Abstract
Overweight and obesity are associated with increased high sensitivity C-reactive protein (hsCRP) levels. The purpose of this study was to determine if weight loss diets differing in fat, protein, or carbohydrate composition differentially reduce hsCRP. POUNDS (Preventing Overweight Using Novel Dietary Strategies) LOST was a two-year trial of overweight and obese adults randomly allocated to one of four weight loss diets with targeted percentages of energy derived from fat, protein, and carbohydrates (20,15,65%;20,25,55%;40,15,45%;40,25,35%, respectively). hsCRP was measured at baseline, 6, and 24 months among 710 participants, and adiposity as measured by dual X-ray absorptiometry (N=340) or abdominal computed tomography (N=126) was correlated with hsCRP change. At 6 months, hsCRP was reduced in all trial participants by −24.7% (IQR +7%,−50%), weight by −6.7% (IQR −3%,−11%), and waist circumference by −6.0% (IQR −3%,−10%) (all P<.002), with no significant differences according to dietary composition. The percent change in hsCRP at 6 and 24 months correlated modestly with change in weight, waist circumference, fasting insulin, fasting glucose, HOMA, and most lipid levels. Reductions in hsCRP persisted despite an approximate 50% regain of weight by 24 months. The percent change in hsCRP at 24 months significantly correlated with changes in total body fat (r=0.42), total abdominal adiposity (r=0.52), subcutaneous abdominal adiposity (r=0.52), visceral adiposity (r=0.47), and hepatic tissue density (r=−0.34) (all P<0.0006). In conclusion, weight loss decreased hsCRP by similar magnitude, irrespective of dietary composition. Clinicians concerned about inflammation and cardiovascular risk should recommend weight loss diets most likely to succeed for their patients.
Introduction
Overweight and obese individuals are more likely to have elevated levels of the inflammatory biomarker high-sensitivity C-reactive protein (hsCRP),1 and both weight loss and statin therapy are known to reduce hsCRP levels.2,3 Following publication of the randomized placebo controlled JUPITER trial demonstrating the efficacy of statin therapy for the primary prevention of cardiovascular events in patients with elevated levels of hsCRP and normal levels of LDL cholesterol,2 some physicians have elected to prescribe statin therapy for cardiovascular disease prevention for patients with isolated hsCRP elevation. However, current recommendations suggest that lifestyle modification including weight loss when appropriate should be first line therapy for primary prevention of cardiovascular disease, even in patients with elevated LDL cholesterol.4 Despite popular interest in “anti-inflammation diets,” few data have been presented describing whether diets differing in fat, protein, or carbohydrate composition significantly modify the effect of weight loss on hsCRP, and there are no clear guidelines about what kind of diet clinicians should recommend to their patients with elevated hsCRP levels. Two small shortterm randomized trials comparing weight loss diets differing in macronutrient composition demonstrated that lower glycemic load diets preferentially decrease hsCRP despite similar weight loss between groups.5,6 In contrast, one study (n=29) demonstrated an increase in hsCRP on a low carbohydrate diet and a decrease in hsCRP on a high carbohydrate diet at one month despite greater weight loss in the low carbohydrate group.7 Other small trials of relatively short duration comparing weight loss diets differing in macronutrient composition have shown no significant differences in hsCRP lowering.8–11 One short-term study demonstrated a significantly greater reduction in hsCRP with higher fat Mediterranean-style diets when compared to a low-fat diet despite minimal weight loss in all groups, suggesting the importance of dietary factors independent of weight loss.12
In the recently completed NIH-funded POUNDS (Preventing Overweight Using Novel Dietary Strategies) LOST trial, overweight individuals were randomly allocated over a 24 month period to one of four weight reduction diets differing in composition of fat, protein, and carbohydrate. As previously reported, after 6 months of intervention, body weight was significantly reduced to a similar degree in all four groups, and there was weight regain at 24 months for the majority of participants.13 Here we report data from the POUNDS LOST trial addressing whether dietary composition impacts the reduction in hsCRP and whether effects of weight loss on hsCRP that might be present at 6 months were sustained for the full 24 month trial period. Further, as a randomly selected subgroup of participants also underwent dual X-ray absorptiometry (DXA) and computed tomography (CT), we also tested whether changes in hsCRP with diet-induced weight loss were preferentially related to changes in total body fat, abdominal fat, and/or hepatic fat.
Methods and Procedures
Participants and trial design
POUNDS LOST was a two-year randomized weight loss trial of 811 overweight and obese (BMI 25–40 kg/m2) volunteers age 30–70, conducted between October 2004-December 2007 at two clinical research sites (Harvard School of Public Health and Brigham and Women’s Hospital, Boston, Massachusetts; and Pennington Biomedical Research Center, Baton Rouge, Louisiana).13 The human subjects committees at both sites approved the study as did a data and safety monitoring board appointed by the National Heart, Lung, and Blood Institute. All subjects gave informed consent in writing.
After the collection of baseline data, participants were randomized into one of four treatment arms with targeted percentages of energy derived from fat, protein, and carbohydrates of 20, 15, 65%; 20, 25, 55%; 40, 15, 45%; and 40, 25, 35%, respectively. The study design allowed for a two-by-two factorial comparison of high fat vs. low-fat as well as high protein vs. average protein diets, as well as a dose-response test of carbohydrate intake ranging from 35 to 65%. The dietary goals for all groups were similar: 8% or less of kilocalories from saturated fat, 150 milligrams or fewer of cholesterol per 1000 kilocalories, and at least 20 grams of fiber daily. All diets adhered to healthy diet principles,14 and included suggestions for low glycemic index carbohydrate-rich foods. Each participant received a tailored diet prescription, based upon a 750 kilocalories/day energy deficit from total energy requirements (as determined by resting energy expenditure measured via metabolic cart and factoring in activity level), and rounded to the nearest 200 kilocalories. The physical activity target was 90 minutes of moderate intensity exercise per week. An intensive behavioral program accompanied all of the diet interventions.
Adherence to diet assignments was assessed in a random sample of 50% of participants using 5-day diet records and 24 hour recalls, and was compared to biomarker data for carbohydrate, protein, and fat intake. As reported in the main paper, there were greater mean differences in macronutrient intake between the four diets at 6 months than has often been seen in previous trials comparing diets of varying macronutrient composition in free-living individuals, and the decreased adherence to macronutrient targets at 24 months is similar to that seen in the few randomized weight loss studies with extended follow-up.15,16
Measurements
Anthropometry and body composition
Measurements of body weight and waist circumference were conducted before breakfast on two different days at baseline, 6 months, and 24 months. About 50% of enrolled participants in the POUNDS LOST trial were randomly selected to receive DXA scans at baseline, 6 months, and 24 months, and about 50% of this subset (25% of participants) were randomly selected to undergo abdominal CT scans at these timepoints.
