Abstract
Stroke is a neurological emergency that carries a risk of morbidity and mortality. Recent studies have shown that the incidence of stroke, while rare, is increasing in pregnant females. In this review, stroke and other vasculopathies in the pregnant and post-partum female are examined. A discussion of the symptoms and clinical presentation of stroke is provided, as well as the current guideline for treatment of stroke in pregnancy. Finally, the data illustrating the recent increases in stroke incidence is outlined.
Keywords: stroke, pregnancy, puerperium, vasculopathy, cerebral sinus venous thrombosis, intracerebral hemorrhage
Stroke in Pregnancy and the Post-Partum Period
A cerebral stroke is a neurological emergency and is a major cause of disability and mortality for women (1–2). The term “stroke” is used to indicate damage to the brain caused by a vascular etiology. By definition, an ischemic stroke occurs when blood flow to the brain is impaired and tissue dies. Causes of ischemic stroke include atherosclerotic disease, embolisms, thrombi and hypotension. Hemorrhagic stroke occurs when a blood vessel ruptures and tissue is damaged by the resulting spread of blood into the brain parenchyma. Factors contributing to hemorrhagic stroke include hypertension, aneurysms, and arterio-venous malformations. Increasing intracranial pressure due to edema and hemorrhage also contribute to tissue damage.
As expected, stroke symptoms depend on the part of the brain affected by the insult. Weakness, numbness, vision and speech abnormalities can all occur. Additionally, changes in mental status can signal both ischemic and hemorrhagic infarctions. Although the overall number of strokes in women of childbearing age is low, pregnant women and women in the post-partum period are at an increased risk, due to a number of different factors that alter the body’s cardiovascular hemodynamics and coagulation mechanisms.
Ischemic Strokes
Much like non-pregnant women and men, the vast majority of strokes in pregnant women are attributed to arterial occlusions from artery-to-artery thromboembolism, cardiac embolism, and intracranial or extracranial atherothrombosis. Cervical artery dissection, either of the carotid or vertebral arteries may lead to ischemic infarction, and can occur during pregnancy. Ischemic cerebral infarction is the most common etiology of stroke in older women and men and accounts for approximately 80% of all strokes (1). As expected, ischemic infarctions due to any of the discussed etiologies, usually present with focal neurological abnormalities, such as weakness, sensory changes and/or cranial nerve abnormalities (Table 1). Symptoms which resolve within 24 hours are classified as a transient ischemic attack or TIA. TIAs are clinically important, as 15% of strokes are preceded by a TIA (3).
Table 1.
Localization of stroke symptoms by vascular territory
| Vessel | Symptoms |
|---|---|
| Middle Cerebral Artery | Contralateral face, arm and leg weakness Contralateral sensory loss Aphasia Ipsilateral gaze deviation |
| Anterior Cerebral Artery | Contralateral leg weakness and sensory loss Apraxia |
| Posterior Cerebral Artery | Contralateral sensory loss Cognitive dysfunction Contralateral homonomous hemianopsia |
| Posterior Inferior Cerebellar Artery | Decreased pain/temperature on ipsilateral face Decreased pain/temperature on contralateral arm/leg Horner’s syndrome Vertigo Dysconjugate gaze/nystagmus Gait, trunk, and limb ataxia |
| Anterior Inferior Cerebellar Artery | Ipsilateral facial weakness, ataxia, deafness Contralateral arm/leg sensory loss |
| Superior Cerebellar Artery | Diplopia Ataxia Contralateral arm/leg sensory loss |
| Basilar Artery & Basilar perforators | Cognitive dysfunction Weakness Cranial nerve palsies Ataxia |
