Abstract
Background
Pathological gambling (PG) frequently co-occurs with anxiety disorders. However, the extent to which the co-occurrence is related to genetic or environmental factors across PG and anxiety disorders is not known.
Method
Data from the Vietnam Twin Registry (n=7869, male twins) were examined in bivariate models to estimate genetic and shared and unique environmental contributions to PG and generalized anxiety disorder (GAD) and PG and panic disorder (PD).
Results
While both genetic and unique environmental factors contributed individually to PG, GAD, and PD, the best fitting model indicated that the relationship between PG and GAD was attributable predominantly to shared genetic contributions (ra =0.53). In contrast, substantial correlations were observed between both the genetic (ra=0.34) and unique environmental (re =0.31) contributions to PG and PD.
Limitations
Results may be limited to middle aged males.
Conclusions
The existence of shared genetic contributions between PG and both GAD and PD suggest that specific genes, perhaps those involved in affect regulation or stress responsiveness, contribute to PG and anxiety disorders. Overlapping environmental contributions to the co-occurrence of PG and PD suggest that common life experiences (e.g., early life trauma) contribute to both PG and PD. Conversely, the data suggest that distinct environmental factors contribute to PG and GAD (e.g., early onset of gambling in PG). Future studies should examine the relationship between PG and anxiety disorders amongst other populations (women, adolescents) to identify specific genetic and environmental influences that account for the manifestation of these disorders and their co-occurrences.
Keywords: Pathological Gambling, Anxiety Disorder, Generalized Anxiety Disorder, Panic Disorder, Twin Study, Vietnam Era
Introduction
Pathological gambling (PG) and anxiety disorders (ADs) have considerable health implications. Whereas PG and ADs each may have unique impacts (e.g., related to incarceration and service utilization), both PG and ADs associate with suicidality and may disrupt social and financial domains (Katzelnick et al., 2001; Argo and Black, 2004). Thus, understanding the contributions to these disorders is clinically relevant.
Both PG and ADs frequently co-occur with each other (Petry et al., 2005). As individuals with co-occurring disorders typically have more substantial illness, fare more poorly in treatment, and require different treatments (Potenza, 2007; Grant and Potenza, 2006), a better understanding of the genetic and environmental factors contributing to PG and ADs is important. As how best to categorize some ADs (e.g., post-traumatic stress disorder) has been debated and given the clinical relevance of GAD and PD, we examined GAD and PD with respect to PG. Within large twin samples (e.g., the Vietnam Era Twin Registry; VET-R), GAD and PD have each been found to have genetic and environmental contributions, some of which are shared across disorders (Scherrer et al., 2000; Chantarujikapong et al., 2001). However, the relative genetic and environmental contributions to the co-occurrence of PG and ADs have not been systematically examined.
The current study used data from the VET-R to investigate whether PG and ADs frequently co-occurred and the hypothesis that, while genetic and unique environmental factors will contribute individually to PG, GAD and PD, the co-occurrences between PG and GAD and PG and PD will be accounted for predominantly by genetic factors as found for PG and depression (Potenza et al., 2005).
Method
Participants
Participants were 10,253 male twins comprising the VET-R; 7,869 (76.7%) were successfully interviewed in 1992 to ascertain DSM-III-R diagnoses. Participants were born between 1939–1957 and served during the Vietnam era (1965–1975). Questionnaires regarding physical appearance and supplemental blood typing were collected, identifying 1,874 monozygotic and 1,498 dizygotic twins (Eisen et al., 1987).
The mean (SD) age of respondents was 42.0 (2.8) years. Participants were predominantly white (93.4%; n=7,349), with individuals acknowledging black (6.2%; n=489) and other (0.4%; n=30) racial identities constituting the remainder. Most participants obtained more than a high-school education (64%, n=4929) and had annual household incomes between $20,000–$40,000 (49.1%, n=3,657).
Measures
Lifetime DSM-III-R diagnoses for PG, GAD, and PD were determined using the Diagnostic Interview Schedule (DIS; Robins and Regier, 1991). Lay interviewers obtained verbal informed consent. This method was approved by the institutional review boards at the participating institutions, as have been data analyses performed in the current study. Criteria for PG were only assessed in participants who gambled 25 times or more in the past year. As previously (Scherrer et al., 2000; Chantarujikapong et al., 2001), D criteria for GAD and C criteria for PD were used. Meeting criteria for GAD involved acknowledging a period of at least one month of worry and the presence of six or more symptoms of GAD during a period of worry. Meeting criteria for PD involved acknowledging a panic attack and four or more symptoms during the panic attack, regardless of whether the attack was associated with phobia.
