Abstract
In this study, we investigated the role of nitric oxide synthase (NOS) isoforms in the enhanced enalapril-evoked hypotension in ethanol-fed female rats by examining the effect of the selective inhibitors of eNOS [N5-(1-iminoethyl)-L-ornithine; L-NIO], nNOS (Nω-propyl-L-arginine; NPLA), or iNOS (1400W) inhibition on the cardiovascular effects of enalapril in ethanol- (5% w/v) fed rats and in their pair-fed controls In liquid diet-fed control rats, enalapril- (10 mg/kg) evoked hypotension was abolished by L-NIO (20 mg/kg), but not by NPLA (1 mg/kg) or 1400W (5 mg/kg), suggesting a preferential role for eNOS in this response. Enalapril had no effect on spectral indices of hemodynamic variability or +dP/dtmax (myocardial contractility). However, in ethanol-fed rats, the greater enalapril-evoked hypotension was associated with reductions in (i) +dP/dtmax, (ii) low-frequency/high-frequency ratio of interbeat intervals (IBILF/HF), suggesting cardiac parasympathetic dominance, and (iii) low-frequency spectral band of systolic blood pressure (BP), a marker of vasomotor sympathetic tone. While NPLA or 1400W attenuated the enalapril-evoked hemodynamic and autonomic responses in ethanol-fed rats, L-NIO virtually abolished the hypotensive response and was more efficacious in rectifying autonomic responses to enalapril. Together, these findings implicate NOS isoforms, particularly eNOS, in the altered cardiovascular autonomic control that leads to the augmented enalapril-evoked hypotension in ethanol-fed female rats.
Keywords: Ethanol, enalapril, hypotension, hemodynamic variability, nitric oxide synthase, female rats
Introduction
Reduction in circulating angiotensin II, due to angiotensin converting enzyme (ACE) inhibition, is the principal mechanism by which ACE inhibitors lower BP (Sepehrdad et al., 2007). The reduced cardiovascular risk and mortality in patients receiving ACE inhibitors also relates to the improved cardiac autonomic control and hemodynamic variability, which may or may not be related to the BP lowering effect (Binkley et al., 2000; Ylitalo et al., 1999). Notably, cardiovascular autonomic neuropathy is associated with impaired regulation of BP, heart rate and heart rate variability (HRV), and increased susceptibility to ventricular arrhythmias and sudden cardiac death (Gerritsen et al., 2001). Further, whereas reductions in cardiac parasympathetic tone predispose to sudden cardiac death (due probably to increased susceptibility to fibrillatory attacks), vagal dominance is coupled with a reduced risk of arrhythmias (Billman, 2002; Sgoifo et al., 1997). Clinical data have also established a relationship between BP variability and the severity of end-organ damage (Mancia and Parati, 2000; Parati et al., 1995).
Our recent study established the first evidence that chronic ethanol exposure potentiates the enalapril-evoked hypotensive response in female rats (El-Mas and Abdel-Rahman, 2011). The underlying molecular mechanism appears to involve ethanol enhancement of Angiotensin II/bradykinin signaling because ethanol-fed rats, when compared to pair-fed control rats, exhibited significantly higher renal Ang II levels and ACE and bradykinin receptor protein expressions. Also, blockade of bradykinin B2 receptors (bradyzide) eliminated the enhanced hypotensive response caused by ACE inhibition in ethanol-fed rats (El-Mas and Abdel-Rahman, 2011).
Notably, reported studies have shown that ethanol does not uniformly potentiate the BP response elicited by antihypertensive medications. Chronic ethanol exposure decreases centrally mediated (clonidine) and increases peripherally mediated (hydralazine) hypotension (El-Mas and Abdel-Rahman, 2003, 2004). Similarly, the mechanism of the BP-lowering effect of antihypertensive drugs determines, at least partly, whether acutely administered ethanol increases or decreases the antihypertensive response (El-Mas and Abdel-Rahman, 1997, 1999a, 1999b). It is imperative to note that all previous studies on the interaction of ethanol with antihypertensive medications were undertaken in male rats (El-Mas and Abdel-Rahman, 1997, 2003, 2004). Therefore, our recent observation that chronic ethanol exposure enhanced the hypotensive action of enalapril in female rats (El-Mas and Abdel-Rahman, 2011) constituted an important step for investigating the interaction of ethanol with antihypertensive therapies in the female population.
In this communication, which extends our previous work (El-Mas and Abdel-Rahman, 2011), we tested the hypothesis that the modulation of cardiovascular autonomic control by NOS mediates the enhancement of the enalapril-evoked hypotension in ethanol-fed female rats. Observations that prompted us to investigate this possibility are (i) NOS upregulation contributes to the cardiovascular effects of ethanol (El-Mas et al., 2008, 2011; Williams et al., 1990) or enalapril (Förstermann and Sessa, 2012; Sahach et al., 2007), and (ii) NOS/NO (nitric oxide) signaling regulates cardiovascular autonomic activity (Heaton et al., 2005; Herring and Paterson, 2001). The present studies were conducted in telemetered female rats at the conclusion of chronic-ethanol (5% w/v) or isocaloric liquid-diet feeding, described in our recent study (El-Mas et al., 2011), to investigate the effect of selective inhibition of constitutive and inducible NOS on the enalapril-evoked changes in BP, +dP/dtmax, and spectral indices of hemodynamic variability. Spectral indices of hemodynamic variability are categorized into low-frequency interbeat intervals (IBI-LF; 0.25–0.75 Hz; reflect the sympathetic drive) and high-frequency interbeat intervals (IBI-HF; 0.75–3 Hz; reflect the cardiac vagal control), along with the ratio of LF to HF interbeat intervals (IBILF/HF), which is a measure of the sympathovagal balance of the heart (El-Mas and Abdel-Rahman, 2012; Thomas, 2011). The ethanol (5% w/v, 8 weeks) or isocaloric liquid diet was provided using a pair-feeding paradigm to ensure similar fluid and nutrient intakes as in our previous studies (El-Mas and Abdel-Rahman 2004; El-Mas et al., 2011). This ethanol paradigm produced blood-ethanol concentrations of 100–130 mg/dL (El-Mas and Abdel-Rahman, 2011; El-Mas et al., 2011), which are comparable to those attained following mild to moderate human consumption of ethanol (Eddleston et al., 2009; Ireland et al., 1984; Schaller et al., 2010).