Total and regional body composition were measured by DXA after an overnight fast using the same QDR 4500A machine (Hologic Inc, Bedford, MA). We previously reported reproducibility of mean (SEM) fat and lean tissue measurements in our laboratory of 1.09% (0.15%) and 0.89% (0.28%), respectively.17 Fat mass was calculated from percent body fat and body weight. CT measurements were performed by a GE High-Light Computed Tomographic scanner (Milwaukee, WI) or GE LightSpeed–VCT (Milwaukee, WI). Eight abdominal images were acquired to assess the area of visceral and subcutaneous adipose tissue (Analyzetm; Lenexa, Kansas), and the sum of individual slices was used to calculate total volume.18 Reader variability (coefficient of variation) averaged 0.9%. For correlations, we excluded participants with incomplete abdominal data (N=26) due to abdominal girth not fitting in the CT field. Hepatic density measured in Hounsfield units (HU) and corrected for spleen density was used to assess hepatic fat infiltration. Given that prior work comparing hepatic quantitative proton magnetic resonance spectroscopy with CT imaging indicates at least 5% hepatic fat infiltration when liver minus spleen density is ≤6.29 HU, we conducted an analysis restricted to participants with at least 5% baseline hepatic fat deposition to examine the association between change in hepatic fat and change in hsCRP. For both CT and DXA measurements, daily phantoms were used to ensure instrument stability over time, and a 3-point body fat phantom was used to verify the accuracy of instruments across study sites.
Biomarkers
Fasting blood samples were obtained at baseline, 6, and 24 months. The procedures for measuring serum lipids, glucose, insulin, glycated hemoglobin (HbA1c), and calculating the HOMA (homeostasis model assessment of insulin sensitivity) in POUNDS LOST have been detailed previously.13 For this analysis of hsCRP, we included 710 participants who provided a baseline blood sample and at least one follow-up blood sample, provided baseline anthropometric data and anthropometric data at the blood sample timepoints, and gave permission for their blood samples to be used for future studies. hsCRP was measured using an immunoturbidimetric assay on the Hitachi 917 analyzer (Roche Diagnostics, Indianapolis, IN) with reagents and calibrators from DiaSorin (Stillwater, MN). The day-to-day variability of the assay at concentrations of 0.91, 3.07, and 13.38 mg/L are 2.81%, 1.61%, and 1.1%, respectively. To relate changes in body composition to changes in hsCRP, we analyzed the data of participants who had been scanned at baseline and at least one follow-up visit for DXA (n=340), abdominal CT (n=137), and CT measured liver density (n=154).
Statistical Analysis
The primary outcome of this study was percent change in hsCRP at 6 and 24 months on diets varying in composition of protein, fat, and carbohydrates. The change was evaluated using ANOVAs, non-parametric Wilcoxon, and Wilcoxon signed rank tests using a two-sided significance level of ≤0.05. Data were pooled for factorial comparisons of high vs. low fat and high vs. average protein, and compared using Kruskal-Wallis tests for non-parametric data. We used Spearman correlations to compare changes in hsCRP with changes in weight loss, waist circumference, risk factors for cardiovascular disease and diabetes, and changes in body composition. As a sensitivity analysis, we re-ran the correlations restricting the analysis to only participants who had data for all three timepoints. All analyses were performed using JMP 9 (SAS Institute Inc., 2011).
Results
Table 1 shows baseline characteristics of study participants. There were no significant differences at baseline between treatment arms for demographic variables, baseline risk factors, or hsCRP levels. Table 2 presents data stratified by diet assignment on weight, waist circumference, and hsCRP at baseline, 6, and 24 months. For the total study population, hsCRP declined by 24.7% at 6 months (P<.0001), an effect that was largely maintained at 24 months (−22.9%, P<.0001 for change from baseline). Consistent with previous reports for the entire cohort,13 weight was reduced by 6.7% at 6 months (P<.0001) and 3.9% at 24 months (P<.0001 for change from baseline), and waist circumference was reduced by 6.0% at 6 months (P<.0001) and 4.7% at 24 months (P<.0001 for change from baseline).
Table 1.
Baseline characteristics of study participants (n=710)
| Characteristic | Low-Fat, Average Protein (n=182) |
Low-Fat, High Protein (n=177) |
High-Fat, Average Protein (n=172) |
High-Fat, High Protein (n=179) |
P value |
Overall (n=710) |
|---|---|---|---|---|---|---|
| Demographics: | ||||||
| Age (yrs) median (IQR) | 52 (46, 58) | 51 (44, 57) | 53 (46, 58) | 52 (46, 57) | 0.40 | 52 (45, 58) |
| Female sex – no. (%) | 108 (59) | 113 (64) | 125 (61) | 112 (63) | 0.76 | 435 (61) |
| Race/ethnic group – no. (%) | ||||||
| White | 144 (79) | 146 (82) | 140 (81) | 149 (83) | 580 (82) | |
| Black | 30 (16) | 23 (13) | 23 (13) | 24 (13) | 100 (14) | |
| Hispanic | 5 (3) | 4 (2) | 8 (5) | 4 (2) | 21 (3) | |
| Other | 3 (2) | 4 (2) | 1 (1) | 2 (1) | 0.80 | 10 (1) |
| Education – no. (%) | ||||||
| High school or less | 22 (12) | 13 (7) | 17 (10) | 17 (10) | 76 (9) | |
| Some college | 40 (22) | 40 (23) | 30 (17) | 32 (17) | 179 (22) | |
