Abstract
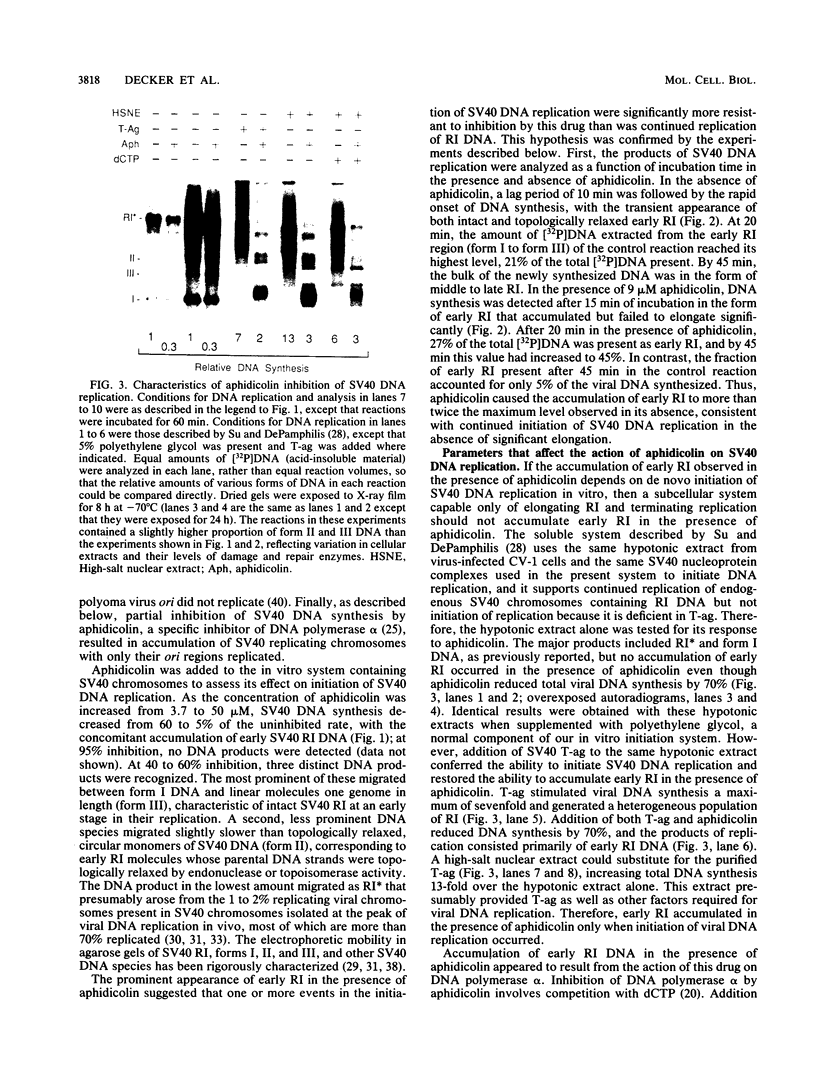
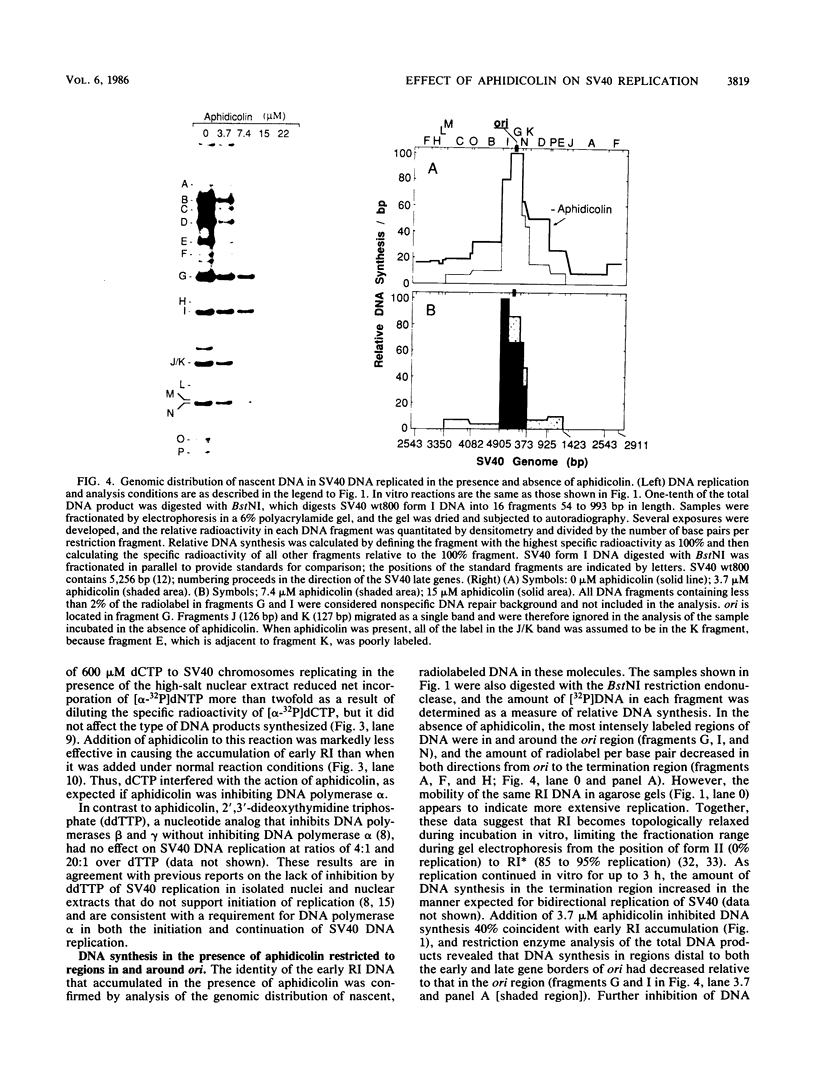
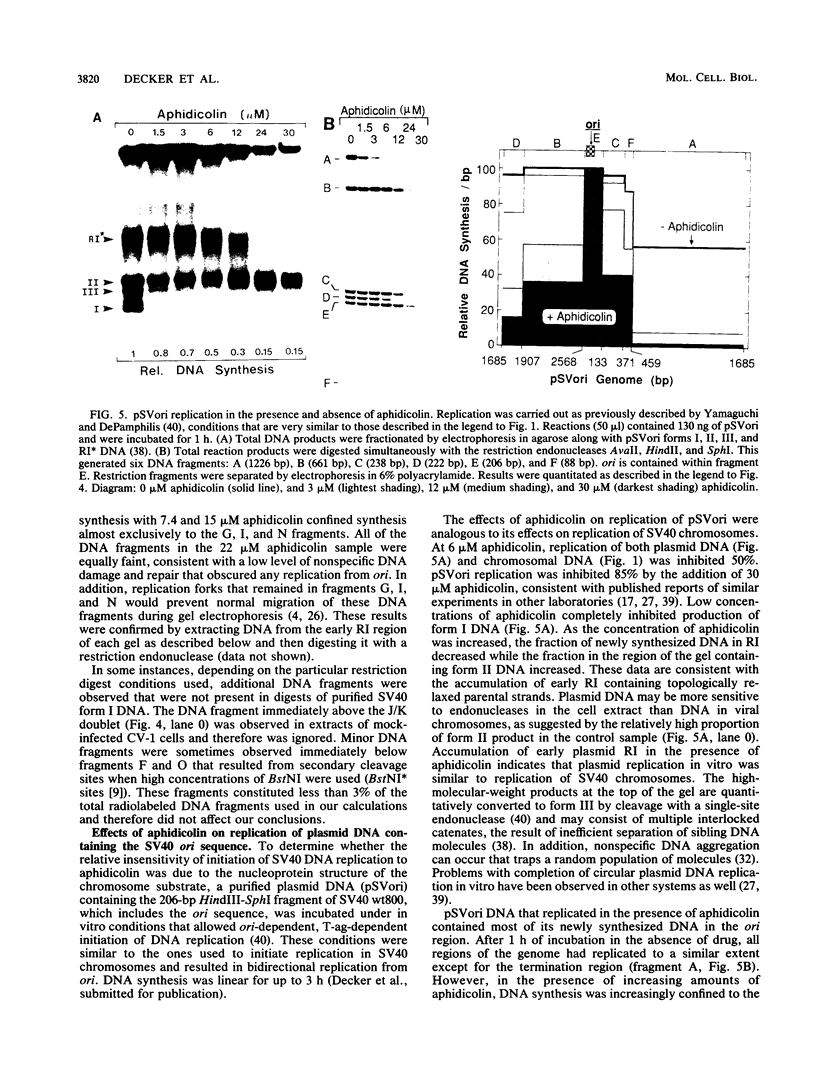
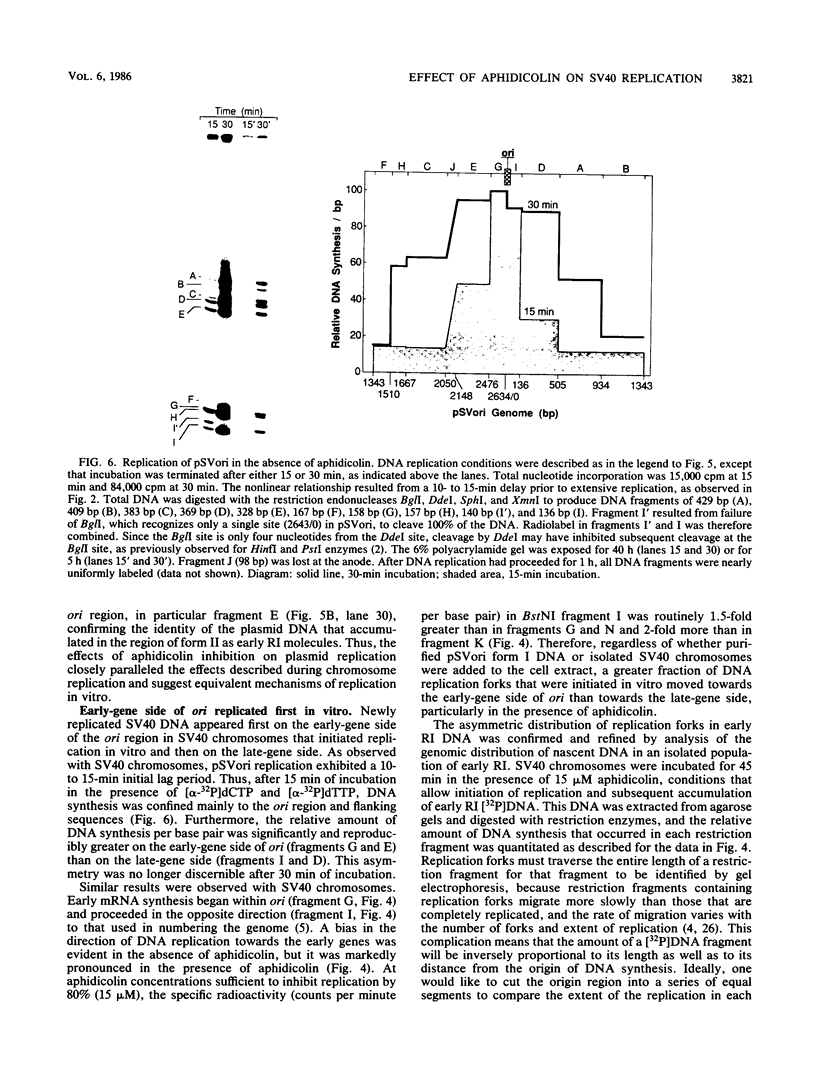
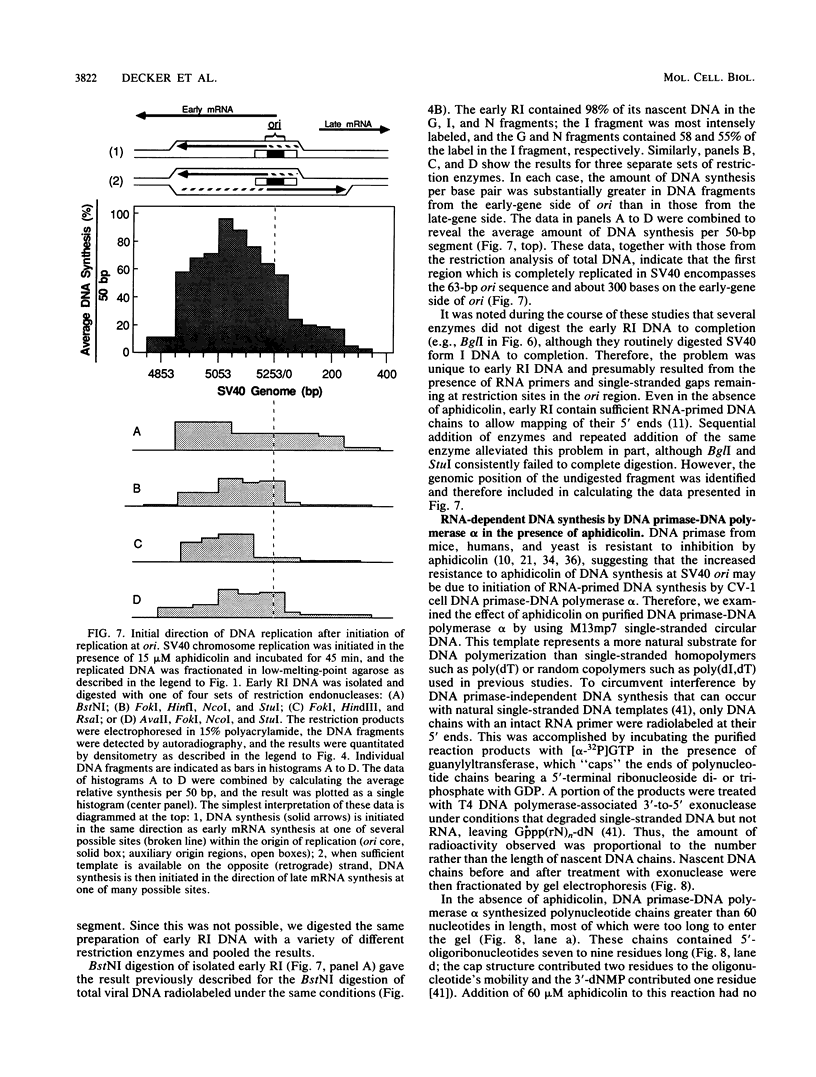
Aphidicolin, a specific inhibitor of DNA polymerase alpha, provided a novel method for distinguishing between initiation of DNA synthesis at the simian virus 40 (SV40) origin of replication (ori) and continuation of replication beyond ori. In the presence of sufficient aphidicolin to inhibit total DNA synthesis by 50%, initiation of DNA replication in SV40 chromosomes or ori-containing plasmids continued in vitro, whereas DNA synthesis in the bulk of SV40 replicative intermediate DNA (RI) that had initiated replication in vivo was rapidly inhibited. This resulted in accumulation of early RI in which most nascent DNA was localized within a 600- to 700-base-pair region centered at ori. Accumulation of early RI was observed only under conditions that permitted initiation of SV40 ori-dependent, T-antigen-dependent DNA replication and only when aphidicolin was added to the in vitro system. Increasing aphidicolin concentrations revealed that DNA synthesis in the ori region was not completely resistant to aphidicolin but simply less sensitive than DNA synthesis at forks that were farther away. Since DNA synthesized in the presence of aphidicolin was concentrated in the 300 base pairs on the early gene side of ori, we conclude that the initial direction of DNA synthesis was the same as that of early mRNA synthesis, consistent with the model proposed by Hay and DePamphilis (Cell 28:767-779, 1982). The data were also consistent with initiation of the first DNA chains in ori by CV-1 cell DNA primase-DNA polymerase alpha. Synthesis of pppA/G(pN)6-8(pdN)21-23 chains on a single-stranded DNA template by a purified preparation