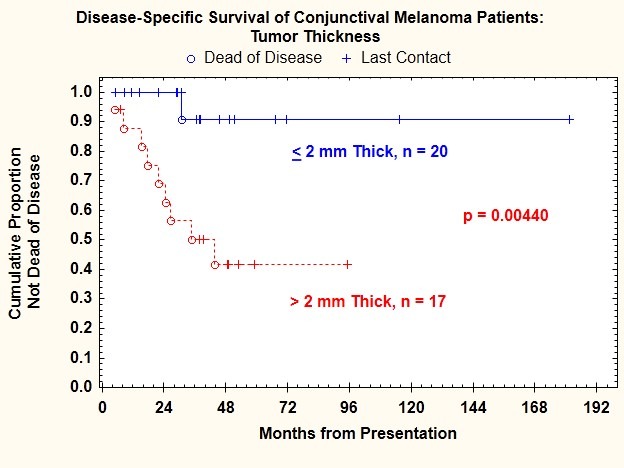
Disease-specific survival for patients with conjunctival melanoma as a function of tumor thickness.
The risk of regional lymph node metastasis was 35.3% for patients with tumors >2 mm thick and only 5% for patients with tumors ≤2 mm thick (Table 1).
TABLE 1.
RELATIONSHIP BETWEEN CHARACTERISTICS OF PRIMARY CONJUNCTIVAL MELANOMA AND RISK OF REGIONAL LYMPH NODE AND DISTANT METASTASIS
| FACTOR | N* | REGIONAL NODAL METASTASIS | DISTANT METASTASIS | ||||
|---|---|---|---|---|---|---|---|
| n | % | P† | n | % | P† | ||
| Tumor location | |||||||
| Bulbar | 20 | 3 | 15.0% | 1.000 | 2 | 10.0% | 0.150 |
| Nonbulbar | 24 | 4 | 16.7% | 7 | 29.2% | ||
| Ulceration | |||||||
| Present | 14 | 6 | 42.9% | 0.008 | 8 | 57.1% | 0.001 |
| Absent | 22 | 1 | 4.5% | 1 | 4.5% | ||
| Tumor thickness | |||||||
| ≤2 mm | 20 | 1 | 5.0% | 0.033 | 1 | 5.0% | 0.005 |
| >2 mm | 17 | 6 | 35.3% | 8 | 47.1% | ||
| Mitotic figures | |||||||
| ≤1 | 16 | 0 | 0.0% | 0.027 | 0 | 0.0% | 0.005 |
| >1 | 21 | 6 | 28.6% | 9 | 42.9% | ||
The total number of patients for some variables does not equal 44 because of missing data.
P values are from 2-tailed Fisher exact tests.
Histologic ulceration was present in 15 tumors (34%). Presence of histologic ulceration was significantly correlated with regional lymph node metastasis (P=.008) (Table 1), regional lymph node or distant metastasis (P=.001), and death from disease (P=.002) (Figure 9). The risk of regional lymph node metastasis was 42.9% for patients with ulceration and only 4.5% for patients without ulceration.
FIGURE 9.
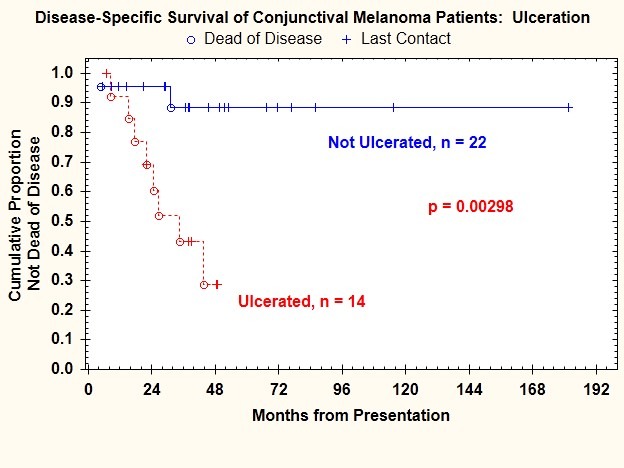
Disease-specific survival for patients with conjunctival melanoma as a function of presence or absence of histologic ulceration.