| Lacunar - Internal Capsule, Corona Radiata | Contralateral motor hemiparesis |
| Lacunar – Thalamus | Contralateral sensory loss |
| Lacunar - Thalamocapsular | Contralateral motor hemiparesis and sensory loss |
| Lacunar – Basis pontis, thalamocapsular, corona radiate | Ipsilateral hemiataxia and hemiparesis |
| Lacunar – Basis pontis | Clumsy Hand/Dysarthria |
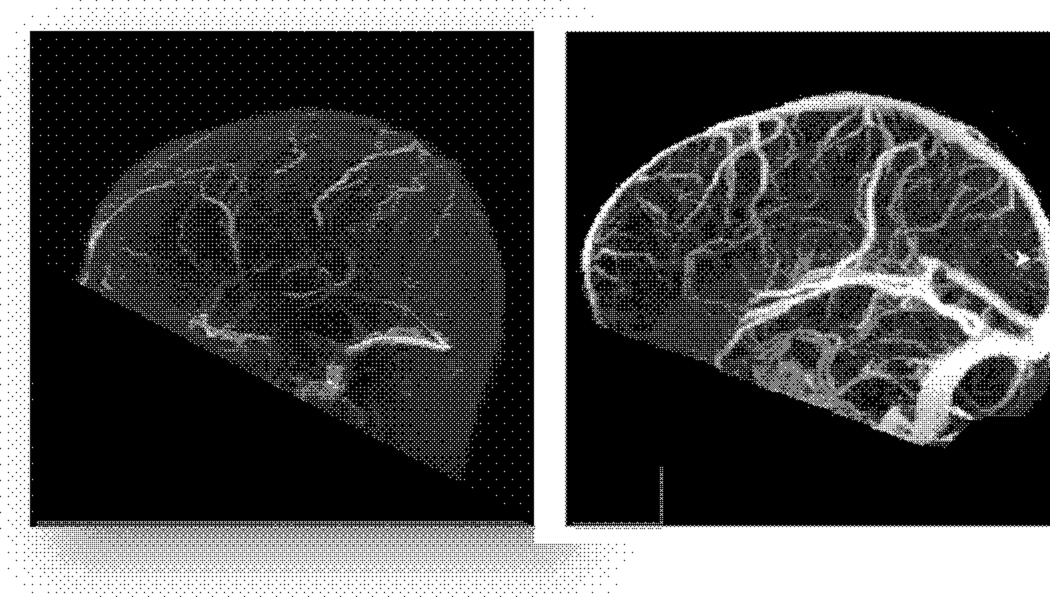
Cerebral venous thrombosis (CVT) is a also a significant cause of stroke in pregnancy, mainly due to hypercoagulability, such as venous stasis (Figure 1). CVT is a diagnosis not to be missed, as it can result in both venous ischemic and hemorrhagic infarctions. The hemorrhage arises from the venous congestion as a result of backflow of blood from the occlusion of a major sinus. CVT often presents as a severe headache with symptoms of increased intracranial pressure, such as nausea and vomiting. Frequently, papilledema is present on physical exam. The standard treatment of CVT is anticoagulation, which can be a difficult issue when there is hemorrhagic conversion of a venous infarct. There are interventional options, such as mechanical thrombectomy, which may pose significant risk, but are often used in the setting of severe hemorrhagic infarcts.
Figure 1.
Superior sagittal thrombosis on MR venography (left). Normal MR venography on right.
Diagnosis of arterial and venous infarctions is made based on clinical signs and radiographic abnormalities. The question of performing radiographic imaging often weighs significantly on the clinician’s mind due to concerns of exposing the developing fetus to radiation and/or magnetic fields. However, in CT imaging of the maternal head, exposure of the fetus to radiation is especially low (4). A stroke workup, including initial head CT to eliminate the possibility of hemorrhagic infarction, should be performed in pregnant women in most cases. An MRI of the brain without contrast is performed since gadolinium is known to cross the placenta, although the effects on the placenta are not known. An MRI of the brain is utilized to determine the location and extent of the stroke and provides evidence as to the acuity, and at times, the etiology of the infarction. Embolic infarcts demonstrate multiple foci that could be localized to one vascular territory, as in artery-to-artery embolus from a carotid or vertebral source. Or, if the infarcts are located in more than one vascular territory (anterior and posterior vessels), and are of different ages, a cardioembolic source may be most likely. Certain sequences of the MRI aid in assessment of the timing of the ischemia, such as the diffusion weighted images (DWI). Within minutes of infarction, water diffusion is restricted in the affected brain regions, which is visible as hyperintense areas on DWI. This is used to differentiate newly infarcted areas of brain from areas of remote infarction and proves to be a valuable resource in determining a time course for infarction.
Hemorrhagic Strokes
Hemorrhagic stroke also affects pregnant women. Subarachnoid hemorrhage (SAH), in which blood collects beneath the arachnoid mater, the tissue covering the brain, usually presents with intense headache (“worst headache of my life”). Other symptoms that may or may not be present include neck stiffness, decreased consciousness, seizures, nausea, vomiting and focal neurological abnormalities. Usually, SAHs are due to ruptured aneurysms or arterio-venous malformations, but can also be due to traumatic injuries. Uninfused head CT is the imaging modality of choice if SAH is suspected. Lumbar puncture to evaluate for xanthochromia can be useful if the CT shows no detectable subarachnoid blood, yet the suspicion for SAH is very high.