of this enzyme was completely resistant to aphidicolin, and further incorporation of deoxynucleotide monophosphates was inhibited. Therefore, in the presence of aphidicolin, this enzyme could initiate RNA-primed DNA synthesis at ori first in the early gene direction and then in the late gene direction, but could not continue DNA synthesis for an extended distance.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ariga H., Sugano S. Initiation of simian virus 40 DNA replication in vitro. J Virol. 1983 Nov;48(2):481–491. doi: 10.1128/jvi.48.2.481-491.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong K. A., Bauer W. R. Site-dependent cleavage of pBR322 DNA by restriction endonuclease HinfI. Nucleic Acids Res. 1983 Jun 25;11(12):4109–4126. doi: 10.1093/nar/11.12.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baril E., Mitchener J., Lee L., Baril B. Action of pancreatic DNase: requirements for activation of DNA as a template-primer for DNA polymerase. Nucleic Acids Res. 1977 Aug;4(8):2641–2653. doi: 10.1093/nar/4.8.2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler-White A. J., Pigiet V. Isolation and characterization of replication forks from discrete regions of the polyoma genome. J Virol. 1982 Nov;44(2):499–508. doi: 10.1128/jvi.44.2.499-508.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinter-Gottlieb G., Kaufmann G. Uncoupling of SV40 tsA replicon activation from DNA chain elongation by temperature shifts and aphidicolin arrest. Nucleic Acids Res. 1982 Jan 22;10(2):763–773. doi: 10.1093/nar/10.2.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R. A., Nathans D. Purification of simian virus 40 large T antigen by immunoaffinity chromatography. J Virol. 1985 Mar;53(3):1001–1004. doi: 10.1128/jvi.53.3.1001-1004.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edenberg H. J., Anderson S., DePamphilis M. L. Involvement of DNA polymerase alpha in simian virus 40 DNA replication. J Biol Chem. 1978 May 10;253(9):3273–3280. [PubMed] [Google Scholar]