Materials and Methods
Female Sprague-Dawley rats (9–10 weeks; 190–225 g; Harlan, Indianapolis, IN) were used in the present study. Upon arrival, rats were housed individually in standard plastic cages and allowed free access to water and rat chow and were maintained on a 12:12-h light-dark cycle with lights off at 4:00 PM. Room temperature was maintained at 22 ± 1°C. After 1 week of acclimatization, rats were fed a standard Lieber-DeCarli high protein liquid diet (Dyets Inc., Bethlehem, PA) for another week before starting the ethanol regimen. Rats received the diet daily at 3:30 PM before the start of the dark cycle. All experiments were approved by the Institutional Animal Care and Use Committee of East Carolina University and were carried out in accordance with the Declaration of Helsinki and with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the U.S. National Institutes of Health.
Ethanol feeding
Two groups of female rats matched for body weight were used. Telemetry transmitters were implanted as detailed below to allow BP recording. Rats in one group (n = 7) were provided a standard Lieber-DeCarli high-protein liquid diet containing 5% w/v ethanol (36% of total caloric intake) for 8 weeks as described in our previous study (El-Mas and Abdel-Rahman, 2011). The other group of rats received an isocaloric liquid diet and served as controls (n = 6). To acclimate rats to the ethanol diet, ethanol was first provided as half-strength (2.5% w/v, 18% of calories intake) for 3 days and then increased to 5% w/v thereafter. The daily ethanol intake amounted to approximately 8–9 g/kg. Control rats were pair-fed and received an isocaloric amount of dextrin/maltose (89.6 g/L) in place of ethanol, which allowed nutrient intake and fluid consumption similar to that of ethanol-fed rats. Fresh diets were prepared every other day and refrigerated until dispensed.
Hemodynamic effects of enalapril in absence or presence of constitutive or inducible NOS inhibitor
At the conclusion of the ethanol/liquid diet feeding described in our recent study (El-Mas et al., 2011), each rat in the ethanol (n = 7) or control (n = 6) group received 5 different i.p. injections at 3-day intervals: (i) saline (1 mL/kg), (ii) enalapril (10 mg/kg), (iii) the eNOS inhibitor L-NIO (20 mg/kg) + enalapril (10 mg/kg), (iv) the nNOS inhibitor NPLA (1 mg/kg) + enalapril (10 mg/kg), and (v) the iNOS inhibitor 1400W (5 mg/kg) + enalapril (10 mg/kg). NOS inhibitors were administered 10 min prior to enalapril. All injections were done at 9:00 AM and hemodynamic monitoring continued for the following 5 hr. The chosen doses of the NOS inhibitors were based on published reports (El-Mas et al., 2008, 2009). Rats were maintained on ethanol or control diet for the duration of the study.
Telemetry transmitter implantation, data acquisition and analysis
The methods used for telemetry transmitter implantation (Data Sciences Int., St. Paul, MN) and the chronic ethanol-feeding regimen are detailed in our previous studies (El-Mas and Abdel-Rahman, 2000, 2003, 2011). Data were collected using a computerized data-acquisition system (Dataquest A.R.T. 4.0, Data Sciences Int.). BP waveforms were sampled at a rate of 1000 Hz for 20 sec every 10 min. The maximum rate of rise of BP waves (+dP/dtmax), which represents myocardial contractility (van den Buuse, 2003), was computed by Data Sciences software. Changes in hemodynamic parameters from baseline values evoked by various drug treatments in pair-fed rats receiving liquid diet with or without ethanol (5%, w/v) were averaged in 40-min blocks (i.e. the average of 4 successive measurements) for analysis as in our previous studies (El-Mas and Abdel-Rahman, 2003, 2011). Baseline hemodynamic values were taken as the average of the 40-min period that preceded saline or drug administration. The interbeat interval (IBI) was calculated from BP waveforms.
Spectral analysis of hemodynamic variability
Spectral hemodynamic fluctuations, which are quantitative indices of cardiovascular autonomic control (El-Mas and Abdel-Rahman, 2011; Stein et al., 1994), were used to detect changes in sympathetic and vagal outflows. Hemodynamic variability was assessed by the frequency domain analysis of systolic blood pressure (SBP) and interbeat interval (IBI) data series as in previous studies including our own (Clifford and Tarassenko, 2004; El-Mas and Abdel-Rahman, 2011). Data Sciences software (Dataquest A.R.T. 4.0) uses the periodogram function of the rectangular window for direct transformation of data points into power spectral density graphs. Data were interpolated to obtain equally spaced samples with an effective sampling frequency of 10 Hz (0.1 s duration). A second-order quadratic equation was employed to fit a smooth curve to the existing data points and produce a smoother visual representation of data. Spectra were integrated into 2 specific frequency bands, LF (0.25–0.75 Hz) and HF (0.75–3 Hz) bands and expressed in normalized units (LFnu and HFnu). The ratio of LF to HF (IBILF/HF) is a measure of the sympathovagal balance of the heart. The LF spectral band of systolic BP (SBPLF) was also measured to mark changes in the vasomotor sympathetic tone (El-Mas and Abdel-Rahman, 2011; Stein et al., 1994). Parameters of hemodynamic variability were averaged every 40 min.
Drugs
Nω-propyl-L-arginine (Tocris Bioscience, Ellisville, MO); N5-(1-iminoethyl)-L-ornithine (Biotium Inc., Hayward, CA); 1400W, Enalapril maleate (Sigma Chemical Co., St. Louis, MO); Ketaject (ketamine), Xylaject (xylazine) (Phoenix Pharmaceuticals Inc., St Joseph, MI); Toradol (ketorolac tromethamine, Abbott Labs, Chicago, IL); Durapen (Penicillin G benzathine and penicillin G procaine, Vedco Inc., Overland Park, KS); and ethanol (Midwest Grain Products Co., Weston, MO) were purchased from commercial vendors.
Statistical analysis
All values are expressed as means ± S.E.M. A repeated measures 2-way analysis of variance (ANOVA) followed by a Newman-Keuls post-hoc test was used to analyze the effects of enalapril on hemodynamic responses in the absence or presence of selective NOS inhibitors. These analyses were performed by SAS Software Release 6.04 (SAS Institute Inc., Cary, NC). Probability levels less than 0.05 were considered significant.