Treatment of Acute Stroke
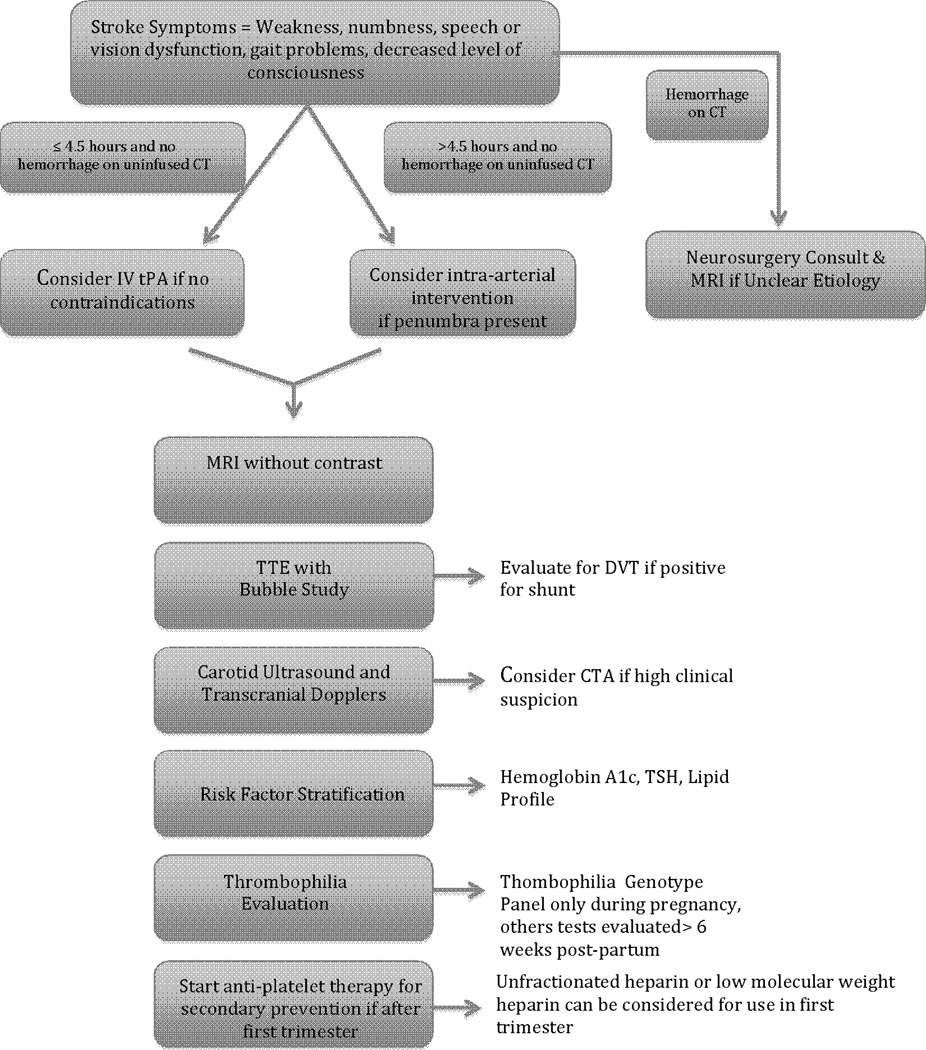
An algorithm for evaluating the pregnant woman with acute stroke symptoms is provided in Figure 2. Recombinant tissue plasminogen activator (rtPA) is a drug that lyses clot when given intravenously or intra-arterially to patients with acute ischemic stroke and is given routinely across the Unites States in patients presenting with an acute stroke. Randomized clinical trial evidence demonstrated that if rtPA is administered within 3 to 4.5 hours of ischemic stroke onset in nonpregnant patients, this drug decreases the risk of mortality and improves outcome at 90 days poststroke compared with placebo (5). However, there is an approximate 6% risk of hemorrhage, and this risk increases with administration greater than 3 h after onset of the stroke symptoms (5). In addition, there are devices that have been approved for mechanical thrombectomy, such as the Merci device (6) or the Penumbra device (7). In some cases, intra-arterial rtPA can be combined with mechanical thrombectomy. Patients tend to have optimal outcomes if the method that is used leads to partial or complete recanalization of the occluded artery in selected patients.
Figure 2.
Algorithm for stroke evaluation and management in the pregnant female. Recommendations for anti-platelet use are from the American Heart Association/American Stroke Association guidelines (20).
There are no clinical trials to evaluate the use of recombinant tissue plasminogen activator in pregnant women with acute stroke; however, it does not cross the placenta and there has been no evidence of teratogenicity in animal studies (8). It is listed as a category C drug and pregnancy is considered a relative contraindication for administration, but there are multiple case reports of successful use in pregnant women (9–13). As with all medications used in pregnant women, risks and benefits should be carefully weighed, but it appears that recombinant tissue plasminogen activator can be used both intravenously and intra-arterially in pregnancy with positive outcomes. There are no published cases of pregnant women receiving mechanical thrombectomy in the literature. However, the risk of systemic bleeding, and thus obstetric bleeding may be lower with mechanical thrombectomy and thus cause less risk to the patient and her unborn child.
