Abstract
Preeclampsia is associated with an increased release of factors from the placental syncytium into maternal blood, including the antiangiogenic factors soluble fms-like tyrosine kinase-1 and soluable endoglin, the antifibrinolytic factor plasminogen activator inhibitor-1, prostanoids, lipoperoxides, cytokines, and microparticles. These factors are suggested to promote maternal endothelium dysfunction and are associated with placental damage in pregnancies also complicated with intrauterine growth restriction (IUGR). In this report, we briefly describe the interaction of syncytial factors with hypoxia, reactive oxygen species, and apoptosis in the pathophysiology of preeclampsia and IUGR. Given the critical role of the syncytium in these complications of pregnancy, we also present a novel methodology in which laser capture microdissection followed by Western blotting is used to assess levels of syncytial Fas ligand, a key protein in the apoptotic cascade.
Keywords: Placenta, syncytiotrophoblast, reactive oxygen species, apoptosis, Fas ligand, pathophysiology of preeclampsia, IUGR, laser capture microdissection
Introduction
Release of Syncytial Factors in Preeclampsia
Although the etiology of the maternal syndrome preeclampsia remains unelucidated, there is agreement that it is preceded by failed conversion of maternal endometrial spiral arteries in the placental bed.1 This is thought to prevent the development of the low-resistance, high-capacitance uteroplacental circulation requisite for normal pregnancy.2,3 Whether this occurs because of inadequate trophoblast invasion, maternal factors, or both is debatable.1 The syncytium is the outer cell layer of the placenta that is in direct contact with maternal blood.4 It is postulated that, in preeclampsia, hypoxia/reperfusion injury and placental damage at the maternal–placental interface promotes increased release of soluble syncytial factors, including cytokines,5,6 eicosanoids,7 peroxides,8,9 the antiangiogenic factors soluble fms-like tyrosine kinase (sFlt)-110 and soluable endoglin,11 as well as syncytiotrophoblast microparticles.12,13 These factors are thought to compromise the function of the maternal endothelium, leading to maternal proteinuria and hypertension, clinical hallmarks of preeclampsia.
Syncytial Hypoxia, Reperfusion Injury, and Apoptosis in Preeclampsia
Hypoxia and/or reperfusion injury associated with preeclampsia occurs in concert with the release of the above-mentioned deleterious compounds by syncytiotrophoblasts.1,8 It is known that superoxide anion (SO), the most common reactive oxygen species (ROS), can be generated during the conversion of xanthine (X) to uric acid by xanthine oxidase (XO).8,14 SO can combine with nitric oxide to produce peroxynitrite anions, which damage proteins.8 Increased placental nitrosylation of proteins and oxidative stress are biochemical markers of preeclampsia.15,16 ROS-associated damage has been noted in syncytiotrophoblasts in pregnancies with preeclampsia and intrauterine growth restriction (IUGR)8,14,16 and may account for higher levels of apoptosis observed in syncytiotrophoblasts in these pregnancy complications.17,18In vitro studies support this idea because hypoxia and reperfusion, but not hypoxia per se, induce apoptosis in syncytiotrophoblasts.19 Our group has studied apoptosis at the maternal–fetal interface in relation to the expression of Fas ligand (FasL),20–22 a member of the tumor necrosis factor family that promotes cell death following binding of its receptor Fas to target cells.23 We found that placental trophoblasts express FasL across gestation, and detected Fas in chorionic trophoblasts, amnion epithelial cells, and the decidua of fetal membranes.20–22 Given that parturition was associated with increased fetal membrane apoptosis,21 this suggested that the Fas/FasL signaling system may play a role in periparturitional remodeling of fetal membranes. In addition, our group has shown that secreted microvesicular trophoblast FasL may play an important function in promoting immune cell apoptosis, affording protection of the fetus across gestation.20
Placental Damage and Plasminogen Activator Inhibitor-1 in Preeclampsia and IUGR
Although fibrin deposition at the syncytial surface is an important part of the physiological repair and differentiation of the placental villous,24 an aberrantly high level of intervillous fibrin is a histological hallmark of pregnancies with preeclampsia and IUGR. Such high levels of intervillous fibrin have been suggested to restrict the flow of nutrients from mother to fetus, resulting in poor neonatal outcomes.25,26 Placental damage (infarct) in pregnancies with preeclampsia and IUGR has been correlated with adverse fetal outcomes and enhanced placental expression of the antifibrinolytic factor plasminogen activator inhibitor-1 (PAI-1).27,28 Immunohistochemistry and in situ hybridization revealed enhanced syncytial expression of PAI-1 in these pregnancies, suggesting that this cell type was responsible for reduced perivillous fibrinolysis in preeclampsia and IUGR.28–30 Notably, dual (maternal + fetal) placental perfusion studies were used by our group to study the syncytial release of PAI-1, microparticles, cytokines, and eicosenoids.31,32 In dual perfusion, the maternal component is perfused via cannulae inserted through the decidual surface directly into the intervillous space, thereby effectively mimicking the process in vivo in which syncytiotrophoblasts release proteins directly into maternal blood. Thus, dual perfusion provides a physiologically relevant model to study the causes of the aberrant release of syncytial factors in preeclampsia.