Results
Hemodynamic and autonomic effects of chronic ethanol feeding
The hemodynamic and autonomic parameters obtained at the conclusion of chronic ethanol (5% w/v) or control liquid diet feeding are shown in Table 1. Compared with pair-fed control rats, chronic ethanol feeding to female rats reduced MAP, +dP/dtmax, LF bands of interbeat intervals (IBILF, 0.25–0.75 Hz) and IBILF/HF ratio (Table 1). On the other hand, the high-frequency bands of interbeat intervals (IBIHF, 0.75–3 Hz) were increased by ethanol (Table 1). No changes in HR or SBP spectra in the LF range were observed (Table 1).
Table 1.
Hemodynamic values at the conclusion of chronic ethanol (5% w/v) or control liquid diet feeding.
| Parameter | Control | Ethanol |
|---|---|---|
| MAP, mmHg | 104 ± 3 | 96 ± 2* |
| HR, beats/min | 345 ± 11 | 331 ± 12 |
| dP/dtmax, mmHg/sec | 1604 ± 105 | 1343 ± 64* |
| IBI-LFnu, sec2/Hz | 0.68 ± 0.01 | 0.56 ± 0.03* |
| IBI-HFnu, sec2/Hz | 0.32 ± 0.01 | 0.43 ± 0.03* |
| IBILF/HF | 2.0 ± 0.2 | 1.3 ± 0.2* |
| SBP-LF, mmHg2/Hz | 0.14 ± 0.01 | 0.14 ± 0.01 |
Values are means ± SEM. P < 0.05 vs. control values.
Hemodynamic effects of enalapril in absence or presence of selective NOS inhibitors in ethanol- and pair-fed control rats
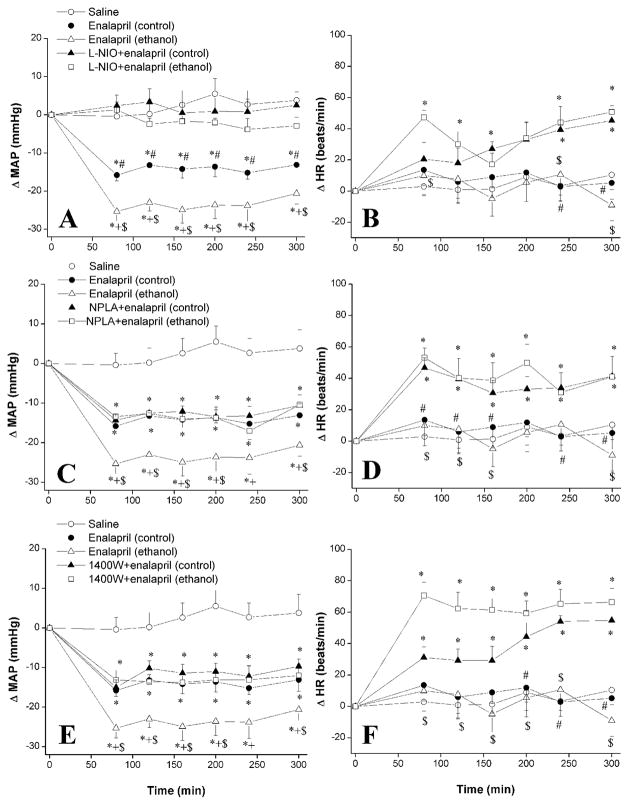
Figures 1 and 2 illustrate the effect of selective inhibition of eNOS, nNOS, or iNOS on MAP, HR and +dP/dtmax responses elicited by enalapril in ethanol-fed rats and pair-fed control rats. In the latter group, and compared with vehicle (saline) values, enalapril (10 mg/kg i.p.) produced significant (P < 0.05) reductions in BP that reached a nadir (−15.8 ± 1.5 mmHg) at 80 min and were sustained during the 5-hr observation period of the study (Fig. 1A). This enalapril-evoked hypotensive response was significantly (P < 0.05) enhanced in ethanol-fed rats (Fig. 1A). Inhibition of eNOS with L-NIO (20 mg/kg) abolished the hypotensive effect of enalapril in ethanol-fed and pair-fed control rats (Fig. 1A). Changes in MAP caused by enalapril in L-NIO-pretreated animals were not statistically different from the changes observed in the corresponding saline-treated animals (Fig. 1A). In contrast, the inhibition of nNOS with NPLA (1 mg/kg, Fig. 1C) and the inhibition of iNOS with 1400W (5 mg/kg, Fig. 1E) had no effect on enalapril-evoked hypotension in control rats but they abrogated the ethanol enhancement of enalapril-evoked hypotension. On the other hand, HR was not influenced by enalapril in ethanol-fed or pair-fed control rats (Fig. 1). The inhibition of eNOS (Fig. 1B), nNOS (Fig. 1D), or iNOS (Fig. 1F) caused similar increases in HR in ethanol-fed and pair-fed control rats.
Figure 1.
The effect of chronic ethanol (5% w/v) or control liquid diet feeding on changes in mean arterial pressure (MAP, left panels) and heart rate (HR, right panels) evoked by i.p. enalapril (10 mg/kg) in the absence or presence of i.p. L-NIO (eNOS inhibitor, 20 mg/kg, panels A and B), NPLA (nNOS inhibitor, 1 mg/kg, panels C and D), or 1400W (iNOS inhibitor, 5 mg/kg, panels E and F) in conscious telemetered female rats. Values are means ± S.E.M. of 6–7 observations. *P < 0.05 vs. saline values, +P < 0.05 vs. enalapril (control) values, #P < 0.05 vs. L-NIO+enalapril (control) values, $P < 0.05 vs. L-NIO+enalapril (ethanol) values.
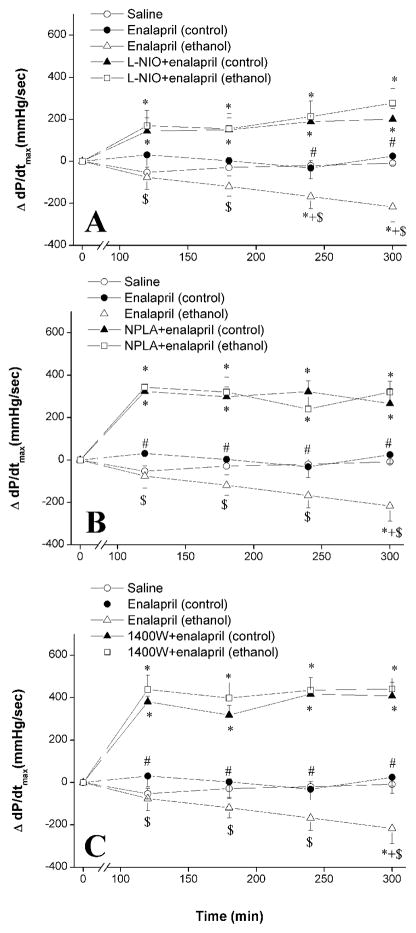
Figure 2.
