Abstract
Background
During embryonic development, endothelial precursor cells (angioblasts) migrate relatively long distances to form the primary vascular plexus. The migratory behavior of angioblasts and localization of the primitive blood vessels is tightly regulated by pro-angiogenic and anti-angiogenic factors encountered in the embryonic environment. Despite the importance of corneal avascularity to proper vision, it is not known when avascularity is established in the developing cornea and how pro- and anti-angiogenic factors regulate this process.
Results and Discussion
Using Tg(tie1:H2B:eYFP) transgenic quail embryos to visualize fluorescently labeled angioblasts, we show that the presumptive cornea remains avascular despite the invasion of cells from the periocular region where migratory angioblasts reside and form the primary vasculature. Semiquantitative RT-PCR analysis and spatiotemporal examination of gene expression revealed that pro- and anti-angiogenic factors were expressed in patterns indicating their potential roles in angioblast guidance.
Conclusions
Our findings show for the first time that chick corneal avascularity is established and maintained during development as the periocular vasculature forms. We also identify potential candidate pro- and anti-angiogenic factors that may play crucial roles during vascular patterning in the anterior eye.
Keywords: Pro-angiogenic, Anti-angiogenic, Angioblast, Vasculogenesis, Ocular development, Periocular vasculature
INTRODUCTION
The absence of blood vessels in the mature cornea is critical for its transparency and function in vision. Avascularity in the adult cornea is actively maintained by a balance between pro-angiogenic and anti-angiogenic factors (Ambati et al., 2007; Ellenberg et al., 2010; Han and Zhang, 2010). Although several studies have focused on elucidating the molecules that maintain corneal angiogenic privilege and the pathological conditions causing its vascularization in adults, it is not clear when corneal avascularity is established during development and whether pro- and anti-angiogenic factors regulate this process.
During embryonic development, endothelial cell precursors (angioblasts) migrate long distances, proliferate, and coalesce into primitive vasculature during a process known as vasculogenesis. This process involves the formation of unobstructed vascular lumens and establishment of vascular networks that transport blood rich in oxygen, nutrients and cells to developing tissues (Risau and Flamme, 1995; Eichmann et al., 2005; Ferguson et al., 2005; Schmidt et al., 2007). As the embryo grows, some primitive blood vessels are pruned, whereas others anastomose with neighboring vascular sprouts to form stable larger vessels, which later invade new regions via a process known as angiogenesis.
To maintain proper vascularization of tissues during embryogenesis and in adults, vascular invasion due to vasculogenesis and angiogenesis are tightly regulated by the complementary action of pro- and anti-angiogenic factors localized within the environment they encounter. Numerous growth factors and regulatory proteins that guide angioblast migration or function in vascular stabilization have been identified (Lindahl et al., 1997; Adams and Alitalo, 2007; Gaengel et al., 2009; Adams and Eichmann, 2010; Hellberg et al., 2010; Tam and Watts, 2010; Crivellato, 2011). The pro-angiogenic factors, including vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), and platelet-derived growth factor B (PDGFB) promote migration, proliferation, and differentiation of angioblasts and endothelial cells. Null mutations of VEGFA, PDGFB or their respective receptors, VEGFR1, VEGFR2, and PDGFR-β, cause vascular and cardiac defects that are embryonic lethal (Tomanek et al., 2001; Bjarnegård et al., 2004). Deficiencies of FGF2, FGFR1, and FGFR2 result in vascular defects and other developmental abnormalities (Blaber et al., 1999; Miller et al., 2000; Murakami et al., 2008). In contrast, the anti-angiogenic factors, including the Semaphorins (Sema3A, and Sema3E), Netrins (Netrin1 and Netrin4), and soluble fms-like tyrosine kinase-1 (sFlt1, a truncated form of VEGFR1) counter the effects of pro-angiogenic factors during vasculogenesis. Semaphorins and Netrins function as repulsive guidance cues, which inhibit endothelial cell migration (Gu, 2005; Guttmann-Raviv et al., 2007; Acevedo et al., 2008; Bouvrée et al., 2008; Lejmi et al., 2008; Sakurai et al., 2010), whereas sFlt1 binds to and sequesters VEGFA in the extracellular environment (Aiello et al., 1995; Ambati et al., 2006).
Blood vessels that supply the adult cornea have been traced to their origin from the ophthalmic artery, which ramifies as it progresses towards the anterior eye to form the ciliary arteries of the pericorneal vascular plexus in the limbus region adjacent to the cornea. The presence of a physical barrier within the transitional limbal region that separates the avascular cornea from the highly vascularized limbus is still a subject of debate (Ellenberg et al., 2010). However, recent studies have challenged this hypothesis by demonstrating that neovascularization can be induced by adding pro-angiogenic factors such as VEGF, FGF, and PDGF into adult corneas (Kenyon et al., 1996; Auerbach et al., 2003; Zhang et al., 2009; Cao et al., 2011). Interestingly, both induced and pathological corneal neovascularization can be inhibited by addition of anti-angiogenic factors (Ambati et al., 2006; Benny et al., 2010; Chen et al., 2010). In addition, our previous study indicated that no such physical barrier exists during cornea development, since neural crest cells from the surrounding periocular region (Hay, 1980; Creuzet et al., 2005) migrate between the ectoderm and lens to form the corneal endothelium and stroma (Lwigale et al., 2005). Currently it is not clear whether angioblasts migrate into the presumptive cornea in concert with the neural crest cells. In addition, it is also not clear if angioblast migration and vasculogenesis are regulated by a balance between pro-and anti-angiogenic factors within the periocular and corneal environment to establish and maintain vasculature that is restricted to the limbal region.
In this study, we initially determined when corneal avascularity is established by characterizing vasculogenesis of the anterior eye. Using Tg(tie1:H2B:eYFP) transgenic quail embryos, we show that during eye development, angioblasts migrate into the anterior eye but avoid the presumptive cornea and form the primary vasculature in the adjacent periocular region. Our data from mRNA expression analysis by RT-PCR and section in situ hybridization show that pro- and anti-angiogenic factors as well as their receptors are expressed in the anterior eye region during ocular vasculogenesis. These data demonstrate for the first time that corneal avascularity is established concomitantly with stroma formation and suggest a potential role for pro-and anti-angiogenic factors during this process.
RESULTS AND DISCUSSION
Vasculogenesis of the Anterior Eye and Corneal Avascularity
There are two possibilities for how corneal avascularity is established: 1) neural crest cells are permitted to migrate into the presumptive cornea, whereas angioblasts are prohibited, or 2) both neural crest cells and angioblasts migrate into the presumptive cornea but the corneal vasculature regresses as observed in the hyaloid vasculature (Latker and Kuwabara, 1981). To characterize the development of the limbal vasculature and determine when corneal avascularity is established, we utilized Tg(tie1:H2B:eYFP) transgenic quail embryos (Sato et al., 2010). Tie1 is an angiopoetin tyrosine kinase receptor that is exclusively expressed by angioblasts and endothelial cells (Iljin et al., 2002; Chan et al., 2008). Expression of the Tg(tie1:H2B:eYFP) allowed us to visualize the nuclear expression of H2B:eYFP in migratory angioblasts and forming blood vessels during eye development. We chose E3, E5, and E7 eyes, which reflect the 3 critical stages of cornea development when neural crest cells surround the presumptive cornea, migrate between the lens and ectoderm to form the corneal endothelium, followed by the corneal stroma (Hay, 1980; Creuzet et al., 2005; Lwigale et al., 2005).
By E3, angioblasts were present in the anterior eye but avoided presumptive cornea (Fig. 1A, D). At this time, individual angioblasts were localized in the periocular mesenchyme adjacent to the optic cup (Fig. 1D). By E5, angioblasts had aggregated to form the tubular temporal and nasal ciliary arteries and a “vascular ring” around the cornea periphery (Fig. 1B). At this stage, the tubular blood vessels are visible under a dissecting microscope. Previous studies visualized the vascular network of the eye following injection of black ink (Hughes, 1934) and by analysis of corrosion casts by electron microscopy (Hiruma and Hirakow, 1995). These methods were not sensitive enough to visualize earlier stages of angioblast migration and aggregation accomplished by the molecular identification used in this study. Interestingly, despite the ongoing migration of cells from the periocular region to the space between the lens and ectoderm to form the cornea endothelium, the angioblasts and primitive vasculature remained in the periocular region (Fig. 1E). By E7, the three major layers of the cornea were formed and surrounded by the newly formed blood vessels. The nasal ciliary artery had regressed (Fig. 1C; asterisk), while the primitive vascular plexus in the temporal region transformed into a network of blood vessels that joined the temporal ciliary artery (Fig. 1C; arrows). Respectively, the pericorneal vascular ring and neural crest cells adjacent to the tip of the optic cup formed the iridial ring artery and stroma of the iris (Fig. 1C, F). Our results show that blood vessels in the anterior region of the eye are generated by vasculogenesis. We also show that cell migration from the periocular region into the cornea is limited to presumptive corneal cells. Since no physical barrier exists between the periocular mesenchyme and presumptive cornea, it is likely that either pro-angiogenic factors do not attract angioblasts into the developing cornea, or anti-angiogenic factors prevent their migration from the periocular region.
Figure 1.