Secondary Stroke Prevention in Pregnant Women
A transthoracic echocardiogram (TTE) should be performed to evaluate for cardioembolic sources of infarction. An agitated saline injection performed during the TTE, known as the bubble study, is utilized to evaluate for a right to left cardiac shunt, such as a patient foramen ovale. In the setting of a lower extremity deep vein thrombosis, a right to left cardiac shunt can result in a cerebral infarction. Thus, should the patient show evidence of a shunt on TTE with bubble study, lower extremity ultrasound should be performed.
Carotid ultrasound with transcranial Dopplers provides information on the hemodynamics of extra and intracranial vessel segments. Both ultrasound examinations function as a screen for carotid, vertebrobasilar, middle and anterior cerebral artery stenosis. However, in young women, these ultrasounds may not adequately detect a vasculopathy, such as posterior reversible encephalopathy syndrome (PRES) or reversible cerebral vasoconstriction syndrome (RCVS), so direct arterial imaging with CT angiography, MR angiography or catheter angiography should be considered if suspicion is high.
Risk stratification with fasting lipid panel, hemoglobin A1c and thyroid function should be performed to evaluate for classic risk factors which increase the risk of stroke, such as hyperlipidemia and diabetes. In some cases, a thrombophilia workup, with evaluation for sickle cell disease, anti-phospholipid antibodies and protein C/S deficiencies, should be pursued. Anti-phospholipid antibodies, including lupus anticoagulant, anti-cardiolipin antibodies and anti-β2-glycoprotein-1 antibodies are associated with an increased risk of both arterial and venous thromboembolism and stroke (14). Additionally, women with antiphospholipid antibody syndrome (APS) are at an increased risk for fetal loss, which is believed to be secondary to antiphospholipid antibody binding to trophoblast cells, leading to faulty placentation (15). The current consensus guidelines recommend treating women with recurrent pregnancy loss, antiphospholipid antibodies and no history of thrombosis with low-dose aspirin in combination with prophylactic or intermediate-dose unfractionated heparin or prophylactic dose low molecular weight heparin, administered in the antepartum period (16). Unfortunately, these studies are often falsely positive during pregnancy and evaluation is postponed until several weeks post-partum. Pregnancy does not influence a genetic thrombophilia panel and this should be ordered in all pregnant women presenting with stroke. Most commercially available panels test for common mutations resulting in factor V deficiency (factor V Leiden mutation), antithrombin deficiency, factor VIII excess, hyperhomocysteinemia, protein C and S deficiency and prothrombin 20210 mutation.
Non-pregnant women and men are treated with anti-platelet therapy following stroke for secondary stroke prevention, providing that no contraindications are present and there is no need for anticoagulation. Regular dose aspirin should not be used during pregnancy because of the risk of maternal and fetal bleeding, oligohydramnios, and premature closure of the ductus arteriosus (17).
Several meta-analyses have shown that low-dose aspirin (60–80 mg/day) can be used safely in select populations of pregnant women. A meta-analysis in 2002 showed no overall increase in risk of congenital malformations associated with aspirin, but determined that there may be an association between aspirin use in the first trimester and gastroschisis (17). However, a subsequent meta-analysis study in 2003 failed to find any increased risk associated with aspirin, including placental abruption, fetal intraventricular hemorrhage or congenital malformations (18). Notably, a recent meta-analysis suggests that low-dose aspirin is beneficial in preventing preeclampsia when started earlier than 16 weeks’ gestation, but not when initiated after 16 weeks (19). In that study, early treatment with aspirin also resulted in a decrease in gestational hypertension and preterm birth.
Secondary to the limited data, current recommendations for stroke prevention in pregnant women are similar to those recommended for thromboprophylaxis for venous thromboembolism. According to the American Heart Association/American Stroke Association guidelines, women at increased risk of stroke in whom antiplatelet therapy would likely be considered outside of pregnancy, may be considered for unfractionated heparin (UFH) or low-molecular-weight heparin (LMWH) during the first trimester, followed by low-dose aspirin in the second and third trimesters (20).
Other Vasculopathies Affecting Pregnant Women
Pre-eclampsia is defined as new-onset hypertension and proteinuria and can be further classified as with mild or severe. Eclampsia is the development of seizures in a patient with pre-eclampsia. Gestational hypertension is described as systolic blood pressure ≥ 140 mm Hg or diastolic blood pressure ≥ 90 mm Hg without proteinuria. Approximately 8% to 12% of all pregnancies are affected by a hypertensive disorder. Preeclampsia and eclampsia have been shown to be independent risk factors in the development of stoke during pregnancy. A 2005 analysis of the Nationwide Inpatient Sample database showed that preeclampsia was associated with a four-fold increase in stroke during pregnancy (21). Females who develop hypertensive disorders during pregnancy are also at a higher risk for development of chronic hypertension and strokes later in life.