The effect of chronic ethanol (5% w/v) or control liquid diet feeding on changes in myocardial contractility index (+dP/dtmax) evoked by i.p. enalapril (10 mg/kg) in the absence or presence of L-NIO (eNOS inhibitor, 20 mg/kg, panel A), NPLA (nNOS inhibitor, 1 mg/kg, panel B), or 1400W (iNOS inhibitor, 5 mg/kg panel C) in conscious telemetered female rats. Values are means ± S.E.M. of 6–7 observations. *P < 0.05 vs. saline values, +P < 0.05 vs. enalapril (control) values, #P < 0.05 vs. L-NIO+enalapril (control) values, $P < 0.05 vs. L-NIO+enalapril (ethanol) values.
Enalapril caused significant (P < 0.05) reductions in +dP/dtmax in ethanol-fed but not in pair-fed control rats (Fig. 2A-C). The enalapril-evoked +dP/dtmax reductions in ethanol-fed rats disappeared following L-NIO (Fig. 2A), NPLA (Fig. 2B), or 1400W (Fig. 2C) administration. The increases in +dP/dtmax observed following the NOS inhibitors were ethanol-independent because they also occurred in pair-fed control rats (Fig. 2).
Cardiovascular autonomic effects of enalapril in absence or presence of selective NOS inhibition in ethanol-fed and pair-fed control rats
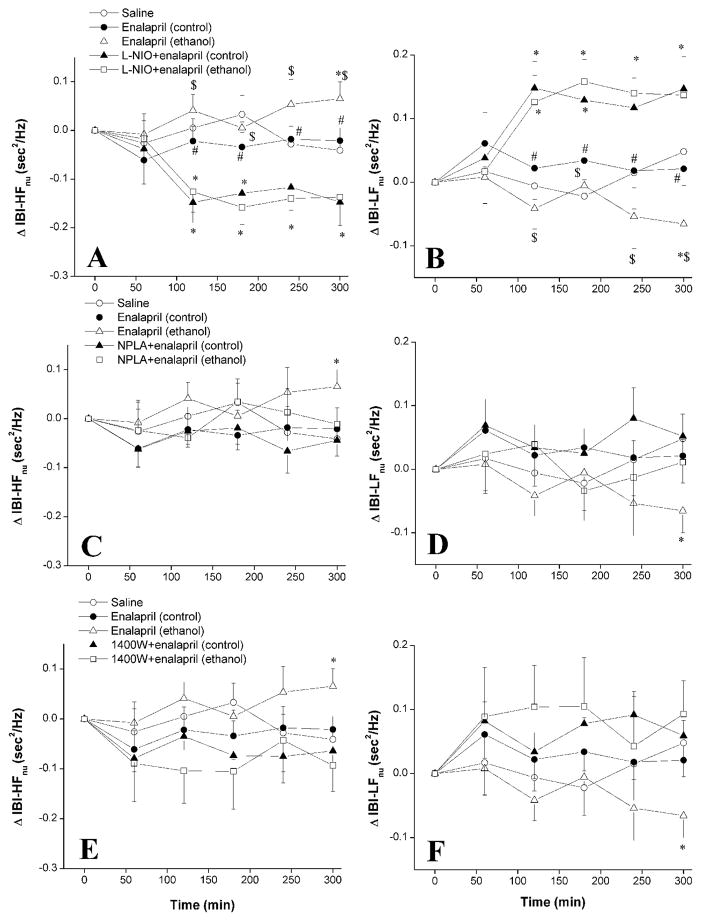
Changes in spectral indices of the cardiovascular autonomic control caused by enalapril in ethanol-fed and pair-fed control rats in the absence or presence of selective NOS inhibition are shown in Figures 3–5. In pair-fed control rats, enalapril had no effect on IBI in the HF or LF range (Fig. 3), the IBILF/HF (Fig. 4) or SBPLF (Fig. 5). However, the L-NIO counteraction of the hypotensive effect of enalapril in these rats was associated with decreases in IBIHF and increases in IBILF (Fig. 3A, B), IBILF/HF (Fig. 4A), and SBPLF (Fig. 5A).
Figure 3.
The effect of chronic ethanol (5% w/v) or control liquid diet feeding on changes in high-frequency (IBI-HFnu, 0.75–3 Hz, left panels) and low-frequency (IBI-LFnu, 0.25–0.75 Hz, right panels) spectral bands of IBI evoked by i.p. enalapril (10 mg/kg) in the absence or presence of i.p. L-NIO (eNOS inhibitor, 20 mg/kg, panels A and B), NPLA (nNOS inhibitor, 1 mg/kg, panels C and D), or 1400W (iNOS inhibitor, 5 mg/kg, panels E and F) in conscious telemetered female rats. Values are means ± S.E.M. of 6–7 observations. *P < 0.05 vs. saline values, #P < 0.05 vs. L-NIO+enalapril (control) values, $P < 0.05 vs. L-NIO+enalapril (ethanol) values.
Figure 5.
The effect of chronic ethanol (5% w/v) or control liquid diet feeding on changes in low-frequency SBP spectral density (SBP-LF, 0.25–0.75 Hz) evoked by i.p. enalapril (10 mg/kg) in the absence or presence of i.p. L-NIO (eNOS inhibitor, 20 mg/kg, panel A), NPLA (nNOS inhibitor, 1 mg/kg, panel B), or 1400W (iNOS inhibitor, 5 mg/kg panel C) in conscious telemetered female rats. Values are means ± S.E.M. of 6–7 observations. *P < 0.05 vs. saline values, +P < 0.05 vs. enalapril (control) values, #P < 0.05 vs. L-NIO+enalapril (control) values, $P < 0.05 vs. L-NIO+enalapril (ethanol) values.