- Fuchs R., Blakesley R. Guide to the use of type II restriction endonucleases. Methods Enzymol. 1983;100:3–38. doi: 10.1016/0076-6879(83)00043-9. [DOI] [PubMed] [Google Scholar]
- Gronostajski R. M., Field J., Hurwitz J. Purification of a primase activity associated with DNA polymerase alpha from HeLa cells. J Biol Chem. 1984 Aug 10;259(15):9479–9486. [PubMed] [Google Scholar]
- Hay R. T., DePamphilis M. L. Initiation of SV40 DNA replication in vivo: location and structure of 5' ends of DNA synthesized in the ori region. Cell. 1982 Apr;28(4):767–779. doi: 10.1016/0092-8674(82)90056-3. [DOI] [PubMed] [Google Scholar]
- Hay R. T., Hendrickson E. A., DePamphilis M. L. Sequence specificity for the initiation of RNA-primed simian virus 40 DNA synthesis in vivo. J Mol Biol. 1984 May 15;175(2):131–157. doi: 10.1016/0022-2836(84)90471-6. [DOI] [PubMed] [Google Scholar]
- Hu S. Z., Wang T. S., Korn D. DNA primase from KB cells. Evidence for a novel model of primase catalysis by a highly purified primase/polymerase-alpha complex. J Biol Chem. 1984 Feb 25;259(4):2602–2609. [PubMed] [Google Scholar]
- Krokan H., Schaffer P., DePamphilis M. L. Involvement of eucaryotic deoxyribonucleic acid polymerases alpha and gamma in the replication of cellular and viral deoxyribonucleic acid. Biochemistry. 1979 Oct 2;18(20):4431–4443. doi: 10.1021/bi00587a025. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laskey R. A. The use of intensifying screens or organic scintillators for visualizing radioactive molecules resolved by gel electrophoresis. Methods Enzymol. 1980;65(1):363–371. doi: 10.1016/s0076-6879(80)65047-2. [DOI] [PubMed] [Google Scholar]
