Abstract
Background
Statins are the most commonly prescribed and effective medications for reducing low-density lipoprotein levels. Some patients experience myopathic symptoms during statin treatment. The etiology is not known, but depletion of mevalonate pathway metabolites, including coenzyme Q10 (CoQ10), has been suggested. CoQ10 supplementation has been recommended to patients who experience myalgic symptoms despite a lack of conclusive evidence supporting its utility.
Objective
The Co-Enzyme Q10 in Statin Myopathy study is designed to examine the effect of CoQ10 supplementation on the extent and intensity of muscle pain during treatment with simvastatin.
Methods
We will recruit patients with a documented history of myalgia during statin treatment. The presence of statin-related myalgia will be confirmed in a crossover run-in trial during which presence and absence of symptoms will be documented during statin and placebo treatment, respectively. Individuals with myalgic symptoms while on statin but not placebo will be randomized to receive simvastatin 20 mg daily plus either 600 mg daily of CoQ10 or placebo. Muscle pain intensity will be documented during weekly phone calls using the Brief Pain Inventory (Short Form) (BPI-SF). Treatment will continue for 8 weeks or until muscle symptoms are reported continuously for one week or become intolerable, and then subjects will crossover to the alternative treatment (CoQ10 or placebo).
Results
This study is an ongoing clinical trial.
Conclusions
This study will determine the utility of CoQ10 for reducing pain intensity in myalgic patients and will provide guidance for clinicians treating patients with hypercholesterolemia who are intolerant to statins.
Keywords: simvastatin, myalgia, ubiquinone, muscle pain, physical performance, strength
INTRODUCTION
Hydroxy-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors or statins are widely-prescribed to reduce low-density lipoprotein (LDL)-cholesterol concentrations and can reduce cardiac events by 20–35% (1). Recommendations by the National Cholesterol Education Program (NCEP) Adult Treatment Panel III advocate more aggressive management of LDL-cholesterol levels (< 70 mg/dL in high-risk patients), which means that statin medications will be prescribed more frequently and in higher doses (2). Statins are generally well-tolerated, but some patients complain of muscular symptoms, including myalgia, muscle cramps, weakness, and in rare circumstances life-threatening rhabdomyolysis (3). The reported incidence of myalgic symptoms with statin treatment ranges from 1% (3) to 25% (4) and muscular complaints may compromise patient compliance (3). How statins produce muscular side effects is not clear, but depletion of ubiquinone or coenzyme Q10 (CoQ10), which is also produced by the cholesterol metabolic pathway, has been suggested as the mechanism (5). Consequently, CoQ10 supplementation is used by many patients and prescribers of statins despite ambiguous study results. CoQ10 blood levels decrease during statin treatment, but CoQ10 is carried in the lower density lipoproteins so most investigators have attributed this decrease to decreases in low density and very low density lipoproteins (5). Muscle biopsy studies have not demonstrated consistent reductions in intramuscular CoQ10 during statin therapy (5), although intramuscular CoQ10 levels were reduced by approximately 30% in subjects with microscopic muscle damage during treatment with 80 mg of simvastatin (6) and intramuscular CoQ10 levels were reduced 2–4 standard deviations below normal in approximately 50% of patients with statin myopathy (Vladitu et al. unpublished work presented at the American College of Rheumatology 2004 Annual Meeting). Thus, intramuscular depletion of CoQ10 may be a viable explanation for statin myopathy.
CoQ10 supplementation and its effects on statin myalgia have not been extensively studied, and results from supplementation trials vary. Treatment with 100 mg/day of CoQ10 reduced pain severity and pain interference with daily activities during statin treatment in patients with a history of myalgia (7). In contrast, 200 mg/day of CoQ10 did not affect myalgia score, adherence to statin treatment, or the number of patients tolerating high-dose simvastatin therapy during upward dose titration of simvastatin from 10 to 40 mg/day (8). The research supporting CoQ10 supplementation during statin treatment is therefore ambiguous, but CoQ10 supplementation is used by many patients and prescribers of statins despite these ambiguous study results. The efficacy of CoQ10 supplementation needs to be verified so that patients with statin intolerance and clinicians can make appropriate recommendations on the potential benefit of this popular supplement.