Hypothesis Testing
Odds ratios (ORs) for GAD in subjects with PG examined the hypothesis that lifetime PG and GAD frequently co-occurred, with a similar approach investigating PG and PD. As twin-pair data violate assumptions of independence, SURVEYLOGISTIC procedure in SAS v9.2 was used which adjusts for error variance of non-independent observations. Both unadjusted and adjusted ORs were examined by logistic regression adjusting for sociodemographic variables (education, age, and income), non-PG and non-GAD psychiatric disorders or non-PD psychiatric disorders, respectively, in a step-wise fashion, controlling first for influences of externalizing disorders (alcohol dependence, nicotine dependence, drug dependence, and antisocial personality disorder).
Tetrachoric correlations examined the hypotheses that diagnoses of PG and GAD, and PG and PD, correlate more strongly within monozygotic twins than in dizygotic twins. Bivariate models fitting the associations between PG and GAD and PG and PD investigated hypotheses regarding genetic and environmental contributions to the co-occurrences of PG and ADs (Slutske et al., 2000). The relationship was deconstructed into three factors: additive genetic (A), shared environmental (C) and unique environmental, including influences of measurement error (E). Using MX software (Neale and Cardon, 1992; Virginia Commonwealth University, Richmond, VA), models of maximum likelihood were fitted. Series of nested models were tested for their goodness of fit against a saturated model that placed no constraints on the elements of the estimated monozygotic and dizygotic twin correlation matrices. The most parsimonious model was selected as best-fitting. Ninety-five-percent confidence-intervals (95% CIs) around the parameter estimates for this model were examined to evaluate whether genetic, shared environment or unique environmental contributions to PG and GAD, and PG and PD, differed significantly from 0 to 1.
Results
Lifetime criteria for PG, GAD and PD were met by 112 (1.4%), 966 (12.3%) and 473 (6.0%) of participants, respectively. In unadjusted models, PG frequently co-occurred with both GAD and PD. After adjusting for sociodemographic and externalizing variables, ORs for GAD and PD remained elevated at 1.91 (95% CI:1.16–3.14) and 2.46 (95% CI:1.42–4.26), respectively. After also adjusting for internalizing factors, the relationship with GAD was no longer significant (OR=1.43; 95% CI:0.84–2.42; Table 1A). If major depression was removed from the model, ORs between PG and GAD (1.72; 95% CI:1.01–2.93) and PG and PD (2.20; 95% CI:1.21–4.02) remained significantly elevated. The OR between PG and PD remained elevated (2.02; 95% CI:1.09–3.73) when controlling for all sociodemographic and psychiatric variables.
Table 1A.
Logistic Regression Model Examining the Relationships Between Pathological Gambling and Generalized Anxiety Disorder and Panic Disorder
| Variable | Odds Ratio (95% CI) |
|---|---|
| Age, yrs | 1.05 (0.97 – 1.15) |
| Income | 0.76 (0.49 – 1.19) |
| High school education | 0.82 (0.36 – 1.86) |
| College | 0.63 (0.28 – 1.43) |
| Alcohol dependence | 2.57 (1.46 – 4.53) |
| Nicotine dependence | 1.12 (0.68 – 1.86) |
| Drug dependence | 1.82 (1.03 – 3.20) |
| Antisocial personality | 2.59 (1.19 – 5.64) |
| Major depression | 1.88 (1.10 – 3.22) |
| Posttraumatic stress disorder | 0.71 (0.38 – 1.32) |
| Generalized anxiety disorder | 1.43 (0.84 – 2.42) |
| Panic disorder | 2.02 (1.09 – 3.73) |
Abbreviations: CI = confidence interval
The standard errors are adjusted for clustering on case.
In the tetrachoric correlations between PG and GAD, and PG and PD, in monozygotic and dizygotic twins (Table 1B, C), within-diagnosis concordance was higher in monozygotic twins than in dizygotic twins, particularly for GAD. The within-twin cross diagnosis correlations were comparable for monozygotic and dizygotic twins in both PG and GAD and PG and PD (Table 1B, C). The cross-diagnosis correlations appeared more strong in monozygotic twins for PG and PD (such correlation was unstable for PG and GAD due to a small sample (n=1) in one cell). Together, these findings suggest a genetic relationship contributing to the co-occurrences of PG and PD and PG and GAD.
Table 1B.