Mitotic figure count ≥1/mm2 was present in 21 tumors (48%). Mitotic figure count ≥1/mm2 was significantly correlated with regional lymph node metastasis (P=.027) (Table 1) and death from disease (P=.0025) (Figure 10). The risk of regional lymph node metastasis was 28.6% for patients with mitotic figure count >1/mm2 and 0% for patients with absence of mitotic figures.
FIGURE 10.
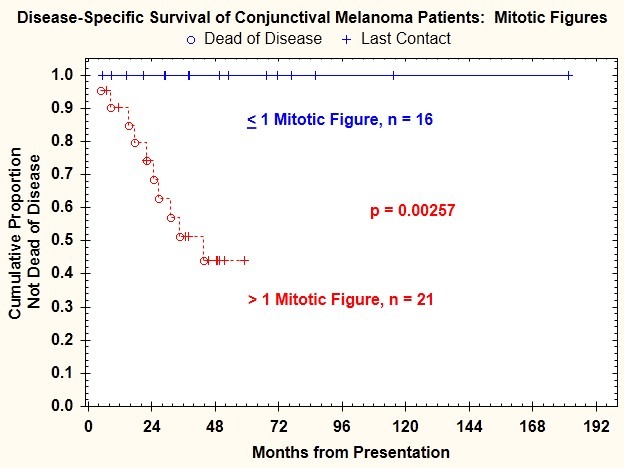
Disease-specific survival for patients with conjunctival melanoma as a function of mitotic figure count.
Log-rank test comparisons of Kaplan-Meier plots of disease-specific survival indicated that in addition to tumor thickness >2 mm, presence of ulceration, and mitotic figure count ≥1/mm2, three additional features were correlated with death from disease: vascular invasion (P=.011) (Figure 11), epithelioid cell type (P=.037) (Figure 12), and microsatellitosis (P=.029) (Figure 13) (Table 2).
FIGURE 11.
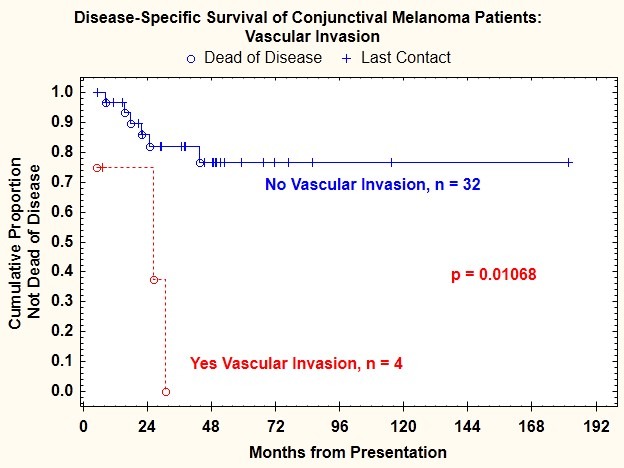
Disease-specific survival for patients with conjunctival melanoma as a function of presence or absence of vascular invasion.
FIGURE 12.
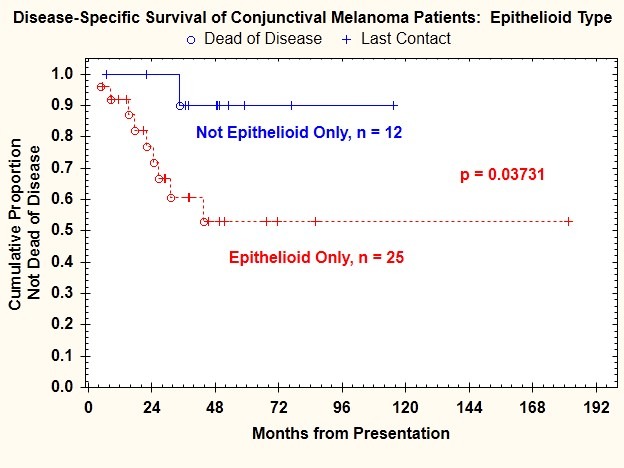
Disease-specific survival for patients with conjunctival melanoma as a function of cell type.
FIGURE 13.