Patterning of the limbal vasculature and expression of pro- and anti-angiogenic factors by the anterior eye during avian corneal development. A–C: Expression of H2B-eYFP by angioblasts and vasculature in whole mount Tg(tie1:H2B:eYFP) quail eyes at E3, E5, and E7. Remodeling of the temporal ciliary artery forms a vascular plexus (arrows) while the nasal ciliary artery regresses (asterisk). D–F: Cross sections through E3, E5, and E7 Tg(tie1:H2B:eYFP) quail eyes showing localization of angioblasts and vasculature in the periocular region. Sections were counterstained with DAPI. G: Semiquantitative reverse transcriptase-polymerase chain reaction (RT-PCR) analysis of tissues isolated from E3, E5, and E7 chick eyes for pro- and anti-angiogenic factors. Abbreviations: C, cornea; vr, vascular ring; tca, temporal ciliary artery; nca, nasal ciliary artery; ir, iridial ring artery; L, lens; OC, optic cup; pm, periocular mesenchyme; ep, corneal epithelium; en, corneal endothelium; st, corneal stroma. Scale bars in (A–C) = 500µm; (D–F) = 50µm.
Expression of Pro- and Anti-angiogenic Factors in the Anterior Eye during Development
Next, we assessed whether pro- and anti-angiogenic factors were present in the anterior eye region during angioblast migration and vasculogenesis. Initially, we performed a semi-quantitative RT-PCR analysis on anterior eye tissues isolated from E3, E5 and E7 embryos. Our results (Fig. 1G) show that mRNA for pro-angiogenic factors including VEGFA and its receptors VEGFR1 and VEGFR2; FGF1, FGF2, and receptors FGFR1 and FGFR2; PDGFB and receptor PDGFR-β; and Sema3G and receptor Npn2 were expressed between E3 and E7. A similar pattern was observed for mRNA encoding anti-angiogenic factors including sFlt1; Sema3E and its receptor PlexinD1; and Netrin1, Netrin4 and receptors Neogenin, Unc5C were also expressed between E3 and E7. With the exception of Netrin4, which was not detected at E3, mRNAs for all pro- and anti-angiogenic factors and their receptors were consistently present during vasculogenesis of the anterior eye and cornea development. Our results indicate a potential role for pro- and anti-angiogenic factors during the patterning of ocular blood vessels and development of avascular cornea.
Spatiotemporal Expression of Pro-angiogenic Factors in the Anterior Eye
Expression of VEGFA, VEGFR1, VEGFR2, and sFlt1
In vertebrates, the VEGF family is comprised of five members (VEGFA, B, C, D, and placental growth factor (PIGF), which differentially signal through the tyrosine-kinase receptors VEGFR1, VEGFR2, and VEGFR3. VEGFA is well known for its role in promoting endothelial cell migration, proliferation, and assembly into primitive vasculature during embryonic development and in adults (Argraves et al., 2002; Ruhrberg et al., 2002; Carmeliet, 2003). VEGFA signaling is transduced by binding to VEGFR1 and VEGFR2 receptors specifically expressed on the surface of endothelial cells (Fong et al., 1995; Shalaby et al., 1995).
VEGFA was vividly expressed in the lens vesicle and optic cup by E3 (Fig. 2A) and its expression was maintained in these tissues at E5 and E7 (Fig. 2B, C). It was also broadly expressed at low levels in the periocular mesenchyme at E3 and E5 (Fig. 2A, B). The expression pattern of VEGFA was maintained in the lens and stroma of the presumptive iris at E7 (Fig. 2C). VEGFR1 was expressed by the angioblasts adjacent to the optic cup at E3 (Fig. 2D, D`), but its expression was diminished in the pericorneal vascular ring at E5 (Fig. 2E, E`). By E7 VEGFR1 expression was not detectable in the iridial ring artery (Fig. 2F), although it remained strong in the posterior retina (data not shown). VEGFR2 was expressed by angioblasts at E3 (Fig. 2G, G`) and maintained in the vascular ring and iridial artery (Fig. 2H, H`, I). The expression pattern of VEGFA in the optic cup and its receptors by angioblasts in the adjacent periocular region is consistent with the pro-angiogenic role of VEGF signaling during vasculogenesis.
Figure 2.
Expression of VEGFA and its receptors in the anterior eye during vasculogenesis and corneal development. A–L: Section in situ hybridization was used to determine the expression of VEGFA (A–C), VEGFR1 (D–F), VEGFR2 (G–I), and sFlt1 (J–L) in the anterior eye at E3, E5, and E7. VEGFR1 and VEGFR2 are strongly expressed by angioblasts at E3 (arrows, D`, G`) and in the vascular ring at E5 (E`, H`). Brown tissue at E5 and E7 indicates natural coloration of the retinal pigmented epithelium (asterisk). Abbreviations: L, lens; OC, optic cup; pm, periocular mesenchyme; pi, presumptive iris; ep, corneal epithelium; en, corneal endothelium; st, corneal stroma; vr, vascular ring; ir, iridial ring artery. Scale bar in (A–L) = 100µm; (D`, E`, G`, H`) = 20 µm.
Although VEGFA is expressed in the lens, angioblasts do not migrate past the tip of the optic cup into the presumptive cornea region. One possibility is that VEGFA-mediated angioblast migration is inhibited. Sequestration of VEGFA by sFlt1 in the adult cornea accounts for one of the mechanisms by which corneal avascularity is maintained (Ambati et al., 2006). Although sFlt1 was present in the anterior eye, expression was at relatively lower levels than VEGFA in the lens and optic cup (Fig. 2J–L), suggesting that sFlt1 alone may not be sufficient to inhibit angioblast migration into the presumptive cornea. In addition, previous studies have shown that another VEGFA receptor, Npn1, is expressed in the periocular region at E3 (Chilton and Guthrie, 2003) overlapping migratory angioblasts and neural crest cells, and later it is expressed by ocular blood vessels at E5 and E6 (Lwigale and Bronner-Fraser, 2009). VEGFA signaling through Npn1 and VEGFR2 is critical for cardiac development and vascular morphogenesis (Kitsukawa et al., 1995; Kawasaki et al., 1999; Yamada et al., 2001; Gu et al., 2003). However, Npn1 is a dual receptor for VEGFA and the cell guidance molecule Sema3A (Soker et al., 1998; Miao et al., 1999; Pan et al., 2007). Given that the Sema3A is strongly expressed by the lens (Lwigale and Bronner-Fraser, 2009; Kubilus and Linsenmayer, 2010) and overlaps with VEGFA (Fig. 2A–C), it is likely that Npn1-expressing angioblasts are inhibited from migrating into the presumptive cornea by Sema3A signaling from the lens. This behavior would be similar to Npn1-expressing periocular neural crest cells, which do not contribute to the cornea (Lwigale and Bronner-Fraser, 2009).
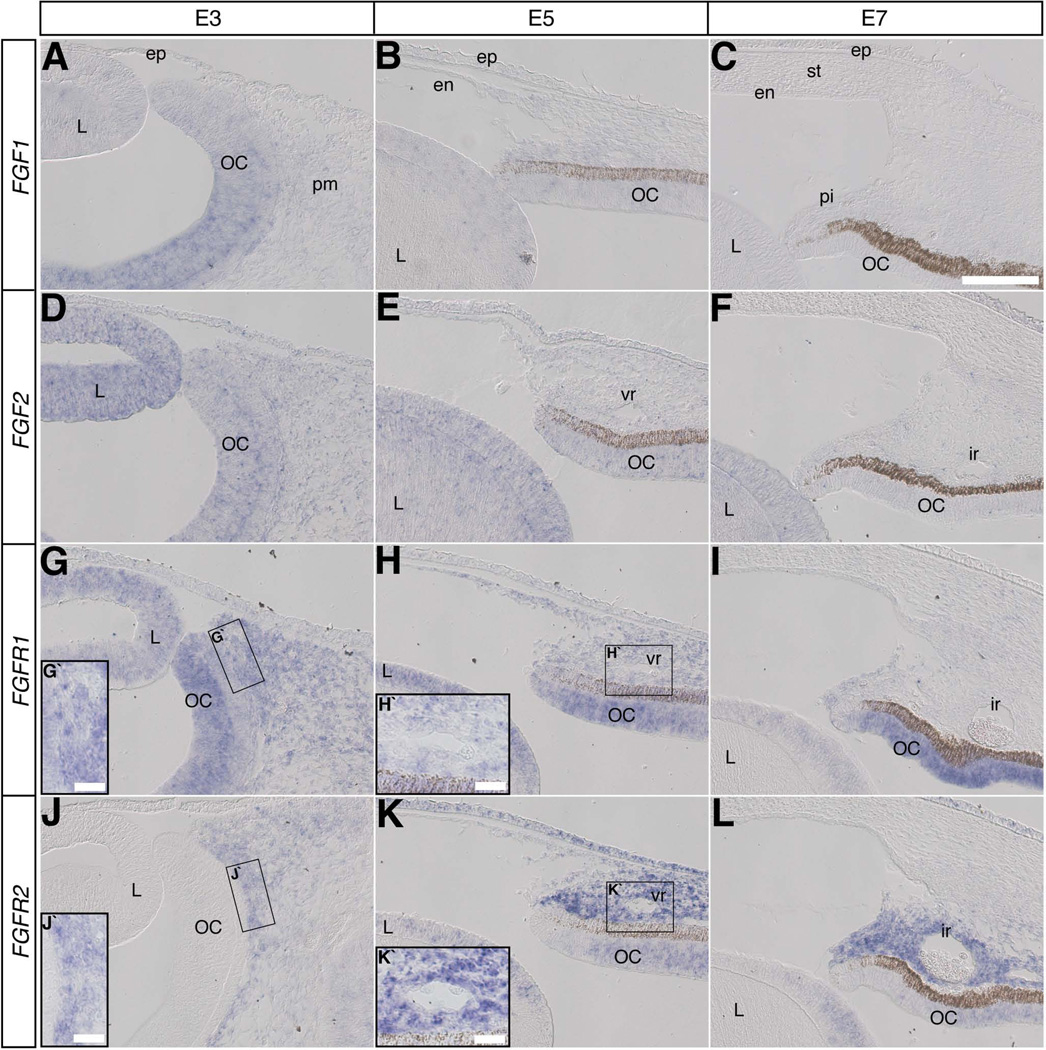
Expression of FGF1, FGF2, FGFR1, and FGFR2
FGF is a large family of morphogens that regulate various processes critical for embryonic development, including angiogenesis, cell differentiation and migration (Moura et al.; McAVOY et al., 1991; Friesel and Maciag, 1995). They signal by differentially binding to four tyrosine-kinase receptors (FGFR1, R2, R3, and R4) and to cell-associated heparin sulfate proteoglycans that act as coreceptors (Pellegrini, 2001; Itoh and Ornitz, 2004). Here we focused on FGF1 and FGF2, which stimulate angiogenesis by signaling through the main receptors FGFR1 and FGFR2 expressed by endothelial cells (Nakamura et al., 2001; Poole et al., 2001; Javerzat et al., 2002; Presta et al., 2005).