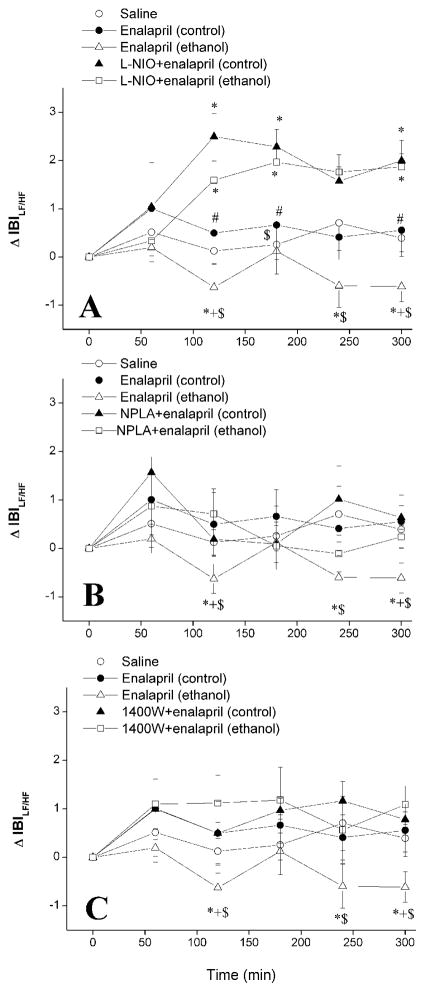
Figure 4.
The effect of chronic ethanol (5% w/v) or control liquid diet feeding on changes in LF/HF ratio of IBI evoked by i.p. enalapril (10 mg/kg) in the absence or presence of i.p. L-NIO (eNOS inhibitor, 20 mg/kg, panel A), NPLA (nNOS inhibitor, 1 mg/kg, panel B), or 1400W (iNOS inhibitor, 5 mg/kg panel C) in conscious telemetered female rats. Values are means ± S.E.M. of 6–7 observations. *P < 0.05 vs. saline values, +P < 0.05 vs. enalapril (control) values, #P < 0.05 vs. L-NIO+enalapril (control) values, $P < 0.05 vs. L-NIO+enalapril (ethanol) values.
In ethanol-fed rats, enalapril increased the IBIHF and decreased the IBILF (Fig. 3), IBILF/HF (Fig. 4), and SBPLF (Fig. 5). These spectral effects of enalapril were abolished in ethanol-fed rats following treatment with individual NOS inhibitors (Figs. 3–5). However, L-NIO was more efficacious than NPLA or 1400W in reversing the effects of enalapril; spectral parameters were increased by L-NIO to levels that were significantly higher than those caused by enalapril in pair-fed control rats (Figs. 3–5).
Discussion
The present study offers several important observations regarding the relative contributions of constitutive and inducible NOS to the hemodynamic and autonomic responses elicited by ACE inhibition in female rats, particularly following chronic ethanol feeding. First, eNOS-derived NO mediated enalapril-evoked hypotension in pair-fed control rats because L-NIO, but not NPLA or 1400W, abolished this response, at least partly, via sympathetic dominance. Second, chronic ethanol feeding exacerbated the enalapril-evoked hypotension as a result of increased cardiac parasympathetic activity and vasomotor sympathoinhibition due to enhanced activity of all three NOS isoforms. Nonetheless, eNOS played the major role because eNOS inhibition (L-NIO) was more efficacious than nNOS (NPLA) or iNOS (1400W) inhibition in attenuating the hypotensive effect of enalapril and the associated autonomic responses in ethanol-fed rats. While these data favor a critical role for eNOS in the exaggerated hypotensive effect of enalapril and underlying autonomic changes in ethanol-fed female rats, the involvement of nNOS and iNOS in the enalapril-ethanol hemodynamic interaction cannot be overlooked.
Despite the established role of enhanced NOS activity in the cardiovascular effects of ACE inhibitors, limited and often contradictory information is available concerning the contribution of constitutive and inducible NOS isoforms to biological responses caused by ACE inhibition. For example, enalapril increases the pancreatic capillary permeability in the fructose-fed rat model via increasing the eNOS but not nNOS expression (Bouffard et al., 2006). On the other hand, the hypotension caused by ACE inhibition in spontaneously hypertensive rats is not coupled with any changes in the abundance of constitutive or inducible NOS in the rat brainstem and midbrain (Hojná et al., 2007). The latter finding sharply contrasts with the notion that upregulation of all NOS isoforms accounts for the favorable effect of ACE inhibition on endothelium dysfunction in aged rats (Sahach et al., 2007). It seems likely that the recruitment of specific NOS isoform(s) depends largely on the studied biological functions. In the current study, we demonstrate for the first time that the enalapril-evoked hypotension in female rats was virtually abolished following pharmacologic inhibition of eNOS (L-NIO) but not following nNOS (NPLA) or iNOS (1400W) inhibition. It is conceivable, therefore, to conclude that eNOS activation is required for the manifestation of the hypotensive action of enalapril in female rats.
Realizing that NO influences cardiovascular functions via the modulation of autonomic activity (Balligand et al., 1993; El-Mas et al., 2011; Kimura et al., 2007), we conducted a power spectral analysis of hemodynamic variability to monitor changes in the cardiovascular autonomic activity caused by enalapril in the absence or presence of NOS inhibitors. We revealed important changes in hemodynamic variability that may account for the counteraction of the hypotensive effect of enalapril by eNOS inhibition (L-NIO) in pair-fed control rats. The increase in the IBILF/HF ratio, which represents the cardiac sympathovagal balance (Stein et al., 1994), and the increased LF oscillations of BP caused by L-NIO, which reflects vasomotor sympathoexcitation (Japundzic-Zigon, 1998; Stein et al., 1994), suggest overall sympathoexcitation in L-NIO/enalapril-treated rats. Together, these findings implicate the sympathetic drive to cardiovascular structures in the L-NIO ability to counteract enalapril-evoked hypotension. Notably, enalapril failed to modify hemodynamic variability, which agrees (Pavithran et al., 2010) and disagrees (Urbancic-Rovan et al., 2007) with reported findings. These discrepancies may relate to differences in the enalapril dose regimen used or the clinical setting.