Postpartum angiopathy is a unique condition associated with pregnancy which is associated with the reversible cerebral vasoconstriction syndromes or RCVS (22). While the pathophysiology is thought to be similar to RCVS, postpartum angiopathy is not confined to patients with history of preeclampsia or eclampsia and mostly occurs in patients who had uncomplicated pregnancies and deliveries with no history of hypertension. Symptoms include thunderclap headache, vomiting, altered mental status and/or focal neurologic deficits. Onset of symptoms classically occur about 5 days after delivery. The neurological symptoms may be transient or may be a result of ischemic stroke or cerebral hemorrhage (22. Diagnosis is made with angiography, which demonstrates multifocal segmental narrowing in the large and medium-sized cerebral arteries, which is often confused with vasculitis. By definition, the process is generally self-limited, with resolution of angiographic abnormalities within 4–6 weeks and typically complete resolution of symptoms (22). It is worth noting, however, that due to its link with ischemic and hemorrhagic infarction, there is a risk of both of morbidity and mortality.
Posterior reversible encephalopathy syndrome (PRES), also referred to as reversible posterior leukoencephalopathy syndrome (RPLS), is associated with reversible vasogenic subcortical edema, typically in the parietal and occipital lobes. Clinical symptoms of PRES include headache, seizure, visual symptoms and global encephalopathy. This vasculopathy can be triggered by hypertensive emergency, exposure to immunosuppressives and preeclampsia/eclampsia (23). Diagnosis is made by clinical symptoms in combination with findings of vasogenic subcortical edema on MRI. Clinical recovery usually occurs within days, with resolution of MRI abnormalities occurring in the range of days to weeks (23).
Incidence and Mortality of Stroke in Pregnancy
Recent data show that in the United States, the incidence of stroke in pregnancy is increasing. In 2011, Kuklina et al. published research that analyzed hospital discharge data from the Nationwide Inpatient Sample of the Healthcare Cost and Utilization Project, which is the largest nationwide all-payer hospital in-patient care database available in the United States (24). Between 1994–1995 to 2006–2007, the rates of antenatal and postpartum hospitalizations for stroke increased by 47% and 83%, respectively. The rate of a diagnosis of stroke during the delivery hospitalization remained the same throughout the time period studied. Specifically, the rates of transient ischemic attacks, CVTs and unspecified strokes were increased in the antenatal hospitalizations and hemorrhagic stroke, CVT and ischemic stroke diagnoses were increased in the postpartum hospitalization group (24). Women with concomitant diagnoses of hypertension and heart disease were more likely to also be diagnosed with a stroke of any type. This association was so strong, that when the authors corrected for hypertension and heart disease in a logistic regression model, the increase in stroke rates from 1994–1995 to 2006–2007 dissipated.
Summarizing the incidence from multiple studies, the rate of stroke is estimated to be 25–34 cases per 100,000 deliveries, whereas the incidence of stroke in non-pregnant woman aged 15–44 years of age is 11 per 100,000 women (21, 25–31). Although a small percentage of pregnant women are diagnosed with stroke, it accounts for 12% of maternal deaths (21) and contributes to significant fetal morbidity and mortality.
Risk Factors for Pregnancy-Related Stroke
As indicated in the aforementioned study by Kuklina et al, hypertensive disorders have been implicated to play a role in stroke in pregnancy. Other studies have shown similar results (21, 25, 28, 30–31). Hypertension is touted by stroke neurologists to be the #1 preventable stroke risk factor, and this is no exception in the pregnant female. Hypertension in pregnancy can be pre-existing, gestational or secondary to pre-eclampsia or eclampsia. Compared with women without hypertension, women with hypertension complicating pregnancy are six- to nine-fold more likely to have stroke (27–28). Other risk factors associated with pregnancy-related stroke include hypertension, diabetes, valvular heart disease, hypercoagulable disorders, sickle cell disease, lupus, abuse of tobacco and other substances, and migraines 21, 28). Complications of pregnancy, labor and delivery have also been associated with increased risk of stroke, including hyperemesis gravidarum, anemia, thrombocytopenia, postpartum hemorrhage, transfusion, fluid, electrolyte and acid-base disorders, and infection (21, 28). Age greater than 35 years increased the odds of stroke twofold, and African–American race-ethnicity increased the odds of stroke by 1.5-fold (21).