The Co-Enzyme Q10 in Statin Myopathy study (NCT01140308) will examine the utility of CoQ10 supplementation by testing the hypothesis that supplementation will reduce the intensity of pain during statin treatment when compared to placebo in patients with a documented history of statin myalgia. The time to onset of myalgia, as well as effects on objective measures of physical performance will be assessed.
METHODS
Study Overview
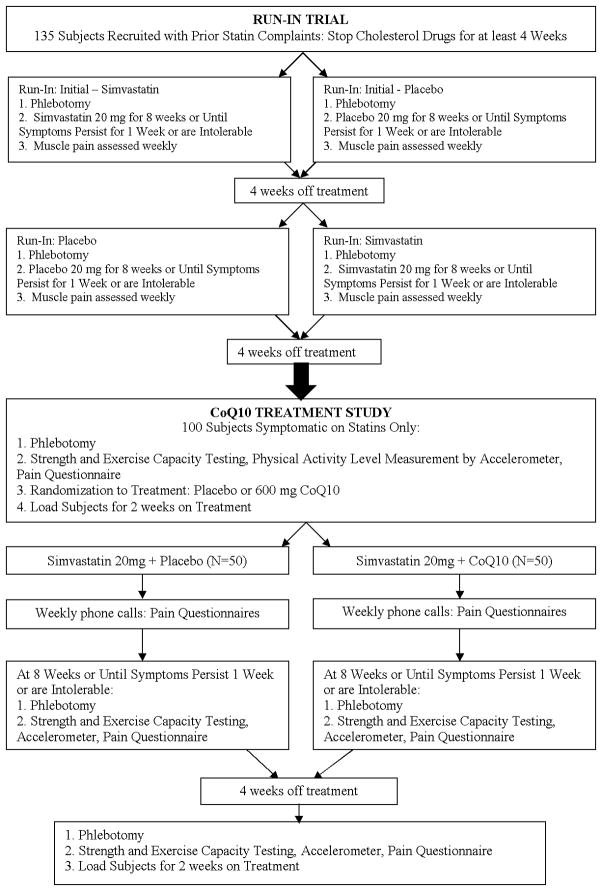
The study is designed to ensure that the CoQ10 section of the study treats only subjects with documented statin myalgia. Consequently, we will recruit patients with a history of myopathic complaints during statin treatment from the Cholesterol Management Center at Hartford Hospital, newspaper and radio advertisements, and contact with physicians’ offices. Recruited subjects will undergo a run-in crossover trial to confirm statin-associated myalgia. Only subjects who develop myalgic symptoms during simvastatin, but not placebo, treatment will then participate in the CoQ10 treatment section of the study to determine if supplementation reduces muscle pain in patients with documented statin myalgia (Figure 1).
Figure 1.
Protocol for Coenzyme Q10 (CoQ10) in Statin Myopathy study. For all study visits, procedures are performed in the order listed. 1Lipids: total cholesterol, low density lipoprotein (LDL)-cholesterol, high density lipoprotein (HDL)-cholesterol, triglycerides. ALT = alanine aminotransferase, CK = creatine kinase, CK-MB = creatine kinase heart-specific isoform, TSH = thyroid-stimulating hormone.
Run-in Trial to Confirm Myalgia
After 4 weeks off statin treatment, recruited subjects will be treated with either 20 mg of simvastatin or placebo daily for 8 weeks or until muscle symptoms persist for 1 week or are intolerable. Muscle symptoms will be documented weekly. After a 4-week wash-out period, subjects will crossover to the alternative treatment (simvastatin 20 mg or placebo). Only subjects who experience new muscle pain on simvastatin but not on placebo that resolves within 4 weeks off treatment will be entered into the CoQ10 section of the study.
