Abstract
Four species of Haemoproteidae were found in Pteropus alecto Temminck, 1837 in Queensland, Australia: i) Johnsprentia copemani, Landau et al., 2012; ii) Sprattiella alecto gen. nov., sp. nov., characterised by schizonts in the renal vessels; iii) Hepatocystis levinei, Landau et al., 1985, originally described from Pteropus poliocephalus Temminck, 1825 and, experimentally from Culicoides nubeculosus and found in this new host and for which features of the hepatic schizonts are reported; iv) gametocytes of Hepatocystis sp. which are illustrated but cannot be assigned to a known species. A tentative interpretation of phylogenetic characters of haemosporidians of bats is provided from the morphology of the gametocytes and localisation of the tissue stages with respect to recent data on the phylogeny of bats.
Keywords: Sprattiella gen. nov., alecto sp. nov., Haemoproteidae, Haemosporidia, Chiroptera
Abstract
Quatre espèces d’hémoproteidés parasitent les Pteropus alecto Temminck, 1837 du Queensland : i) Johnsprentia copemani, Landau et al., 2012 ; ii) Sprattiella alecto gen. nov., sp. nov., caractérisée par des schizontes siégeant dans la lumière des vaisseaux du rein ; iii) Hepatocystis levinei, Landau et al., 1985, décrite chez Pteropus poliocephalus et, expérimentalement, chez Culicoides nubeculosus ; l’espèce est retrouvée chez ce nouvel hôte et des compléments à la description des schizontes hépatiques sont apportés ; iv) Hepatocystis sp., dont les gamétocytes sont figurés mais n’ont pas pu être rattachés à une espèce particulière. Une tentative d’interprétation des caractères évolutifs des Hémosporidies de Chiroptères en fonction de la morphologie des gamétocytes, de la localisation des stades tissulaires et des données récentes sur la phylogénie des Chauve-souris, est ici présentée.
Keywords: Sprattiella gen. nov., alecto sp. nov., hémoprotéidés, hémosporidies, chiroptères
Introduction
Gametocytes of haemoproteids have frequently been reported in flying foxes (Pteropodidae). In the absence of information on the localization of their schizonts, they have been placed in the genus Hepatocystis. This genus was defined by (Garnahm, 1966) as having “megaloschizonts” in the liver and gametogony stages in the blood. In reality, the haemoproteids of bats are highly ivvariable with respect to such characters and their representatives in Pteropus alecto Temminck, 1837 from Queensland, provide an excellent example of this diversity. They are:
Hepatocystis levinei described by Landau et al. (1985) from Pteropus poliocephalus Temminck, 1825. The life cycle was completed using Culicoides nubeculosus under laboratory conditions (Landau et al., 1985). This species was found in four of 11 P. alecto examined from Townsville, Queensland, Australia, and we here provide additional data on the hepatic schizonts;
Johnsprentia copemani described by Landau et al. (2012) in P. alecto from Queensland. Co-infection with other haemoproteids was reported in the same paper;
Hepatocystis sp. represented by gametocytes in the blood of four of the 11 bats examined in the present study, but no schizonts were found which could be attributed to this species;
Sprattiella alecto gen. nov., sp. nov., herein described, gametocytes in the blood of six of 11 bats examined and characteristic schizonts in vessels of the kidney in one of the bats examined.
Recent advances of phylogeny studies of the Pteropodidae based on molecular methods prompted us to reevaluate the morphological features of haemoproteids of bats and to compare them with the molecular data currently available for the hosts.
Materials and Methods
The methods used were essentially the same as those used in the description of Johnsprentia copemani by Landau et al. (2012).
Eleven P. alecto, captured at Townsville using a mist net and exhibiting a parasitaemia with Haemoproteidae, were transported to Paris, to the Muséum National d’Histoire Naturelle, shortly after their capture, arriving on the 15.12.1978 and the 07.06.1979, respectively. Blood samples from each animal were collected by pricking the radial vein and the blood was smeared onto a slide, air dried, fixed with absolute methanol and stained using Giemsa stain (8 % in phosphate buffer, pH 7.4). The bats were examined over a period of several months. At autopsy, internal organs were fixed in Carnoy’s fluid and serial sections of each organ were stained by the giemsa-colophonium method (Bray & Garnham, 1962; Garnahm, 1966) and examined for tissue stages of the parasites. Type material has been deposited in the Muséum National d’Histoire Naturelle (MNHN), Paris.
Bat number 850-932PX in which we found renal schizonts was autopsied on the 03-01-1979, after 19 days in captivity.
Systematics
Phylum: Apicomplexa (Sporozoa).
Class: Coccidea.
Order: Haemosporida.
Family: Haemoproteidae.
Genus: Sprattiella gen. nov.
Etymology: named after Dr David Spratt.
Definition: Haemoproteidae of Pteropodidae with relatively small elongated schizonts, not producing colloid, inside a hypertrophied bi-nucleated host cell, free in the large vessels of the kidneys; gametocytes in erythrocytes.
Type species: Sprattiella alecto sp. nov.
Etymology: named after the host specific name.