At E3, FGF1 was expressed in the optic cup in a diminishing gradient towards the anterior eye region (Fig. 3A). Although FGF1 expression was maintained at high levels in the posterior retina between E5 and E7 (data not shown), it was not expressed in the anterior eye region (Fig. 3B, C). In contrast, FGF2 expression was strong in the lens but low and diffused in the optic cup and periocular mesenchyme at E3 (Fig. 3D). At E5, FGF2 expression was maintained at similar levels in the lens, optic cup, and periocular mesenchyme (Fig 3E). By E7, FGF2 was faint in the lens and absent in the cornea and optic cup (Fig. 3F).
Figure 3.
Expression of FGF1, FGF2, and their receptors in the anterior eye during vasculogenesis and corneal development. A–L: Section in situ hybridization was used to determine the expression of FGF (A–C), FGF2 (D–F), FGFR1 (G–I), and FGFR2 (J–L) in the anterior eye at E3, E5, and E7. Abbreviations: L, lens; OC, optic cup; pm, periocular mesenchyme; pi, presumptive iris; ep, corneal epithelium; en, corneal endothelium; st, corneal stroma; vr, vascular ring; ir, iridial ring artery. Scale bar in (A–L) = 100µm; (G`, H`, J`, K`) = 20 µm.
FGFR1 was expressed at E3 in the lens, optic cup, and periocular mesenchyme (Fig. 3G, G`). At E5 it appeared in the forming cornea endothelium (Fig. 3H), but it was expressed at low levels in the vascular ring compared to the surrounding periocular mesenchyme (Fig. 3H`). By E7, expression of FGFR1 disappeared from the cornea endothelium, vasculature, and periocular mesenchyme, but it persisted at low levels in the lens and iris stroma, and remained vivid in the optic cup (Fig. 3I). FGFR2 was expressed in the periocular mesenchyme, including the region of the migratory angioblasts at E3 (Fig. 3J, J`). By E5 FGFR2 was expressed in the lens, optic cup, ectoderm, and in the periocular mesenchyme, including the pericorneal vascular ring, but absent in the cornea endothelium (Fig. 3K, K`). At E7, FGFR2 was highly expressed in the neural crest mesenchyme of the presumptive iris and the iridial ring artery, but maintained at low levels in the lens and optic cup (Fig. 3L). The complementary expression patterns of FGFR1 and FGFR2 at E7 suggest that they perform different developmental roles at this stage.
The localization of FGF1 suggests that it is involved in patterning events in the retina, possibly through FGFR1 (Matsushima et al., 1996; Rousseau et al., 2000). The dynamic expression of FGF2 suggests its potential role during early anterior eye development by signaling through FGFR1 and FGFR2 to regulate angioblast migration and proliferation. Also, FGF2 signals through only FGFR2 at a later stage to regulate tubulogenesis (Zhou et al., 1998).
Expression of PDGFB and PDGFR-²
Platelet-derived growth factor (PDGF) is a family of heparin binding growth factors (PDGF-A, -B, -C, and -D) that bind to PDGF receptors -α and -β resulting in activation of a receptor tyrosine kinase and signal transduction. PDGF signaling plays significant roles during embryonic development, including angiogenesis (Andrae et al., 2008). In this group, PDGFB signaling via PDGFR-β promotes differentiation of endothelial cell precursors into endothelial cells (Rolny et al., 2006). Furthermore, PDGFB signaling recruits mural cells to newly formed blood vessels that play an important role during vascular remodeling and angiogenesis (Hellström et al., 1999; Bjarnegård et al., 2004; Armulik et al., 2005; Zhang et al., 2009)
PDGFB was expressed in the lens from E3-E7 (Fig. 4A–C). Expression of PDGFB in the periocular mesenchyme adjacent to the developing vasculature was evident at E5 and E7 (Fig. 4B–C). PDGFR-β was not visible in the angioblasts at E3 (Fig. 4D, D`), but was localized in the mesenchyme around the developing vasculature of the presumptive iris at E5 and E7 (Fig. 4E, E`, F). Based on these expression patterns it is likely that PDGFB signaling plays a role during development and maturation of the vascular plexus in the anterior eye. In addition, expression of PDGFB in the lens could be involved in cell proliferation and differentiation via PDGFR-α (Reneker and Overbeek, 1996; Kok et al., 2002).
Figure 4.
Expression of PDGFB and PDFGR-β in the anterior eye during vasculogenesis and corneal development. A–F: Section in situ hybridization was used to determine the expression of PDGFB (A–C), and PDGFR-β (D–F) in the anterior eye at E3, E5, and E7. Abbreviations: L, lens; OC, optic cup; pm, periocular mesenchyme; pi, presumptive iris; ep, corneal epithelium; en, corneal endothelium; st, corneal stroma; vr, vascular ring; ir, iridial ring artery. Scale bar in (A–F) = 100µm; (D`, E`) = 20 µm.
Spatiotemporal Expression of Anti-angiogenic Factors in the Anterior Eye
Expression of Sema3E/PlexinD1 and Sema3G/Npn2
Semaphorins are a group of secreted and membrane-associated proteins that play important roles during embryonic development and adulthood. All vertebrate semaphorins (semaphorins 3–7) signal by directly binding plexins (plexinA-D) or through tyrosine kinase receptors Npn1 and Npn2 with plexin co-receptors (Callander et al., 2007; Bagri et al., 2009). In this group, the secreted Sema3A, Sema3E, and Sema3G have been associated with vascular development and patterning (Bates et al., 2003; Serini et al., 2003; Gitler et al., 2004; Gu, 2005; Kutschera et al., 2011). The expression pattern and possible role of Sema3A/Npn-1 signaling was discussed earlier in relation to VEGF signaling. Therefore, we will focus on the expression of Sema3E/PlexinD1 and Sema3G/Npn2.
In the anterior eye region, Sema3E was expressed in the optic cup at all stages (Fig. 5A–C) and in the periocular mesenchyme in the region of the iridocorneal angle at E7 (Fig. 5C; arrowheads). The anti-angiogenic signaling by Sema3E is mediated by binding directly to plexinD1 (Gu et al., 2005). PlexinD1 was expressed by angioblasts (Fig. 5D, D`) and maintained in the forming blood vessels (Fig. 5E, E`, F), and in the mesenchyme of the presumptive iris at E5 and E7 (Fig. 5E–F; long arrows). The complementary pattern of Sema3E and PlexinD1 expression in the eye is similar to that observed in the trunk region, where Sema3E signaling by the somites guides angioblast migration via PlexinD1, leading to the formation of intersegemental vessels (Torres-Vázquez et al., 2004; Gu, 2005). It is therefore possible that Sema3E/PlexinD1 signaling in the eye plays a role in preventing vascularization of the anterior optic cup and in the migration of the PlexinD1-expressing angioblasts and neural crest mesenchyme past the tip of the optic cup into the presumptive cornea. Furthermore the expression of Sema3E in the iridocorneal angle may serve as a barrier to vascular ingrowth into the cornea at later stages of development.
Figure 5.
Expression of Sema3E, Sema3G and their respective receptors PlexinD1 and Npn2 in the anterior eye during vasculogenesis and corneal development. A–L: Section in situ hybridization was used to determine the expression of Sema3E (A–C), PlexinD1 (D–F), Sema3G (G–I), and Npn2 (J–L) in the anterior eye at E3, E5, and E7. Sema3E is expressed in the mesenchyme of the iridocorneal angle at E7 (arrowheads). PlexinD1 and Sema3G are strongly expressed by angioblasts at E3 (arrows, D`, G`) and in the vascular ring at E5 (E`, H`). PlexinD1 is also expressed in the mesenchyme of the presumptive iris at E5 and E7 (long arrows). Abbreviations: L, lens; OC, optic cup; pm, periocular mesenchyme; pi, presumptive iris; ep, corneal epithelium; en, corneal endothelium; st, corneal stroma; vr, vascular ring; ir, iridial ring artery; ca, ciliary artery. Scale bar in (A–L) = 100µm; (D`, E`, G`, H`, J`, K`) = 20 µm.