Role of Syncytial Products in Preeclampsia and IUGR
In FIGURE 1 we present a model that incorporates the roles of syncytial hypoxia/reperfusion and ROS in the activation of the maternal endothelium and damage to the placenta associated with preeclampsia and IUGR in pregnancy. We postulate that hypoxia alone, or in combination with reperfusion and ROS generation, is associated with an apoptotic/necrotic cascade in syncytiotrophoblasts that results in the release of syncytial products, including sFlt-1, soluable endoglin, eicosanoids, peroxides, and syncytiotrophoblast microparticles. These factors aberrantly activate maternal endothelium and promote the maternal syndrome of preeclampsia. In addition, we suggest that, when preeclampsia occurs in combination with enhanced levels of syncytial PAI-1 and intervillous fibrin deposition, this may critically reduce the flow of nutrients from mother to fetus, leading to IUGR.
FIGURE 1.
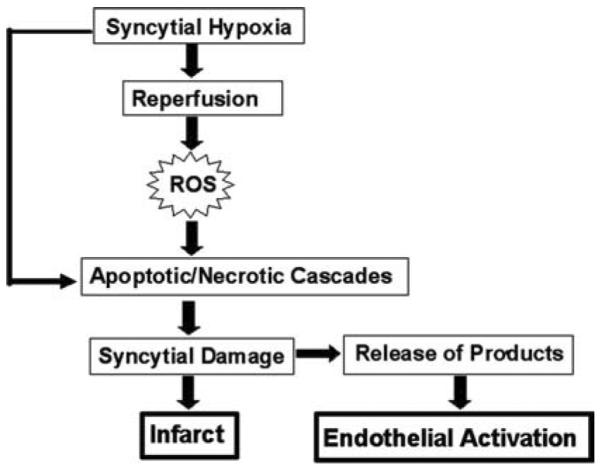
Model of the putative role of the placental syncytium in the pathophysiology of preeclampsia and IUGR.
In light of the clear association between alterations in syncytial integrity and function and the occurrence of preeclampsia and IUGR during pregnancy, we now present a new methodology to analyze syncytial protein expression.