Our postulate that the ethanol alterations of enalapril-evoked cardiovascular and autonomic effects might be NOS-dependent was based on the key role for NOS in autonomic control (Heaton et al., 2005; Herring and Paterson, 2001) and in the cardiovascular effects of ethanol (El-Mas et al., 2008, 2011; Williams et al., 1990) or enalapril (Förstermann and Sessa, 2012; Sahach et al., 2007). Several important observations emerged with regard to the role of NOS isoforms in the ethanol-dependent cardiovascular and autonomic effects of enalapril. The exaggerated hypotensive effect of enalapril in ethanol-fed rats, which agrees with our earlier report (El-Mas and Abdel-Rahman, 2011), was coupled with cardiac (IBILF/HF) and vasomotor (SBPLF) sympathoinhibition. Obviously, these autonomic effects of enalapril are ethanol-dependent because they were not observed in enalapril-treated pair-fed control rats. The interesting possibility should be considered that the reductions in BP and IBILF/HF caused by ethanol feeding per se (Table 1) might have contributed to the enalapril effect on these parameters. Interestingly, while eNOS was the only isoform implicated in pair-fed rats, all three NOS isoforms contributed to enalapril effects in ethanol-fed rats. Nonetheless, eNOS inhibition (L-NIO) abolished while nNOS (NPLA) or iNOS inhibition (1400W) only reduced (approx. 50%) enalapril-evoked hypotension (Fig. 1). Further, compared with NPLA or 1400W, L-NIO caused greater increases in cardiac (IBILF/HF) and vasomotor (SBPLF) sympathetic activity. Collectively, eNOS, and to a lesser extent nNOS and iNOS, participate in the exaggerated hypotensive and underlying autonomic actions of enalapril in ethanol-fed rats. Notably, the dose of enalapril employed in the current study (10 mg/kg) has been used in many reported studies and its biological effects mimicked those seen in humans (Andrade et al., 2007; De Gennaro Colonna et al., 2005; Melnick et al., 1998). Nonetheless, the enalapril dose-hemodynamic effect relationship and its interaction with ethanol need to be investigated in future studies.
It is important to comment on the role of myocardial contractility (+dP/dtmax) in the ethanol-enalapril interaction. We show that enalapril reduced +dP/dtmax in ethanol-fed but not in pair-fed control rats, which implicates the reduced myocardial contractility in the exaggerated enalapril-evoked hypotension in ethanol-fed rats. This conclusion gains support from the findings that NOS inhibition abrogated the enalapril-evoked hypotension as well as the associated decreases in +dP/dtmax (Fig. 2), and that cardiac NOS activity correlates with reductions in myocardial contractility, cardiac output, and subsequently BP (El-Mas and Abdel-Rahman, 2007; El-Mas et al., 2008). It might be argued that the similar increases in +dP/dtmax caused by the inhibition of the three NOS isoforms in enalapril-treated ethanol-fed and pair-fed control rats cast doubt about the contribution of the reduction in cardiac contractility to the exaggerated enalapril-evoked hypotension in ethanol-fed rats. It is imperative to note that because enalapril significantly reduced +dP/dtmax only in ethanol-fed rats, the magnitude of the +dP/dtmax increase was substantially greater following NOS inhibition in enalapril-treated ethanol-fed rats (Fig. 2). Nonetheless, more studies are apparently needed to clarify the precise role of cardiac dynamics in the NOS-dependent ethanol-enalapril interaction.
It is important to comment on the possibility that alterations in pharmacokinetic characteristics might have contributed to the ethanol-enalapril hemodynamic interaction. ACE inhibitors have been shown to reduce ethanol intake, due probably to taste aversions (Hubbell et al., 1992; Sommer et al., 2007). It is notable that the decrease in alcohol intake caused by ACE inhibitors occurs without concomitant changes in the overall fluid balance, or the distribution or metabolism of alcohol (Spinosa et al., 1988). To our knowledge, there are no studies on whether the metabolic profiles of ethanol and enalapril could be altered upon their simultaneous administration; this issue needs to be investigated in future studies.
In summary, this study showed that ethanol feeding modifies the cardiovascular and spectral autonomic effects of enalapril in female rats. The hypotensive action of enalapril is enhanced in ethanol-fed rats as a consequence of the altered autonomic control of cardiac (increased vagal and reduced sympathetic activity) and vasomotor tone (sympathoinhibition). The activation of eNOS, and to a lesser extent nNOS and iNOS, mediates the exacerbated cardiovascular actions of enalapril in ethanol-fed female rats. Clinically, when using enalapril and perhaps other ACE inhibitors as cardioprotective or renoprotective agents in women (Bartels et al., 1999), the concomitant use of alcohol should be avoided or at least minimized to avoid unnecessary or excessive decreases in BP. Alternatively, lower doses of enalapril may be recommended when the drug is used as a cardioprotective, renoprotective, or antihypertensive medication in female alcoholic patients.