Cesarean delivery has been associated with peripartum stroke, although a causal relationship has not been well established (32). Historically, cesarean delivery has been recommended for women with ICH, particularly recent subarachnoid hemorrhage, untreated ruptured arteriovenous malformation (AVM) or unclipped ruptured aneurysm, to circumvent potential risks during labor and delivery (33). However, studies suggest that outcomes of vaginal and cesarean delivery are probably equivalent after ICH (34–35).
Although CVT occurs due to thrombosis of the sinuses, cerebral veins or jugular veins, and ischemic stroke occurs as a result of an arterial thrombosis or hemodynamic cause, there is quite a bit of overlap in the risk factors for both types of strokes during pregnancy. The primary causes for both types of strokes are thought to be influenced by the prothrombotic state of pregnancy itself, often in the setting of dehydration or an underlying predisposition for thrombophilia (36). The physiologic changes during pregnancy that may lead to arterial or venous thrombo embolism include decreases in circulating antithrombotic factors, venous stasis or sudden reduction in blood volume after delivery (36).
Pregnancy Factors that Can Influence Stroke Risk
Hemodynamic Factors
During pregnancy, the body’s total volume of water increases, beginning about 10 weeks after gestation. Throughout the preganancy, a female has about 50% increase in total body water, which begins to decline 2 weeks postpartum. Furthermore, cardiac output, stroke volume and heart rate all increase due to increased demand from the fetus and placenta and the chronic hypervolemic state. Blood pressure is decreased secondary to decreased systemic vascular resistance. Venous compliance increases throughout pregnancy, which steers the body towards decreased blood flow and increased venous stasis. Thus, physiological changes during pregnancy predispose to a state of hypervolemia, increased circulatory demands, decreased blood pressure and increased venous stasis, thereby changing the hemodynamic landscape (37).
Changes in coagulability
Physiological changes during pregnancy are believed to shift the balance towards a more hypercoagable state. As the pregnancy approaches term, many of the clotting factors increase, including procoagulant factors I, VII, VIII, IX, X, XII, and XIII (38). This is likely in expectation of the displacement of the placenta with subsequent release of prothrombotic factors to prevent hemorrhage (38). These changes in prothrombotic factors are greatest in the third trimester and return to baseline at three weeks postpartum.
Connective Tissue Changes
Several studies in animal models have suggested that cerebral arterial architecture changes during pregnancy (39–40), leading to decreased collagen, elasticity and distensibility. It is unclear how these changes translate to humans, but in the setting of hypervolemia and increased cardiac demands, the cerebral arteries may not be able to compensate, leading to theoretical increased risk of hemorrhagic infarctions (41).
In summary, stroke during pregnancy is rare, but the incidence is increasing, perhaps due to an increase in hypertension in young women before and during the child-bearing years. Therefore, identification of risk factors for stroke during pregnancy is critical in order to prevent this rare, and often devastating condition.
Acknowledgments
Funding support: Dr. Bushnell is supported by NIH/NINDS K02 NS058760.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roger V, Go A, Lloyd-Jones D, et al. Heart disease and stroke statistics 2011 update: a report from the American Heart Association. Circulation. 2010 doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seshadri S, Beiser AS, Kelly-Hayes M, et al. The lifetime risk of stroke. Estimates from the Framingham Study. Stroke. 2006;37:345–350. doi: 10.1161/01.STR.0000199613.38911.b2. [DOI] [PubMed] [Google Scholar]
- 3.Bushnell C, Rexrode K. Women and Health. Second Edition. San Diego: Academic Press; 2013. Cerebrovascular disease in women; pp. 1003–1020. [Google Scholar]
- 4.National Radiological Protection Board (NRPB) Diagnostic medical exposures: exposure to ionizing radiation of pregnant women. Doc NRPB. 1993;4:5–14. [Google Scholar]