Materials and Methods
Frozen sections of placenta delivered from uncomplicated pregnancies at term were used for this study (n=3). Following staining with Mayer's hematoxylin, laser capture microdissection (LCMD, Leica Microsystems, Wetzlar, Germany) of an intact terminal villus was carried out using a focused laser pulse directed to the area of interest. In the first round of microdissection, the placental villus core, consisting of fibroblast, macrophages, fetal vessels, and connective tissue, was removed. In the second round of LCMD, the syncytial layer of that same villus was removed and collected in lysis buffer containing detergent and protease inhibitors, and the number of nuclei per sample was recorded. This procedure was repeated until syncytial tissue from 10–15 villi was collected. Electrophoretic separation of lysate proteins was then carried out, and Western blotting and immunodetection using an anti-FasL antibody (mouse Mab clone 33, Transduction Laboratories, Lexington, KY) was performed.20 Incubation with anti-mouse secondary antibody and detection of FasL using enhanced checiluminescent technology was carried out as we have previously described.20,22
Results
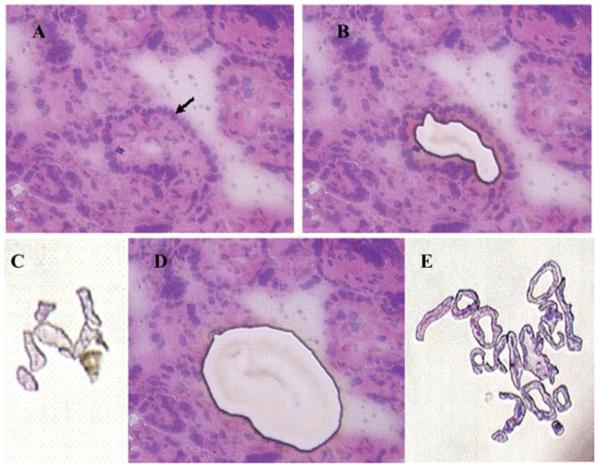
For this study, a single intact terminal villus was chosen (arrow, FIG. 2A). In the first round of microdissection, the placental villus core, consisting of fibroblasts, macrophages, fetal vessels, and connective tissue, was removed and saved for further analysis (FIG.2B). In the second round of LCMD, the syncytial layer of that same villus was removed and collected for further study (FIG. 2D). This procedure was repeated 14 times until core (FIG. 2C) and syncytial (FIG. 2E) tissue from approximately 15 villi were collected. We examined levels of the pro apoptotic protein FasL as a model for study because we had previously demonstrated its expression in extracts of placental tissue and isolated cells22 and it is a protein of low to moderate abundance in placenta. For this study, core sections of hematoxylin and eosin–stained villi were initially removed by LCMD, and then syncytial layers from 10–15 villi were captured and pooled. Four specimens of microdissected tissue containing approximately 5000 (FIG.3, Lane 1), 2000 (Lane 2), 1000 (Lane 3), and 500 (Lane 4) nuclei were captured in buffer containing a protease inhibitor cocktail. Electrophoretic separation of lysate proteins was then performed, and Western blotting and immunodetection using anti-FasL antibody was then carried out. It was noted that FasL migrated as a 37-kD species as we have previously observed20,22 and, most importantly, it could be reliably detected in specimens containing as few as 1000 nuclei. Because FasL is a cytokine of relatively moderate abundance in placenta, this suggests that we will be able to apply LCMD and Western blotting methodology to the study of syncytial protein expression.
FIGURE 2.
LCMD of human term placental tissue. Tissue prior to microdissection of a single villus (arrow) (A); tissue following the removal of the core of one villus (B); pooled core tissue from approximately 15 villi (C); tissue following removal of the core and the syncytium of one villus (D); pooled syncytial tissue from approximately 15 villi (E).
FIGURE 3.
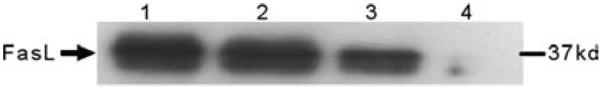
Western blot detection of FasL from microdissected syncytia. Results from extracts of tissue containing the following number of nuclei: Lane 1, 5000; Lane 2, 2000; Lane 3, 1000; Lane 4, 500.
Summary
In this paper, we briefly describe the connection between the disruption of syncytial structure resulting from hypoxia and/or reperfusion injury and the occurrence of preeclampsia and IUGR. We present evidence that the maternal syndrome of preeclampsia is associated with, and potentially promoted by, the increased release of specific syncytial products into maternal blood that occurs in association with trophoblast apoptosis. We suggest that these released factors negatively impact the function of the maternal endothelium and promote the hallmark clinical manifestations of preeclampsia. In addition, we posit that, when significant placental damage and placental infarction is associated with preeclampsia, this reduces adequate placental transport, leading to IUGR. Finally, we describe a novel methodology involving LCMD and Western blotting; this method may be used to analyze changes in the expression of syncytial proteins that may play a critical role in the pathophysiology of preeclampsia and IUGR.