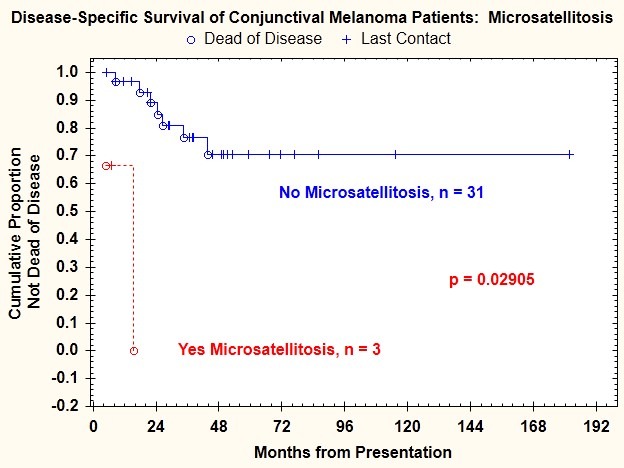
Disease-specific survival for patients with conjunctival melanoma as a function of presence or absence of microsatellitosis.
TABLE 2.
COX PROPORTIONAL HAZARD RATIOS FOR EACH VARIABLE AND THE MULTIVARIATE MODEL FOR DISEASE-SPECIFIC SURVIVAL FOR PATIENTS WITH CONJUNCTIVAL MELANOMA
| VARIABLE | DISEASE-SPECIFIC SURVIVAL | |||
|---|---|---|---|---|
| Log-rankP | Cox Proportional hazards ratio | Lower 95% CI | Upper 95% CI | |
| Tumor thickness >2 mm | .004 | 10.816 | 1.370 | 85.416 |
| Ulceration | .003 | 7.797 | 1.641 | 37.049 |
| Mitotic figures >1/mm2 | .003 | Not calculable | ||
| Vascular invasion | .011 | 7.409 | 1.753 | 31.309 |
| Epithelioid cell type | .037 | 6.264 | 0.789 | 49.712 |
| Microsatellites | .029 | 41.768 | 3.596 | 485.178 |
| Nonbulbar location | .092 | 3.497 | 0.742 | 16.494 |
| Model | Significance | |||
| Overall | <.001 | |||
| Ulceration | .010 | 16.625 | 1.981 | 139.525 |
| Microsatellites | .022 | 17.203 | 1.510 | 196.010 |
None of the other relationships examined between histologic features and outcomes revealed a significant correlation.
INDEPENDENT PREDICTORS OF DISEASE-SPECIFIC MORTALITY
On analysis using 2-tailed Fisher exact tests, all of the significant high-risk histologic features described above except microsatellitosis were found to be correlated, indicating that these features were not independently associated with disease-specific survival (Table 2). A model that contained the presence of ulceration and microsatellitosis was significantly correlated with higher disease-specific mortality (P<.001).
DISCUSSION
In this study, we confirmed previously reported predictors and identified new predictors of regional lymph node metastasis and disease-specific survival in patients with conjunctival melanoma. Our findings confirmed our hypothesis except that two features that we hypothesized would be predictive of survival were not.
We found that three histologic features were significantly correlated with regional lymph node metastasis: tumor thickness >2 mm, histologic evidence of ulceration, and mitotic figure count >1/mm2. Several previous studies by other investigators and by our group have shown that tumor thickness is an important predictor of lymph node metastasis for conjunctival melanoma.3,5,6,13–24 However, none of these previous studies showed that histologic ulceration or mitotic figure count >1/mm2 was correlated with the risk of regional lymph node metastasis for conjunctival melanoma. We previously reported our preliminary observations indicating that histologic evidence of ulceration was correlated with a positive sentinel lymph node in patients with ocular adnexal (conjunctival or eyelid) melanoma and with local recurrence in patients with conjunctival melanoma treated surgically.17,18 In the current study of a larger cohort of patients, we confirmed that ulceration is correlated with regional lymph node metastasis in conjunctival melanoma in general.
Thicker tumor, histologic ulceration, and mitotic figure count >1/mm2 are all well known to correlate with the risk of lymph node metastasis for cutaneous melanoma. The relationships have been confirmed in several studies published in the last several decades,9–12 and these features are incorporated into the American Joint Committee on Cancer’s (AJCC) primary tumor classification for cutaneous melanoma described in the 7th edition of the AJCC Cancer Staging Manual, which was published in 2009.19 However, to our knowledge, the current study is the first to correlate these histologic features with outcomes for conjunctival melanoma. If additional studies in conjunctival melanoma confirm our findings, it may be reasonable to incorporate histologic ulceration and mitotic figure count >1 mm2 into future editions of the AJCC’s primary tumor classification for conjunctival melanoma,20 particularly as factors that may be correlated with lymph node metastasis in early-stage disease.