Tetrachoric Correlations Between Pathological Gambling and Generalized Anxiety Disorder in Monozygotic and Dizygotic Twins
| Zygosity | Within-Diagnosis Tetrachoric Correlations (SE) | Cross-Diagnosis Tetrachoric Correlations Between PG and GAD (SE) | ||
|---|---|---|---|---|
| PG | GAD | Within-twin | Cross-twin | |
| Monozygotic | 0.62 (0.10); (n=6) | 0.39 (0.05); (n=64) | 0.21 (0.10); (n=15) | 0.24 (0.10); (n=15) |
| Dizygotic | 0.40 (0.16); (n=2) | 0.13 (0.06); (n=33) | 0.32 (0.10); (n=19) | unstable*; (n=5) |
N’s in table 1B indicate the number of twins meeting each indicated criterion. In cases where two tetrachoric correlations were derived and contributed to the tabulated values (e.g., for cross-twin, cross-diagnosis values where twin A with GAD and twin B with PG and twin A with PG and twin B with GAD), the tabulated correlations and SEs represent average values and n’s indicate total numbers of subjects.
There exists an unstable relationship due to a small sample (n=1 in one cell), yielding an estimated average value of −0.04(0.15).
Table 1C.
Tetrachoric Correlations Between Pathological Gambling and Panic Disorder in Monozygotic and Dizygotic Twins
| Zygosity | Within-Diagnosis Tetrachoric Correlations (SE) | Cross-Diagnosis Tetrachoric Correlations Between PG and PD (SE) | ||
|---|---|---|---|---|
| PG | PD | Within-twin | Cross-twin | |
| Monozygotic | 0.62 (0.10); (n=6) | 0.47(0.06); (n=27) | 0.29 (0.11); (n=12) | 0.18 (0.13); (n=7) |
| Dizygotic | 0.40 (0.16); (n=2) | 0.05 (0.10); (n=6) | 0.33 (0.11); (n=12) | 0.06 (0.15); (n=2)* |
N’s in table 1C indicate the number of twins meeting each indicated criterion. In cases where two tetrachoric correlations were derived and contributed to the tabulated values, the tabulated correlations and SEs represent average values and n’s indicate total numbers of subjects.
This estimate was derived from one correlation as the cell for cases in one of the two contributing correlations was empty (n=0).
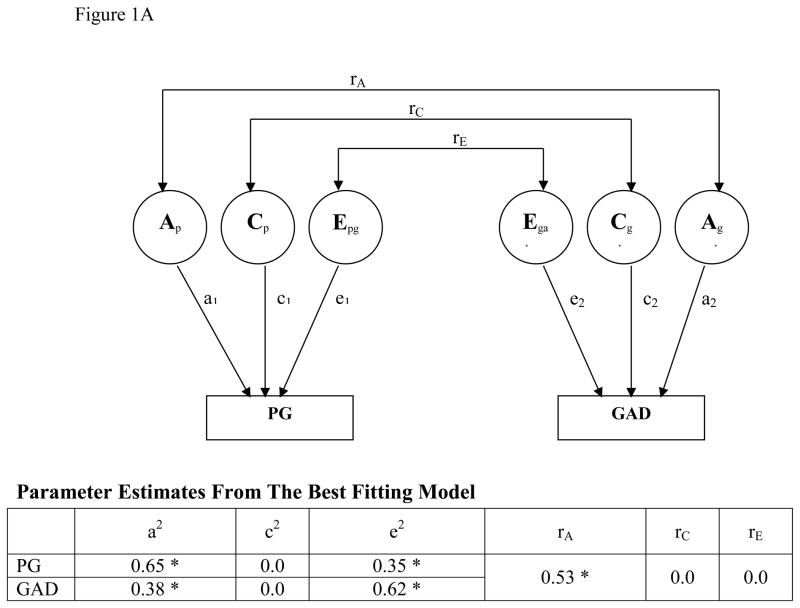
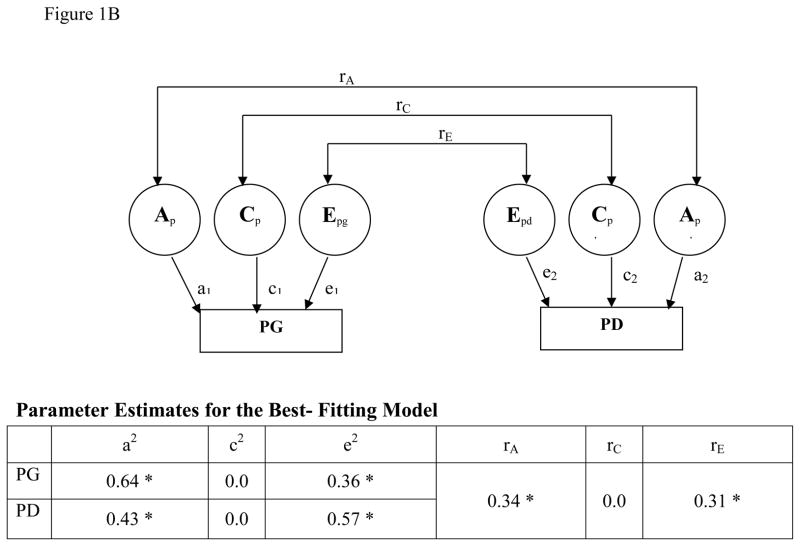
For PG, GAD, and PD individually, parameter estimates suggest significant genetic and unique environmental contributions (Figure 1A, B). For the relationship between PG and GAD, the best-fitting bivariate model (Table 1D) had correlations for both shared and unique environmental factors set to 0 (rC=0, rE=0) and estimated correlations for the additive genetic factors (rA) at 0.53 (95% CI:0.42–0.73). The best-fitting bivariate model for the relationship between PG and PD had the shared environmental factor set to 0 (Table 1E) and demonstrated significant correlations in the unique environmental (rE=0.31; 95% CI:0.29–0.62) and additive genetic (rA=0.34; 95% CI:0.05–0.63) domains.