Sema3G was expressed by angioblasts in the periocular region at E3 (Fig. 5G, G`; arrows), and this expression was maintained during vasculogenesis between E5 and E7 (Fig. 5H, H`, I). Sema3G was also strongly expressed in the posterior neural retina and blood vessels adjacent to the retinal pigment epithelium at E7 (data not shown). Npn2 was expressed in the optic cup, lens epithelium, and in a few angioblasts and periocular mesenchyme of the posterior eye at E3 (Fig. 5J, J`). Also at this stage Npn2 was strongly expressed in the newly formed cranial blood vessels in the posterior eye region where it persisted until later stages (data not shown). By E5, expression of Npn2 was strong in the periocular mesenchyme and presumptive corneal endothelium, and evident in the forming vasculature (Fig. 5K, K`). Npn2 expression in the lens and optic cup was dramatically diminished at E5. At E7, expression of Npn2 was strong in the blood vessels and periocular mesenchyme, stroma of the presumptive iris, and in the corneal stroma (Fig. 5L). These expression patterns are consistent with previous observations in developing murine arterial blood vessels, where Sema3G is expressed by endothelial cells and Npn2 is expressed by the adjacent smooth muscle cells (Kutschera et al., 2011). Since Npn2 is expressed by the periocular blood vessels and adjacent mesenchyme, it is possible that autocrine signaling by Sema3G in endothelial cells stabilizes developing blood vessels, whereas paracrine signaling stimulates migration and incorporation of pericytes and smooth muscle cells in the formation of mature blood vessels.
Expression of Netrin1, Netrin4, Neogenin, and Unc5B
Netrins are secreted extracellular proteins that are well known for their role in axon guidance, angiogenesis, and in various developmental processes including cell migration and differentiation (Jiang et al., 2003; Lu et al., 2004; Carmeliet and Tessier-Lavigne, 2005). Netrins are structurally related to laminins, with netrins1–3 bearing homology to the γ-chain and netrin4 to the β-chain. Signaling by netrins is mediated via activation of receptors belonging to the uncoordinated 5 (Unc5) family, which consist of Unc5A–D, and by deleted in colorectal cancer (DCC), comprised of DCC and Neogenin (Rajasekharan and Kennedy, 2009). The Unc5B and Neogenin receptors have been implicated in mediating anti-angiogenic netrin signaling during endothelial cell migration and vascular sprouting. Netrin1/unc5B signaling inhibits sprouting angiogenesis and endothelial migration in vitro (Lu et al., 2004; Larrivée et al., 2007; Bouvrée et al., 2008). Netrin4 signaling through neogenin plays a similar role (Lejmi et al., 2008).
The vascular-specific receptor Unc5B was expressed by the angioblasts and forming periocular blood vessels between E3 and E7 (Fig. 6A–C). As previously reported (Adler and Belecky-Adams, 2002; Harada et al., 2007), Netrin1 was expressed in the ventral region of the optic cup at E3 (Fig. 6G, H). By E5, Netrin1 expression was confined to the region of the choroid fissure (Fig. 6G, I; asterisk) and adjacent to the optic stalk (Fig. 6G, I; arrowhead). In mammals and zebrafish, angioblast migration through the choroid fissure leads to the formation of the transient hyaloid vasculature (Saint-Geniez and D’Amore, 2004; Morcillo et al., 2006; Weiss et al., 2012). In birds angioblasts do not migrate into the optic cup and the hyaloid vasculature does not from, but instead a pecten artery develops from the optic nerve region in the posterior eye (Hiruma, 1996). Based on the expression patterns above, Netrin1/unc5B signaling may play a role in preventing angioblast migration into the chick eye via the choroid fissure.
Figure 6.
Expression of Netrin1, Netrin4, Neogenin, and Unc5 in the anterior eye during vasculogenesis and corneal development. A–F, H–K: Section in situ hybridization was used to determine the expression of Unc5B (A–C), Neogenin (D–F), Netrin1 (H–I), and Netrin4 (J–K) in the anterior eye at E3, E5, and E7. Unc5B is strongly expressed by angioblasts at E3 (arrows). Neogenin is expressed in the iridocorneal angle (long arrows). G: Schematic representation of the expression patterns of Netrin1 and Netrin4 in the eye. Netrin1 is expressed at the optic cup along the choroid fissure (asterisk). Cross-section in (I) is perpendicular to the choroid fissure, as depicted in (G, dotted line). J`, K`: Netrin4 is expressed in the optic stalk (arrowheads), as depicted in G. Abbreviations: L, lens; OC, optic cup; pm, periocular mesenchyme; pi, presumptive iris; ep, corneal epithelium; en, corneal endothelium; st, corneal stroma; vr, vascular ring; ir, iridial ring artery. Scale bar in (A–F, H, J, J`, K, K`) = 100 µm; (A`, B`, D`, E`) = 20 µm; (I) = 500 µm.
Neogenin was strongly expressed by the optic cup and at relatively low levels in the lens and periocular mesenchyme at E3 and E5 (Fig. 6D–E). By E7, Neogenin expression was diminished in the lens and optic cup, but remained vivid in the mesenchyme of the iridocorneal angle region of presumptive ocular muscles (Fig. 6F; long arrows) and neural retina (data not shown). Netrin4 was expressed at very low levels in the lens compared to the optic stalk region of the neural retina (Fig. 6J,J’ and 6K, K’). Therefore, Netrin4/Neogenin signaling may play a role during angioblast migration but is not required later during vasculogenesis.
Summary
We have shown that corneal avascularity is established early and maintained during avian eye development as neural crest cells migrate from the periocular region to form the corneal endothelium and stroma. Our gene expression results and potential involvement in ocular vasculogenesis are summarized in the schematic representation of the anterior eye (Fig. 7). Transcripts encoding both pro- and anti-angiogenic factors are expressed in the anterior eye during cornea development, whereas their receptors are expressed by migratory angioblasts and newly formed ocular vasculature. Expression of the major proangiogenic factors VEGFA, FGF2, PDGFB and anti-angiogenic factors sFlt1, Netrin1, Netrin4, Sema3E, and Sema3A (not described in the current study), overlap in the lens and optic cup. At later stages of eye development, VEGFA and Sema3E also overlap in the periocular mesenchyme of the presumptive iridocorneal angle. Given that all the above factors are secreted within the environment of the angioblasts and forming vasculature, lack of angioblast migration into the presumptive cornea suggests that the anti-angiogenic signals dominate in this region and prevent its vascularization. In contrast, pro-angiogenic signals secreted from the lens, optic cup, and periocular mesenchyme may dominate the periocular region and permit angioblast migration and vascular development. Expression of Sema3E in the anterior region of the optic cup suggests that Sema3E/PlexinD1 signaling provides additional inhibitory cues to prevent angioblast migration past the tip of the optic cup. Expression of Sema3G and its receptor Npn2 by the forming vasculature suggests that autocrine Sema3G signaling prevents angioblast migration as an initial step towards vasculogenesis. Additional Sema3G signaling to the surrounding Npn2-positive periocular neural crest could be involved in the formation of pericytes and vascular smooth muscle, which stabilize the newly formed ocular blood vessels. Expression of Netrin1 in the optic cup suggests that it plays a role in preventing the development of retinal blood vessels. In addition to vascular patterning, these factors may play other roles such as cell guidance in the neural retina as well as proliferation and differentiation of lens and periocular neural crest cells. Altogether, our results suggest the presence of pro-angiogenic and anti-angiogenic factors in the anterior eye with potential to orchestrate the formation of the periocular blood vessels while maintaining corneal avascularity during development. A similar pattern can be suggested from the growing evidence that pro- and anti-angiogenic factors are maintained in a balance under normal physiological conditions of the adult cornea, and play a vital role in its angiogenic privilege and transparency. In addition to pro-and anti-angiogenic factors, other players such as miRNAs, which are increasingly growing in importance as regulators of the angiogenic process, might be involved in ocular vasculogenesis (Suárez and Sessa, 2009; Xu, 2009). Understanding their expression patterns during ocular development and their interactions with pro- and anti-angiogenic angiogenic factors will benefit future functional studies of ocular vasculogenesis and the gene networks involved in this process.
Figure 7.
Schematic diagram summarizing gene expression and the putative role of pro- and anti-angiogenic factors during vasculogenesis of the anterior eye. Pro- and anti-angiogenic factors are expressed in the lens, optic cup, and periocular mesenchyme (blue and red, respectively). These expression patterns suggest that corresponding proteins are secreted into the presumptive cornea and periocular region (blue and red arrows, respectively). Angioblasts, blood vessels (green), and periocular neural crest cells (gray) express receptors for the pro- and anti-angiogenic factors. Absence of angioblast migration and vasculogenesis in the developing cornea suggest a strong response to anti-angiogenic factors probably secreted by the lens and optic cup. In contrast, there is a strong response to angiogenic factors in the periocular region, which favor angioblast migration and proliferation. Brown coloration in the optic cup represents the retinal pigment epithelium. Abbreviations: pc, presumptive cornea; PM, periocular mesenchyme; OC, optic cup; A/BV, angioblasts and blood vessels; NC, neural crest cells.