We found that six histologic features were correlated with worse disease-specific survival in our cohort of patients with conjunctival melanoma: tumor thickness >2 mm, histologic evidence of ulceration, mitotic figure count >1/mm2, lymphovascular invasion, predominantly epithelioid histology, and microsatellitosis. To our knowledge, the only other investigators who have systematically evaluated clinicopathologic features related to mortality in conjunctival melanoma are Tuomaala and colleagues.5 They evaluated the impact of tumor location, tumor thickness, cell type, mitotic count, assessment of nucleoli, tumor-infiltrating lymphocytes, and microvascular loops and networks on disease-specific mortality. These investigators concluded that only tumor thickness and nonlimbal location were correlated with higher melanoma-related mortality. The same investigators have published several other studies that come to the same conclusion that tumor thickness and nonlimbal location are the only high-risk features predictive of mortality.3,6,23 In none of the publications by these esteemed colleagues has there been a mention of histologic ulceration or lymphovascular invasion as features correlated with melanoma-related mortality.
We found that tumor location (bulbar vs nonbulbar) was not significantly correlated with regional lymph node metastasis or with conjunctival melanoma-related mortality. This is in contrast with several other published reports.2–6,14,15 We wonder if this difference in findings may be partly explainable by the fact that the practice of the principal investigator for the current study, who is an oculoplastics and orbit specialist, attracts more patients who have complex and advanced melanomas involving not only the bulbar conjunctiva but also multiple other anatomic units on the ocular surface, such as the palpebral and tarsal conjunctiva and the caruncle (Figure 14); in the current study, 55% of the tumors were designated as nonbulbar and 39% of the tumors had both bulbar and nonbulbar (forniceal, palpebral conjunctival, or caruncular) components. Thus, the proportions of tumors in different anatomic categories may be different in the current study compared with the previously published studies. In the patients included in the study by Tuomaala and colleagues,5 which have been the subject of many publications by these investigators, only 36% of the lesions were nonlimbal.3,5,6 In the recent study of Shields and colleagues,4 48% of tumors were labeled as involving the fornix or palpebral or caruncular conjunctiva. Another possible explanation for the difference in findings between our study and previous studies with respect to the prognostic value of tumor location is the heterogeneity of the classifications and designations of the anatomic sites involved. Some studies designated tumors as limbal vs nonlimbal,3,5,6 some classified tumors as lesions touching the limbus vs not,2 and some classified tumors as bulbar vs palpebral vs forniceal vs caruncular.4 The most recently published AJCC staging system for conjunctival melanoma divides tumor location into bulbar vs nonbulbar (which includes forniceal, palpebral, and caruncular).20 Furthermore, it is not clear from the review of the published literature or from the recent AJCC designation how lesions that involve both the bulbar conjunctiva and other structures would be classified in terms of location. In the current study, any lesion that had any extension beyond the bulbar conjunctiva was counted as “nonbulbar” even if it had a bulbar conjunctival component.
FIGURE 14.

Example of a conjunctival melanoma not only involving large parts of the bulbar conjunctiva (small arrows) but also involving the palpebral conjunctiva (large arrows) and caruncle (dotted arrow). The surgical specimen was submitted over a drawing of the lesion on a Telfa sheet. Note that the canaliculi and medial upper and lower eyelids were resected as a composite specimen with the bulbar and caruncular lesion because the melanoma involved not just the bulbar or palpebral conjunctiva or caruncle but all three components. In our study, this lesion was labeled as “nonbulbar” in line with the American Joint Committee on Cancer’s current designation. It is not clear how this lesion would have been designated in other previously reported studies (original magnification ×5).3–6
A more uniform use of the AJCC classification for conjunctival tumors by all investigators and practitioners may help standardize reporting of outcomes for conjunctival melanoma in future publications. In addition, perhaps future modifications of the AJCC staging system can address the issue of how to designate lesions that involve not only the bulbar conjunctiva but also the palpebral conjunctiva, fornix, and/or caruncle (Figure 14); addressing this issue would help ensure that reported data can be compared across various studies of this rare cancer.