Figure 1.
Figure 1A. BIVARIATE MODEL: PG and GAD
*Parameters are statistically significant (the 95% CI does not include 0 or 1).
A schematic diagram for the bivariate biometric model examining the relationship between pathological gambling (PG) and generalized anxiety disorder (GAD). Factors including PG and GAD include additive genetic factors (A), shared environment (C), and unique environment plus error (E). Correlations between these factors across disorders are represented as rA, rC, and rE respectively. The contributions of each of these factors to PG (aPG, CPG, ePG) and GAD (aGAD, cGAD, eGAD) are also indicated. CI indicates confidence interval and lowercase a, c, and e refer to path loading for factors A, C, and E respectively.
Figure 1B. BIVARIATE MODEL: PG and PD
*Parameters are statistically significant (the 95% CI does not include 0 or 1).
A schematic diagram for the bivariate biometric model examining the relationship between pathological gambling (PG) and panic disorder (PD). Factors including PG and PD include additive genetic factors (A), shared environment (C), and unique environment plus error (E). Correlations between these factors across disorders are represented as rA, rC, and rE respectively. The contributions of each of these factors to PG (aPG, Cpg, ePG) and PD (aPD, cPD, ePD) are also indicated. CI indicates confidence interval and lowercase a, c, and e refer to path loading for factors A, C, and E respectively.
Table 1D.
Bivariate Model Fitting Statistics for GAD
| Model Fits Statistics
| |||||||
|---|---|---|---|---|---|---|---|
| Model | −2 Log Likelihood | DF | −2 Log Likelihood Difference | DF Difference | P-value | AIC | |
| 0 | full | 6917.210 | 15687 | n/a | n/a | n/a | n/a |
| 1 | rA=0 | 6930.960 | 15688 | 13.750 | 1 | 0.000 | 11.750 |
| 2 | rA=1 | 6932.780 | 15688 | 15.570 | 1 | 0.000 | 13.570 |
| 3 | rC=0 | 6917.210 | 15688 | 0.000 | 1 | 1.000 | −2.000 |
| 4 | rC=1 | 6917.210 | 15688 | 0.000 | 1 | 1.000 | −2.000 |
| 5 | rE=0 | 6917.283 | 15688 | 0.073 | 1 | 0.788 | −1.927 |
| 6 | rE=1 | 792.002 | 15688 | 2874.792 | 1 | 0.000 | 2872.792 |
| 7 | c1 = c2= rC=0 | 6917.210 | 15690 | 0.00 | 3 | 1.00 | −6.00 |
| 8 | rA=c1 = c2= rC=0 | 6930.960 | 15691 | 13.750 | 4 | 0.008 | 5.750 |
| 9 | rA=1, c1 = c2= rC=0 | 6932.780 | 15691 | 15.570 | 4 | 0.004 | 7.570 |
| 10 | c1 = c2= rC= rE=0 | 6917.283* | 15691* | 0.073* | 4* | 0.999* | −7.927* |
| 11 | c1 = c2= rC= 0, rE=1 | 9792.002 | 15691 | 2874.792 | 4 | 0.000 | 2866.792 |
Abbreviations: A = Additive genetics; AIC = Akaike information criterion; C = Shared environment; E = Unique environment plus error; GAD = Generalized Anxiety Disorder as defined in DSM-III-R; NA = not applicable; PG= pathological gambling as defined in DSM-III-R; rA = Correlation between APG and AGAD; rC = Correlation between CPG and CGAD; rE = Correlation between EPG and EGAD;
= Best Fitting model
Table 1E.