| College grad | 120 (66) | 124 (70) | 125 (73) | 130 (73) | 0.57 | 554 (68) |
| Household income – no. (%) | ||||||
| <50K | 50 (27) | 38 (21) | 39 (23) | 40 (22) | 195 (24) | |
| 50–100K | 72 (40) | 72 (41) | 66 (38) | 72 (40) | 321 (40) | |
| 100–150K | 39 (21) | 39 (22) | 37 (22) | 30 (17) | 164 (20) | |
| >150K | 26 (10) | 26 (15) | 29 (17) | 36 (19) | 120 (15) | |
| Refused | 2 (1) | 2 (1) | 1 (1) | 3 (2) | 0.70 | 9 (1) |
| Cardiac risk factors/modifiers: | ||||||
| Smoking status – no (%) | ||||||
| Current smoker | 7 (4) | 3 (2) | 4 (2) | 9 (5) | 30 (4) | |
| Former smoker | 74 (41) | 51 (29) | 69 (40) | 66 (37) | 298 (37) | |
| Never smoked | 101 (55) | 123 (69) | 99 (58) | 104 (58) | 0.07 | 481 (59) |
| Use of lipid-lowering agents no. (%) | 29 (16) | 42 (24) | 36 (21) | 29 (16) | 0.17 | 136 (19) |
| Use of anti-hypertensives no. (%) | 51 (28) | 52 (29) | 51 (30) | 56 (31) | 0.93 | 210 (30) |
| Post-menopausal status among women – no. (%) | 57 (53) | 58 (51) | 62 (50) | 64 (57) | 0.74 | 241 (55) |
| Hormone use among postmenopausal women – no. (%) | 24 (42) | 20 (34) | 18 (29) | 18 (28) | 0.35 | 80 (33) |
| Baseline pedometer steps median (IQR) n=475 | 6750 (4596, 9173) | 6481 (4535, 8612) | 6469 (4366, 9196) | 6748 (4781, 9196) | 0.68 | 6720 (4540, 8682) |
| Metabolic/Lipids: | ||||||
| Fasting glucose (mg/dl) median (IQR) | 91 (86, 96) | 90 (85, 97) | 91 (85, 97) | 90 (83, 98) | 0.92 | 90 (85, 97) |
| Fasting insulin (uU/ml) median (IQR) | 11 (7, 16) | 11 (7, 15) | 11 (7, 17) | 10 (7, 15) | 0.35 | 11 (7, 16) |
| HOMA median (IQR) | 2.3 (1.5, 3.8) | 2.4 (1.5, 3.3) | 2.6 (1.6, 4.0) | 2.3 (1.5, 3.5) | 0.36 | 2.4 (1.5, 3.7) |
| HbA1c (%) median (IQR) | 5.3 (5.1, 5.6) | 5.4 (5.2, 5.6) | 5.4 (5.1, 5.6) | 5.3 (5.1, 5.6) | 0.89 | 5.3 (5.1, 5.6) |
| Total cholesterol (mg/dl) median (IQR) | 201 (176, 222) | 203 (176, 225) | 198 (176, 225) | 200 (180, 228) | 0.82 | 200 (178, 225) |
| LDL cholesterol (mg/dl) median (IQR) | 122 (105, 146) | 124 (100, 147) | 122 (102, 150) | 124 (104, 143) | 0.99 | 123 (103, 146) |
| HDL cholesterol (mg/dl) median (IQR) | 47 (38, 57) | 45 (40, 56) | 46 (39, 53) | 50 (39, 61) | 0.14 | 47 (39, 57) |
| Triglycerides (mg/dl) median (IQR) | 112 (82, 157) | 129 (90, 186) | 128 (97, 189) | 119 (81, 185) | 0.16 | 123 (86, 181) |
| Inflammatory: | ||||||
| hsCRP (mg/l) median (IQR) | 1.70 (0.79, 3.53) | 1.98 (0.86, 3.44) | 1.72 (0.90, 3.80) | 2.08 (1.15, 3.50) | 0.37 | 1.84 (0.89, 3.53) |
Table 2.
Body fat, Weight, BMI, Waist Circumference, and hsCRP at baseline, 6, and 24 months by Macronutrient Assignment in POUNDS LOST*
| Sample | Median | Baseline value |
6m value (Median % change from baseline) (N=675) |
Comparison within groups for change from baseline at 6m |
24m value (Median % change from baseline) (N=568) |
Comparison within groups for change from baseline at 24m |
|---|---|---|---|---|---|---|
| Entire Sample (N=710) | Wt (kg) | 92.5 | 85.8 (−6.7%) | p<.0001 | 87.9 (−3.9%) | p<.0001 |
| Waist circ. (cm) | 103.7 | 96.8 (−6.0%) | p<.0001 | 97.8 (−4.7%) | p<.0001 | |
| hsCRP (mg/L) | 1.8 | 1.4 (−24.7%) | p<.0001 | 1.3 (−22.9%) | p<.0001 | |
| Low-fat, Avg. protein (N=182) | Wt (kg) | 93.5 | 89.5 (−6. 8%) | p<.0001 | 89.3 (−2.7%) | p<.0001 |
| 20% Fat, 15% Pro, 65% CHO | Waist circ. (cm) | 104.8 | 99.7 (−5.6%) | p<.0001 | 98.4 (−3.7%) | p<.0001 |
| hsCRP (mg/L) | 1.7 | 1.2 (−29.9%) | p<.0001 | 1.1 (−21.1%) | p<.0001 | |
| Low-fat, High protein (N=177) | Wt (kg) | 91.9 | 83.4 (−7.3%) | p<.0001 | 85.1 (−5.1%) | p<.0001 |
| 20% Fat, 25% Pro, 55% CHO | Waist circ. (cm) | 103.0 | 93.8 (−6.0%) | p<.0001 | 95.7 (−3.7%) | p<.0001 |
| hsCRP (mg/L) | 2.0 | 1.5 (−23.4%) | p<.0001 | 1.4 (−22.7%) | p=.0015 | |
| High-fat, Avg. protein (N=172) | Wt (kg) | 92.5 | 85.0 (−6.3%) | p<.0001 | 88.6 (−3.8%) | p<.0001 |
| 40% Fat, 15% Pro, 45% CHO | Waist circ. (cm) | 103.3 | 97.6 (−5.9%) | p<.0001 | 100.2 (−4.4%) | p<.0001 |
| hsCRP (mg/L) | 1.7 | 1.2 (−24.8%) | p<.0001 | 1.2 (−23.9%) | p<.0001 | |
| High-fat, High protein (N=179) | Wt (kg) | 93.1 | 87.5 (−6.4%) | p<.0001 | 89.3 (−3.8%) | p<.0001 |
| 40% Fat, 25% Pro, 35% CHO | Waist circ. (cm) | 103.6 | 95.2 (−6.3%) | p<.0001 | 97.8 (−4.9%) | p<.0001 |
| hsCRP (mg/L) | 2.1 | 1.5 (−23.5%) | p<.0001 | 1.2 (−23.5%) | p<.0001 | |
| Low-fat (N=359) | Wt (kg) | 92.2 | 85.8 (−7.0%) | p<.0001 | 88.8 (−3.8%) | p<.0001 |
| Waist circ. (cm) | 104.0 | 97.3 (−5.8%) | p<.0001 | 99.1 (−4.8%) | p<.0001 | |
| hsCRP (mg/L) | 1.8 | 1.4 (−25.3%) | p<.0001 | 1.3 (−21.6%) | p<.0001 | |
| High Fat (N=351) | Wt (kg) | 92.7 | 86.1 (−6.3%) | p<.0001 | 88.8 (−3.8%) | p<.0001 |
| Waist circ. (cm) | 103.4 | 96.6 (−6.1%) | p<.0001 | 99.1 (−4.6%) | p<.0001 | |
| hsCRP (mg/L) | 1.9 | 1.3 (−23.7%) | p<.0001 | 1.2 (−23.7%) | p<.0001 | |
| Average protein (N=354) | Wt (kg) | 92.8 | 86.6 (−6.7%) | p<.0001 | 88.7 (−3.3%) | p<.0001 |
| Waist circ. (cm) | 104.3 | 98.7 (−5.9%) | p<.0001 | 99.4 (−4.0%) | p<.0001 | |
| hsCRP (mg/L) | 1.7 | 1.2 (−27.7%) | p<.0001 | 1.1 (−22.7%) | p<.0001 | |
| High protein (N=356) | Wt (kg) | 92.2 | 84.6 (−6.9%) | p<.0001 | 86.5 (−4.6%) | p<.0001 |
| Waist circ. (cm) | 103.4 | 94.7 (−6.3%) | p<.0001 | 96.5 (−5.2%) | p<.0001 | |
| hsCRP (mg/L) | 2.0 | 1.5 (−23.5%) | p<.0001 | 1.3 (−23.1%) | p<.0001 |
No significant changes were seen between groups at baseline, 6 months, or 24 months.