Descriptions
Sprattiella alecto sp. nov. (figs 1-8, 11–24)
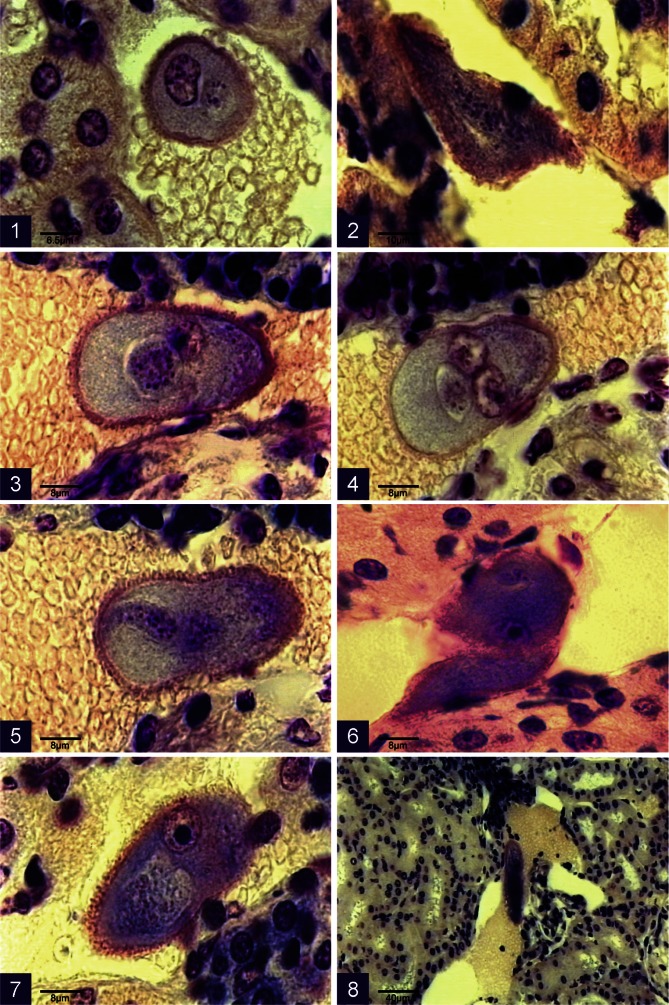
Figs 1–8.
Microphotographs of schizonts of Sprattiella alecto in the blood vessels of kidney sections from Pteropus alecto
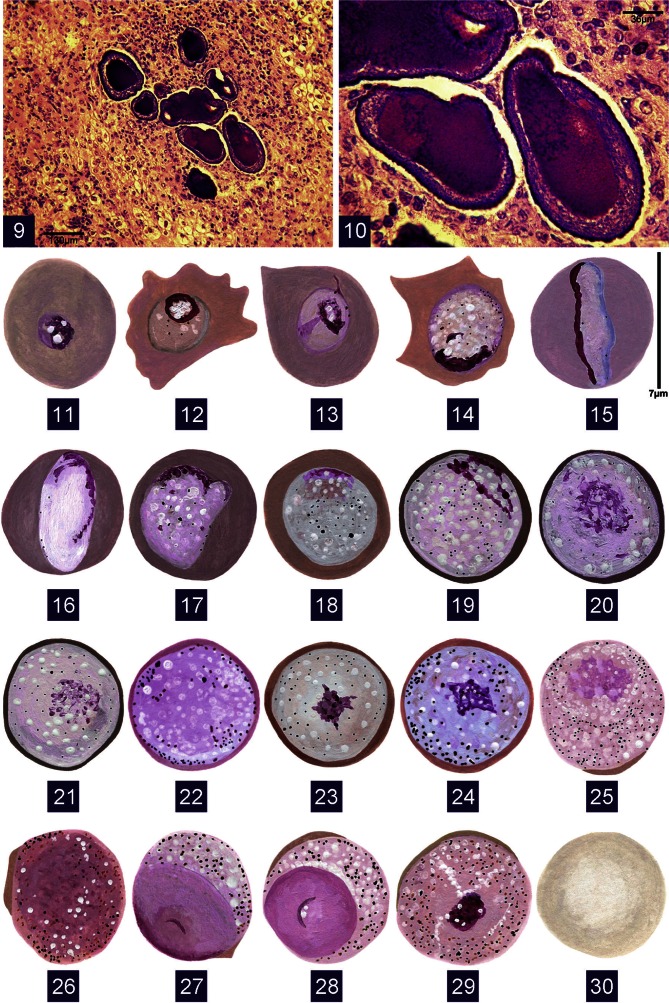
Figs 9–10 & 11–30.
Fig. 9: microphotograh of schizonts of Hepatocystis levinei in liver sections from Pteropus alecto; Fig. 10: detail of Fig. 9; Figs 11-30: giemsa stain; Figs 11-24: drawings of gametocytes of Sprattiella alecto; Fig. 11: very young trophozoite; Figs 12-18: young trophozoites; Figs 18-21: immature microgametocytes; Fig. 22: mature microgametocyte; Figs 23-24: macrogametocytes; Figs 25-28: microgametocytes of Hepatocystis sp.; Fig. 29: Microgametocyte “en cocarde” of Hepatocystis levinei; Fig. 30: uninfected RBC
Holotype material: schizont in section of kidney from P. alecto (850-932PX), MNHN P2 XXXI 10 (Fig. 7). Paratypes: blood smears and sections of schizonts in kidney, deposited in MNHN.