Bivariate Model Fitting Statistics for PD
| Model Fits Statistics
| |||||||
|---|---|---|---|---|---|---|---|
| Model | −2 Log Likelihood | DF | −2 Log Likelihood Difference | DF Difference | P-value | AIC | |
| 0 | Full | 4653.530 | 15685 | n/a | n/a | n/a | n/a |
| 1 | rA=0 | 4658.097 | 15686 | 4.566 | 1 | 0.033 | 2.566 |
| 2 | rA=1 | 4671.186 | 15686 | 17.656 | 1 | 0.000 | 15.656 |
| 3 | rC=0 | 4653.530 | 15686 | 0.000 | 1 | 1.000 | −2.000 |
| 4 | rC=1 | 4653.530 | 15686 | 0.000 | 1 | 1.000 | −2.000 |
| 5 | rE=0 | 4656.847 | 15686 | 3.317 | 1 | 0.069 | 1.317 |
| 6 | rE=1 | 4662.219 | 15686 | 8.689 | 1 | 0.003 | 6.689 |
| 7 | c1 = c2= rC=0* | 4653.530* | 15688* | 0.00* | 3* | 1.00* | −6.00* |
| 8 | rA=c1 = c2= rC=0 | 4658.536 | 15689 | 5.006 | 4 | 0.287 | −2.994 |
| 9 | rA=1, c1 = c2= rC=0 | 4671.186 | 15689 | 17.656 | 4 | 0.001 | 9.656 |
| 10 | c1 = c2= rC= rE=0 | 4656.847 | 15689 | 3.317 | 4 | 0.506 | −4.683 |
| 11 | c1 = c2= rC= 0, rE=1 | 4662.219 | 15689 | 8.689 | 4 | 0.069 | 0.689 |
Abbreviations: A = Additive genetics; AIC = Akaike information criterion; C = Shared environment; E = Unique environment plus error; PD = Panic Disorder as defined in DSM-III-R; NA = not applicable; PG= pathological gambling as defined in DSM-III-R; rA = Correlation between APG and APD; rC = Correlation between CPG and CPD; rE = Correlation between EPG and EPD;
= Best Fitting model
When comparing the additive genetic component in PG and GAD, the genetic component accounted for 65% (95% CI, 45–80%) of the variance observed in PG and 38% (95% CI, 28%–47%) of that for GAD. Of the 65% genetic variance contributing to PG, approximately one-third (18%; 95% CI, 8%–43%) was shared with GAD and two-thirds (47%; 95% CI, 21% – 66%) was not. Of the 38% genetic variance contributing to GAD, approximately one third (11%; 95% CI, 5%–25%) was shared with PG and about two thirds (27%; 95% CI, 13%–39%) was not. The overlap between PG and GAD was accounted for by additive genetic factors.
When comparing PG and PD, of the 64% genetic variance observed in PG, 7% (95% CI, 0.1–31%) was shared with PD. Of the 43% genetic variance contribution to PD, 5% (95% CI, 0.1–22%) was shared with PG. In PG and PD, the unique environmental component accounted for 36% (95% CI, 21–57%) of the variance in PG and 57% (95% CI, 45–57%) of that observed in PD. Of the 36% of the unique environmental component contributing to PG, 3% (95% CI, 2–22%) was shared with PD. Of the 57% of the unique environmental component contributing to PD, 5% (95% CI, 4–22%) was shared.
Discussion
The hypothesis that both ADs would co-occur frequently with PG was largely confirmed, particularly in unadjusted models. Whereas ORs between PG and PD remained elevated following adjustments, those between PG and GAD remained significant when accounting for sociodemographic and externalizing psychiatric variables, but not after adjusting for internalizing psychiatric variables. When removing major depression from the model, the OR between PG and GAD remained elevated, suggesting that some of the variance between PG and GAD is attributable to depression, consistent with the notion of internalizing disorders (Krueger, 1999).
The results from the tetrachoric correlations and bivariate models for PG and GAD are consistent with the hypothesis that the relationship between PG and GAD is substantially genetic in nature, suggesting that different unique environmental influences contribute to PG and GAD. In contrast, the relationship between PG and PD appeared accounted for by both shared genetic and unique environmental influences. These findings suggest different etiologies to the co-occurrences between PG and GAD and PG and PD, respectively.