As shown in Table 2, reductions in hsCRP over time were of similar magnitude across all dietary composition groups. Specifically, hsCRP levels were reduced at 6 months by 29.9%, 23.4%, 24.8%, and 23.5%, among those in the low-fat/average protein, low-fat/high protein, high-fat/average protein, and high-fat/high protein groups, respectively (P between groups=0.76). Similarly, hsCRP was reduced at 6 months by 25.3% and 23.7% in the low-fat and high-fat groups, respectively (P=0.83), and by 27.7% and 23.5% in the average protein and high protein groups, respectively (P=0.41). There was no significant difference (P=0.50) between the change in hsCRP at 6 months for the lowest (−23.5%) and highest carbohydrate groups (−29.9%). Effects on hsCRP persisted at 24 months in all groups, despite regain of weight and waist circumference.
As reductions in hsCRP were not significantly different according to randomized dietary interventions, we combined the study sample for subsequent analyses seeking to understand correlates of hsCRP change. Table 3 shows baseline hsCRP as well as changes in hsCRP and weight over time in various subgroups. We noted significant differences in hsCRP at baseline between men and women, Caucasians and African-Americans, overweight and obese participants, participants using lipid lowering therapy vs. those who were not, and postmenopausal women using hormones vs. those who were not. Despite these significant differences at baseline, there was no difference in hsCRP lowering at 6 and 24 months except for postmenopausal women using hormones. Compared to women not on hormone therapy, postmenopausal women on hormone therapy had a significantly greater reduction in hsCRP at 24 months (P=0.0139).
Table 3.
Change from baseline in hsCRP and body weight at 6 and 24 months by subgroup among participants (n=710)
| Characteristic | Baseline CRP median (IQR) |
P value between groups at baseline |
6m median hsCRP change from baseline in mg/L (median % change) |
P value between groups for % hsCRP change |
6m median change from baseline in wt in kg (median % change) |
P value between groups for % wt change |
24 median hsCRP change from baseline in mg/L (median % change) |
P value between groups for % hsCRP change |
24m median change from baseline in wt in kg (median % change) |
P value between groups for % wt change |
|---|---|---|---|---|---|---|---|---|---|---|
| Gender: | ||||||||||
| Female (n=435) | 2.2 (1.1,4.3) | −0.4 (−24%) | −5.8 (−6.5%) | −0.3 (−20%) | −2.8 (−3.2%) | |||||
| Male (n=275) | 1.6 (0.8,2.7) | <.0001 | −0.4 (−27%) | 0.31 | −6.8 (−6.8%) | 0.81 | −0.3 (−28%) | 0.08 | −3.9 (−3.7%) | 0.28 |
| Race: | ||||||||||
| Caucasian (n=579) | 1.8 (0.9,3.4) | −0.4 (−25%) | −6.6 (−7.2%) | −0.3 (−24%) | −3.7 (−4.1%) | |||||
| Black (n=100) | 2.4 (1.2,4.8) | 0.0032 | −0.5 (−24%) | 0.65 | −3.9 (−4.2%) | <.0001 | −0.4 (−21%) | 0.70 | −1.0 (−1.1%) | .0011 |
| Age: | ||||||||||
| ≤50 years old (n=295) | 1.8 (0.8,3.6) | −0.4 (−23%) | −5.6 (−6.0%) | −0.2 (−23%) | −2.8 (−3.0%) | |||||
| >50 years old (n=415) | 1.9 (0.9,3.5) | 0.64 | −0.4 (−28%) | 0.34 | −6.5 (−7.2%) | 0.0149 | −0.4 (−23%) | 0.32 | −3.5 (−4.0%) | .0362 |
| BMI: | ||||||||||
| <30 (n=180) | 1.2 (0.6,2.4) | −0.2 (−24%) | −6.0 (−7.3%) | −0.2 (−21%) | −2.8 (−3.6%) | |||||
| ≥30 (n=493) | 2.2 (1.1,4.1) | <.0001 | −0.5 (−25%) | 0.34 | −6.4 (−6.5%) | 0.72 | −0.4 (−23%) | 0.21 | −3.5 (−3.5%) | 0.67 |
| Lipid lowering medicine: | ||||||||||
| Yes (n=135) | 1.4 (0.7,2.6) | −0.3 (−32%) | −6.6 (−6.8%) | −0.2 (−22%) | −4.0 (−4.3%) | |||||
| No (n=575) | 2.0 (0.9,3.8) | 0.0019 | −0.4 (−24%) | 0.38 | −6.1 (−6.7%) | 0.48 | −0.4 (−22%) | 0.87 | −2.9 (−3.3%) | 0.26 |
| Use of hormones among post-menopausal women: | ||||||||||
| Yes (n=80) | 2.9 (1.5,4.7) | −0.8 (−26%) | −7.0 (−8.6%) | −0.6 (−37%) | −2.1 (−2.2%) | |||||
| No (n=161) | 1.8 (1.0,3.4) | 0.0029 | −0.4 (−24%) | 0.64 | −5.7 (−6.4%) | 0.17 | −0.3 (−19%) | 0.0139 | −3.7 (−4.7%) | 0.55 |
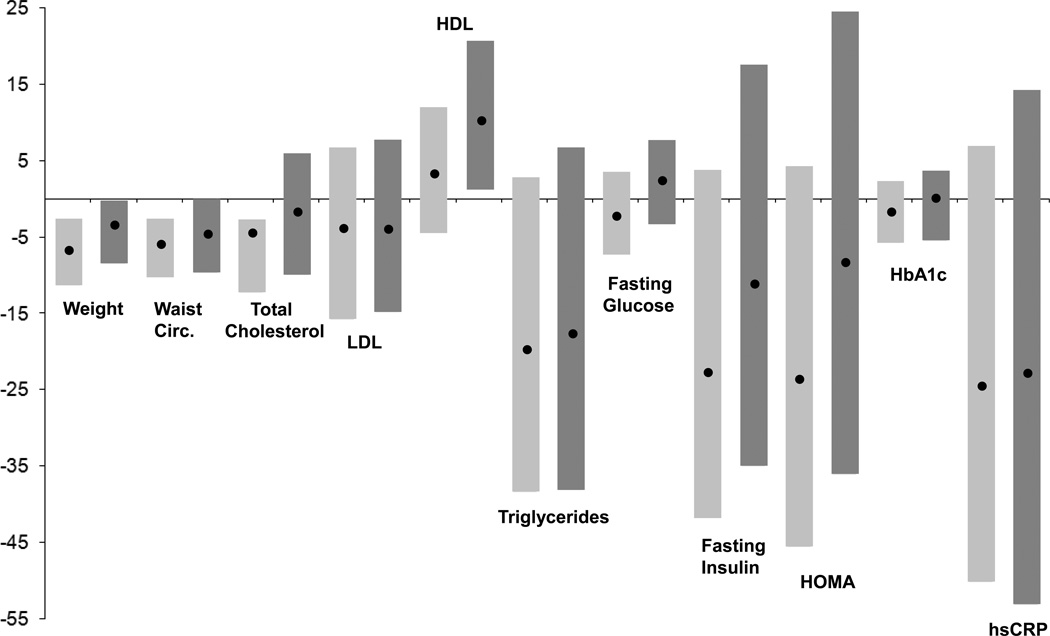