EXPERIMENTAL PROCEDURES
Embryos
Tg(tie1:H2B:eYFP) transgenic quail eggs were purchased from Ozark Egg Company (Stover, MO). Eggs were incubated at 37 °C for 3, 5, and 7 days to obtain embryos at the three main stages of cornea formation (Hay, 1980; Lwigale et al., 2005). Embryos were screened using a dissecting microscope and a 488 filter for fluorescence of angioblasts and blood vessels before further processing. White Leghorn chicken eggs were obtained from Texas A&M Poultry Center (College Station, TX). Similarly, chick eggs were incubated for 3, 5, and 7 days. All animal studies were approved by the Institutional Animal Care and Use Committee (IACUC) at Rice University.
Immunostaining
Tg(tie1:H2B:eYFP) quail embryos were decapitated and the heads were fixed overnight at 4 °C in 4% paraformaldehyde (PFA). In some cases whole heads of E3 and anterior hemispheres of E5 and E7 eyes were immunostained using standard protocol with a rabbit anti-GFP antibody (used at 1:2000, Covance) followed by AlexaFluor 488 secondary antibody (used at 1:200, Invitrogen) to enhance the fluorescence signal. After whole-mount imaging, tissues were embedded in gelatin and sectioned at 8–10 µm. Sections were rinsed in phosphate buffer solution (PBS), counterstained with 4,6-diamidino-2-phenylindole (DAPI), and coverslipped prior to imaging.
RNA Isolation and RT-PCR
Total RNA was isolated from E3 chick heads, anterior hemispheres of E5 and E7 eyes using Trizol reagent (Ambion). cDNA was synthesized by superscript lll reverse transcriptase (Invitrogen). Transcription of pro- and anti-angiogenic factors was analyzed by semi-quantitative PCR reaction using hotstart polymerase (Sigma), and gene specific primers (Table1; Steffensky et al., 2006). Analysis of PCR products was done by standard 2% Agarose gels. GAPDH transcripts were used as an equal loading control.
TABLE 1.
Primers used for section in situ hybridization and RT-PCR
| In situ Hybridization Primers | ||
|---|---|---|
| Forward | Reverse | |
| * VEGFA | 5’ CCCTGTGGATGTGTACAACGTCAC 3’ | 5’TTTCCGCTGCTCACCGTCTC 3’ |
| * VEGFR1 | 5’ GCCTGAAGCTCTAAGTAAGGACAGC 3’ | 5’CACTCCAGCATGGCAGAGTTAC 3’ |
| * VEGFR2 | 5’ TCAGCTACGCAGGCATGGTC 3’ | 5’GTGCCATGGGCTCTTGAACAG 3’ |
| * sFlt1 | 5’ AAGGGAACATATTCACGGGGAAA 3’ | 5’ CAAGATGATATGAGACACCTGATACTG 3’ |
| * FGF1 | 5’ GGGGAGATAACCACCTTCACC 3’ | 5’ CTCAGTCAGCCGACACCG 3’ |
| FGF2 | 5' CAAGCAGAAGAAAGAGGAG 3' | 5' TGTCCAGTCCTTTTCAGTG 3' |
| FGFR1 | Construct provided by Marianne Bronner (McCabe et al., 2007) | |
| FGFR2 | Construct provided by Marianne Bronner (McCabe et al., 2007) | |
| * PDGFB | 5’ CATGAATTTCGGCGTGGTCTTC 3’ | 5’ CTATGCTATGAGGATTTCTTTC 3’ |
| * PDGFRB | 5’ TATCTGTGAAATCTCCTCCAAG 3’ | 5’ AAACTGGAGGTTATTCCTGG 3’ |
| Sema3E | 5' AGGACAGCGGATCGTTGTGA 3' | 5' ATCGCTCCTCAGTCTTCTCC 3' |
| Sema3G | 5' AGGTAAAGACAGATGAGCGG 3' | 5' CTCGGCCTCTTTATTTGTGG 3' |
| PlexinD1 | 5’ GACCTCAGCCGCAATGACAGC 3’ | 5’ CCTTCGGGGATCTTGTAATGTGC 3’ |
| Npn2 | Construct provided by Marianne Bronner (Gammill et al., 2008) | |
| Netrin1 | 5’ AACATGTTCACCGTGCGAC 3’ | 5’ GACAGCTTGTACAGCTCGATGTT 3’ |
| Netrin4 | 5’ AGGGCTTCTCAGCTCTGCGGC 3’ | 5’ GCAGGCTCATCGTCAACATGAA 3’ |
| * Neogenin | 5’ CTCCAGTGCCAGATCCATCTCCC 3’ | 5’ ATGGGGACTGTTACTGCCGC 3’ |
| Unc5B | 5’ CTGCCTGCACAATAAAAGAGTTC 3’ | 5’ CTCTCTGCCTTGTTGATGAC 3’ |
| RT-PCR Primers | ||
|---|---|---|
| Forward | Reverse | |
| FGF2 | 5' TTCTTCCTGCGCATCAAC 3' | 5' GGATAGCTTTCTGTCCAG 3' |
| FGFR1 | 5' AAGTGGCTGTAAAGATGCTC 3' | 5' ATCTTCATCACGTTGTCCTC 3' |
| FGFR2 | 5' CTATTGGACACACACAGACA 3' | 5' CCTCTCTACGTGTTTTATCC 3' |
| Sema3E | 5' AGAGTACAATAGCACCCTTC 3' | 5' GGAAGTTGCTGTAACCTATC 3' |
| Sema3G | 5' CTCTGTCAAGGCCAAAAGC 3' | 5' GAAGGTGCTGTTGTGCTCCA 3' |
| PlexinD1 | 5’ GAAAACCAAGACCATGTGAC 3’ | 5’ CTTCAAACATCCTCTCCATC 3’ |
| † Npn2 | 5' GGCAACTCTGAACCAAGTCCCGA 3' | 5' CCAGCTTCGAGTTTCCAGTATC 3' |
| Netrin1 | 5’ AACATGGAGCTGTACAAGCTGTCG 3’ | 5’ TTGGAGGCTTTGCAGTACGAGTCA 3’ |
| Netrin4 | 5’ GACTGATTTAACCCTTCCTG 3’ | 5’ GGCAGGTAGTAAAGTGCTTT 3’ |
| Unc5B | 5' TAGAAGAGATGGGCAAGAGC 3' | 5' AAGGAGGAGGAGAACCAGAT 3' |
Primers used for in situ hybridization and RT-PCR
Primers previously described (Steffensky et al., 2006)
Section in situ Hybridization
Freshly isolated E3 chick heads and anterior hemispheres of E5 and E7 eyes were fixed overnight at 4°C in modified Carnoy's fixative (60% ethanol, 30% formaldehyde, and 10% glacial acetic acid). Tissues were dehydrated in an increasing ethanol series, embedded in paraffin, and sectioned at 10–12 µm. Riboprobes were prepared by amplifying cDNA templates for genes of interest from a chick cDNA pool and cloning into TOPO vector with dual promoters (Invitrogen). Primers used for amplification of template cDNA fragments from a chicken cDNA pool are listed in Table1. Plasmids encoding cDNA for Npn2, FGFR1, and FGFR2 were kindly provided by Marianne Bronner (Gammill et al., 2006; McCabe et al., 2007). Sense and anti-sense digoxygenin labeled riboprobes were generated by polymerase reactions. Section in situ hybridization was performed as described (Etchevers et al., 2001). Sense probes were used in parallel for each gene as negative controls.
Imaging
Differential interference contrast (DIC) and fluorescent images of whole-mount and sections were captured using a CCD camera (Axiocam; Carl Zeiss AG, Oberkochen, Germany) mounted on a fluorescence microscope (AxioImager 2; Carl Zeiss AG) with a slider module (ApoTome; Carl Zeiss AG).
Supplementary Material
Supplemental Figure1. (Data not shown). Expression of pro- and anti-angiogenic factors in the posterior eye region and retina during development. A–E: Section in situ hybridization was used to determine the expression of VEGFR1 (A), FGF1 (B), Sema3G (C), Neogenin (D), and Unc5B (E) in the posterior retina. VEGFR1 and Sema3G are expressed in blood vessels outside the retinal pigment epithelium at E5 and E7, respectively (arrows). Neogenin is expressed in the presumptive ocular muscle region (arrowheads). F–H: Npn2 is expressed in the mesenchyme and blood vessels located in the posterior eye region (arrows), and by the neural retina. Abbreviations: OC, optic cup; pm, periocular mesenchyme. Scale bar = 100 µm.
Supplemental Figure2. A. Double in situ hybridization on a cross section through an E3 eye showing the expression of VEGFR1 and PlexinD1 by angioblasts in the periocular region (arrows). B. Control section without in situ hybridization shows autofluorescence of angioblasts (arrows).
Key findings.
Angioblasts migrate into the periocular region of the eye during development, but avoid the presumptive cornea.
Pro- and anti-angiogenic factors are expressed within the vicinity of the developing cornea, whereas angioblasts, periocular mesenchyme and forming ocular vasculature express their receptors.
Expression profile of pro- and anti-angiogenic factors and their receptors suggest their potential roles in ocular vasculogenesis and corneal avascularity during development.