CoQ10 Treatment Study
After a 4-week wash-out period following the run-in trial, subjects qualifying for the CoQ10 protocol will be randomized into groups treated with 20 mg of simvastatin with either 600 mg of CoQ10 or placebo for 8 weeks, until muscle symptoms are experienced continuously for 1 week, or until symptoms are intolerable. We selected an 8-week treatment period for both the run-in and treatment study because in the largest clinical trial the median time to onset of symptoms was 1 month (9), and symptoms are typically provoked sooner with statin re-challenge (3). After 4 weeks off treatment, subjects will cross over to the alternative group: statin/CoQ10 or statin/placebo. Subjects will be first loaded for 2 weeks with either CoQ10 or placebo to ensure adequate tissue levels before beginning simvastatin treatment. Pain intensity will be recorded weekly, and subjects will undergo additional testing including a blood draw, muscle performance and exercise capacity, physical activity level monitoring, and a pain questionnaire at the beginning and end of each treatment phase.
Study Monitoring
The Coenzyme Q10 in Statin Myopathy study is approved by the Institutional Review Board at Hartford Hospital. A Data Safety and Monitoring Board (DSMB) composed of two physicians and a statistician will oversee the project with biannual meetings. The purpose of the DSMB is to conduct periodic assessments of data quality and timeliness, participant recruitment, accrual and retention, participant risk versus benefit, performance of trial sites, and other factors that can affect study outcomes. In addition, significant adverse event reports as well as safety data [creatine kinase (CK) and alanine aminotransaminase (ALT) values] will be provided to the DSMB at each meeting. Members will discuss and analyze these data to determine whether the trial should be stopped. Stopping rules are as follows: a) The presence of a significantly higher frequency of adverse events related to the drug, and b) the emergence of unexpected serious adverse experience(s) not specified in the study. Findings and recommendations of the DSMB will be reported regularly to the Institutional Review Board and National Institutes of Health.
Study Drug Preparation
The study pharmacists will compound identical simvastatin and placebo capsules. Simvastatin tablets will be obtained from a single supplier (Cardinal Health, Dublin, Ohio). The tablets will be cut, covered with lactose secundumartem, and fit into opaque capsules. The placebo tablets will be filled with lactose alone. A random sample of capsules will be weighed during the compounding process to ensure the weight of the simvastatin and placebo capsules is similar (within 15%). CoQ10 and placebo will be prepared and obtained in identical matching 300 mg softgelatin capsules from Tishcon Corporation (Waterbury, New York).
Study Population
We will recruit an equal number of men and women ≥20 yrs of age (Table I). We will not exclude individuals with diagnosed coronary artery disease, peripheral vascular disease, or an elevated CK of treatment <10 upper normal limit (UNL) because spontaneous elevations in CK levels are normal in the general population. Subjects previously using supplemental CoQ10 will discontinue supplementation 2 months prior to the study. Subjects will follow a lipid-lowering diet during the study. Subjects’ use of medications, including over the counter agents, will be recorded throughout the study. Lipid-lowering medications will be discontinued prior to participation. Subjects with elevated CK levels (> 10 times UNL) during study treatment will be taken off the statin and removed from the study immediately.
Table I.
Criteria for inclusion and exclusion of study participants.
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
| |
|
|
Abbreviations : ALT: alanine aminotransferase; CK: creatine kinase; TSH: thyroid stimulating hormone; UNL: upper normal limit
Serologic Markers
Blood will be drawn at each testing time point during the run-in and CoQ10 section of the study for measurement of serum lipids (total, HDL, and LDL-cholesterol and triglycerides), CK, vitamin D, and ALT levels. In addition, creatinine and thyroid stimulating hormone (TSH) levels will be measured at baseline in the run-in trial, and CoQ10 levels will be measured at each point during the CoQ10 trial. A 4 ml sample will be stored for future white blood cell analysis for genetic testing.