Acknowledgment
This work was supported in part by NIH Grant HD33909.
Footnotes
Conflict of Interest The authors declare no conflicts of interest.
References
- 1.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308:1592–1594. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 2.Kaufmann P, et al. Endovascular trophoblast invasion: implications for the pathogenesis of intrauterine growth retardation and preeclampsia. Biol. Reprod. 2003;69:1–7. doi: 10.1095/biolreprod.102.014977. [DOI] [PubMed] [Google Scholar]
- 3.Stepan H, et al. New insights into the biology of preeclampsia. Biol. Reprod. 2006;74:772–776. doi: 10.1095/biolreprod.105.045997. [DOI] [PubMed] [Google Scholar]
- 4.Benirschke K. Remarkable placenta. Clin. Anat. 1998;11:194–205. doi: 10.1002/(SICI)1098-2353(1998)11:3<194::AID-CA8>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 5.Benyo DF, et al. Expression of inflammatory cytokines in placentas from women with preeclampsia. J. Clin. Endocrinol. Metab. 2001;86:2505–2512. doi: 10.1210/jcem.86.6.7585. [DOI] [PubMed] [Google Scholar]
- 6.Conrad KP, et al. Circulating levels of immunoreactive cytokines in women with preeclampsia. Am. J. Reprod. Immunol. 1998;40:102–111. doi: 10.1111/j.1600-0897.1998.tb00398.x. [DOI] [PubMed] [Google Scholar]
- 7.Walsh SW, et al. Placental isoprostane is significantly increased in preeclampsia. Faseb. J. 2000;14:1289–1296. doi: 10.1096/fj.14.10.1289. [DOI] [PubMed] [Google Scholar]
- 8.Myatt L, Cui X. Oxidative stress in the placenta. Histochem. Cell. Biol. 2004;122:369–382. doi: 10.1007/s00418-004-0677-x. [DOI] [PubMed] [Google Scholar]
- 9.Sikkema JM, et al. Placental superoxide is increased in pre-eclampsia. Placenta. 2001;22:304–308. doi: 10.1053/plac.2001.0629. [DOI] [PubMed] [Google Scholar]
- 10.Maynard SE, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J. Clin. Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Venkatesha S, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat. Med. 2006;12:642–649. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 12.Goswami D, et al. Excess syncytiotrophoblast microparticle shedding is a feature of early-onset pre-eclampsia, but not normotensive intrauterine growth restriction. Placenta. 2006;27:56–61. doi: 10.1016/j.placenta.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 13.Knight M, et al. Shedding of syncytiotrophoblast microvilli into the maternal circulation in pre-eclamptic pregnancies. Br. J. Obstet. Gynaecol. 1998;105:632–640. doi: 10.1111/j.1471-0528.1998.tb10178.x. [DOI] [PubMed] [Google Scholar]
- 14.Poston L, Raijmakers MT. Trophoblast oxidative stress, antioxidants and pregnancy outcome—a review. Placenta. 2004;25(Suppl A):S72–78. doi: 10.1016/j.placenta.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Many A, et al. Invasive cytotrophoblasts manifest evidence of oxidative stress in preeclampsia. Am. J. Pathol. 2000;156:321–331. doi: 10.1016/S0002-9440(10)64733-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Myatt L, et al. Nitrotyrosine residues in placenta. Evidence of peroxynitrite formation and action. Hypertension. 1996;28:488–493. doi: 10.1161/01.hyp.28.3.488. [DOI] [PubMed] [Google Scholar]
- 17.Ishihara N, et al. Increased apoptosis in the syncytiotrophoblast in human term placentas complicated by either preeclampsia or intrauterine growth retardation. Am. J. Obstet. Gynecol. 2002;186:158–166. doi: 10.1067/mob.2002.119176. [DOI] [PubMed] [Google Scholar]