The current study has multiple clinical implications. First, in preliminary studies, candidate genetic allelic variants (e.g., MAO-A and 5-HTTLPR) have been individually associated with PG and GAD (Perez de Castro et al., 1999; Ibáñez et al., 2000; Perez de Castro et al., 2002; Tadic et al., 2003; You et al., 2005), whereas allelic variants of DAT1 and TPH have been associated individually with PG and PD (Comings et al., 2001; Mossner et al., 2006; Bae et al., 2007; Yoon et al., 2008). The extent to which these and other candidate genes might contribute to the co-occurrence of PG and ADs should be investigated. Second, the genetic overlaps between PG and GAD and between PG and PD suggest that biological mechanisms implicated in PG may also underlie ADs. For example, genetic and biological factors contributing to reward processing, impulsivity, decision-making, neuroticism, and stress responsiveness that have been implicated in PG may hold relevance for ADs (Mackintosh et al., 2006; Potenza et al., 2003; Brewer and Potenza, 2008). Third, future research should attempt to define the specific environmental contributions contributing to the co-occurrence of PG and PD. By understanding better such environmental factors that have been implicated in PG and PD (for e.g., childhood trauma), more targeted interventions can be implemented (Leskin and Sheikh, 2002; Petry, 2005; Scherrer et al., 2007; Sareen et al., 2007). Fourth, as treatments for PG have not been as extensively studied as treatments for ADs, it may be beneficial to investigate the efficacy and tolerability of pharmacological and behavioral treatments for ADs in individuals with PG and co-occurring ADs (Grant and Potenza, 2006). Fifth, the findings of shared genetic contributions to PG and ADs raises questions regarding how best to categorize PG. In DSM-IV-TR, PG is classified as an impulse control disorder and being considered as an addiction in DSM-V (Potenza, 2006; Potenza, 2008; Holden, 2010). Future studies are warranted to examine how PG best fits into the structure of psychiatric disorders.
Limitations and Strengths
Study limitations include the potential applicability of findings to the general population. As this study used data from the VET-R consisting of male twin pairs who were largely well-educated, served in the army, middle-aged, and predominantly white, the results may not extend to women or populations that are educationally and ethnically diverse. Data analyzed were collected close to twenty years earlier (when different diagnostic criteria were employed and different environments (e.g., with respect to availability of legalized gambling) existed). Additional studies using more recent information are important to examine the extent to which the findings extend to the present time. Strengths of the study include the use of a large, well-characterized sample of twins with formal diagnostic information.
Conclusions
The significant genetic overlap observed between PG and GAD and PG and PD indicates the need for future investigations that identify specific genetic factors that may contribute to this relationship. Further, the identification of shared environmental contributions to PG and PD indicates the need to identify specific environmental factors (e.g., early life trauma) that have been implicated in each disorder. Such information could aid in advancing prevention and treatment strategies for PG and ADs.
Acknowledgments
Role of Funding Source: This work was supported in part by the NIH (R01 DA019039, RC1 DA028279, MH60426), the VA VISN1 MIRECC, and a Center of Excellence in Gambling Research Award from the National Center for Responsible Gaming and its affiliated Institute for Research on Gambling Disorders. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Center for Responsible Gaming or the Institute for Research on Gambling Disorders or any of the other funding agencies. The NIH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
We would like to acknowledge the continual cooperation and participation of the VET-R members and their families. We greatly appreciate their assistance and without them this research would not have been possible. We would like to thank the numerous organizations that have provided assistance for the conductance of this study, including the Department of Defense (Washington, DC); National Personnel Records Center (St Louis, Mo); National Archives and Records Administration (College Park, Md); Internal Revenue Service (Washington); National Opinion Research Center (Chicago, III); National Research Council (Washington); National Academy of Sciences (Washington); and Institute for Survey Research, Temple University (Philadelphia, Pa). We would like to acknowledge David Reiss for helpful comments and discussions.
Footnotes
Contributors: Seth Eisen participated in the initial study design and oversight of data collection. Hong Xian, Jeffrey Scherrer, Seth Eisen and Marc Potenza participated in data analytical planning. Hong Xian performed the statistical analyses, Justine Giddens wrote the first draft of the manuscript, and Marc Potenza worked closely with Justine Giddens in revising the manuscript. All authors participated in the drafting of the manuscript and have approved the submitted version.
Conflict of Interest: Dr. Potenza has received financial support or compensation for the following: Dr. Potenza consults for and is an advisor to Boehringer Ingelheim; has financial interests in Somaxon; has received research support from the National Institutes of Health, Veteran’s Administration, Mohegan Sun Casino, the National Center for Responsible Gaming and its affiliated Institute for Research on Gambling Disorders, and Forest Laboratories pharmaceuticals; has participated in surveys, mailings or telephone consultations related to drug addiction, impulse control disorders or other health topics; has consulted for law offices on issues related to addictions or impulse control disorders; has provided clinical care in the Connecticut Department of Mental Health and Addiction Services Problem Gambling Services Program; has performed grant reviews for the National Institutes of Health and other agencies; has guest-edited journal sections; has given academic lectures in grand rounds, CME events and other clinical or scientific venues; and has generated books or book chapters for publishers of mental health texts. All authors reported no conflict of interest in the content of this paper.