Measurement of Muscle Pain
Subjects in the experimental and placebo groups will be contacted weekly throughout each phase of the run-in and CoQ10 sections of the study to inquire about muscle symptoms using the Brief Pain Inventory (Short Form) (BPI-SF) (10). The BPI-SF will be administered in an identical fashion to experimental and placebo groups to avoid bias while measuring the intensity and location of muscle pain and the extent that muscle pain interferes with daily activities. A pain severity score (PSS) will be calculated by averaging scores on 4 pain-intensity items, and a pain interference score (PIS) will be calculated by averaging scores on 7 pain-interference items. PIS indicates the extent that muscle pain interferes with aspects of daily life, including mood, walking, working, sleeping, and relationships during the previous 24 hours based on 7 items rated 0–10 (“0” = does not interfere, “10” = completely interferes). During each phase of the study, if a subjects reports symptoms that have persisted for 1 week or are intolerable, treatment will be stopped and testing will be performed. Otherwise, testing will be performed after 8 weeks of treatment.
Anthropometric Measurements
Body weight will be measured using a calibrated balance beam scale and height will be determined using a tape measure mounted on a wall. Skinfold thickness will be measured at the subscapular, triceps, biceps, abdomen, and thigh using calipers. Waist circumference will be measured as the circumference of the abdomen halfway between the lower rib and umbilicus using a non-distensible tape measure.
Muscle Strength and Endurance
Muscle strength and exercise performance will be measured at the beginning of the CoQ10 treatment study during two visits (one practice and one test session) and at the end of each treatment phase (statin/CoQ10 or statin/placebo) in one visit. Muscle strength will be assessed by handgrip strength using a handgrip dynanomometer, and elbow flexor and knee extensor isometric and isokinetic force using the Biodex System 3 isokinetic dynamometer (Biodex Medical, Shirley, New York). All measurements will be taken on the dominant hand side of the body. Knee extensor muscular endurance will also be assessed using the Biodex isokinetic dynamometer. Measurements will be performed according to our previously used procedures (11).
Oxygen Uptake Kinetics
Oxygen uptake kinetics will be measured by assessing changes in oxygen uptake at the onset of steady-state exercise. Subjects will perform a submaximal bicycle test with breath-by-breath gas analysis using a Parvomedics TrueOne 2400 metabolic cart (ParvoMedics Corp., Sandy, Utah). Subjects will warm up for 3 minutes at a workload of 20W (very light cycling) and a speed of 60 rpm. After 3 minutes, cadence will be maintained and workload will be increased to 60W for 5 min. Subjects will rest 10 minutes and repeat the protocol. Heart rate will be monitored continuously with a 12 lead electrocardiogram to assess heart rate kinetics simultaneously with oxygen uptake kinetics. The bike protocol will be performed after the strength testing and before aerobic exercise performance.
Measurement of Aerobic Exercise Performance
Aerobic exercise performance and the ventilatory threshold will be measured using a breath-by-breath gas analysis method and a Parvomedics system. A physician-supervised treadmill test with electrocardiographic monitoring will be performed using a modified Astrand protocol at the beginning of the CoQ10 section and after each treatment phase (statin/CoQ10 or statin/placebo)
Physical Activity Determination
Subjects’ physical activity levels will be measured at each testing time point during the CoQ10 section of the study using an Actiwatch (Actigraph, Pensicola, Florida) accelerometer recording device to determine if more active subjects are more prone to statin-related muscle symptoms. Subjects will wear the Actiwatch for 4 consecutive days, and the number of counts will be recorded and averaged over the 4 day period
Medication Compliance Assessment
Subjects will be instructed to take three study pills (1 simvastatin and either 2 CoQ10 or 2 placebo tablets ) daily. If a dose is missed, subjects will take that dose before noon on the next day. Subjects will be queried regarding missed pills and problems taking medications during weekly phone calls during both the run-in trial and CoQ10 section of the study and this will be recorded. Subjects will be required to return any unused pills, which will be counted.
Study Unblinding
Subjects will be unblinded whenever their personal physician or study physicians determine that knowledge of drug use is necessary to protect the health of the subjects. Otherwise, the entire study will be unblinded once the last of the required 100 subjects has completed the protocol.