Comparison of the rates of metastasis and disease-related death between our study and previous studies yields some important insights.3–5,14,15,25–32 Sixteen percent of the patients in our cohort had regional lymph node metastasis, and 20% had documented distant organ metastasis. At the most recent follow-up, 10 patients (23%) had died of disease. The 3-year disease-specific survival rate was 74.7% (Figure 6). In the previously mentioned study of Shields and colleagues,4 which reports on the largest published series to date of conjunctival melanoma, with 382 consecutive patients, the 5-year and 10-year melanoma-related death rates were 5% and 9%. The investigators reported that metastasis was found in 19% of patients, but they did not specify whether metastasis was regional nodal or distant. They also did not report the tumor thickness breakdown for these 382 patients. Thus, it is quite possible that the risk profile of their large group of patients was very different from the risk profile of our cohort. In the study by Tuomaala and colleagues,5 the estimated 10-year cumulative incidence of initial regional lymph node metastasis was 11%, and this risk was higher for patients with tumor thickness >2 mm (18%).3 These investigators further reported that the incidence of systemic metastasis was 18%, and it was higher for patients with nonlimbal tumors (38% vs 7%) and for tumors >2 mm thick (28% vs 6%). The real incidence of regional lymph node metastasis in our cohort—16%—is similar to the estimated risk reported by Tuomaala and colleagues: 11%. In addition, our real incidence of distant metastasis—20%—is similar to the estimated risk of 18% reported by Tuomaala and colleagues. Any differences in reported risks in subcategories is most likely due to the higher risk profile of the tumors in our series than of the tumors in previous reports. Werschnik and colleagues,15 from Germany, reported a tumor-related death rate of 22.3%, similar to the 3-year melanoma-related mortality rate of 25.3% in our series. Missotten and colleagues14 reviewed the experience with conjunctival melanomas in the Netherlands and reported a 21% incidence of regional lymph node metastasis and a 25% incidence of distant organ metastasis. They correlated these risks with tumor thickness and tumor diameter.
We particularly concur with Tuomaala and colleagues5 that tumor thickness >2 mm is an important prognostic factor for regional lymph node metastasis, but other histologic features, such as ulceration and mitotic figures, also correlate with regional lymph node metastasis and should be considered for patient selection for sentinel lymph node biopsy. Histologic evidence of ulceration and mitotic figures was not analyzed specifically in the report by Tuomaala and colleagues in relation to regional lymph node metastasis.
In summary, the current study suggests that several new histologic features should be routinely evaluated and studied in conjunctival melanoma because of their impact on the risk of regional lymph node metastasis and thus (1) the decision to consider sentinel lymph node biopsy or not, (2) counseling of patients regarding their prognosis, and (3) determination of the best frequency of surveillance of lymph nodes and distant organs to rule out metastasis. To aid in determining the risk of lymph node metastasis, we advocate specific reporting of histologic ulceration and mitotic figure count >1/mm2 in addition to exact measurement of tumor thickness at the time of evaluation of the primary tumor. Our study also suggests that several histologic factors correlate with melanoma-related mortality; these are tumor thickness >2 mm, histologic ulceration, mitotic figure count >1/mm2, lymphovascular invasion, predominantly epithelioid histology, and microsatellitosis. We recommend recording of all these features routinely and in a systematic, standardized fashion for all melanomas of the conjunctiva so that our findings in this study can be validated in future studies and so that it can be determined whether these features should be incorporated into future refinements of the AJCC classification for conjunctival melanoma. We also believe that there is a need for a better definition of tumor location for conjunctival melanoma, and we advocate a more uniform use of the AJCC classification to enable better determination of the impact of tumor location on outcomes. There is also a need for clarification of how to categorize conjunctival melanomas that encompass not only the bulbar conjunctiva but also other anatomic units on the ocular surface.
Acknowledgments
Funding/Support: This research was supported in part by the National Institutes of Health through MD Anderson’s Cancer Center Support Grant CA016672.
Financial Disclosures: None.
Author Contributions: Design of the study (B.E.); Conduct of the study (B.E.); Collection, management, analysis, and interpretation of the data (B.E.); Preparation of the manuscript (B.E.); Approval of the final manuscript (B.E., D.R., M.F., H.C., S.K., M.R., V.P.).
Other Acknowledgements: I would like to thank my long-time mentor in oculoplastics and orbit, the late Dr Bartley Frueh, for nominating me for membership to the American Ophthalmological Society. His unexpected passing made my work on this thesis a bittersweet endeavor.