References
- Argo TR, Black DW. Clinical characteristics. In: Grant JE, Potenza MN, editors. Pathological gambling: A clinical guide to treatment. Arlington, VA: American Psychiatric Publishing; 2004. pp. 39–54. [Google Scholar]
- Bae SM, Lim SW, Oh KS, Lee MS. Association between panic disorder and dopamine transporter gene (DAT1) Polymorphism. J Korean Society Biol Psychiatry. 2007;14(1):55–60. [Google Scholar]
- Brewer JA, Potenza MN. The neurobiology and genetics of impulse control disorders: Relationships to drug addictions. Biochem Pharmacol. 2008;75(1):63–75. doi: 10.1016/j.bcp.2007.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantarujikapong SI, Scherrer JF, Xian H, Eisen SA, Lyons MJ, Goldberg J, Tsuang M, True WR. A twin study of generalized anxiety disorder symptoms, panic disorder symptoms and post-traumatic stress disorder in men. Psychiatry Res. 2001;103:133–145. doi: 10.1016/s0165-1781(01)00285-2. [DOI] [PubMed] [Google Scholar]
- Comings DE, Gade-Andavolu R, Gonzalez N, Wu S, Muhleman D, Chen C, Koh P, Farwell K, Blake H, Dietz G, MacMurray JP, Lesieur HR, Rugle LJ, Rosenthal RJ. The additive effect of neurotransmitter genes in pathological gambling. Clin Genet. 2001;60:107–116. doi: 10.1034/j.1399-0004.2001.600204.x. [DOI] [PubMed] [Google Scholar]
- Eisen S, True W, Goldberg J, Henderson W, Robinette CD. The Vietnam Era Twin Registry (VET): Method of construction. Acta Genet Med Gemellol (Roma) 1987;36:61–66. doi: 10.1017/s0001566000004591. [DOI] [PubMed] [Google Scholar]
- Grant JE, Potenza MN. Escitalopram treatment of pathological gambling with co-occurring anxiety: An open-label pilot study with double-blind discontinuation. Int Clin Psychopharmacol. 2006;21(4):203–209. doi: 10.1097/00004850-200607000-00002. [DOI] [PubMed] [Google Scholar]
- Holden C. Behavioral addictions debut in proposed DSM-V. Science. 2010;327:935. doi: 10.1126/science.327.5968.935. [DOI] [PubMed] [Google Scholar]
- Ibáñez A, Pérez de Castro I, Fernández-Piqueras J, Sáiz-Ruiz J. Association between the low-functional MAO-A gene promoter and pathological gambling. Am J Med Genet. 2000;96(4):464–465. [Google Scholar]
- Katzelnick DJ, Koback KA, DeLeire T, Henk HJ, Greist JH, Davidson JRT, Schneier FR, Stein MB, Helstad CP. Impact of generalized social anxiety disorder in managed care. Am J Psychiatry. 2001;158(12):1999–2007. doi: 10.1176/appi.ajp.158.12.1999. [DOI] [PubMed] [Google Scholar]
- Kruger RF. The structure of common mental health disorders. Arch Gen Psychiatry. 1999;56:921–926. doi: 10.1001/archpsyc.56.10.921. [DOI] [PubMed] [Google Scholar]
- Leskin GA, Sheikh JI. Lifetime trauma history and panic disorder: Findings from the National. 2002. [DOI] [PubMed] [Google Scholar]
- Mackintosh MA, Gatz M, Wetherell JL, Pedersen NL. A twin study of lifetime generalized anxiety disorder (GAD) in older adults: Genetic and environmental influences shared by neuroticism and GAD. Twin Res Human Gen. 2006;9(1):30–37. doi: 10.1375/183242706776402902. [DOI] [PubMed] [Google Scholar]
- Mossner R, Freitag CM, Gutknecht L, Reif A, Tauber R, Franke P, Fritze J, Wagner G, Peikert G, Wenda B, Sand P, Rietschel M, Garritsen H, Jacob C, Lesch KP, Deckert J. The novel brain-specific tryptophan hydroxylase-2 gene in panic disorder. J Psychopharmacol. 2006;20(4):547–552. doi: 10.1177/0269881106059704. [DOI] [PubMed] [Google Scholar]
- Neale MC, Cardon LR. Methodology for genetic studies of twins and families. Kluwer Academic Publishers; Dordrecht, The Netherlands: 1992. [Google Scholar]
- Petry NM, Stinson FS, Grant BF. Comorbidity of DSM–IV pathological gambling and other psychiatric disorders: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2005;66:564–574. doi: 10.4088/jcp.v66n0504. [DOI] [PubMed] [Google Scholar]
