Abstract
K(ATP) channels play critical roles in many cellular functions by coupling cell metabolic status to electrical activity. First discovered in cardiomyocytes,1 KATP channels (comprised of Kir6.x and SUR subunits) have since been found in many other tissues, including pancreatic beta cells, skeletal muscle, smooth muscle, brain, pituitary and kidney. By linking cellular metabolic state with membrane potential, KATP channels are able to regulate a number of cellular functions such as hormone secretion, vascular tone and excitability. Specifically, a reduction in metabolism causes a decrease in the ATP:ADP ratio, opening of KATP channels, K+ efflux, membrane hyperpolarization, and suppression of electrical activity. Conversely, increased cellular metabolism causes an increase in the ATP:ADP ratio that leads to closure of the KATP channel, membrane depolarization, and stimulation of cell electrical activity.
Keywords: ankyrin, spectrin, trafficking, targeting, cytoskeleton, diabetes
Numerous studies in isolated cells and tissues, as well as genetically-modified mice or patients with mutations in KATP channel genes, have demonstrated that KATP channels play important roles in a wide range of physiological processes.2 Their contribution to glucose homeostasis is well-documented with regard to K ATP-dependent regulation of insulin secretion by beta cells,3 glucagon secretion from pancreatic alpha cells,4 somatostatin secretion from pancreatic delta cells,5 and glucagon-like peptide 1 (GLP-1) secretion from L-cells.6 Moreover, KATP channels in ventromedial hypothalamic neurons mediate the counter-regulatory response to glucose,7 while KATP channels in the arcuate nucleus are hypothesized to play a role in appetite regulation.8 Interestingly, these glucose-sensing cell types express KATP channels that are open under resting conditions. In other tissues, however, KATP channels are closed under physiological conditions and open only in response to ischemia, neurotransmitters, or hormonal stimulation. For example, in cardiac muscle and central neurons, opening of KATP channels reduces electrical activity to protect against cardiac stress and seizures.9–12 Moreover, KATP channels are involved in the phenomenon of ischemic preconditioning in the heart13 and in the regulation of vascular smooth muscle tone.14,15 Additionally, KATP channels modulate electrical activity and synaptic neurotransmitter release in the hippocampus, substantia nigra and the hypothalamus.8,16–19
Macroscopic KATP channel current is the highly-coordinated product of open probability, single channel conductance, and the total number of membrane channels. KATP channel open probability in vivo is tightly regulated by ATP levels ([ATP]:[ADP] ratios). In fact, even minor changes in cellular [ATP]:[ADP] ratios may result in dramatic alterations in KATP channel opening, cellular excitability, and tissue function.20 The importance of normal KATP channel ATP sensitivity for normal metazoan physiology is illustrated by human mutations in Kir6.2 or SUR genes that affect KATP channel ATP sensitivity and result in neonatal diabetes.21 Likewise, human gene variants that affect KATP channel biosynthesis, targeting, or membrane expression may result in abnormal beta cell excitability and human disease.22,23
In 2006, Shyng and colleagues identified human KATP channel gene variants that resulted in neonatal diabetes due to defects in both gating and membrane targeting. For example, Lin et al. specifically demonstrated that Kir6.2 mutants R201C and R201H showed both decreased membrane expression (conductance) as well as abnormal gating phenotypes resulting in diabetes.24 In a recent publication, our group linked a human disease mutant, Kir6.2 E322K, with a dual mechanistic phenotype (alterations in both gating and membrane targeting).25 Specifically, our findings identified a critical motif in the C-terminal domain of Kir6.2 that is essential not only for normal Kir6.2 membrane targeting, but also metabolic regulation by ATP.25 The Kir6.2/SUR KATP channel complex directly interacts with the membrane adaptor protein ankyrin-B26,27 and Kir6.2 targeting is significantly reduced when the Kir6.2 motif is disrupted or when ankyrin is depleted from the cell. Interestingly, ankyrin plays a key role in regulating ATP sensitivity, as Kir6.2 ATP sensitivity is decreased in the presence of peptides that disrupt the ankyrin-B:Kir6.2 interaction. Finally, we demonstrated that a previously identified human neonatal diabetes mutation in Kir6.2 (E322K) blocks the association of ankyrin-B with Kir6.2, resulting in a complex beta cell phenotype.