ACKNOWLEDGMENTS
We thank Drs Cathy Mathews and Charles Stewart for critical reading of the manuscript, Dr Marianne Bronner for the plasmids encoding cDNA for Npn2, FGFR1, and FGFR2, and members of the Lwigale lab for discussions of the raw data and editing the early version of the manuscript. This work was supported by NIH grants EY018050 and EY022158 to PYL.
REFERENCES
- Acevedo LM, Barillas S, Weis SM, Göthert JR, Cheresh DA. Semaphorin 3A suppresses VEGF-mediated angiogenesis yet acts as a vascular permeability factor. Blood. 2008;111:2674–2680. doi: 10.1182/blood-2007-08-110205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol. 2007;8:464–478. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- Adams RH, Eichmann A. Axon guidance molecules in vascular patterning. Cold Spring Harb Perspect Biol. 2010;2:1–18. doi: 10.1101/cshperspect.a001875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler R, Belecky-Adams TL. The role of bone morphogenetic proteins in the differentiation of the ventral optic cup. Development. 2002;129:3161–3171. doi: 10.1242/dev.129.13.3161. [DOI] [PubMed] [Google Scholar]
- Aiello LP, Pierce EA, Foley ED, Takagi H, Chen H, Riddle L, Ferrara N, King GL, Smith LE. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc Natl Acad Sci U S A. 1995;92:10457–10461. doi: 10.1073/pnas.92.23.10457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambati BK, Nozaki M, Singh N, Takeda A, Jani PD, Suthar T, Albuquerque RJ, Richter E, Sakurai E, Newcomb MT, Kleinman ME, Caldwell RB, Lin Q, Ogura Y, Orecchia A, Samuelson DA, Agnew DW, St Leger J, Green WR, Mahasreshti PJ, Curiel DT, Kwan D, Marsh H, Ikeda S, Leiper LJ, Collinson JM, Bogdanovich S, Khurana TS, Shibuya M, Baldwin ME, Ferrara N, Gerber HP, De Falco S, Witta J, Baffi JZ, Raisler BJ, Ambati J. Corneal avascularity is due to soluble VEGF receptor-1. Nature. 2006;443:993–997. doi: 10.1038/nature05249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambati BK, Patterson E, Jani P, Jenkins C, Higgins E, Singh N, Suthar T, Vira N, Smith K, Caldwell R. Soluble vascular endothelial growth factor receptor-1 contributes to the corneal antiangiogenic barrier. Br J Ophthalmol. 2007;91:505–508. doi: 10.1136/bjo.2006.107417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22:1276–1312. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argraves WS, Larue AC, Fleming PA, Drake CJ. VEGF signaling is required for the assembly but not the maintenance of embryonic blood vessels. Dev Dyn. 2002;225:298–304. doi: 10.1002/dvdy.10162. [DOI] [PubMed] [Google Scholar]
- Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97:512–523. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- Auerbach R, Lewis R, Shinners B, Kubai L, Akhtar N. Angiogenesis assays: a critical overview. Clin Chem. 2003;49:32–40. doi: 10.1373/49.1.32. [DOI] [PubMed] [Google Scholar]
- Bagri A, Tessier-Lavigne M, Watts RJ. Neuropilins in tumor biology. Clin Cancer Res. 2009;15:1860–1864. doi: 10.1158/1078-0432.CCR-08-0563. [DOI] [PubMed] [Google Scholar]
- Bates D, Taylor GI, Minichiello J, Farlie P, Cichowitz A, Watson N, Klagsbrun M, Mamluk R, Newgreen DF. Neurovascular congruence results from a shared patterning mechanism that utilizes Semaphorin3A and Neuropilin-1. Dev Biol. 2003;255:77–98. doi: 10.1016/s0012-1606(02)00045-3. [DOI] [PubMed] [Google Scholar]
- Bjarnegård M, Enge M, Norlin J, Gustafsdottir S, Fredriksson S, Abramsson A, Takemoto M, Gustafsson E, Fässler R, Betsholtz C. Endothelium-specific ablation of PDGFB leads to pericyte loss and glomerular, cardiac and placental abnormalities. Development. 2004;131:1847–1857. doi: 10.1242/dev.01080. [DOI] [PubMed] [Google Scholar]
- Blaber SI, Culajay JF, Khurana A, Blaber M. Reversible thermal denaturation of human FGF-1 induced by low concentrations of guanidine hydrochloride. Biophys J. 1999;77:470–477. doi: 10.1016/S0006-3495(99)76904-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvrée K, Larrivée B, Lv X, Yuan L, DeLafarge B, Freitas C, Mathivet T, Bréant C, Tessier-Lavigne M, Bikfalvi A, Eichmann A, Pardanaud L. Netrin-1 inhibits sprouting angiogenesis in developing avian embryos. Dev Biol. 2008;318:172–183. doi: 10.1016/j.ydbio.2008.03.023. [DOI] [PubMed] [Google Scholar]
- Callander DC, Lamont RE, Childs SJ, McFarlane S. Expression of multiple class three semaphorins in the retina and along the path of zebrafish retinal axons. Dev Dyn. 2007;236:2918–2924. doi: 10.1002/dvdy.21315. [DOI] [PubMed] [Google Scholar]
- Cao R, Lim S, Ji H, Zhang Y, Yang Y, Honek J, Hedlund EM, Cao Y. Mouse corneal lymphangiogenesis model. Nat Protoc. 2011;6:817–826. doi: 10.1038/nprot.2011.359. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Tessier-Lavigne M. Common mechanisms of nerve and blood vessel wiring. Nature. 2005;436:193–200. doi: 10.1038/nature03875. [DOI] [PubMed] [Google Scholar]
- Chan B, Yuan HT, Ananth Karumanchi S, Sukhatme VP. Receptor tyrosine kinase Tie-1 overexpression in endothelial cells upregulates adhesion molecules. Biochem Biophys Res Commun. 2008;371:475–479. doi: 10.1016/j.bbrc.2008.04.091. [DOI] [PubMed] [Google Scholar]
- Chilton JK, Guthrie S. Cranial expression of class 3 secreted semaphorins and their neuropilin receptors. Dev Dyn. 2003;228:726–733. doi: 10.1002/dvdy.10396. [DOI] [PubMed] [Google Scholar]
- Creuzet S, Couly G, Le Douarin NM. Patterning the neural crest derivatives during development of the vertebrate head: insights from avian studies. J Anat. 2005;207:447–459. doi: 10.1111/j.1469-7580.2005.00485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crivellato E. The role of angiogenic growth factors in organogenesis. Int J Dev Biol. 2011;55:365–375. doi: 10.1387/ijdb.103214ec. [DOI] [PubMed] [Google Scholar]
- Eichmann A, Yuan L, Moyon D, Lenoble F, Pardanaud L, Breant C. Vascular development: from precursor cells to branched arterial and venous networks. Int J Dev Biol. 2005;49:259–267. doi: 10.1387/ijdb.041941ae. [DOI] [PubMed] [Google Scholar]
- Ellenberg D, Azar DT, Hallak JA, Tobaigy F, Han KY, Jain S, Zhou Z, Chang JH. Novel aspects of corneal angiogenic and lymphangiogenic privilege. Prog Retin Eye Res. 2010;29:208–248. doi: 10.1016/j.preteyeres.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchevers HC, Vincent C, Douarin NML, Couly GF. The cephalic neural crest provides pericytes and smooth muscle cells to all blood vessels of the face and forebrain. Development. 2001;128:1059–1068. doi: 10.1242/dev.128.7.1059. [DOI] [PubMed] [Google Scholar]
- Ferguson JE, Kelley RW, Patterson C. Mechanisms of endothelial differentiation in embryonic vasculogenesis. Arterioscler Thromb Vasc Biol. 2005;25:2246–2254. doi: 10.1161/01.ATV.0000183609.55154.44. [DOI] [PubMed] [Google Scholar]
- Fong GH, Rossant J, Gertsenstein M, Breitman ML. Role of the FLT-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature. 1995;376:66–70. doi: 10.1038/376066a0. [DOI] [PubMed] [Google Scholar]
- Friesel R, Maciag T. Molecular mechanisms of angiogenesis: fibroblast growth factor signal transduction. FASEB J. 1995;9:919–925. doi: 10.1096/fasebj.9.10.7542215. [DOI] [PubMed] [Google Scholar]
- Gaengel K, Genové G, Armulik A, Betsholtz C. Endothelial-mural cell signaling in vascular development and angiogenesis. Arterioscler Thromb Vasc Biol. 2009;29:630–638. doi: 10.1161/ATVBAHA.107.161521. [DOI] [PubMed] [Google Scholar]
- Gammill LS, Gonzales C, Bronner-Fraser M. Neuropilin 2/Semaphorin 3F signaling is essential for cranial neural crest migration and trigeminal ganglion condensation. Dev Neurobiol. 2006;67:47–56. doi: 10.1002/dneu.20326. [DOI] [PubMed] [Google Scholar]
- Gitler AD, Lu MM, Epstein JA. PlexinD1 and semaphorin signaling are required in endothelial cells for cardiovascular development. Dev Cell. 2004;7:107–116. doi: 10.1016/j.devcel.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Gu C. Semaphorin 3E and Plexin-D1 control vascular pattern independently of neuropilins. Science. 2005;307:265–268. doi: 10.1126/science.1105416. [DOI] [PubMed] [Google Scholar]