• Renal schizonts
Found in the lumen of renal veins, inside hypertrophied cells invariably with two nuclei (Fig. 4); nuclei also hypertrophied and modified. Host cell nuclei ellipsoidal, up to 10.5 μm with chromatin concentrated around the periphery and a large nucleolus. Host cell surrounded by fibrous, bright pink substance, up to 2 μm in thickness.
Parasitized cells free in vessels in most sections (Fig. 1), but in some serial sections, apparently attached to the walls of veins (Fig. 2) or wedged by a shrinkage of vessels (Figs 5, 6); round or oval depending on the angle of the section, sometimes deformed when trapped in shrunken vessel (Fig. 6).
The smallest schizont observed was in an enlarged, binucleate rounded cell measuring 16.5 μm, with a dense basophilic cytoplasm. The cell was surrounded by an irregular, pink, tufted layer. The schizont, by contrast, was small, 4 μm in diameter, containing a dozen small nuclei with little, pale cytoplasm. The schizont develops as cordons, at first straight with small well-spaced nuclei (Figs 2, 5), then reflected upon themselves (Figs 7, 8). The enlarged host cell (Fig. 3) is pyriform with a uniformly granular, grey cytoplasm, containing distinctive nuclei: oval 2-2.5 μm long with a clear centre and a thin membrane with masses of chromatin attached to its inner surface. These nuclei subsequently fragment and the oldest schizont observed (Fig. 8) measured 83 × 21 μm with elongated masses within the host cell with a basophilic cytoplasm and numerous nuclei.
• Gametocytes (Figs 11-24)
In the serial blood films examined, gametocytes were more numerous in the middle and terminal thirds of the films than in other regions. They lodge in red blood cells (RBC) and from the young trophozoite stage undergo significant transformation. The RBC is generally rounded but may become deformed with young parasites either irregularly (Fig. 12) or pyriform (Fig. 13). The parasite is either a clear reddish-brown, or more commonly darker, reddish or brick-red. The gametocytes increase in size slightly (7 μm); the intact host cell is still visible surrounding the gametocyte which against the dark background appears clear, measuring between 6 and 7 μm.
The microgametocytes (Figs 19-22) are pale pink or pink with several vacuoles and a more or less elongated zone of chromatin forming small, loosely attached masses. The macrogametocyte (Figs 23, 24) is pale blue to almost white against the darkly staining red blood cell background; its nucleus is smaller, and more dense than that of the microgametocyte. Pigment in both micro- and macrogametocytes is fine, scattered and more abundant towards the periphery. The microgametocytes of Sprattiella belong within the vivax group (vide infra).
• Relationship between the gametocytes of Sprattiella and renal schizonts
In multiple infections it is often difficult to associate gametocytes with their corresponding schizonts. In the blood of bat 850-932PX, we observed the gametocytes of four species of Haemoproteidae. We already know the gametocytes of three species: Johnsprentia copemani, Hepatocystis levinei and Hepatocystis sp. It is therefore logical to associate the renal schizonts with the distinctive gametocytes described here. Consequently, the holotype of Sprattiella alecto has been designated as the renal schizont and the name is therefore attached to this stage.
• Differential diagnosis
The gametocytes of Sprattiella differ from those of the other three known species present in P. alecto due to their striking effect on the host cell and the following morphological features: the nucleus of the microgametocyte is not peripheral in contrast to the other species; in addition, the nucleus of Sprattiella is diffuse in contrast to that of Johnsprentia which is in the form of a crescent and that of H. levinei which has a condensed center surrounded by a clear space.
Sprattiella alecto is also compared with the other two haemoproteids in which the schizont and its hypertrophied host cell are found in the lumen of medium to large blood vessels.
i) The schizonts of Dionisia bunoi are found in the blood vessels of the liver of the microchiropteran Hipposideros cyclops (Landau, Chabaud, et al., 1980b). They exhibit several characters in common with the present parasite: an intravascular localisation, host cell and nucleus hypertrophied and the presence of a hirsute pink substance around the periphery. However, they are exclusively hepatic and not renal. The schizont of Dionisia is anchored by prolongations from the host cell to the hepatic capillaries of the surrounding tissue while that of Sprattiella is free. The schizont of Dionisia forms a solid rounded mass while that of Sprattiella consists of elongated cords. The unique host nucleus of Dionisia becomes very large and highly modified while those of Sprattiella only undergo a moderate increase in volume. Finally and of most significance, the microgametocytes of Dionisia are of the “falciparum” type and the microgametocytes of the “malariae” type; those from Pteropus – host are of the “vivax” type. ii) The schizonts of Polychromophilus are found in the lumen of vessel, primarily in the lung, but also in other organs (liver, kidney, adrenals) (Landau, Rosin, et al., 1980a); the host cell is only visible in very young schizonts and is reduced in older forms to a slightly hypertrophied nucleus and a peripheral band of homogeneous, bright red ‘colloid’, different to the tufted band in Sprattiella. The gametocytes of Polychromophilus are of the “malariae” type.