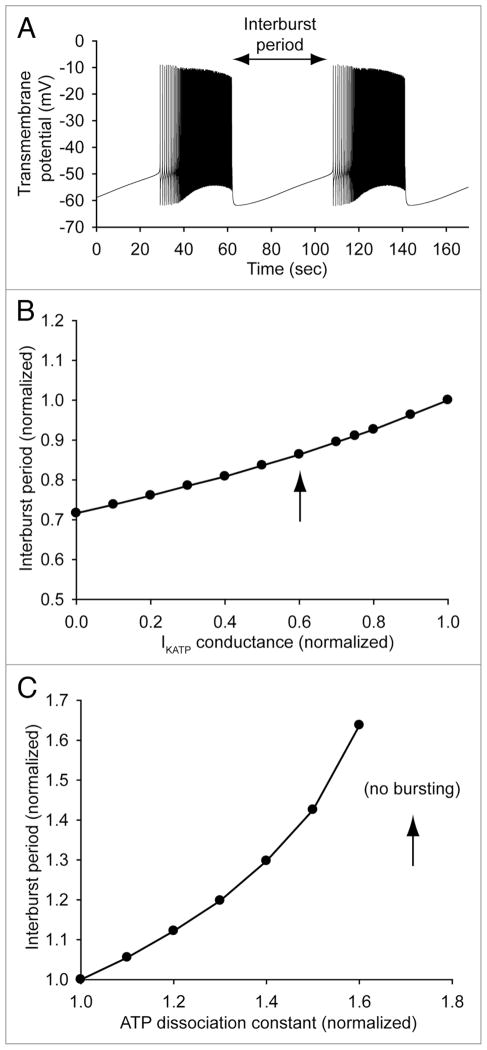
While KATP channel function (and therefore cell excitability) is rather predictably affected by gene mutations that alter ATP sensitivity or channel membrane expression alone, it is more difficult to predict how KATP channel will be affected by a mutation that alters several channel properties simultaneously.24,25 In our study, we used mathematical modeling of beta cell excitability (Fig. 1) to help gain important insight into how complex changes in KATP channel properties result in the ultimate cellular phenotype and disease.25 Interestingly, computational modeling predicts that beta cell excitability (measured as inter-burst period in Fig. 1) is much more sensitive to changes in KATP channel ATP sensitivity than it is to changes in channel conductance (slope of linear regression = 1.01 and 0.28, respectively). In fact, the most severe trafficking defect associated with the E322K mutant channel resulted in only about a 14% decrease in inter-burst period (arrow in Fig. 1B), while decreased ATP sensitivity due to the mutation (arrow in Fig. 1C) completely eliminated spontaneous electrical activity. Thus, even though fewer mutant channels traffic to the membrane (loss-of-function), the decreased ATP sensitivity of those channels that do localize properly is enough to produce a net gain-of-function. Together, these findings demonstrate a key role of the cytoskeleton in KATP channel function as well as illustrate the complex cellular phenotypes that have evolved in metazoans to modulate channel function and cellular excitability.
Figure 1.
Simulated beta cell electrical activity as a function of KATP channel conductance and ATP sensitivity. (A) Pancreatic beta cell electrical activity is simulated using the mathematical model developed by Fridyland et al.28 (B) Pancreatic beta cell inter-burst period decreases (excitability increases) as IKATP conductance is reduced. However, the relationship between inter-burst period and IKATP conductance is relatively flat. In fact, a 40% decrease in IKATP conductance (corresponding to the more severe trafficking defect associated with ankyrin-B loss in HEK cells25) results in only a 14% decrease in inter-burst period (arrow). (C) In contrast, beta cell inter-burst period is steeply dependent on the ATP sensitivity of the KATP channel. A 70% increase in the ATP dissociation constant of the KATP channel (corresponding to the E322K heterozygous mutant25) results in a complete loss of spontaneous beta cell firing (arrow).
Acknowledgments
We acknowledge support from the NIH (HL084583, HL083422 to Peter J. Mohler, HL096805 to Thomas J. Hund), and Pew Scholars Trust (Peter J. Mohler).