- Gu C, Rodriguez ER, Reimert DV, Shu T, Fritzsch B, Richards LJ, Kolodkin AL, Ginty DD. Neuropilin-1 conveys semaphorin and VEGF signaling during neural and cardiovascular development. Developmental Cell. 2003;5:45–57. doi: 10.1016/s1534-5807(03)00169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttmann-Raviv N, Shraga-Heled N, Varshavsky A, Gulmaraes-Sternberg C, Kessler O, Neufeld G. Semaphorin-3A and Semaphorin-3F work together to repel endothelial cells and to inhibit their survival by induction of apoptosis. J Biol Chem. 2007;282:26294–26305. doi: 10.1074/jbc.M609711200. [DOI] [PubMed] [Google Scholar]
- Han X, Zhang MC. Potential anti-angiogenic role of Slit2 in corneal neovascularization. Exp Eye Res. 2010;90:742–749. doi: 10.1016/j.exer.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Harada T, Harada C, Parada LF. Molecular regulation of visual system development: more than meets the eye. Genes Dev. 2007;21:367–378. doi: 10.1101/gad.1504307. [DOI] [PubMed] [Google Scholar]
- Hay ED. Development of the vertebrate cornea. Int Rev Cytol. 1980;63:263–322. doi: 10.1016/s0074-7696(08)61760-x. [DOI] [PubMed] [Google Scholar]
- Hellberg C, Ostman A, Heldin CH. PDGF and vessel maturation. Recent Results Cancer Res. 2010;180:103–114. doi: 10.1007/978-3-540-78281-0_7. [DOI] [PubMed] [Google Scholar]
- Hellström M, Kalén M, Lindahl P, Abramsson A, Betsholtz C. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development. 1999;126:3047–3055. doi: 10.1242/dev.126.14.3047. [DOI] [PubMed] [Google Scholar]
- Hiruma T. Formation of the ocular arteries in the chick embryo: observations of corrosion casts by scanning electron microscopy. Anat Embryol. 1996;193:585–592. doi: 10.1007/BF00187930. [DOI] [PubMed] [Google Scholar]
- Hiruma T, Hirakow R. Formation of the pharyngeal arch arteries in the chick embryo. Observations of corrosion casts by scanning electron microscopy. Anat Embryol. 1995;191:415–423. doi: 10.1007/BF00304427. [DOI] [PubMed] [Google Scholar]
- Hughes AFW. On the development of the blood vessels in the head of the chick. Philos T Roy Soc B. 1934;224:75–130. [Google Scholar]
- Iljin K, Petrova TV, Veikkola T, Kumar V, Poutanen M, Alitalo K. A fluorescent Tie1 reporter allows monitoring of vascular development and endothelial cell isolation from transgenic mouse embryos. FASEB J. 2002;16:1764–1774. doi: 10.1096/fj.01-1043com. [DOI] [PubMed] [Google Scholar]
- Itoh N, Ornitz DM. Evolution of the FGF and FGFR gene families. Trends Genet. 2004;20:563–569. doi: 10.1016/j.tig.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Javerzat S, Auguste P, Bikfalvi A. The role of fibroblast growth factors in vascular development. Trends Mol Med. 2002;8:483–489. doi: 10.1016/s1471-4914(02)02394-8. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Liu M, Gershon MD. Netrins and DCC in the guidance of migrating neural crest-derived cells in the developing bowel and pancreas. Dev Biol. 2003;258:364–384. doi: 10.1016/s0012-1606(03)00136-2. [DOI] [PubMed] [Google Scholar]
- Kawasaki T, Kitsukawa T, Bekku Y, Matsuda Y, Sanbo M, Yagi T, Fujisawa H. A requirement for Neuropilin-1 in embryonic vessel formation. Development. 1999;126:4895–4902. doi: 10.1242/dev.126.21.4895. [DOI] [PubMed] [Google Scholar]
- Kenyon BM, Voest EE, Chen CC, Flynn E, Folkman J, D’Amato RJ. A model of angiogenesis in the mouse cornea. IOVS. 1996;37:1625–1632. [PubMed] [Google Scholar]
- Kitsukawa T, Shimono A, Kawakami A, Kondoh H, Fujisawa H. Overexpression of a membrane protein, neuropilin, in chimeric mice causes anomalies in the cardiovascular system, nervous system and limbs. Development. 1995;121:4309–4318. doi: 10.1242/dev.121.12.4309. [DOI] [PubMed] [Google Scholar]
- Kok A, Lovicu FJ, Chamberlain CG, McAvoy JW. Influence of platelet-derived growth factor on lens epithelial cell proliferation and differentiation. Growth Factors. 2002;20:27–34. doi: 10.1080/08977190290022202. [DOI] [PubMed] [Google Scholar]
- Kubilus JK, Linsenmayer TF. Developmental guidance of embryonic corneal innervation: Roles of Semaphorin3A and Slit2. Dev Biol. 2010;344:172–184. doi: 10.1016/j.ydbio.2010.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutschera S, Weber H, Weick A, De Smet F, Genove G, Takemoto M, Prahst C, Riedel M, Mikelis C, Baulande S, Champseix C, Kummerer P, Conseiller E, Multon MC, Heroult M, Bicknell R, Carmeliet P, Betsholtz C, Augustin HG. Differential endothelial transcriptomics identifies Semaphorin 3G as a vascular class 3 semaphorin. Arterioscler Thromb Vasc Biol. 2011;31:151–159. doi: 10.1161/ATVBAHA.110.215871. [DOI] [PubMed] [Google Scholar]
- Larrivée B, Freitas C, Trombe M, Lv X, Delafarge B, Yuan L, Bouvrée K, Bréant C, Del Toro R, Bréchot N, Germain S, Bono F, Dol F, Claes F, Fischer C, Autiero M, Thomas JL, Carmeliet P, Tessier-Lavigne M, Eichmann A. Activation of the Unc5B receptor by Netrin-1 inhibits sprouting angiogenesis. Genes Dev. 2007;21:2433–2447. doi: 10.1101/gad.437807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latker CH, Kuwabara T. Regression of the tunica vasculosa lentis in the postnatal rat. Invest Ophthalmol Vis Sci. 1981;21:689–699. [PubMed] [Google Scholar]
- Lejmi E, Leconte L, Pédron-Mazoyer S, Ropert S, Raoul W, Lavalette S, Bouras I, Feron JG, Maitre-Boube M, Assayag F, Feumi C, Alemany M, Jie TX, Merkulova T, Poupon MF, Ruchoux MM, Tobelem G, Sennlaub F, Plouët J. Netrin-4 inhibits angiogenesis via binding to Neogenin and recruitment of Unc5B. Proc Natl Acad Sci U S A. 2008;105:12491–12496. doi: 10.1073/pnas.0804008105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl P, Johansson BR, Levéen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277:242–245. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- Lu X, Le Noble F, Yuan L, Jiang Q, De Lafarge B, Sugiyama D, Bréant C, Claes F, De Smet F, Thomas JL, Autiero M, Carmeliet P, Tessier-Lavigne M, Eichmann A. The netrin receptor Unc5B mediates guidance events controlling morphogenesis of the vascular system. Nature. 2004;432:179–186. doi: 10.1038/nature03080. [DOI] [PubMed] [Google Scholar]
- Lwigale PY, Bronner-Fraser M. Semaphorin3A/Neuropilin-1 signaling acts as a molecular switch regulating neural crest migration during cornea development. Dev Biol. 2009;336:257–265. doi: 10.1016/j.ydbio.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lwigale PY, Cressy PA, Bronner-Fraser M. Corneal keratocytes retain neural crest progenitor cell properties. Dev Biol. 2005;288:284–293. doi: 10.1016/j.ydbio.2005.09.046. [DOI] [PubMed] [Google Scholar]
- Matsushima M, Ogata N, Takada Y, Tobe T, Yamada H, Takahashi K, Uyama M. FGF receptor 1 expression in experimental choroidal neovascularization. Jpn J Ophthalmol. 1996;40:329–338. [PubMed] [Google Scholar]
- McAvoy JW, Chamberlain CG, de Iongh RU, Richardson NA, Lovicu FJ. The role of fibroblast growth factor in eye lens development. Ann N Y Acad Sci. 1991;638:256–274. doi: 10.1111/j.1749-6632.1991.tb49036.x. [DOI] [PubMed] [Google Scholar]
- McCabe KL, Shiau CE, Bronner-Fraser M. Identification of candidate secreted factors involved in trigeminal placode induction. Dev Dyn. 2007;236:2925–2935. doi: 10.1002/dvdy.21325. [DOI] [PubMed] [Google Scholar]
- Miao HQ, Soker S, Feiner L, Alonso JL, Raper JA, Klagsbrun M. Neuropilin-1 mediates Collapsin-1/Semaphorin III inhibition of endothelial cell motility: functional competition of Collapsin-1 and vascular endothelial growth factor-165. J Cell Biol. 1999;146:233–241. doi: 10.1083/jcb.146.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DL, Ortega S, Bashayan O, Basch R, Basilico C. Compensation by fibroblast growth factor 1 (FGF1) does not account for the mild phenotypic defects observed in FGF2 null mice. Mol Cell Biol. 2000;20:2260–2268. doi: 10.1128/mcb.20.6.2260-2268.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morcillo J, Martínez-Morales JR, Trousse F, Fermin Y, Sowden JC, Bovolenta P. Proper patterning of the optic fissure requires the sequential activity of Bmp7 and Shh. Development. 2006;133:3179–3190. doi: 10.1242/dev.02493. [DOI] [PubMed] [Google Scholar]