Hepatocystis levinei
• Additions to the description of the hepatic schizonts Schizonts and gametocytes belonging to H. levinei were found in the blood and in liver sections of several P. alecto, having first been reported from Pteropus poliocephalus by Landau et al. (1985). We had not, at the time, observed all of the hepatic schizogonic stages, in particular the intermediate stage between the young, stellate schizont lacking colloid and the mature schizont. Here, we illustrate (Fig. 29) a characteristic microgametocyte with the condensed center and a mature schizont in a section through several lobes, each lobe comprising a peripheral zone with numerous small nuclei and a central zone filled with purple colloid. In the section in which the parasite is largest, the parasite and the surrounding zone of inflammation measure 1,700 × 2,700 μm; the parasite itself measures 630 × 370 μm.
Hepatocystis sp.
The microgametocytes of this species are of the “vivax” type with a diffuse nucleus (Figs 25-28). Macrogametocytes could not be distinguished from those of other species.
Discussion
The different types of haemosporidian gametocytes in mammals
The morphology of haemosporidian gametocytes in mammals is generally considered to be of limited taxonomic interest. By contrast, we consider that because of their stability and their archaic nature (they represent a fundamental primitive stage in the cycle of all coccidiomorphs), they are probably of considerable importance from a phyletic and systematic perspective.
Schizogony, be it haematogenous or in tissues, is a superimposed character with numerous morphological variants including convergences between distant species. Landau et al. (1976a), based on the morphology of the gametocytes of mammalian haemosporidians, divided them into three types (Table I) which were designated by the name of the best known species in each group (“falciparum”, considered the most archaic, then “malariae” and finally “vivax”).
Table I.
Morphological characteristics of microgametocytes of mammalian haemosporidians (Landau et al., 1976, modified).
| Type of microgametocyte |
|||
|---|---|---|---|
| Morphological characteristic | falciparum | malariae | vivax |
| Filling completely or almost the host RBC | no | no | yes |
| Form | ellipsoidal or elongate | rounded | rounded |
| Eccentric nucleus | no | no | yes |
| Distinct limit between nucleus and cytoplasm | no | yes | yes |
| Accessory chromatin dot | frequent | frequent | absent |
| Distinct border between nucleus and pigmented zone | no | no | yes |
| Pigment granules | few and coarse | Few and coarse | fine and abundant |
| Distribution of pigment | irregular, often grouped | irregular, often grouped | dispersed |
The classification is based on the morphology of the microgametocytes which are the most characteristic and most readily recognizable features of Plasmodium.
The principal morphological characters of the three types are summarised in Table I and their distribution in different types of bats is presented in Table II. In addition, a dichotomous key for the identification of the genera of haemoproteids in bats is presented in Table III.
Table II.
Haemosporidia of Chiroptera.
| Host | Genus | Species | Authors | Gametocyte type | Schizont location | |
|---|---|---|---|---|---|---|
| Nycteridae | Nycteris arge | Nycteria | erardi | Rosin , Landau et al., 1978 | M | HEP |
| Nycteris capensis | Nycteria | medusiformis | Garnahm & Heisch, 1953 | M | HEP | |
| Nycteris nana | Nycteria | houini | Rosin , Landau et al., 1978 | M | HEP | |
| Nycteris thebaica | Nycteria | medusiformis | Garnahm & Heisch, 1953 | M | HEP | |
| Vespertilionidae | Eptesicus fuscus | Bioccala | deanei | (Garnahm Lainson et al., 1971,)** | F | RES |
| Vespertilio murinus | Bioccala | murinus | (Dionisi, 1899) | F | RES | |
| Miniopteridae | Miniopterus minor | Polychromophilus | adami | Landau, Rosin, et al., 1980a | M | RES |
| Miniopterus minor | Bioccala | murinus | (Dionisi, 1899) | F | RES | |
| Miniopterus schreibersi | Polychromophilus | melaniferus | (Dionisi, 1899) | M | RES | |
| Miniopterus schreibersi | Polychromophilus | corradettii | Landau, Rosin, et al., 1980a | M | RES | |
| Miniopterus schreibersi | Bioccala | murinus | (Dionisi, 1899) | F | RES | |
| Myotis myotis | Bioccala | murinus | (Dionisi, 1899) | F | RES | |
| Myotis natterei | Bioccala | murinus | (Dionisi, 1899) | F | RES | |
| Myotis nigricans | Bioccala | deanei | (Garnahm, Lainson et al., 1971) ** | F | RES | |
| Pteropodidae | Cynopterus sphinx | Hepatocystis | garnhami | Landau, 1981 | V | HEP |