Acknowledgments
Supported by Grant R01 AA014441-6 from the National Institute on Alcohol Abuse and Alcoholism. The authors thank Kui Sun for her technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrade TU, Pinto VD, Medeiros AR, Abreu GR, Moysés MR, Sampaio KN, Bissoli NS. Effect of enalapril treatment on the sensitivity of cardiopulmonary reflexes in rats with myocardial infarction. Clin Exp Pharmacol Physiol. 2007;34:606–611. doi: 10.1111/j.1440-1681.2007.04613.x. [DOI] [PubMed] [Google Scholar]
- Balligand JL, Kelly RA, Marsden PA, Smith TW, Michel T. Control of cardiac muscle cell function by an endogenous nitric oxide signaling system. Proc Natl Acad Sci USA. 1993;90:347–351. doi: 10.1073/pnas.90.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels GL, van den Heuvel FM, van Veldhuisen DJ, van der Ent M, Remme WJ. Acute anti-ischemic effects of perindoprilat in men with coronary artery disease and their relation with left ventricular function. Am J Cardiol. 1999;83:332–336. doi: 10.1016/s0002-9149(98)00863-7. [DOI] [PubMed] [Google Scholar]
- Billman GE. Aerobic exercise conditioning: a nonpharmacological antiarrhythmic intervention. J Appl Physiol. 2002;92:446–454. doi: 10.1152/japplphysiol.00874.2001. [DOI] [PubMed] [Google Scholar]
- Binkley PF, Nunziata E, Haas GJ, Starling RC, Leier CV, Cody RJ. Dissociation between ACE activity and autonomic response to ACE inhibition in patients with heart failure. Am Heart J. 2000;140:34–42. doi: 10.1067/mhj.2000.107180. [DOI] [PubMed] [Google Scholar]
- Bouffard L, Papirakis ME, Maheux P. Enalapril increases the local extravasation of macromolecules and nitric oxide synthase in pancreas of the fructose-fed insulin-resistant rat model. Pancreas. 2006;33:418–424. doi: 10.1097/01.mpa.0000236729.01123.7d. [DOI] [PubMed] [Google Scholar]
- Clifford GD, Tarassenko L. Segmenting cardiac-related data using sleep stages increases separation between normal subjects and apnoeic patients. Physiol Meas. 2004;25:N27–35. doi: 10.1088/0967-3334/25/6/n03. [DOI] [PubMed] [Google Scholar]
- De Gennaro Colonna V, Rigamonti A, Fioretti S, Bonomo S, Manfredi B, Ferrario P, Bianchi M, Berti F, Muller EE, Rossoni G. Angiotensin-converting enzyme inhibition and angiotensin AT1-receptor antagonism equally improve endothelial vasodilator function in L-NAME-induced hypertensive rats. Eur J Pharmacol. 2005;516:253–259. doi: 10.1016/j.ejphar.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Eddleston M, Gunnell D, von Meyer L, Eyer P. Relationship between blood alcohol concentration on admission and outcome in dimethoate organophosphorus self-poisoning. Br J Clin Pharmacol. 2009;68:916–919. doi: 10.1111/j.1365-2125.2009.03533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Mas MM, Abdel-Rahman AA. Contrasting effects of urethane, ketamine, and thiopental anesthesia on ethanol-clonidine hemodynamic interaction. Alcohol Exp Clin Res. 1997;21:19–27. [PubMed] [Google Scholar]
- El-Mas MM, Abdel-Rahman AA. Ethanol counteraction of II-imidazoline but not alpha-2 adrenergic receptor-mediated reduction in vascular resistance in conscious spontaneously hypertensive rats. J Pharmacol Exp Ther. 1999a;288:455–462. [PubMed] [Google Scholar]
- El-Mas MM, Abdel-Rahman AA. Role of the sympathetic control of vascular resistance in ethanol-clonidine hemodynamic interaction in SHRs. J Cardiovasc Pharmacol. 1999b;34:589–596. doi: 10.1097/00005344-199910000-00017. [DOI] [PubMed] [Google Scholar]
- El-Mas MM, Abdel-Rahman AA. Ovariectomy alters the chronic hemodynamic and sympathetic effects of ethanol in radiotelemetered female rats. Clin Exp Hypertens. 2000;22:109–126. doi: 10.1081/ceh-100100066. [DOI] [PubMed] [Google Scholar]
- El-Mas MM, Abdel-Rahman AA. Effects of chronic ethanol feeding on clonidine-evoked reductions in blood pressure, heart rate, and their variability: Time-domain analyses. J Pharmacol Exp Ther. 2003;306:271–278. doi: 10.1124/jpet.102.048355. [DOI] [PubMed] [Google Scholar]
- El-Mas MM, Abdel-Rahman AA. Contrasting effects of chronic ethanol feeding on centrally and peripherally evoked hypotension in telemetered female rats. Vascul Pharmacol. 2004;41:59–66. doi: 10.1016/j.vph.2004.05.001. [DOI] [PubMed] [Google Scholar]
- El-Mas MM, Abdel-Rahman AA. Role of myocardial contractility and autonomic control in the hypotensive response to a limited access ethanol paradigm in SHRs. Alcohol Clin Exp Res. 2007;31:1071–1079. doi: 10.1111/j.1530-0277.2007.00395.x. [DOI] [PubMed] [Google Scholar]
- El-Mas MM, Abdel-Rahman AA. Bradykinin B2 receptor-dependent enhancement of enalapril-evoked hypotension in ethanol-fed female rats. J Cardiovasc Pharmacol. 2011;57:72–78. doi: 10.1097/FJC.0b013e3181fef9e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Mas MM, Abdel-Rahman AA. Exacerbation of myocardial dysfunction and autonomic imbalance contribute to the estrogen-dependent chronic hypotensive effect of ethanol in female rats. Eur J Pharmacol. 2012;679:95–100. doi: 10.1016/j.ejphar.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Mas MM, Fan M, Abdel-Rahman AA. Endotoxemia-mediated induction of cardiac inducible nitric-oxide synthase expression accounts for the hypotensive effect of ethanol in female rats. J Pharmacol Exp Ther. 2008;324:368–375. doi: 10.1124/jpet.107.127498. [DOI] [PubMed] [Google Scholar]
- El-Mas MM, Fan M, Abdel-Rahman AA. Facilitation of myocardial PI3K/Akt/nNOS signaling contributes to ethanol-evoked hypotension in female rats. Alcohol Clin Exp Res. 2009;33:1158–1168. doi: 10.1111/j.1530-0277.2009.00939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Mas MM, Fan M, Abdel-Rahman AA. Upregulation of cardiac NOS due to endotoxemia and vagal overactivity contributes to the hypotensive effect of chronic ethanol in female rats. Eur J Pharmacol. 2011;650:317–323. doi: 10.1016/j.ejphar.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Förstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33:829–37. 837a–837d. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerritsen J, Dekker JM, TenVoorde BJ, Kostense PJ, Heine RJ, Bouter LM, Heethaar RM, Stehouwer CD. Impaired autonomic function is associated with increased mortality, especially in subjects with diabetes, hypertension, or a history of cardiovascular disease: the Hoorn Study. Diabetes Care. 2001;24:1793–1798. doi: 10.2337/diacare.24.10.1793. [DOI] [PubMed] [Google Scholar]