- Moura RS, Coutinho-Borges JP, Pacheco AP, daMota PO, Correia-Pinto J. FGF signaling pathway in the developing chick lung: expression and inhibition studies. PLoS One. 2011;6:e17660. doi: 10.1371/journal.pone.0017660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M, Nguyen LT, Zhang ZW, Moodie KL, Carmeliet P, Stan RV, Simons M. The FGF system has a key role in regulating vascular integrity. J Clin Invest. 2008;118:3355–3366. doi: 10.1172/JCI35298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Mochizuki Y, Kanetake H, Kanda S. Signals via FGF receptor 2 regulate migration of endothelial cells. Biochem Biophys Res Commun. 2001;289:801–806. doi: 10.1006/bbrc.2001.6046. [DOI] [PubMed] [Google Scholar]
- Pan Q, Chathery Y, Wu Y, Rathore N, Tong RK, Peale F, Bagri A, Tessier-Lavigne M, Koch AW, Watts RJ. Neuropilin-1 binds to VEGF121 and regulates endothelial cell migration and sprouting. J Biol Chem. 2007;282:24049–24056. doi: 10.1074/jbc.M703554200. [DOI] [PubMed] [Google Scholar]
- Pellegrini L. Role of heparan sulfate in fibroblast growth factor signalling: a structural view. Curr Opin Struct Biol. 2001;11:629–634. doi: 10.1016/s0959-440x(00)00258-x. [DOI] [PubMed] [Google Scholar]
- Poole TJ, Finkelstein EB, Cox CM. The role of FGF and VEGF in angioblast induction and migration during vascular development. Dev Dyn. 2001;220:1–17. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1087>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Presta M, Dell’Era P, Mitola S, Moroni E, Ronca R, Rusnati M. Fibroblast growth factor/fibroblast growth factor receptor system in angiogenesis. Cytokine Growth Factor Rev. 2005;16:159–178. doi: 10.1016/j.cytogfr.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Rajasekharan S, Kennedy TE. The netrin protein family. Genome Biol. 2009;10:239–239. doi: 10.1186/gb-2009-10-9-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reneker LW, Overbeek PA. Lens-specific expression of PDGF-A alters lens growth and development. Dev Biol. 1996;180:554–565. doi: 10.1006/dbio.1996.0328. [DOI] [PubMed] [Google Scholar]
- Risau W, Flamme I. Vasculogenesis. Annu Rev Cell Dev Biol. 1995;11:73–91. doi: 10.1146/annurev.cb.11.110195.000445. [DOI] [PubMed] [Google Scholar]
- Rolny C, Nilsson I, Magnusson P, Armulik A, Jakobsson L, Wentzel P, Lindblom P, Norlin J, Betsholtz C, Heuchel R, Welsh M, Claesson-Welsh L. Platelet-derived growth factor receptor-β promotes early endothelial cell differentiation. Blood. 2006;108:1877–1886. doi: 10.1182/blood-2006-04-014894. [DOI] [PubMed] [Google Scholar]
- Rousseau B, Dubayle D, Sennlaub F, Jeanny JC, Costet P, Bikfalvi A, Javerzat S. Neural and angiogenic defects in eyes of transgenic mice expressing a dominant-negative FGF receptor in the pigmented cells. Exp Eye Res. 2000;71:395–404. doi: 10.1006/exer.2000.0892. [DOI] [PubMed] [Google Scholar]
- Ruhrberg C, Gerhardt H, Golding M, Watson R, Ioannidou S, Fujisawa H, Betsholtz C, Shima DT. Spatially restricted patterning cues provided by heparin-binding VEGF-A control blood vessel branching morphogenesis. Genes Dev. 2002;16:2684–2698. doi: 10.1101/gad.242002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Geniez M, D’Amore PA. Development and pathology of the hyaloid, choroidal and retinal vasculature. Int J Dev Biol. 2004;48:1045–1058. doi: 10.1387/ijdb.041895ms. [DOI] [PubMed] [Google Scholar]
- Sakurai A, Gavard J, Annas-Linhares Y, Basile JR, Amornphimoltham P, Palmby TR, Yagi H, Zhang F, Randazzo PA, Li X, Weigert R, Gutkind JS. Semaphorin 3E initiates antiangiogenic signaling through Plexin D1 by regulating Arf6 and R-Ras. Mol Cell Biol. 2010;30:3086–3098. doi: 10.1128/MCB.01652-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y, Poynter G, Huss D, Filla MB, Czirok A, Rongish BJ, Little CD, Fraser SE, Lansford R. Dynamic analysis of vascular morphogenesis using transgenic quail embryos. PLoS ONE. 2010;5:e12674. doi: 10.1371/journal.pone.0012674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A, Brixius K, Bloch W. Endothelial precursor cell migration during vasculogenesis. Circ Res. 2007;101:125–136. doi: 10.1161/CIRCRESAHA.107.148932. [DOI] [PubMed] [Google Scholar]
- Serini G, Valdembri D, Zanivan S, Morterra G, Burkhardt C, Caccavari F, Zammataro L, Primo L, Tamagnone L, Logan M, Tessier-Lavigne M, Taniguchi M, Püschel AW, Bussolino F. Class 3 semaphorins control vascular morphogenesis by inhibiting integrin function. Nature. 2003;424:391–397. doi: 10.1038/nature01784. [DOI] [PubMed] [Google Scholar]
- Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, Schuh AC. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92:735–745. doi: 10.1016/s0092-8674(00)81402-6. [DOI] [PubMed] [Google Scholar]
- Steffensky M, Steinbach K, Schwarz U, Schlosshauer B. Differential impact of semaphorin 3E and 3A on CNS axons. Int J Dev Neurosci. 2006;24:65–72. doi: 10.1016/j.ijdevneu.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Suárez Y, Sessa WC. MicroRNAs as novel regulators of angiogenesis. Circ Res. 2009;104:442–454. doi: 10.1161/CIRCRESAHA.108.191270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam SJ, Watts RJ. Connecting vascular and nervous system development: angiogenesis and the blood-brain barrier. Annu Rev Neurosci. 2010;33:379–408. doi: 10.1146/annurev-neuro-060909-152829. [DOI] [PubMed] [Google Scholar]
- Tomanek RJ, Sandra A, Zheng W, Brock T, Bjercke RJ, Holifield JS. Vascular endothelial growth factor and basic fibroblast growth factor differentially modulate early postnatal coronary angiogenesis. Circ Res. 2001;88:1135–1141. doi: 10.1161/hh1101.091191. [DOI] [PubMed] [Google Scholar]
- Torres-Vázquez J, Gitler AD, Fraser SD, Berk JD, Van N Pham, Fishman MC, Childs S, Epstein JA, Weinstein BM. Semaphorin-Plexin signaling guides patterning of the developing vasculature. Dev Cell. 2004;7:117–123. doi: 10.1016/j.devcel.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Weiss O, Kaufman R, Michaeli N, Inbal A. Abnormal vasculature interferes with optic fissure closure in lmo2 mutant zebrafish embryos. Dev Biol. 2012;369:191–198. doi: 10.1016/j.ydbio.2012.06.029. [DOI] [PubMed] [Google Scholar]
- Xu S. MicroRNA expression in the eyes and their significance in relation to functions. Prog Retin Eye Res. 2009;28:87–116. doi: 10.1016/j.preteyeres.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Yamada E, Tobe T, Yamada H, Okamoto N, Zack DJ, Werb Z, Soloway PD, Campochiaro PA. TIMP-1 promotes VEGF-induced neovascularization in the retina. Histol Histopathol. 2001;16:87–97. doi: 10.14670/HH-16.87. [DOI] [PubMed] [Google Scholar]
- Zhang J, Cao R, Zhang Y, Jia T, Cao Y, Wahlberg E. Differential roles of PDGFR-α and PDGFR-β in angiogenesis and vessel stability. FASEB J. 2009;23:153–163. doi: 10.1096/fj.08-113860. [DOI] [PubMed] [Google Scholar]
- Zhou M, Sutliff RL, Paul RJ, Lorenz JN, Hoying JB, Haudenschild CC, Yin M, Coffin JD, Kong L, Kranias EG, Luo W, Boivin GP, Duffy JJ, Pawlowski SA, Doetschman T. Fibroblast growth factor 2 control of vascular tone. Nat Med. 1998;4:201–207. doi: 10.1038/nm0298-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure1. (Data not shown). Expression of pro- and anti-angiogenic factors in the posterior eye region and retina during development. A–E: Section in situ hybridization was used to determine the expression of VEGFR1 (A), FGF1 (B), Sema3G (C), Neogenin (D), and Unc5B (E) in the posterior retina. VEGFR1 and Sema3G are expressed in blood vessels outside the retinal pigment epithelium at E5 and E7, respectively (arrows). Neogenin is expressed in the presumptive ocular muscle region (arrowheads). F–H: Npn2 is expressed in the mesenchyme and blood vessels located in the posterior eye region (arrows), and by the neural retina. Abbreviations: OC, optic cup; pm, periocular mesenchyme. Scale bar = 100 µm.
Supplemental Figure2. A. Double in situ hybridization on a cross section through an E3 eye showing the expression of VEGFR1 and PlexinD1 by angioblasts in the periocular region (arrows). B. Control section without in situ hybridization shows autofluorescence of angioblasts (arrows).