| Dobsonia moluccensis | Hepatocystis | pteropi manwelli | Garnahm, 1966 | V | HEP | |
| Epomops franqueti | Hepatocystis | brosseti | Miltgen, Landau et al., 1977 | V | HEP | |
| Epomops franqueti | Hepatocystis | epomophori | (Rodhain, 1926) | V | HEP | |
| Hypsignatus monstrosus | Hepatocystis | carpenteri | Miltgen, Landau et al., 1980 | V | HEP | |
| Lyssonycteris smithi | Plasmodium | voltaicum | Van der Kaay, 1964 | V | HEP | |
| Myonycteris torquata | Hepatocystis | perronae | Landau and Adam, 1971 | V | HEP | |
| Pteropus alecto | Johnsprentia | copemani | Landau, Chavatte et al., 2012 | V | RES | |
| Pteropus alecto | Sprattiella | alecto | Landau, Chavatte et al., 2012 | V | RES | |
| Pteropus alecto | Hepatocystis | levinei | Landau, Humpherey-Smith et al., 1985 | V | HEP | |
| Pteropus gouldii | Hepatocystis | pteropi | (Breinl, 1913) | V | HEP | |
| Pteropus poliocephalus | Hepatocystis | levinei | Landau, Humpherey-Smith et al., 1985 | V | HEP | |
| Roussetus leachi | Plasmodium | rousseti | Van Riel, Herniaux-L’Hoëst et al., 1951 | V | HEP | |
| Hipposideridae | Hipposideros armiger | Hepatocystis | sp. | Manwell & Kuntz, 1966 | V | HEP |
| Hipposideros bicolor | Bioccala | sp. | Eyles, Dunn et al., 1962 | F | RES* | |
| Hipposideros cyclops | Dionisia | bunoi | Landau, Chabaud et al., 1980b | ♀F ♂V | RES* | |
| Hipposideros cyclops | Plasmodium | cyclopsi | Landau & Chabaud, 1978 | M | HEP | |
| Hipposideros galeritus | Hepatocystis | bainae | Mialhe & Landau, 1977 | V | HEP | |
| Hipposideros galeritus | Hepatocystis | rodhaini | Landau, Miltgen et al., 1976b | V | HEP | |
| Hipposideros larvatus | Biguetiella | minuta | Landau, Baccam et al., 1984 | M | HEP | |
| Hipposideros larvatus | Hepatocystis | sp. | Duval, Robert et al., 2007 | V | HEP* | |
| Hipposideros larvatus | Nycteria | brucechwatti | Landau, Baccam et al., 1984 | M | RES | |
| Rhinolophidae | Rhinolophus hildebrandti | Nycteria | congolensis | (Krampitz & Anciaux de Faveaux, 1960) | M | HEP |
| Rhinolophus sylvestris | Nycteria | gabonensis | Rosin, Landau et al., 1978 | M | HEP | |
| Rhinolophus sp. | Nycteria | krampitzi | Rosin, Landau et al., 1978 | M | HEP | |
Type of gametocytes: M = “malariae”, F = “falciparum” and V = “vivax”; site of schizogony: HEP = hepatocyte and RES = reticulo-endothelial system.
indicate the potential location of schizogony which is unknown;
redescribed from Eptesicus fuscus by Marinkelle (1995) who change the genus name Polychromophilus by Bioccala. The reference of the authors of each taxa are listed in the references.
Table III.
Key for the identification of the Haemoproteidae of Chiroptera.
| Characteristic | Haemoproteidae | ||
|---|---|---|---|
| 1–(4) | Elongated or ellipsoid gametocytes type F | ||
| 2–(3) | RES: small schizonts spread through the organism ...................................................................................... (Miniopteridae, Vespertilionidae) |
Bioccala | |
| 3–(2) | HEP: small schizonts in the liver .................................................................................................................... (Hipposiderosidae) |
Biguetiella | |
| 4–(1) | Roundish gametocytes | ||
| 5–(10) | Gametocytes type M | ||
| 6–(9) | RES | ||
| 7–(8) | RES: large schizonts spread in different organs ............................................................................................ (Miniopteridae, Vespertilionidae) |
Polychromophilus | |
| 8–(7) | RES: small schizonts, in the vessels of the liver ............................................................................................ (Hipposideros cyclops) |
Dionisia | |
| 9–(6) | HEP: large schizonts in the hepatocytes ........................................................................................................ (Nycteridae, Rhinolophidae) |
Nycteria | |
| 10–(5) | Gametocytes type V | ||
| 11–(14) | RES | ||
| 12–(13) | RES: large schizonts in the lungs ................................................................................................................... (Pteropodidae) |
Johnsprentia | |
| 13–(12) | RES: small schizonts, in the vessels of the kidney ......................................................................................... (Pteropodidae) |
Sprattiella | |
| 14–(11) | HEP: large schizonts (megaloschizonts) in the liver ....................................................................................... (Pteropodidae, Rhinolophidae) |
Hepatocystis | |
M = “malariae”, F = “falciparum” and V = “vivax”. HEP = hepatocyte, and RES = reticulo-endothelial system cells.