Figure 1 presents the change in anthropometric measurements, lipid-related risk factors, metabolic risk factors, and hsCRP for the entire study population. There were minimal changes in fasting blood glucose and HbA1c over the course of the study, and total cholesterol, LDL, and HDL changed by less than 10%. In contrast, the 20% reduction in triglycerides, fasting insulin, and HOMA was similar to the change in hsCRP. These effects persisted for triglycerides and hsCRP at 24 months but were largely attenuated for fasting insulin and HOMA. Spearman correlation coefficients relating the percent change in hsCRP and the change in weight, waist circumference, lipids, and metabolic variables are presented in Table 4. The strongest correlations with change in hsCRP were with percent change in weight (r=0.48), waist circumference (r=0.42), insulin (r=0.32), and HOMA (r=0.32). Smaller but statistically significant correlations were also observed for percent change in lipid levels and HbA1c, particularly at the end of 24 months of follow-up. These correlations were not substantially altered when the analysis was restricted to participants with data at all three timepoints.
Figure 1.
The median percent change from baseline at 6 months (light gray bars) and 24 months (dark gray bars) among all participants for weight, BMI, waist circumference, total cholesterol, low-density lipoprotein, high-density lipoprotein, triglycerides, fasting glucose, fasting insulin, HOMA, HbA1c, and hsCRP. The dot represents the medians, and the bars show the range from the 25th to the 75th percentile.
Table 4.
Spearman correlation coefficients for the percent change in hsCRP and the percent change in weight, waist circumference, lipids, metabolic factors, percent change in total body fat, and absolute change in total abdominal fat, visceral fat, subcutaneous abdominal fat, and hepatic fat at 6 and 24 months
| Measurement | Percent change in hsCRP at 6 months |
Percent change in hsCRP at 24 months |
|---|---|---|
| Percent change in body weight | 0.31* | 0.48* |
| (n=675) | (n=568) | |
| Percent change in waist circumference | 0.25* | 0.42* |
| (n=675) | (n=568) | |
| Percent change in total cholesterol | 0.11* | 0.10* |
| (n=675) | (n=568) | |
| Percent change in LDL | 0.08* | 0.10* |
| (n=675) | (n=568) | |
| Percent change in HDL | −0.01 | −0.21* |
| (n=675) | (n=568) | |
| Percent change in triglycerides | 0.08* | 0.20* |
| (n=675) | (n=568) | |
| Percent change in fasting glucose | 0.11* | 0.19* |
| (n=675) | (n=568) | |
| Percent change in fasting insulin | 0.15* | 0.32* |
| (n=675) | (n=568) | |
| Percent change in HOMA | 0.16* | 0.32* |
| (n=675) | (n=568) | |
| Percent change in HbA1c | 0.03 | 0.15* |
| (n=675) | (n=568) | |
| Percent change in total body fat (DXA) | 0.25* | 0.42* |
| (n=330) | (n=235) | |
| Change in total abdominal adipose | 0.36* | 0.52* |
| tissue (CT) | (n=117) | (n=89) |
| Change in visceral abdominal adipose | 0.33* | 0.47* |
| tissue (CT) | (n=117) | (n=89) |
| Change in subcutaneous abdominal | 0.35* | 0.52* |
| adipose tissue (CT) | (n=117) | (n=89) |
| Change in hepatic density (CT) | −0.30* | −0.34* |
| (n=147) | (n=112) |
Significant at p<.05
Among participants who underwent DXA scanning (n=340), median fat loss was 4.7 kg (−2.6%) and 3.1 kg (−1.6%) at 6 and 24 months (P<.0001 for both timepoints), respectively. For participants who underwent abdominal CT scanning, median total abdominal fat loss was −2.9 kg at 6 months and −2.1 kg at 24 months. The loss of visceral abdominal fat tissue was −0.9 kg at 6 months and −0.7 kg at 24 months, while abdominal subcutaneous fat losses were −1.9 kg at 6 months and −1.3 kg at 24 months (P<.0001 for change at all timepoints). At 6 and 24 months there was a significant (P<.0001) increase in hepatic density (signifying a decrease in fatty infiltration) of +2.4 HU (IQR 0.2, 6.2) and +3.6 HU (IQR −0.5, +7.7), respectively. Table 4 shows that the percent change in hsCRP correlated significantly with changes in total body fat, total abdominal adiposity, subcutaneous abdominal adiposity, visceral adiposity, and hepatic tissue density (all P<.0006). In the participants with at least 5% hepatic fat infiltration present at baseline (53/154, 34%), the correlations between percent change in hsCRP and change in hepatic fat were magnified, (6 months: r=−0.36, P=0.0076; 24 months r=−0.47, P=0.0030).