REFERENCES
- 1.Shildkrot Y, Wilson MW. Conjunctival melanoma: pitfalls and dilemmas in management. Curr Opin Ophthalmol. 2010;21:380–386. doi: 10.1097/ICU.0b013e32833b7aab. [DOI] [PubMed] [Google Scholar]
- 2.Shields CL, Shields JA, Gündüz K, et al. Conjunctival melanoma: risk factors for recurrence, exenteration, metastasis, and death in 150 consecutive patients. Arch Ophthalmol. 2000;118:1497–1507. doi: 10.1001/archopht.118.11.1497. [DOI] [PubMed] [Google Scholar]
- 3.Tuomaala S, Kivelä T. Metastatic pattern and survival in disseminated conjunctival melanoma: implications for sentinel lymph node biopsy. Ophthalmology. 2004;111:816–821. doi: 10.1016/j.ophtha.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Shields CL, Markowitz JS, Belinsky I, et al. Conjunctival melanoma outcomes based on tumor origin in 382 consecutive cases. Ophthalmology. 2011;118:389–395. doi: 10.1016/j.ophtha.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 5.Tuomaala S, Toivonen P, Al-Jamal R, Kivelä T. Prognostic significance of histopathology of primary conjunctival melanoma in Caucasians. Curr Eye Res. 2007;32:939–952. doi: 10.1080/02713680701648019. [DOI] [PubMed] [Google Scholar]
- 6.Tuomaala S, Eskelin S, Tarkkanen A, Kivelä T. Population-based assessment of clinical characteristics predicting outcome of conjunctival melanoma in whites. Invest Ophthalmol Vis Sci. 2002;43:3399–3408. [PubMed] [Google Scholar]
- 7.Breslow A. Thickness, cross-sectional areas and depth of invasion in the prognosis of cutaneous melanoma. Ann Surg. 1970;172:902–908. doi: 10.1097/00000658-197011000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark WH, Jr, From L, Bernardino EA, Mihm MC. The histogenesis and biologic behavior of primary human malignant melanomas of the skin. Cancer Res. 1969;29:705–727. [PubMed] [Google Scholar]
- 9.Balch CM, Wilkerson JA, Murad TM, Soong SJ, Ingalls AL, Maddox WA. The prognostic significance of ulceration of cutaneous melanoma. Cancer. 1980;45:3012–3017. doi: 10.1002/1097-0142(19800615)45:12<3012::aid-cncr2820451223>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 10.Ross MI. Early-stage melanoma: staging criteria and prognostic modeling. Clin Cancer Res. 2006;12(7 Pt 2):2312s–2319s. doi: 10.1158/1078-0432.CCR-05-2643. [DOI] [PubMed] [Google Scholar]
- 11.Balch CM, Soong SJ, Atkins MB, et al. An evidence-based staging system for cutaneous melanoma. CA Cancer J Clin. 2004;54:131–149. doi: 10.3322/canjclin.54.3.131. [DOI] [PubMed] [Google Scholar]
- 12.Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paridaens AD, Minassian DC, McCartney AC, Hungerford JL. Prognostic factors in primary malignant melanoma of the conjunctiva: a clinicopathological study of 256 cases. Br J Ophthalmol. 1994;78:252–259. doi: 10.1136/bjo.78.4.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Missotten GS, Keijser S, De Keizer RJ, De Wolff-Rouendaal D. Conjunctival melanoma in the Netherlands: a nationwide study. Invest Ophthalmol Vis Sci. 2005;46:75–82. doi: 10.1167/iovs.04-0344. [DOI] [PubMed] [Google Scholar]
- 15.Werschnik C, Lommatzsch PK. Long-term followup of patients with conjunctival melanoma. Am J Clin Oncol. 2002;25:248–255. doi: 10.1097/00000421-200206000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Esmaeli B, Wang X, Youssef A, Gershenwald JE. Patterns of regional and distant metastasis in patients with conjunctival melanoma: experience at a cancer center over four decades. Ophthalmology. 2001;108:2101–2105. doi: 10.1016/s0161-6420(01)00782-5. [DOI] [PubMed] [Google Scholar]