Planned Statistical Analyses
Caso et al. 2007 found that concurrent CoQ10 supplementation decreased the pain severity of myalgia in statin users by 40% (7), whereas Young et al. found no effect of CoQ10 supplementation on muscle outcomes in myalgic patients (8). Averaging these two results, we based our sample size estimates on an expected 20% between-group difference in pain severity (statin users on CoQ10 vs. statin users on placebo). We determined that we needed to study 40 subjects on CoQ10 and placebo to reject the null hypothesis that the population means for pain severity of the experimental and control groups are equal, and to detect possible interactions with gender, with probability (power) 0.9, and Type I error probability of 0.05. Therefore, we aimed to enter 100 true myalgics from the run-in trial into the CoQ10 study (50 in placebo group and 50 in the CoQ10 group). At study conception, we intended to recruit 135 myalgic subjects to be entered into the run-in trial. We conservatively estimated that 75% of recruited subjects would have true statin-myalgia, which would yield approximately 100 subjects with confirmed myalgia to be randomly assigned to either COQ10 (experimental) or placebo (control) groups, ensuring that we could adequately address our primary outcomes with sufficient statistical power while accounting for drop-outs. However, the run-in trial yielded fewer confirmed myalgics than anticipated. Therefore, we added a cross-over phase to the CoQ10 treatment study, wherein confirmed myalgics are treated with both CoQ10 and placebo, allowing for within-subjects comparisons and reducing the necessary sample size to detect a treatment effect.
Standard diagnostics will be performed to ensure that basic statistical assumptions, including variance homogeneity, normality, and the presence of outliers, are met prior to data analyses. Resultant statistical analyses on the outcome variables (pain severity index score, pain interference score, muscle performance and exercise capacity measures) will be conducted with a linear mixed-effects model that accounts for the correlation among repeated measurements on an individual. In this repeated measures cross over design there are two within subject factors (drug treatment and time). We will examine the influence of other potential variables of interest (baseline serum CoQ10 levels, changes in serum CoQ10 levels, ethnicity, body mass index) as either between-subject factors with interaction terms in the Analysis of Variance (ANOVA) model or as continuous predictors in an Analysis of Covariance (ANCOVA) model. To address the potential issue of missing data points, we will use multiple imputation techniques, average physiological measurements when a subject withdraws, or develop a statistical model to predict dropout based on treatment or any other relevant covariates.
RESULTS
This study is funded by the National Center for Complementary and Alternative Medicine (NCCAM), and data collection began in October, 2009. To date, 115 subjects with a history of statin intolerance have been enrolled in the study and 37 (12 females and 25 males) out of 43 subjects with confirmed statin-related myalgia in the run-in trial have completed at least one arm of the CoQ10 treatment study.
DISCUSSION
This study is the first to our knowledge to determine the effects of CoQ10 supplementation on myopathic symptoms during statin treatment in patients with a history of statin myalgia which has been confirmed via a novel cross-over, run-in trial prior to the CoQ10 treatment phase. In addition to determining if CoQ10 supplementation prevents or lessens statin associated myalgia,, this study should also fill several gaps in our knowledge of statin myalgia including the percentage of patients with a history of self-reported myalgia who develop symptoms during placebo treatment as well as the percentage of self-reporting myalgics with confirmed myalgia only during simvastatin treatment. Examining the clinical characteristics and pre-study serum concentrations for such factors as Vitamin D and CoQ10 should help develop a clinical profile of these “true myalgics” and may also help identify factors contributing to statin myalgia. The muscle and exercise performance measures used in this study should also provide information on whether or not patients with true myalgia also have changes in muscle strength and performance.