Affinities with the haemospiridians of sauropsids
(Garnahm, 1966), in his treatise of the Haemosporidia, separated them into two distinct lineages, those which multiplied in the reticulo-endothelial system including the haemosporidians of birds and reptiles, and in mammals Bioccala, designated at the time as a species of Polychromophilus; the others multiplying in hepatocytes, comprising Nycteria and Hepatocystis. Development in the reticulo-endothelial system is close to the primitive model seen in reptiles and birds and the evolution of schizonts in hepatocytes, is in our view a specialisation.
The gametocytes of the “malariae” and “falciparum” groups have morphological characteristics in common with parasites of Sauropsidae and are therefore, according to Landau et al. (1976a), more archaic than those of the “vivax” group. Thus, Bioccala, a parasite of vepertilionids and miniopterids is, for us, the genus closest to the primitive species. Its gametocyte is ellipsoidal, more or less elongate, does not fill the erythrocyte, the pigment consists of large, irregularly grouped granules, the nucleus is poorly defined, and an accessory chromatin granule is present; the schizonts are small, invasive and are found in the reticuloendothelial system.
The “malariae” group is represented by two different genera: Polychromophilus in Vespertilionidae and Miniopteridae, and Nycteria in Nycteris and Rhinolophus. The archaic characters of the gametocytes are the fact that the erythrocyte remains partially visible when the gametocyte is mature, the presence of an accessory chromatin granule and the irregularly grouped, large chromatin granules. However, in contrast to Bioccala, the nucleus is well delimited and is rounded. The two genera also differ in their schizonts which are relatively large and non-invasive, in the reticulo-endothelial system for Polychromophilus and in hepatocytes for Nycteris.
The gametocytes of the “vivax” group are homogeneous morphologically and differ from the preceding forms. At maturity, they fill or nearly fill the erythrocyte, lack an accessory chromatin granule, have fine, abundant pigment, dispersed in mature forms, and a distinct boundary between nucleus and cytoplasm. Two species are reticulo-endothelial parasites (Johnsprentia copemani and Sprattiella alecto), but most of the species known from the Pteropodidae have megaloschizonts, which develop in hepatocytes (Hepatocystis)
The chromatin in the nucleus of some Hepatocystis species instead of being diffuse is aggregated in the center of a sometimes large clear zone (nucleus “en cocarde”). Otherwise they conform to the other species of the group.
Molecular data and host spectrum
Molecular data from the different groups of animal Haemosporidia are fragmentary and sometimes contradictory for several reasons: polyparasitism of the natural hosts, imprecise identifications... However, some recent molecular data on bat Haemosporidia (Megali et al., 2011) appear consistent with the morphology and the host spectrum: Polychromophilus (= P. melanipherus) and Bioccalla (= P. murinus) form two distinct but close taxonomic groups associated with the Vespertilionidae. The Hepatocystis of Peropodidae, in this same work, appear as a sister group of both the two previous groups.
Teeling et al. (2005) and Simmons (2005) have proposed, using molecular techniques, a phylogenetic tree and dates for the appearance of the different genera of bats. The hosts of haemosporidians of bats belong to five families:
The Nycteridae harbour parasites of the “malariae” group. They separated from other Emballonuroidea 58-47 mya.
The Miniopteridae and Vespertilionidae harbour a pair of cosmopolitan parasite genera, Bioccala and Polychromophilus, often in the same individual, which belong respectively to the “falciparum” and “malariae” groups. These two families are also ancient and separated according to (Miller-Butterworth et al. 2007) 48-39 mya.
The Rhinolophidae, where Rhinolophus harbours species of the “malariae” group and Hipposideros harbours species of three groups, “vivax”, “malariae” and “falciparum”. They diversified more recently, 39 mya.
The Pteropodidae, which harbour only species of the “vivax” group are even more recent and diverged from the preceding families 24 mya.
Conclusion
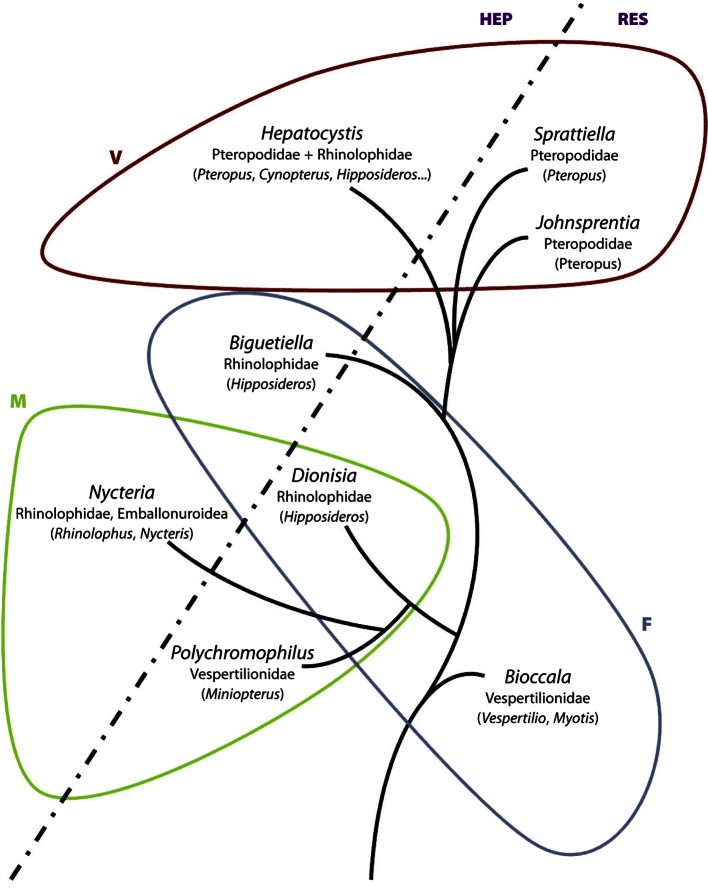
In conclusion, these results appear to be compatible with our hypotheses: the microchiropterans which are, for the most part, older than the megachiropterans, harbour with a few exceptions (in Hipposideros) parasites of the “falciparum” and “malariae” types, which we consider to be the most archaic, while megachiropterans harbour parasites of the “vivax” group. A hypothetical line of ascent of haemoproteid parasites of bats synthesising these results is provided in Fig. 31.