Discussion
In this randomized trial of four weight reduction diets, we observed the association between weight loss and substantial reductions in hsCRP, an effect that was independent of macronutrient composition. Further, among those randomized to imaging studies, we observed that the percent reduction in hsCRP correlated similarly with changes in all measures of body fat including total fat, abdominal fat, and intrahepatic fat.
Our data confirm prior work from smaller studies demonstrating that diet-induced weight loss substantially reduces hsCRP.3,19,20 In a systematic review of 28 lifestyle weight loss studies ranging from 14 to 90 participants, the weighted correlation between change in hsCRP and change in weight was 0.30,3 similar to our 6 month correlation coefficient of 0.31. The decrease in hsCRP with weight loss in our study is also similar to that observed among participants with impaired glucose tolerance in the Diabetes Prevention Program (DPP), where a 6.7% reduction in body mass was associated with a 30% reduction in hsCRP,21 and among participants with diabetes participating in the Look AHEAD trial, where 8.8% weight loss at one year was associated with a 44% decrease in hsCRP.22 Similarly, studies of bariatric surgery show substantial reductions in hsCRP with weight loss.23,24 Thus, our current data from POUNDS LOST in conjunction with other randomized evidence demonstrate that weight reduction decreases hsCRP by amounts similar to that reported with statin therapy.
Despite evidence for the effect of weight loss on hsCRP, there are scant prior data about the optimal content of weight loss diets that should be prescribed to decrease inflammation, and studies demonstrate conflicting results.5–11 In our large randomized trial, the macronutrient composition of four different weight loss diets did not affect the change in hsCRP at 6 or 24 months. Similarly, there was no difference between high and average protein diets, between high and low fat diets, or when the highest carbohydrate diet was compared to the lowest carbohydrate diet. These findings suggest that macronutrient composition is unlikely to have a differential effect on the reduction of hsCRP with weight loss.
In our trial, there was no difference in the percent change in hsCRP at 6 and 24 months between African-Americans and Caucasians despite significant differences in percent weight loss over time. Similarly, the percent reduction in hsCRP did not differ between overweight and obese individuals or by gender. As anticipated, participants using lipid lowering therapy at baseline had significantly lower baseline hsCRP values. However, as also observed in the LOOK AHEAD trial,22 statin users had similar reductions in hsCRP with weight loss as non-statin users. Taken together, these findings suggest that weight loss will further decrease hsCRP in patients who are already experiencing a reduction in hsCRP from taking statins.
Consistent with other studies examining anthropometric, cardiovascular, and metabolic correlates of hsCRP, we found the largest correlation coefficients for change in weight and waist circumference.21 In addition, several cross-sectional analyses suggest that high hsCRP levels are related to measures of insulin resistance, including fasting insulin and HOMA-IR.25,26 In our study, changes in HOMA and insulin were more correlated with changes in hsCRP than changes in LDL, especially at 24 months. This may be because of the relatively greater impact of weight loss and a healthy diet on hsCRP and insulin levels than upon LDL.
While some cross-sectional studies suggest that visceral fat is more highly correlated with hsCRP than abdominal subcutaneous fat,27,28 other cross-sectional studies suggest that hsCRP is correlated similarly with abdominal subcutaneous fat and visceral fat.29,30 However, few studies have examined how changes in these fat depots with weight loss relate to changes in hsCRP over time.31 In our randomized trial, the percent change in hsCRP correlated similarly with changes in all measures of body fat, including total body fat, and total abdominal, subcutaneous abdominal, and visceral fat. This similar effect is likely because of the similar fat reduction in all compartments in this study.
Hepatic steatosis is associated with both cardiovascular disease risk32 and elevated hsCRP.33 Previous studies demonstrate that hepatic fat infiltration can be reversed with weight loss,34 but few studies have examined how changes in hepatic steatosis with weight loss relate to changes in hsCRP.35 In our study we note a reversal of fat infiltration with weight loss which is significantly correlated with the change in hsCRP over time, a correlation which was magnified in those individuals who had significant hepatic steatosis at baseline.
In this trial, hsCRP levels did not rebound, despite significant weight re-gain by 24 months, an observation seen previously in other studies.21,22 Similarly, in one six month weight maintenance study of 932 adults, hsCRP levels continued to decrease despite slight weight regain, although this effect was more pronounced in the low glycemic index maintenance diets.36 The persistent changes in hsCRP noted in our study may reflect remodeling at the level of adipose tissue, including changes in adipocyte size and/or gene expression,37,38 that could be contributing to a decrease in hsCRP production. Given that all participants were placed on healthy diets that adhere to current recommendations, including fiber intake, carbohydrate quality, and type of fat, it is also possible that participants continued a healthier diet overall despite weight re-gain, regardless of their diet assignment. This is consistent with some studies demonstrating the importance of diet quality, particularly the Mediterranean diet, for reducing inflammation, possibly by modifying inflammatory pathways.19,39 Alternatively, it may be that a particular threshold of weight loss needs to be maintained. Further studies should examine the mechanisms responsible for this effect.
Strengths of our trial include the large diverse population as well as a larger proportion of men (39%), lower drop out rate (20%), and longer follow-up (2 years) than most weight loss studies. The addition of imaging measures of body fat allowed us to examine how changes in body composition relate to changes in hsCRP. Nonetheless, limitations of our analysis merit consideration. Not all of our participants had data at all three timepoints. However, a sensitivity analysis conducted among subjects with complete data demonstrated that the correlations were not substantially different. In addition, since all participants were advised to choose carbohydrate-rich foods with a low glycemic index, the study does not allow for a true comparison between low and high glycemic index diets. However, the fact that there were no significant differences in hsCRP between those assigned to 35% and 65% carbohydrate diets suggests that total carbohydrate content does not significantly affect hsCRP. Finally, as all groups in POUNDS LOST had similar exercise recommendations, we cannot evaluate the effect of physical activity separately from weight loss. However, the recent INFLAME study found that exercise training without weight loss is not associated with a reduction in hsCRP.40
In conclusion, our analysis indicates that diet induced weight loss results in a substantial reduction of hsCRP that is of similar magnitude to statin therapy and is independent of macronutrient composition. As such, physicians concerned about elevated hsCRP levels in their patients should emphasize the importance of weight loss, and suggest that patients choose a weight loss diet that would be most likely to lead to success, regardless of macronutrient composition.