There are several limitations with the study design. We chose to use simvastatin as the statin for the study because simvastatin was generic at the start of the study and therefore widely used. In addition, simvastatin was suspected by many statin experts as having more adverse muscle effects than other statins at similar LDL-lowering doses. Nevertheless, the use of only one statin restricts the generalizability of the study. The run-in study protocol used to determine if subjects have true statin myalgia also has limitations. The dose of simvastatin used is only 20 mg daily to avoid elevations in CK with higher doses and because we were concerned that patients with documented statin myalgia would not participate if higher doses were used. Most statin-intolerant patients develop symptoms even at low doses of medication and a lower dosage will hopefully allow patients to remain on treatment for a longer period so that we may better detect an effect of CoQ10 if one exists. Both the low dose and short treatment period may limit our ability to detect myalgic patients who would develop symptoms on higher doses or with longer-term therapy. Nevertheless, these issues should not limit our ability to detect the effect of CoQ10 supplementation on the symptoms of statin myalgia. Previous studies that used CoQ10 doses between 100 and 200 mg daily to treat statin myalgia (7, 8) have been criticized for not ensuring adequate tissue levels throughout the trial. We chose to study a high dose of CoQ10, 600 mg daily, to reduce the risk of erroneously concluding that CoQ10 is ineffective because of using too low a dose. Consequently, we will not be able to determine the minimal effective dose, but that can be determined in subsequent studies.
Conclusions
Statins are widely prescribed and effective medications for the treatment of hypercholesterolemia. However some patients who would benefit from lipid-lowering therapy are intolerant to statin treatment due to myopathic symptoms. These individuals would greatly benefit from a treatment that could reduce muscle pain during statin treatment. CoQ10 supplementation is frequently recommended by practitioners during statin treatment despite the lack of conclusive findings regarding its efficacy. This study will address the lack of conclusive scientific evidence for the utility of CoQ10 supplementation by examining its effects on the severity of muscle pain during statin treatment in patients with confirmed statin myalgia. In addition, we will examine the effects of supplementation on muscle performance and exercise capacity. The findings of this study will be clinically important since it will either identify an effective intervention for statin myalgia or indicate that CoQ10 supplementation is ineffective, thus preventing an unnecessary expense for patients.
Figure 2.
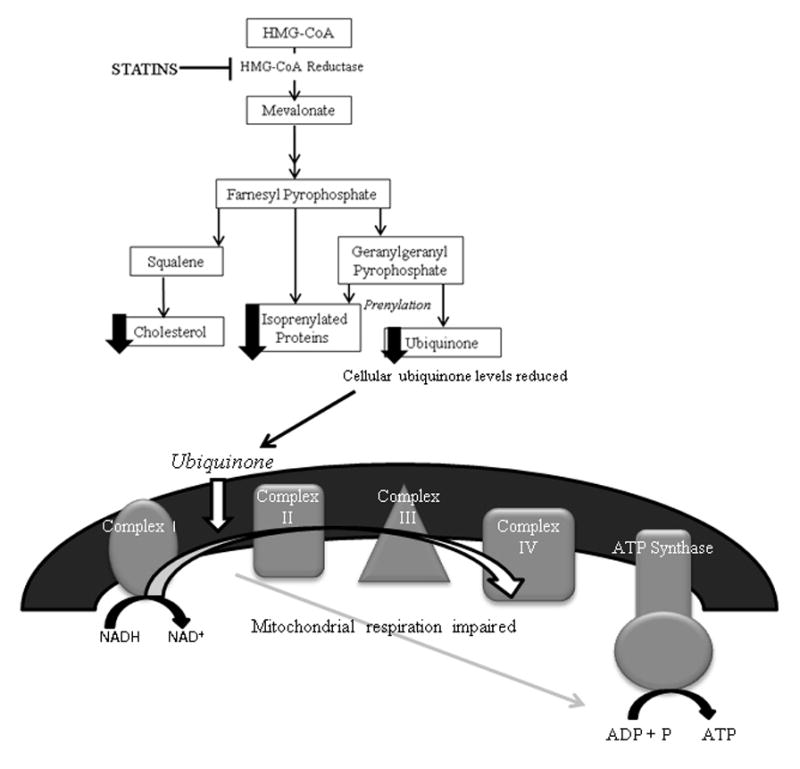
Illustration of the proposed theory explaining statin myopathy as related to cellular ubiquinone depletion. Statins inhibit hydroxy-methylglutaryl-coenzyme A (HMG-CoA) reductase leading to reduced production of mevalonate pathway metabolites including ubiquinone or Coenzyme Q10. Ubiquinone is an essential coenzyme in the process of mitochondrial respiration facilitating the transfer of electrons between complex I and II of the respiratory chain. Consequently, depletion of ubiquinone may impair mitochondrial respiration and cellular energy production within skeletal muscle. Abbreviations: NADH = nicotinamide adenine dinucleotide (oxidized form); NAD+ = nicotinamide adenine dinucleotide (reduced form); ADP: adenosine diphosphate; ATP: adenosine triphosphate; P: phosphate.