Fig. 31.
Hypothetical line of ascent of haemoproteid parasites of bats. The oblique doted line separates species with schizonts in the reticuloendothelial system (RES) on the right and the species with schizonts in hepatocytes (HEP) on the left. The green line (low left) encircles parasites with gametocytes of the “malariae” type, the blue line (low right) gametocytes of the “falciparum” type and the red line (top) gametocytes of the “vivax” type
References
- Breinl A.Report of the year 1911. Australian Institute of Tropical Medicine, Townsville, 1911 [Google Scholar]
- Dionisi A.La malaria di alcune specie di pipistrelli. Atti della Società per gli Studi della Malaria, 1899, 1, 133–175 [Google Scholar]
- Duval L., Robert V., Csorba G., Hassanin A., Randrianarivelojosia M., Walston J., Nhim T., Goodman S.M. & Ariey F.Multiple host-switching of Haemosporidia parasites in bats. Malaria Journal, 2007, 6, 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyles D.E., Dunn F.L. & Liat L.B.Blood parasites of Malayan bats. Medical Journal of Malaya, 1962, 17, 87–88 [Google Scholar]
- Garnahm P.C.C.Malaria parasites and other Haemosporidia. Blackwell, Oxford, 1966, 1114 p. [Google Scholar]
- Garnahm P.C.C. & Heisch R.B.On a new blood parasite of insectivorous bats. Transaction of the Royal Society of Tropical Medicine and Hygiene, 1953, 47, 357–363 [DOI] [PubMed] [Google Scholar]
- Garnahm P.C.C., Lainson R. & Shaw J.J.A contribution to the study of the haematozoon parasites of the bats. A new mammalian haemoproteid, Polychromophilus deanei n. sp. Memórias do Instituto Oswaldo Cruz, 1971, 69, 119–125 [DOI] [PubMed] [Google Scholar]
- Krampitz H.E. & Anciaux De Faveaux F.M.Über einiger Haemosporidien aus Fledermaüsen der Höhlen des Berglandes Katanga. Zeitschrift für Tropenmedizin und Parasitologie, 1960, 11, 391–400 [PubMed] [Google Scholar]
- Landau I. & Adam J.P.Description de schizontes de rechute chez un nouvel Haemoproteidae, Hepatocystis perronae n. sp., parasite de Megachiroptères africains. Cahier ORSTOM., Série Entomologie Médicale et Parasitologie, 1971, 9, 373–378 [Google Scholar]
- Landau I., Miltgen F. & Chabaud A.G.Les différents types de gamétocytes chez les hémosporidies de mammifères. Corrélations avec la morphologie des schizontes tissulaires. Hypothèses sur l’évolution du groupe. Annales de Parasitologie Humaine et Comparée, 1976a, 51, 175–187 [PubMed] [Google Scholar]
- Landau I., Miltgen F., Le Bail O. & Yap L.F.Hepatocystis de Malaisie IV. Description d’Hepatocystis rodhaini n. sp. parasite de Microchiroptères. Annales de Parasitologie Humaine et Comparée, 1976b, 51, 303–307 [PubMed] [Google Scholar]
- Landau I. & Chabaud A.G.Description de Plasmodium cyclopsi n. sp. parasite du microchiroptère Hipposideros cyclops à Makokou (Gabon), Annales de Parasitologie Humaine et Comparée, 1978, 53, 247–253 [DOI] [PubMed] [Google Scholar]
- Landau I., Rosin G., Miltgen F., Hugot J.P., Leger N., Beveridge I. & Baccam D.Sur le genre Polychromophilus (Haemoproteidae, parasite de microchiroptères). Annales de Parasitologie Humaine et Comparée, 1980a, 55, 13–32 [Google Scholar]
- Landau I., Chabaud A.G., Miltgen F. & Baccam D.Dionisia bunoi n. gen., n. sp. Haemoproteidae parasite du microchiroptère Hipposideros cyclops au Gabon. Annales de Parasitologie Humaine et Comparée, 1980b, 55, 271–280 [DOI] [PubMed] [Google Scholar]
- Landau I.Description d’Hepatocystis garnhami n. sp., parasite du chiroptère Cynopterus sphinx (Vahl) en Thaïlande, in: Parasitological topics. A presentation volume to P.C.C. Garnham, F.R.S., on the occasion of his 80th Birthday, 1981. Canning E.U. (ed.), Society of Protozoologists, Allen Press, Inc., Lawrence, Kansas, 1981. Special Publication No.1, 166–169 [Google Scholar]