References
- 1.Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983;305:147–8. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- 2.Seino S, Miki T. Gene targeting approach to clarification of ion channel function: studies of Kir6. x null mice. J Physiol. 2004;554:295–300. doi: 10.1113/jphysiol.2003.047175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashcroft FM, Harrison DE, Ashcroft SJ. Glucose induces closure of single potassium channels in isolated rat pancreatic beta-cells. Nature. 1984;312:446–8. doi: 10.1038/312446a0. [DOI] [PubMed] [Google Scholar]
- 4.Gopel SO, Kanno T, Barg S, Weng XG, Gromada J, Rorsman P. Regulation of glucagon release in mouse-cells by KATP channels and inactivation of TTX-sensitive Na+ channels. J Physiol. 2000;528:509–20. doi: 10.1111/j.1469-7793.2000.00509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gopel SO, Kanno T, Barg S, Rorsman P. Patch-clamp characterisation of somatostatin-secreting-cells in intact mouse pancreatic islets. J Physiol. 2000;528:497–507. doi: 10.1111/j.1469-7793.2000.00497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gribble FM, Williams L, Simpson AK, Reimann F. A novel glucose-sensing mechanism contributing to glucagon-like peptide-1 secretion from the GLUTag cell line. Diabetes. 2003;52:1147–54. doi: 10.2337/diabetes.52.5.1147. [DOI] [PubMed] [Google Scholar]
- 7.Miki T, Liss B, Minami K, Shiuchi T, Saraya A, Kashima Y, et al. ATP-sensitive K+ channels in the hypothalamus are essential for the maintenance of glucose homeostasis. Nature neuroscience. 2001;4:507–12. doi: 10.1038/87455. [DOI] [PubMed] [Google Scholar]
- 8.Wang R, Liu X, Hentges ST, Dunn-Meynell AA, Levin BE, Wang W, et al. The regulation of glucose-excited neurons in the hypothalamic arcuate nucleus by glucose and feeding-relevant peptides. Diabetes. 2004;53:1959–65. doi: 10.2337/diabetes.53.8.1959. [DOI] [PubMed] [Google Scholar]
- 9.Zingman LV, Hodgson DM, Bast PH, Kane GC, Perez-Terzic C, Gumina RJ, et al. Kir6. 2 is required for adaptation to stress. Proc Natl Acad Sci USA. 2002;99:13278–83. doi: 10.1073/pnas.212315199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamada K, Ji JJ, Yuan H, Miki T, Sato S, Horimoto N, et al. Protective role of ATP-sensitive potassium channels in hypoxia-induced generalized seizure. Science. 2001;292:1543–6. doi: 10.1126/science.1059829. [DOI] [PubMed] [Google Scholar]
- 11.Hernandez-Sanchez C, Basile AS, Fedorova I, Arima H, Stannard B, Fernandez AM, et al. Mice transgenically overexpressing sulfonylurea receptor 1 in fore-brain resist seizure induction and excitotoxic neuron death. Proc Natl Acad Sci USA. 2001;98:3549–54. doi: 10.1073/pnas.051012898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzuki M, Sasaki N, Miki T, Sakamoto N, Ohmoto-Sekine Y, Tamagawa M, et al. Role of sarcolemmal K(ATP) channels in cardioprotection against ischemia/reperfusion injury in mice. J Clin Invest. 2002;109:509–16. doi: 10.1172/JCI14270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gumina RJ, Pucar D, Bast P, Hodgson DM, Kurtz CE, Dzeja PP, et al. Knockout of Kir6. 2 negates ischemic preconditioning-induced protection of myocardial energetics. Am J Physiol Heart Circ Physiol. 2003;284:2106–13. doi: 10.1152/ajpheart.00057.2003. [DOI] [PubMed] [Google Scholar]
- 14.Daut J, Klieber HG, Cyrys S, Noack T. KATP channels and basal coronary vascular tone. Cardiovasc Res. 1994;28:811–7. doi: 10.1093/cvr/28.6.811. [DOI] [PubMed] [Google Scholar]
- 15.Miki T, Minami K, Zhang L, Morita M, Gonoi T, Shiuchi T, et al. ATP-sensitive potassium channels participate in glucose uptake in skeletal muscle and adipose tissue. Am J Physiol Endocrinol Metab. 2002;283:1178–84. doi: 10.1152/ajpendo.00313.2002. [DOI] [PubMed] [Google Scholar]