- 5.The National Institute of Neurological Disorders. Stroke rt-PA Stroke Study Group tissue plasminogen activator for acute ischemic stroke. N. Engl. J. Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 6.Smith WS, Sung G, Saver J, et al. for the Multi MERCI Investigators: Mechanical thrombectomy for acute ischemic stroke. Final results of the multi MERCI trial. Stroke. 2008;39:1205–121. doi: 10.1161/STROKEAHA.107.497115. [DOI] [PubMed] [Google Scholar]
- 7.The Penumbra Stroke Trial Investigators The Penumbra Pivotal Stroke Trial. Safety and effectiveness of a new generation of mechanical devices for clot removal in intracranial large vessel occlusive disease. Stroke. 2009;40:2761–2768. doi: 10.1161/STROKEAHA.108.544957. [DOI] [PubMed] [Google Scholar]
- 8.DeKeyser J, Gdovinova Z, Uyttenboogaart M, Vroomen P, Jan Luijckx G. Intravenous alteplase for stroke. Beyond the guidelines and in particular clinical situations. Stroke. 2007;38:2612–2618. doi: 10.1161/STROKEAHA.106.480566. [DOI] [PubMed] [Google Scholar]
- 9.Johnson D, Kramer D, Cohen E, et al. Thrombolytic therapy for acute stroke in late pregnancy withintra-arterial recombinant tissue plasminogen activator. Stroke. 2005;36:E53–E55. doi: 10.1161/01.str.0000166203.27135.27. [DOI] [PubMed] [Google Scholar]
- 10.Murugappan A, Coplin W, Al-Sadat A, et al. Thrombolytic therapy of acute ischemic stroke during pregnancy. Neurology. 2006;66:768–770. doi: 10.1212/01.wnl.0000201272.90216.15. [DOI] [PubMed] [Google Scholar]
- 11.Elford K, Leader A, Wee R, Stys P. Stroke in ovarian hyperstimulation syndrome in early pregnancy treated with intra-arterial rt-PA. Neurology. 2002;59:1270–1272. doi: 10.1212/01.wnl.0000032492.77156.35. [DOI] [PubMed] [Google Scholar]
- 12.Wiese K, Talkad A, Mathews M, Wang D. Intravenous recombinant tissue plasminogen activator in a pregnant woman withcardioembolic stroke. Stroke. 2006;37:2168–2169. doi: 10.1161/01.STR.0000230286.95513.c2. [DOI] [PubMed] [Google Scholar]
- 13.Dapprich M. Fibrinolysis with alteplase in a pregnant woman with stroke. Cerebrovasc. Dis. 2002;13:290. doi: 10.1159/000057859. [DOI] [PubMed] [Google Scholar]
- 14.Lim W. Antiphospholipid syndrome. Hematology. 2009;2009:233–239. doi: 10.1182/asheducation-2009.1.233. [DOI] [PubMed] [Google Scholar]
- 15.Di Simone N, Luigi MP, Marco D, et al. Pregnancies complicated with antiphospholipid syndrome: the pathogenic mechanism of antiphospholipid antibodies: as review of the literature. Ann NY Acad Sci. 2007;1108:505–514. doi: 10.1196/annals.1422.054. [DOI] [PubMed] [Google Scholar]
- 16.Bates SM, Greer IA, Pabinger I, et al. Venous thromboembolism, thrombophilia, antithrombotic therapy and pregnancy: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133:844S–886S. doi: 10.1378/chest.08-0761. [DOI] [PubMed] [Google Scholar]
- 17.Kozer E, Nikfar S, Costei A, et al. Aspirin consumption during the first trimester of pregnancy and congenital anomalies: a meta-analysis. Am. J. Obstet. Gynecol. 2002;187:1623–1630. doi: 10.1067/mob.2002.127376. [DOI] [PubMed] [Google Scholar]
- 18.Coomarasamy A, Honest H, Papaioannou S, Gee H, Khan K. Aspirin for prevention of preeclampsia in women with historical risk factors: a systematic review. Obstet. Gynecol. 2003;101:1319–1332. doi: 10.1016/s0029-7844(03)00169-8. [DOI] [PubMed] [Google Scholar]
- 19.Bujold E, Roberge S, Lacasse Y, et al. Prevention of preeclampsia and intrauterine growth restriction with aspirin starte in early pregnancy. Obstet. Gynecol. 2010;116:402–414. doi: 10.1097/AOG.0b013e3181e9322a. [DOI] [PubMed] [Google Scholar]
- 20.Furie K, Kasner S, Adams R, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack. A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010 [Google Scholar]
- 21.James AH, Bushnell CD, Jamison MG, et al. Incidence and risk factors for stroke in pregnancy and the puerperium. Obstetrics & Gynecology. 2005;106:509–516. doi: 10.1097/01.AOG.0000172428.78411.b0. [DOI] [PubMed] [Google Scholar]
- 22.Calabrese L, Dodick D, Schwedt T, et al. Narrative review: reversible cerebral vasoconstriction syndrome. Ann Int Med. 2007;146:34–44. doi: 10.7326/0003-4819-146-1-200701020-00007. [DOI] [PubMed] [Google Scholar]