Acknowledgements
We thank the POUNDS LOST participants without whom this study would not be possible. We thank Russell de Souza PhD, RD (Clinical Nutrition and Risk Factor Modification Center, University of Toronto, Toronto, Ontario), for his review of the manuscript and Jeremy Furtado, ScD (Department of Nutrition, Harvard School of Public Health), for his assistance with coordinating the blood samples for this project.
Funding
The POUNDS LOST study was supported by grants from the National Heart, Lung, and Blood Institute (HL073286) and the General Clinical Research Center, National Institutes of Health (RR-02635). Dr. Nicklas was supported by an Institutional National Research Service Award #T32AT000051 from the National Center for Complementary and Alternative Medicine (NCCAM) at the National Institutes of Health.
Footnotes
Disclosure
Dr Ridker is listed as a co-inventor on patents held by the Brigham and Women's Hospital that relate to the use of inflammatory biomarkers in cardiovascular disease. Dr. Ridker has received research funding support from Astra-Zeneca, Novartis, and Sanofi-Aventis unrelated to this project. No other relationships disclosed.
References
- 1.Nguyen XM, Lane J, Smith BR, Nguyen NT. Changes in inflammatory biomarkers across weight classes in a representative US population: a link between obesity and inflammation. J Gastrointest Surg. 2009;13(7):1205–1212. doi: 10.1007/s11605-009-0904-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 3.Selvin E, Paynter NP, Erlinger TP. The effect of weight loss on C-reactive protein: a systematic review. Arch Intern Med. 2007;167(1):31–39. doi: 10.1001/archinte.167.1.31. [DOI] [PubMed] [Google Scholar]
- 4.Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 5.Pereira MA, Swain J, Goldfine AB, Rifai N, Ludwig DS. Effects of a low-glycemic load diet on resting energy expenditure and heart disease risk factors during weight loss. JAMA. 2004;292:2482–2490. doi: 10.1001/jama.292.20.2482. [DOI] [PubMed] [Google Scholar]
- 6.Pittas AG, Roberts SB, Das SK, et al. The effects of dietary glycemic load on type 2 diabetes risk factors during weight loss. Obesity. 2006;14(12):2200–2209. doi: 10.1038/oby.2006.258. [DOI] [PubMed] [Google Scholar]
- 7.Rankin JW, Turpyn AD. Low carbohydrate, high fat diet increases C-reactive protein during weight loss. J Am Coll Nutr. 2007;26(2):163–169. doi: 10.1080/07315724.2007.10719598. [DOI] [PubMed] [Google Scholar]
- 8.O’Brien KD, Brehm BJ, Seeley RJ, et al. Diet-induced weight loss is associated with decreases in plasma serum amyloid a and C-reactive protein independent of dietary macronutrient composition in obese subjects. J Clin Endocrinol Metab. 2005;90(4):2244–2249. doi: 10.1210/jc.2004-1011. [DOI] [PubMed] [Google Scholar]
- 9.Noakes M, Foster PR, Keogh JB, James AP, Mamo JC, Clifton PM. Comparison of isocaloric very low carbohydrate/high saturated fat and high carbohydrate/low saturated fat diets on body composition and cardiovascular risk. Nutr Metb. 2006;3:7. doi: 10.1186/1743-7075-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tay J, Brinkworth GD, Noakes M, Keogh J, Clifton PM. Metabolic effects of weight loss on a very-low-carbohydrate diet compared with an isocaloric high-carbohydrate diet in abdominally obese subjects. J Am Coll Cardiol. 2008;51(1):59–67. doi: 10.1016/j.jacc.2007.08.050. [DOI] [PubMed] [Google Scholar]
- 11.Brinkworth GD, Noakes M, Buckley JD, Keogh JB, Clifton PM. Long-term effects of a very-low-carbohydrate weight loss diet compared with an isocaloric low-fat diet after 12 mo. Am J Clin Nutr. 2009;90(1):23–32. doi: 10.3945/ajcn.2008.27326. [DOI] [PubMed] [Google Scholar]
- 12.Estruch R, Martinez-Gonzalez MA, Corella D, et al. Effects of a Mediterranean-style diet on cardiovascular risk factors. Ann Int Med. 2006;145(1):1–11. doi: 10.7326/0003-4819-145-1-200607040-00004. [DOI] [PubMed] [Google Scholar]
- 13.Sacks FM, Bray GA, Carey VJ, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Eng J Med. 2009;360(9):859–873. doi: 10.1056/NEJMoa0804748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American Heart Association Nutrition Committee. Lichtenstein AH, Appel LJ, et al. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114(1):82–96. doi: 10.1161/CIRCULATIONAHA.106.176158. [DOI] [PubMed] [Google Scholar]
- 15.Dansinger ML, Gleason JA, Griffith JL, Selker JP, Schaefer EJ. Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: a randomized trial. JAMA. 2005;293:43–53. doi: 10.1001/jama.293.1.43. [DOI] [PubMed] [Google Scholar]
- 16.Gardner CD, Kiazand A, Alhassan S, et al. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: the A to Z Weight Loss Study: a randomized trial. JAMA. 2007;297:969–977. doi: 10.1001/jama.297.9.969. [Erratum, JAMA 2007; 298:178.] [DOI] [PubMed] [Google Scholar]
- 17.LeBoff MS, Kohlmeier L, Hurwitz S, Franklin J, Wright J, Glowacki J. Occult vitamin D deficiency in postmenopausal US women with acute hip fracture. JAMA. 1999;281(16):1505–1511. doi: 10.1001/jama.281.16.1505. [DOI] [PubMed] [Google Scholar]
- 18.Kvist H, Chowdhury B, Grangard U, Tylen U, Sjostrom L. Total and visceral adipose-tissue volumes derived from measurements with computed tomography in adult men and women: predictive equations. Am J Clin Nutr. 1988;48:1351–1361. doi: 10.1093/ajcn/48.6.1351. [DOI] [PubMed] [Google Scholar]
- 19.Esposito K, Pontillo A, Di Palo C, et al. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: A randomized controlled trial. JAMA. 2003;289:1799–1804. doi: 10.1001/jama.289.14.1799. [DOI] [PubMed] [Google Scholar]