- 16.Zawar C, Plant TD, Schirra C, Konnerth A, Neumcke B. Cell-type specific expression of ATP-sensitive potassium channels in the rat hippocampus. J Physiol. 1999;514:327–41. doi: 10.1111/j.1469-7793.1999.315ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griesemer D, Zawar C, Neumcke B. Cell-type specific depression of neuronal excitability in rat hippocampus by activation of ATP-sensitive potassium channels. Eur Biophys J. 2002;31:467–77. doi: 10.1007/s00249-002-0241-3. [DOI] [PubMed] [Google Scholar]
- 18.Schmid-Antomarchi H, Amoroso S, Fosset M, Lazdunski M. K+ channel openers activate brain sulfonylurea-sensitive K+ channels and block neurosecretion. Proc Natl Acad Sci USA. 1990;87:3489–92. doi: 10.1073/pnas.87.9.3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amoroso S, Schmid-Antomarchi H, Fosset M, Lazdunski M. Glucose, sulfonylureas and neurotransmitter release: role of ATP-sensitive K+ channels. Science. 1990;247:852–4. doi: 10.1126/science.2305257. [DOI] [PubMed] [Google Scholar]
- 20.Koster JC, Marshall BA, Ensor N, Corbett JA, Nichols CG. Targeted overactivity of beta cell K(ATP) channels induces profound neonatal diabetes. Cell. 2000;100:645–54. doi: 10.1016/s0092-8674(00)80701-1. [DOI] [PubMed] [Google Scholar]
- 21.Girard CA, Shimomura K, Proks P, Absalom N, Castano L, Perez de Nanclares G, et al. Functional analysis of six Kir6. 2 (KCNJ11) mutations causing neonatal diabetes. Pflugers Arch. 2006;453:323–32. doi: 10.1007/s00424-006-0112-3. [DOI] [PubMed] [Google Scholar]
- 22.Reimann F, Huopio H, Dabrowski M, Proks P, Gribble FM, Laakso M, et al. Characterisation of new K ATP-channel mutations associated with congenital hyperinsulinism in the Finnish population. Diabetologia. 2003;46:241–9. doi: 10.1007/s00125-002-1014-3. [DOI] [PubMed] [Google Scholar]
- 23.Marthinet E, Bloc A, Oka Y, Tanizawa Y, Wehrle-Haller B, Bancila V, et al. Severe congenital hyperinsulinism caused by a mutation in the Kir6. 2 subunit of the adenosine triphosphate-sensitive potassium channel impairing trafficking and function. The Journal of clinical endocrinology and metabolism. 2005;90:5401–6. doi: 10.1210/jc.2005-0202. [DOI] [PubMed] [Google Scholar]
- 24.Lin CW, Lin YW, Yan FF, Casey J, Kochhar M, Pratt EB, et al. Kir6. 2 mutations associated with neonatal diabetes reduce expression of ATP-sensitive K+ channels: implications in disease mechanism and sulfonylurea therapy. Diabetes. 2006;55:1738–46. doi: 10.2337/db05-1571. [DOI] [PubMed] [Google Scholar]
- 25.Kline CF, Kurata HT, Hund TJ, Cunha SR, Koval OM, Wright PJ, et al. Dual role of KATP channel C-terminal motif in membrane targeting and metabolic regulation. Proc Natl Acad Sci USA. 2009;106:16669–74. doi: 10.1073/pnas.0907138106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mohler PJ, Hoffman JA, Davis JQ, Abdi KM, Kim CR, Jones SK, et al. Isoform Specificity among Ankyrins: An amphipathic alpha-helix in the divergent regulatory domain of ankyrin-B interacts with the molecular co-chaperone Hdj1/Hsp40. J Biol Chem. 2004;279:25798–804. doi: 10.1074/jbc.M401296200. [DOI] [PubMed] [Google Scholar]
- 27.Cunha SR, Mohler PJ. Cardiac ankyrins: Essential components for development and maintenance of excitable membrane domains in heart. Cardiovasc Res. 2006;71:22–9. doi: 10.1016/j.cardiores.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 28.Fridlyand LE, Tamarina N, Philipson LH. Modeling of Ca2+ flux in pancreatic beta-cells: role of the plasma membrane and intracellular stores. Am J Physiol Endocrinol Metab. 2003;285:138–54. doi: 10.1152/ajpendo.00194.2002. [DOI] [PubMed] [Google Scholar]