- 17.Savar A, Esmaeli B, Ho H, Liu S, Prieto VG. Conjunctival melanoma: local-regional control rates, and impact of high-risk histopathologic features. J Cutan Pathol. 2011;38:18–24. doi: 10.1111/j.1600-0560.2010.01625.x. [DOI] [PubMed] [Google Scholar]
- 18.Savar A, Ross MI, Prieto VG, Ivan D, Kim S, Esmaeli B. Sentinel lymph node biopsy for ocular adnexal melanoma: experience in 30 patients. Ophthalmology. 2009;116:2217–2223. doi: 10.1016/j.ophtha.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 19.Edge SB, Byrd DR, Compton CC, et al., editors. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer; 2009. Melanoma of the skin; pp. 325–344. [Google Scholar]
- 20.Edge SB, Byrd DR, Compton CC, et al., editors. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer; 2009. Melanoma of the conjunctiva; pp. 531–537. [Google Scholar]
- 21.Esmaeli B, Eicher S, Popp J, Delpassand E, Prieto VG, Gershenwald JE. Sentinel lymph node biopsy for conjunctival melanoma. Ophthal Plast Reconstr Surg. 2001;17:436–442. doi: 10.1097/00002341-200111000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Ho VH, Ross MI, Prieto VG, Khaleeq A, Kim S, Esmaeli B. Sentinel lymph node biopsy for sebaceous cell carcinoma and melanoma of the ocular adnexa. Arch Otolaryngol Head Neck Surg. 2007;133:820–826. doi: 10.1001/archotol.133.8.820. [DOI] [PubMed] [Google Scholar]
- 23.Tuomaala S, Kivelä T. Sentinel lymph node biopsy guidelines for conjunctival melanoma. Melanoma Res. 2008;18:235. doi: 10.1097/CMR.0b013e3282fafd21. [DOI] [PubMed] [Google Scholar]
- 24.Lim M, Tatla T, Hersh D, Hungerford J. Patterns of regional head and neck lymph node metastasis in primary conjunctival malignant melanoma. Br J Ophthalmol. 2006;90:1468–1471. doi: 10.1136/bjo.2006.099754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harooni H, Schoenfield LR, Singh AD. Current appraisal of conjunctival melanocytic tumors: classification and treatment. Future Oncol. 2011;7:435–446. doi: 10.2217/fon.11.12. [DOI] [PubMed] [Google Scholar]
- 26.Zimmermann P, Dietrich T, Bock F, et al. Tumor-associated lymphangiogenesis in conjunctival malignant melanoma. Br J Ophthalmol. 2009;93:1529–1534. doi: 10.1136/bjo.2008.147355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Damato B, Coupland SE. Conjunctival melanoma and melanosis: a reappraisal of terminology, classification and staging. Clin Exp Ophthalmol. 2008;36:786–795. doi: 10.1111/j.1442-9071.2008.01888.x. [DOI] [PubMed] [Google Scholar]
- 28.Maly A, Epstein D, Meir K, Pe’er J. Histologic criteria for grading of atypia in melanocytic conjunctival lesions. Pathology. 2008;40:676–681. doi: 10.1080/00313020802436428. [DOI] [PubMed] [Google Scholar]
- 29.Novais GA, Fernandes BF, Belfort RN, Castiglione E, Cheema DP, Burnier MN., Jr Incidence of melanocytic lesions of the conjunctiva in a review of 10675 ophthalmic specimens. Int J Surg Pathol. 2010;18:60–63. doi: 10.1177/1066896908319775. [DOI] [PubMed] [Google Scholar]
- 30.Shields JA, Shields CL, Mashayekhi A, et al. Primary acquired melanosis of the conjunctiva: risks for progression to melanoma in 311 eyes. The 2006 Lorenz E. Zimmerman lecture. Ophthalmology. 2008;115:511–519. doi: 10.1016/j.ophtha.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 31.Sugiura M, Colby KA, Mihm MC, Jr, Zembowicz A. Low-risk and high-risk histologic features in conjunctival primary acquired melanosis with atypia: clinicopathologic analysis of 29 cases. Am J Surg Pathol. 2007;31:185–192. doi: 10.1097/01.pas.0000213339.32734.64. [DOI] [PubMed] [Google Scholar]
- 32.Esmaeli B. Regional lymph node assessment for conjunctival melanoma: sentinel lymph node biopsy and positron emission tomography. Br J Ophthalmol. 2008;92:443–445. doi: 10.1136/bjo.2007.131615. [DOI] [PubMed] [Google Scholar]