- Heaton DA, Golding S, Bradley CP, Dawson TA, Cai S, Channon KM, Paterson DJ. Targeted nnos gene transfer into the cardiac vagus rapidly increases parasympathetic function in the pig. J Mol Cell Cardiol. 2005;39:159–164. doi: 10.1016/j.yjmcc.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Herring N, Paterson DJ. Nitric oxide-cGMP pathway facilitates acetylcholine release and bradycardia during vagal nerve stimulation in the guinea-pig in vitro. J Physiol. 2001;535:507–518. doi: 10.1111/j.1469-7793.2001.00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojná S, Kadlecová M, Dobesová Z, Valousková V, Zicha J, Kunes J. The participation of brain NO synthase in blood pressure control of adult spontaneously hypertensive rats. Mol Cell Biochem. 2007;297:21–29. doi: 10.1007/s11010-006-9318-0. [DOI] [PubMed] [Google Scholar]
- Hubbell CL, Chrisbacher GA, Bilsky EJ, Reid LD. Manipulations of the renin-angiotensin system and intake of a sweetened alcoholic beverage among rats. Alcohol. 1992;9:53–61. doi: 10.1016/0741-8329(92)90010-8. [DOI] [PubMed] [Google Scholar]
- Ireland MA, Vandongen R, Davidson L, Beilin LJ, Rouse IL. Acute effects of moderate alcohol consumption on blood pressure and plasma catecholamines. Clin Sci. 1984;66:643–648. doi: 10.1042/cs0660643. [DOI] [PubMed] [Google Scholar]
- Japundzic-Zigon N. Physiological mechanisms in regulation of blood pressure fast frequency variations. Clin Exp Hypertens. 1998;20:359–388. doi: 10.3109/10641969809053219. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Hirooka Y, Sagara Y, Sunagawa K. Long-acting calcium channel blocker, azelnidipine, increases endothelial nitric oxide synthase in the brain and inhibits sympathetic nerve activity. Clin Exp Hypertens. 2007;29:13–21. doi: 10.1080/10641960601096745. [DOI] [PubMed] [Google Scholar]
- Mancia G, Parati G. Ambulatory blood pressure monitoring and organ damage. Hypertension. 2000;36:894–900. doi: 10.1161/01.hyp.36.5.894. [DOI] [PubMed] [Google Scholar]
- Melnick JZ, Preisig PA, Haynes S, Pak CY, Sakhaee K, Alpern RJ. Converting enzyme inhibition causes hypocitraturia independent of acidosis or hypokalemia. Kidney Int. 1998;54:1670–1674. doi: 10.1046/j.1523-1755.1998.00150.x. [DOI] [PubMed] [Google Scholar]
- Parati G, Ulian L, Santucciu C, Omboni S, Mancia G. Blood pressure variability, cardiovascular risk and antihypertensive treatment. J Hypertens. 1995;13(suppl 4):S27–34. doi: 10.1097/00004872-199512002-00005. [DOI] [PubMed] [Google Scholar]
- Pavithran P, Prakash ES, Dutta TK, Madanmohan T. Effect of antihypertensive drug therapy on short-term heart rate variability in newly diagnosed essential hypertension. Clin Exp Pharmacol Physiol. 2010;37:e107–113. doi: 10.1111/j.1440-1681.2009.05295.x. [DOI] [PubMed] [Google Scholar]
- Sahach VF, Baziliuk OV, Stepanenko LH, Korkach IuP, Kotsiuruba AV. Effect of enalapril on nitric oxide synthesis, oxidative metabolism, and vascular tone in aging rats. Fiziol Zh. 2007;53:15–26. [PubMed] [Google Scholar]
- Schaller G, Kretschmer S, Gouya G, Haider DG, Mittermayer F, Riedl M, Wagner O, Pacini G, Wolzt M, Ludvik B. Alcohol acutely increases vascular reactivity together with insulin sensitivity in type 2 diabetic men. Exp Clin Endocrinol Diabetes. 2010;118:57–60. doi: 10.1055/s-0029-1233453. [DOI] [PubMed] [Google Scholar]
- Sepehrdad R, Frishman WH, Stier CT, Jr, Sica DA. Direct inhibition of renin as a cardiovascular pharmacotherapy: focus on aliskiren. Cardiol Rev. 2007;15:242–256. doi: 10.1097/CRD.0b013e318093e43a. [DOI] [PubMed] [Google Scholar]
- Sgoifo A, de Boer SF, Westenbroek C, Maes FW, Beldhuis H, Suzuki T, Koolhaas JM. Incidence of arrhythmias and heart rate variability in wild-type rats exposed to social stress. Am J Physiol. 1997;273:H1754–1760. doi: 10.1152/ajpheart.1997.273.4.H1754. [DOI] [PubMed] [Google Scholar]
- Sommer WH, Rimondini R, Marquitz M, Lidström J, Siems WE, Bader M, Heilig M. Plasticity and impact of the central renin-angiotensin system during development of ethanol dependence. J Mol Med (Berl) 2007;85:1089–1097. doi: 10.1007/s00109-007-0255-5. [DOI] [PubMed] [Google Scholar]
- Spinosa G, Perlanski E, Leenen FH, Stewart RB, Grupp LA. Angiotensin converting enzyme inhibitors: animal experiments suggest a new pharmacological treatment for alcohol abuse in humans. Alcohol Clin Exp Res. 1998;12:65–70. doi: 10.1111/j.1530-0277.1988.tb00134.x. [DOI] [PubMed] [Google Scholar]
- Stein PK, Bosner MS, Kleiger RE, Conger BM. Heart rate variability: a measure of cardiac autonomic tone. Am Heart J. 1994;127:1376–1381. doi: 10.1016/0002-8703(94)90059-0. [DOI] [PubMed] [Google Scholar]
- Thomas GD. Neural control of the circulation. Adv Physiol Educ. 2011;35:28–32. doi: 10.1152/advan.00114.2010. [DOI] [PubMed] [Google Scholar]
- Urbancic-Rovan V, Meglic B, Stefanovska A, Bernjak A, Azman-Juvan K, Kocijancic A. Incipient cardiovascular autonomic imbalance revealed by wavelet analysis of heart rate variability in Type 2 diabetic patients. Diabet Med. 2007;24:18–26. doi: 10.1111/j.1464-5491.2007.02019.x. [DOI] [PubMed] [Google Scholar]
- van den Buuse M. Acute effects of antipsychotic drugs on cardiovascular responses to stress. Eur J Pharmacol. 2003;464:55–62. doi: 10.1016/s0014-2999(03)01369-4. [DOI] [PubMed] [Google Scholar]
- Williams SP, Adams RD, Mustafa SJ. The effects of chronic ethanol treatment on endothelium-dependent responses in rat thoracic aorta. Alcohol. 1990;7:121–127. doi: 10.1016/0741-8329(90)90072-k. [DOI] [PubMed] [Google Scholar]
- Ylitalo A, Airaksinen KE, Sellin L, Huikuri HV. Effects of combination antihypertensive therapy on baroreflex sensitivity and heart rate variability in systemic hypertension. Am J Cardiol. 1999;83:885–889. doi: 10.1016/s0002-9149(98)01067-4. [DOI] [PubMed] [Google Scholar]