- Li J. J., Kelly T. J. Simian virus 40 DNA replication in vitro. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6973–6977. doi: 10.1073/pnas.81.22.6973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oguro M., Suzuki-Hori C., Nagano H., Mano Y., Ikegami S. The mode of inhibitory action by aphidicolin on eukaryotic DNA polymerase alpha. Eur J Biochem. 1979 Jul;97(2):603–607. doi: 10.1111/j.1432-1033.1979.tb13149.x. [DOI] [PubMed] [Google Scholar]
- Plevani P., Foiani M., Valsasnini P., Badaracco G., Cheriathundam E., Chang L. M. Polypeptide structure of DNA primase from a yeast DNA polymerase-primase complex. J Biol Chem. 1985 Jun 10;260(11):7102–7107. [PubMed] [Google Scholar]
- Richter A., Scheu R., Otto B. Replication of simian virus 40 chromatin in vitro depends on the amount of DNA polymerase alpha associated with replicating chromatin. Eur J Biochem. 1980 Aug;109(1):67–73. doi: 10.1111/j.1432-1033.1980.tb04768.x. [DOI] [PubMed] [Google Scholar]
- Shuman S., Hurwitz J. Mechanism of mRNA capping by vaccinia virus guanylyltransferase: characterization of an enzyme--guanylate intermediate. Proc Natl Acad Sci U S A. 1981 Jan;78(1):187–191. doi: 10.1073/pnas.78.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simanis V., Lane D. P. An immunoaffinity purification procedure for SV40 large T antigen. Virology. 1985 Jul 15;144(1):88–100. doi: 10.1016/0042-6822(85)90308-3. [DOI] [PubMed] [Google Scholar]
- Spotila L. D., Huberman J. A. Method of mapping DNA replication origins. Mol Cell Biol. 1985 Jan;5(1):85–92. doi: 10.1128/mcb.5.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillman B. W., Gluzman Y. Replication and supercoiling of simian virus 40 DNA in cell extracts from human cells. Mol Cell Biol. 1985 Aug;5(8):2051–2060. doi: 10.1128/mcb.5.8.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su R. T., DePamphilis M. L. Simian virus 40 DNA replication in isolated replicating viral chromosomes. J Virol. 