- 20.Shai I, Schwarzfuchs D, Henkin Y, et al. Dietary Intervention Randomized Controlled Trial (DIRECT) Group. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med. 2008;359:229–241. doi: 10.1056/NEJMoa0708681. [DOI] [PubMed] [Google Scholar]
- 21.Haffner S, Temprosa M, Crandall J, et al. Intensive lifestyle intervention or metformin on inflammation and coagulation in participants with impaired glucose tolerance. Diabetes. 2005;(5):1566–1572. doi: 10.2337/diabetes.54.5.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belacazar LM, Reboussin DM, Haffner SM, et al. A 1-year lifestyle intervention for weight loss in individuals with type 2 diabetes reduces high C-reactive protein levels and identifies metabolic predictors of change: from the Look AHEAD (Action for Health in Diabetes) study. Diabetes Care. 2010;33(11):2297–2303. doi: 10.2337/dc10-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laimer M, Ebenbichler CF, Kaser S, et al. Markers of chronic inflammation and obesity: a prospective study on the reversibility of this association in middle-aged women undergoing weight loss by surgical intervention. Int J Obes. 2002;26:659–662. doi: 10.1038/sj.ijo.0801970. [DOI] [PubMed] [Google Scholar]
- 24.Cugno M, Castelli R, Mari D, et al. Inflammatory and prothrombotic parameters in normotensive nondiabetic obese women: effect of weight loss obtained by gastric banding. Intern Emerg Med. 2011:1–6. doi: 10.1007/s11739-011-0522-x. [DOI] [PubMed] [Google Scholar]
- 25.Yudkin JS, Stehouwer CDA, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction. Arterioscler Thromb Vasc Biol. 1999;19:972–978. doi: 10.1161/01.atv.19.4.972. [DOI] [PubMed] [Google Scholar]
- 26.Festa A, D’Agostino R, Howard G, Mykkanen L, Tracy RP, Haffner SM. Chronic subclinical inflammation as part of the insulin resistance syndrome. Circulation. 2000;102:42–47. doi: 10.1161/01.cir.102.1.42. [DOI] [PubMed] [Google Scholar]
- 27.Beasley LE, Koster A, Newman AB, et al. Inflammation and race and gender differences in computerized tomography-measured adipose depots. Obesity. 2009;17:1062–1069. doi: 10.1038/oby.2008.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park HS, Park JY, Yu R. Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF-alpha and IL-6. Diabetes Res Clin Pract. 2005;69:29–35. doi: 10.1016/j.diabres.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 29.Pou KM, Massaro JM, Hoffmann U, et al. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: the Framingham Heart Study. Circulation. 2007;116:1234–1241. doi: 10.1161/CIRCULATIONAHA.107.710509. [DOI] [PubMed] [Google Scholar]
- 30.Piche ME, Lemieux S, Weisnagel SJ, et al. Relation of high-sensitivity C-reactive protein, interleukin-6, tumor necrosis factor-alpha, and fibrinogen to abdominal adipose tissue, blood pressure, and cholesterol and triglyceride levels in healthy postmenopausal women. Am J Cardiol. 2005;96:92–97. doi: 10.1016/j.amjcard.2005.02.051. [DOI] [PubMed] [Google Scholar]
- 31.Fisher G, Hyatt TC, Hunter GR, Oster RA, Desmond RA, Gower BA. Effect of diet with and without exercise training on markers of inflammation and fat distribution in overweight women. Obesity. 2011;(6):1131–1136. doi: 10.1038/oby.2010.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McKimmie RL, Daniel KR, Carr JJ, et al. Hepatic steatosis and subclinical cardiovascular disease in a cohort enriched for type 2 diabetes: the Diabetes Heart Study. Am J Gastroenterol. 2008;103(12):3029–3035. doi: 10.1111/j.1572-0241.2008.02188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zimmerman E, Anty R, Tordjman J, et al. C-reactive protein levels in relation to various features of non-alcoholic fatty liver disease among obese patients. J Hepatol. 2011;55(3):660–665. doi: 10.1016/j.jhep.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 34.Goodpaster BH, DeLany JP, Otto AD, et al. Effects of diet and physical activity interventions on weight loss and cardiometabolic risk factors in severly obese adults. JAMA. 2010;304(16):1795–1802. doi: 10.1001/jama.2010.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Larson-Meyer DE, Newcomer BR, Heilbronn LK, et al. Effect of 6-month calorie restriction and exercise on serum and liver lipids and markers of liver function. Obesity. 2008 Jun;16(6):1355–1362. doi: 10.1038/oby.2008.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gögebakan O, Kohl A, Osterhoff MA, et al. Effects of weight loss and long-term weight maintenance with diets varying in protein and glycemic index on cardiovascular risk factors: the diet, obesity, and genes (DiOGenes) study: a randomized, controlled trial. Circulation. 2011 Dec 20;124(25):2829–2838. doi: 10.1161/CIRCULATIONAHA.111.033274. [DOI] [PubMed] [Google Scholar]
- 37.Rizkalla SW, Prifti E, Cotillard A, et al. Differential effects of macronutrient content in 2 energy-restricted diets on cardiovascular risk factors and adipose tissue cell size in moderately obese individuals: a randomized controlled trial. Am J Clin Nutr. 2012 Jan;95(1):49–63. doi: 10.3945/ajcn.111.017277. [DOI] [PubMed] [Google Scholar]
- 38.Clément K, Viguerie N, Poitou C, et al. Weight loss regulates inflammation-related genes in white adipose tissue of obese subjects. FASEB J. 2004 Nov;18(14):1657–1669. doi: 10.1096/fj.04-2204com. [DOI] [PubMed] [Google Scholar]
- 39.Giugliano D, Ceriello A, Esposito K. The effects of diet on inflammation: Emphasis on the metabolic syndrome. J Am Coll Cardiol. 2006;48:677–685. doi: 10.1016/j.jacc.2006.03.052. [DOI] [PubMed] [Google Scholar]
- 40.Church TS, Earnest CP, Thompson AM, et al. Exercise without weight loss does not reduce Creactive protein: The INFLAME study. Med Sci Sports Exerc. 2010;(4):708–716. doi: 10.1249/MSS.0b013e3181c03a43. [DOI] [PMC free article] [PubMed] [Google Scholar]