- Landau I., Baccam D., Ratanaworabhan N., Yenbutra S., Boulard Y. & Chabaud A.G.Nouveaux Haemoproteidae parasites de Chiroptères en Thaïlande. Annales de Parasitologie Humaine et Comparée, 1984, 59, 437–447 [PubMed] [Google Scholar]
- Landau I., Humpherey-Smith I., Chabaud A.G., Miltgen F., Copeman B. & Boulard Y.Description et transmission expérimentale de l’haemoproteidé Hepatocystis levinei n. sp. Annales de Parasitologie Humaine et Comparée, 1985, 60, 373–382 [Google Scholar]
- Landau I., Chavatte J.M. & Beveridge I.Johnsprentia copemani gen. nov., sp. nov. (Haemoproteidae), a parasite of the flying-fox, Pteropus alecto (Pteropodidae), from Queensland. Memoirs of the Queensland Museum, 2012, 56, 61–66 [Google Scholar]
- Manwell R. & Kuntz R.E.Hepatocystis of Formosan Mammals with description of a new species. Journal of Protozoology, 1966, 13, 670–672 [Google Scholar]
- Marinkelle C.J.The haemoproteid parasite, Bioccala deanei, from a Colombian bat, Eptesicus fuscus (Vespertilionidae). Annals of Tropical Medicine and Parasitology, 1995, 89, 89–91 [DOI] [PubMed] [Google Scholar]
- Megali A., Yannic G. & Christe P.Disease in the dark: molecular characterization of Polychromophilus murinus in temperate zone bats revealed a worldwide distribution of this malaria-like disease. Molecular Ecology, 2011, 20, 1039–1048 [DOI] [PubMed] [Google Scholar]
- Mialhe E. & Landau I.Description d’Hepatocystis bainae n. sp. (Haemoproteidae) parasite d’Hipposideros galeritus, Microchiroptère de Malaisie. Annales de Parasitologie Humaine et Comparée, 1977, 52, 385–390 [PubMed] [Google Scholar]
- Miller-Butterwoth C.M., Murphy W.J., O’Brien S.J., Jacobs D.S., Springer M.S. & Teeling E.C.A family matter: conclusive resolution of the taxonomic position of the long-fingered bats, Miniopterus. Molecular Biology and Evolution, 2007, 24, 1553–1561 [DOI] [PubMed] [Google Scholar]
- Miltgen F., Landau I., Rosin G. & Erard C.Hepatocystis brosseti n. sp., Haemoproteidae parasite d’Epomops franqueti, Pteropinae, au Gabon. Annales de Parasitologie Humaine et Comparée, 1977, 52, 589–596 [DOI] [PubMed] [Google Scholar]
- Miltgen F., Landau I. & Bradbury J.Hepatocystis d’Hypsignathus monstrosus (Pteropinae) au Gabon. Description d’Hepatocystis carpenteri n. sp. Annales de Parasitologie Humaine et Comparée, 1980, 485, 55–490 [PubMed] [Google Scholar]
- Rodhain J.Plasmodium epomophori n. sp., parasite commun des roussettes épaulières au Congo Belge. Bulletin de la Société de Pathologie Exotique, 1926, 8, 726–729 [Google Scholar]
- Rosin G., Landau I. & Hugot J.P.Considérations sur le genre Nycteria (Haemoproteidae) parasite de Microchiroptères africains avec description de 4 espèces nouvelles. Annales de Parasitologie Humaine et Comparée, 1978, 53, 447–459 [PubMed] [Google Scholar]
- Simmons N.B., An Eocene big bang for bats, Science, 2005, 307, 527–528 [DOI] [PubMed] [Google Scholar]
- Teeling E.C., Spinger M.S., Madsen O., Bates P., O’brien S.J. & Murphy W.J.A molecular phylogeny for bats illuminates biogeography and the fossil record. Science, 2005, 307, 580–584 [DOI] [PubMed] [Google Scholar]
- Van der Kaay H.J.Description of a new Plasmodium: Plasmodium voltaicum sp. nov. found in a fruit bat, Roussetus smithi in Ghana. Annals of Tropical Medicine and Parasitology, 1964, 58, 261–264 [DOI] [PubMed] [Google Scholar]
- Van Riel J., Herniaux-L’hoest D. & Herniaux-L’hoest J.Description of a Plasmodium found in bat, Roussetus leachi. Parasitology, 1951, 41, 270–273 [DOI] [PubMed] [Google Scholar]