1978 Oct;28(1):53–65. doi: 10.1128/jvi.28.1.53-65.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundin O., Varshavsky A. Arrest of segregation leads to accumulation of highly intertwined catenated dimers: dissection of the final stages of SV40 DNA replication. Cell. 1981 Sep;25(3):659–669. doi: 10.1016/0092-8674(81)90173-2. [DOI] [PubMed] [Google Scholar]
- Tack L. C., DePamphilis M. L. Analysis of simian virus 40 chromosome-T-antigen complexes: T-antigen is preferentially associated with early replicating DNA intermediates. J Virol. 1983 Oct;48(1):281–295. doi: 10.1128/jvi.48.1.281-295.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapper D. P., Anderson S., DePamphilis M. L. Distribution of replicating simian virus 40 DNA in intact cells and its maturation in isolated nuclei. J Virol. 1982 Mar;41(3):877–892. doi: 10.1128/jvi.41.3.877-892.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapper D. P., DePamphilis M. L. Discontinuous DNA replication: accumulation of Simian virus 40 DNA at specific stages in its replication. J Mol Biol. 1978 Apr 15;120(3):401–422. doi: 10.1016/0022-2836(78)90427-8. [DOI] [PubMed] [Google Scholar]
- Tapper D. P., DePamphilis M. L. Preferred DNA sites are involved in the arrest and initiation of DNA synthesis during replication of SV40 DNA. Cell. 1980 Nov;22(1 Pt 1):97–108. doi: 10.1016/0092-8674(80)90158-0. [DOI] [PubMed] [Google Scholar]
- Tseng B. Y., Ahlem C. N. DNA primase activity from human lymphocytes. Synthesis of oligoribonucleotides that prime DNA synthesis. J Biol Chem. 1982 Jul 10;257(13):7280–7283. [PubMed] [Google Scholar]
- Wang T. S., Hu S. Z., Korn D. DNA primase from KB cells. Characterization of a primase activity tightly associated with immunoaffinity-purified DNA polymerase-alpha. J Biol Chem. 1984 Feb 10;259(3):1854–1865. [PubMed] [Google Scholar]