- 23.Pedraza R, Marik PE, Varon J. Posterior Reversible Encephalopathy Syndrome: A Review. Crit Care & Shock. 2009;12:135–143. [Google Scholar]
- 24.Kuklina EV, Tong X, Bansil P, et al. Trends in pregnancy hospitalizations that included a stroke in the United States from 1994 to 2007. Stroke. 2011;42:2564–2570. doi: 10.1161/STROKEAHA.110.610592. [DOI] [PubMed] [Google Scholar]
- 25.Kittner SJ, Stern BJ, Feeser BR, et al. Prenancy and the risk of stroke. N. Eng. J. Med. 1996;335:768–774. doi: 10.1056/NEJM199609123351102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lanska DJ, Kryscio RJ. Peripartum stroke and intracranial venous thrombosis in the National Hospital Discharge Survey. Obstet. Gynecol. 1997;89:413–418. doi: 10.1016/S0029-7844(96)00516-9. [DOI] [PubMed] [Google Scholar]
- 27.Lanska DJ, Kryscio RJ. Stroke and intracranial venous thrombosis during pregnancy and puerperium. Neurology. 1998;51:1622–1628. doi: 10.1212/wnl.51.6.1622. [DOI] [PubMed] [Google Scholar]
- 28.Lanska DJ, Kryscio RJ. Risk factors for peripartum and postpartum stroke and intracranial venous thrombosis. Stroke. 2000;31:1274–1282. doi: 10.1161/01.str.31.6.1274. [DOI] [PubMed] [Google Scholar]
- 29.Petitti D, Sidney S, Quesenberry CJ, Bernstein A. Incidenceof stroke and myocardial infarction in women of reproductive age. Stroke. 1997;28:280–283. doi: 10.1161/01.str.28.2.280. [DOI] [PubMed] [Google Scholar]
- 30.Bateman B, Schumacher H, Bushnell CD, et al. Intracerebral hemorrhage in pregnancy. Frequency, risk factors, and outcome. Neurology. 2006;67:424–429. doi: 10.1212/01.wnl.0000228277.84760.a2. [DOI] [PubMed] [Google Scholar]
- 31.Sharshar T, Lamy C, Mas J. Incidence and causes of strokes associated with pregnancy and puerperium. A study in public hospitals of Ile de France. Stroke. 1995;26:930–936. doi: 10.1161/01.str.26.6.930. [DOI] [PubMed] [Google Scholar]
- 32.Lin SY, Hu CJ, Lin HC. Increased risk of stroke in patients who undergo cesareandelivery: a nationwide population-based study. Am J Obstet Gynecol. 2008:391.e1–391.e7. doi: 10.1016/j.ajog.2007.10.789. [DOI] [PubMed] [Google Scholar]
- 33.Cohen-Gadol AA, Friedman JA, Friedman JD, et al. Neurosurgical management of intracranial lesions in the pregnant patient: a 36-year institutional experience and review of the literature. Journal of Neurosurgery. 2009;111:1150–1157. doi: 10.3171/2009.3.JNS081160. [DOI] [PubMed] [Google Scholar]
- 34.Treadwell S, Thanvi B, Robinson T. Stroke in pregnancy and the puerperium. Postgrad. Med. J. 2008;84:238–245. doi: 10.1136/pgmj.2007.066167. [DOI] [PubMed] [Google Scholar]
- 35.Wilterdink JL, Feldmann E. Intracranial hemorrhage. Adv. Neurol. 2002;90:63–74. [PubMed] [Google Scholar]
- 36.Saposnik G, Barinagarrementeria F, Brown R, et al. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(4):1158–1192. doi: 10.1161/STR.0b013e31820a8364. [DOI] [PubMed] [Google Scholar]
- 37.Tettenborn B. Stroke and pregnancy. Neurol Clin. 2012;30:913–924. doi: 10.1016/j.ncl.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 38.Cerneca F, Ricci G, Simeone R, et al. Coagulation and fibrinolysis changes in normal pregnancy. Increased levels of procoagulants and reduced levels of inhibitors during pregnancy indice a hypercoagulable state, combined with a reactive fibrinolysis. European Journal of Obstetrics & Gynecology and Reproductive Biology. 1997;73:31–36. doi: 10.1016/s0301-2115(97)02734-6. [DOI] [PubMed] [Google Scholar]
- 39.Chillon JM, Baumbach GL. Effects of chronic nitric oxide synthase inhibition on cerebral arterioles in Wistar-Kyoto rats. J Hypertens. 2004;22:529–534. doi: 10.1097/00004872-200403000-00015. [DOI] [PubMed] [Google Scholar]
- 40.Aukes AM, Vitullo L, Zeeman GG, et al. Pregnancy prevents hypertensive remodeling and decreases myogenic reactivity in posterior cerebral arteries from Dahl salt-sensitive rats: a role in eclampsia. AJP – Heart. 2006;292:H1071–H1076. doi: 10.1152/ajpheart.00980.2006. [DOI] [PubMed] [Google Scholar]
- 41.Cipolla MJ, Vitullo L, McKinnon J. Cerebral artery reactivity changes during pregnancy and the postpartum period: a role in eclampsia? Am J Physiol Heart Circ Physiol. 2004;286:H2127–H2132. doi: 10.1152/ajpheart.01154.2003. [DOI] [PubMed] [Google Scholar]