- Pérez de Castro I, Ibáñez A, Sáiz-Ruiz J, Fernández-Piqueras J. Genetic contribution to pathological gambling: Association between a functional DNA polymorphism at the serotonin transporter gene (5-HTT) and affected males. Pharmacogenetics. 1999;9:397–400. [PubMed] [Google Scholar]
- Pérez de Castro I, Ibáñez A, Saiz-Ruiz J, Fernández-Piqueras J. Concurrent positive association between Pathological Gambling and functional DNA polymorphisms at the MAO-A and the 5-HT transporter genes. Mol Psychiatry. 2002;7:927–928. doi: 10.1038/sj.mp.4001148. [DOI] [PubMed] [Google Scholar]
- Potenza MN. Should addictive disorders include non-substance- related conditions? Addiction. 2006;101(Suppl 1):142–151. doi: 10.1111/j.1360-0443.2006.01591.x. [DOI] [PubMed] [Google Scholar]
- Potenza MN. Impulse Control Disorders and Co-Occurring Disorders: Dual Diagnosis Considerations. J Dual Diagnosis. 2007;3(2):47–57. [Google Scholar]
- Potenza MN. The neurobiology of pathological gambling and drug addiction: an overview and new findings. Philosophical Transactions of the Royal Society of London Series B: Biological Sciences, (London) 2008;363:3181–3189. doi: 10.1098/rstb.2008.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza MN, Steinberg MA, Skudlarski P, Fulbright RK, Lacadie CM, Wilber MK, Rounsaville BJ, Gore JC, Wexler BE. Gambling urges in pathological gambling a Functional magnetic resonance imaging study. Arch Gen Psychiatry. 2003;60:828–836. doi: 10.1001/archpsyc.60.8.828. [DOI] [PubMed] [Google Scholar]
- Potenza MN, Xian H, Shah K, Scherrer JF, Eisen SA. Shared genetic contributions to pathological gambling and major depression in men. Arch Gen Psychiatry. 2005;62:1015–1021. doi: 10.1001/archpsyc.62.9.1015. [DOI] [PubMed] [Google Scholar]
- Robins LN, Regier DA. Psychiatric disorders in America. Free Press; New York: 1991. [Google Scholar]
- Sareen J, Cox BJ, Afifi TO, Stein MB, Belik S, Meadows G, Asmundson GJG. Combat and peacekeeping operations in relation to prevalence of mental disorders and perceived need for mental health care findings from a large representative sample of military personnel. Arch Gen Psychiatry. 2007;64(7):843–852. doi: 10.1001/archpsyc.64.7.843. [DOI] [PubMed] [Google Scholar]
- Scherrer JF, True WR, Xian H, Lyons MJ, Eisen SA, Goldberg J, Lin N, Tsuang MT. Evidence for genetic influences common and specific to symptoms of generalized anxiety and panic. J Affect Disord. 2000;57:25–35. doi: 10.1016/s0165-0327(99)00031-2. [DOI] [PubMed] [Google Scholar]
- Scherrer JF, Xian H, Krygiel Kapp JM, Waterman B, Shah KR, Volberg R, Eisen SA. Association between exposure to childhood and lifetime traumatic events and lifetime pathological gambling in a twin cohort. J Nerv Ment Dis. 2007;195:72–78. doi: 10.1097/01.nmd.0000252384.20382.e9. [DOI] [PubMed] [Google Scholar]
- Slutske WS, Eisen S, True WR, Lyons MJ, Goldberg J, Tsuang M. Common genetic vulnerability for pathological gambling and alcohol dependence in men. Arch Gen Psychiatry. 2000;57:666–674. doi: 10.1001/archpsyc.57.7.666. [DOI] [PubMed] [Google Scholar]
- Tadic A, Rujescu D, Szegedi A, Giegling I, Singer P, Moller HJ, Dahmen N. Association of a MAOA gene variant with generalized anxiety disorder, but not with panic disorder or major depression. Am J Med Gen Part B: Neuropsychiatric Genetics. 2003;117:1–6. doi: 10.1002/ajmg.b.10013. [DOI] [PubMed] [Google Scholar]
- Yoon HK, Yang JC, Lee BH, Kim YK. The association between serotonin-related gene polymorphisms and panic disorder. J Anxiety Disord. 2008;22(8):1529–1534. doi: 10.1016/j.janxdis.2008.03.006. [DOI] [PubMed] [Google Scholar]
- You JS, Hu SY, Chen B, Zhang HG. Serotonin transporter and tryptophan hydroxylase gene polymorphisms in Chinese patients with generalized anxiety disorder. Psychiatr Genet. 2005;15:7–11. doi: 10.1097/00041444-200503000-00002. [DOI] [PubMed] [Google Scholar]