- Waqar M. A., Evans M. J., Burke J. F., Tsubota Y., Plummer M. J., Huberman J. A. In vitro DNA synthesis by an alpha-like DNA polymerase bound to replicating simian virus 40 chromosomes. J Virol. 1983 Oct;48(1):304–308. doi: 10.1128/jvi.48.1.304-308.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver D. T., Fields-Berry S. C., DePamphilis M. L. The termination region for SV40 DNA replication directs the mode of separation for the two sibling molecules. Cell. 1985 Jun;41(2):565–575. doi: 10.1016/s0092-8674(85)80029-5. [DOI] [PubMed] [Google Scholar]
- Wobbe C. R., Dean F., Weissbach L., Hurwitz J. In vitro replication of duplex circular DNA containing the simian virus 40 DNA origin site. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5710–5714. doi: 10.1073/pnas.82.17.5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M., DePamphilis M. L. DNA binding site for a factor(s) required to initiate simian virus 40 DNA replication. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1646–1650. doi: 10.1073/pnas.83.6.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M., Hendrickson E. A., DePamphilis M. L. DNA primase-DNA polymerase alpha from simian cells. Modulation of RNA primer synthesis by ribonucleoside triphosphates. J Biol Chem. 1985 May 25;260(10):6254–6263. [PubMed] [Google Scholar]
- Yamaguchi M., Hendrickson E. A., DePamphilis M. L. DNA primase-DNA polymerase alpha from simian cells: sequence specificity of initiation sites on simian virus 40 DNA. Mol Cell Biol. 1985 May;5(5):1170–1183. doi: 10.1128/mcb.5.5.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Vliet P. C., Kwant M. M. Role of DNA polymerase gamma in adenovirus DNA replication. Nature. 1978 Nov 30;276(5687):532–534. doi: 10.1038/276532a0. [DOI] [PubMed] [Google Scholar]