- 18.Levy R, et al. Trophoblast apoptosis from pregnancies complicated by fetal growth restriction is associated with enhanced p53 expression. Am. J. Obstet. Gynecol. 2002;186:1056–1061. doi: 10.1067/mob.2002.122250. [DOI] [PubMed] [Google Scholar]
- 19.Hung TH, et al. Hypoxia-reoxygenation: a potent inducer of apoptotic changes in the human placenta and possible etiological factor in preeclampsia. Circ. Res. 2002;90:1274–1281. doi: 10.1161/01.res.0000024411.22110.aa. [DOI] [PubMed] [Google Scholar]
- 20.Abrahams VM, et al. First trimester trophoblast cells secrete Fas ligand which induces immune cell apoptosis. Mol. Hum. Reprod. 2004;10:55–63. doi: 10.1093/molehr/gah006. [DOI] [PubMed] [Google Scholar]
- 21.Runic R, et al. Apoptosis and Fas expression in human fetal membranes. J. Clin. Endocrinol. Metab. 1998;83:660–666. doi: 10.1210/jcem.83.2.4600. [DOI] [PubMed] [Google Scholar]
- 22.Runic R, et al. Expression of Fas ligand by human cytotrophoblasts: implications in placentation and fetal survival. J. Clin. Endocrinol. Metab. 1996;81:3119–3122. doi: 10.1210/jcem.81.8.8768884. [DOI] [PubMed] [Google Scholar]
- 23.Neale DM, Mor G. The role of Fas mediated apoptosis in preeclampsia. J. Perinat. Med. 2005;33:471–477. doi: 10.1515/JPM.2005.085. [DOI] [PubMed] [Google Scholar]
- 24.Humphrey RG, et al. Fibrin enhances differentiation, but not apoptosis, and limits hypoxic injury of cultured term human trophoblasts. Placenta. 2005;26:491–497. doi: 10.1016/j.placenta.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 25.Salafia CM, et al. Intrauterine growth restriction in infants of less than thirty-two weeks' gestation: associated placental pathologic features. Am. J. Obstet. Gynecol. 1995;173:1049–1057. doi: 10.1016/0002-9378(95)91325-4. [DOI] [PubMed] [Google Scholar]
- 26.Salafia CM, et al. Placental pathology of absent and reversed end-diastolic flow in growth-restricted fetuses. Obstet. Gynecol. 1997;90:830–836. doi: 10.1016/S0029-7844(97)00473-0. [DOI] [PubMed] [Google Scholar]
- 27.Estelles A, et al. Changes in the plasma levels of type 1 and type 2 plasminogen activator inhibitors in normal pregnancy and in patients with severe preeclampsia. Blood. 1989;74:1332–1338. [PubMed] [Google Scholar]
- 28.Estelles A, et al. Altered expression of plasminogen activator inhibitor type 1 in placentas from pregnant women with preeclampsia and/or intrauterine fetal growth retardation. Blood. 1994;84:143–150. [PubMed] [Google Scholar]
- 29.Estelles A, et al. Abnormal expression of plasminogen activator inhibitors in patients with gestational trophoblastic disease. Am. J. Pathol. 1996;149:1229–139. [PMC free article] [PubMed] [Google Scholar]
- 30.Grancha S, et al. Decreased expression of PAI-2 mRNA and protein in pregnancies complicated with intrauterine fetal growth retardation. Thromb. Haemost. 1996;76:761–767. [PubMed] [Google Scholar]
- 31.Di Santo S, et al. Dual in vitro perfusion of an isolated cotyledon as a model to study the implication of changes in the third trimester placenta on preeclampsia. Placenta. 2007;28(Suppl A):S23–32. doi: 10.1016/j.placenta.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 32.Guller S, et al. Differential release of plasminogen activator inhibitors (PAIs) during dual perfusion of human placenta: implications in preeclampsia. Placenta. 2007;28:278–285. doi: 10.1016/j.placenta.2006.05.005. [DOI] [PubMed] [Google Scholar]