Glossary of Abbreviations
- CoQ10
coenzyme Q10
- UNL
upper normal limit
- BPI-SF
Brief Pain Inventory Short-Form
- HDL
high-density lipoprotein
- LDL
low-density lipoprotein
- CK
creatine kinase
- CK-MB
creatine kinase heart specific isoform
- TSH
Thyroid stimulating hormone
- ALT
alanine aminotransferase
- PSS
pain severity score
- PIS
pain interference score
- HMG-CoA
hydroxy-methylglutaryl-coenzyme A
- NCEP
National Cholesterol Education Program
- DSMB
Data safety monitoring board
- ANOVA
Analysis of variance
- ANCOVA
Analysis of covariance
- NCCAM
National Center for Complementary and Alternative Medicine
Footnotes
Financial Disclosure The Co ENZYME Q10 in STATN MYOPATHY study is funded by NHLBI/NIH grant RO1 1RC1AT005836-01 (P. Thompson). Dr. Paul Thompson is also a consultant for Astra Zenica International, Merck & Company, Inc., The Schering-Plough Corporation, Roche, Esperion, Lupin Pharaceuticals, Pfizer and Genomas and is a member of the speaker’s bureau for Merck & Company, Inc., Pfizer, Inc., Abbott Labs, Astra Zenica International, and Glaxo Smith Kline. Dr. Gregory, Dr. Parker, Mrs. Lorson, Dr. Polk, and Dr. White do not have any conflict of interest or financial disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Heart Protection Study Collaborative G. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22. doi: 10.1016/S0140-6736(02)09327-3. [DOI] [PubMed] [Google Scholar]
- 2.Grundy SM, Cleeman JI, Merz CN, Brewer HB, Jr, Clark LT, Hunninghake DB, Pasternak RC, Smith SC, Stone NJ. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. J Am Coll Cardiol. 2004;44:720–32. doi: 10.1016/j.jacc.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Thompson PD, Clarkson P, Karas RH. Statin-associated myopathy. JAMA. 2003;289:1681–90. doi: 10.1001/jama.289.13.1681. [DOI] [PubMed] [Google Scholar]
- 4.Phillips PS, Haas RH, Bannykh S, Hathaway S, Gray NL, Kimura BJ, Vladitiu GD, England JD. Statin-associated myopathy with normal creatine kinase levels. Ann Intern Med. 2002;137:581–5. doi: 10.7326/0003-4819-137-7-200210010-00009. [DOI] [PubMed] [Google Scholar]
- 5.Marcoff L, Thompson PD. The role of coenzyme Q10 in statin-associated myopathy: a systematic review. J Am Coll Cardiol. 2007;49:2231–7. doi: 10.1016/j.jacc.2007.02.049. [DOI] [PubMed] [Google Scholar]
- 6.Paiva H, Thelen KM, Van Coster R, Smet J, De Paepe B, Mattila K, Laakso J, Lehtimaki T, von Bergmann K, Lutjohann D, Laaksosen R. High-dose statins and skeletal muscle metabolism in humans: a randomized, controlled trial. Clin Pharmacol Ther. 2005;78:60–8. doi: 10.1016/j.clpt.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 7.Caso G, Kelly P, McNurlan MA, Lawson WE. Effect of coenzyme q10 on myopathic symptoms in patients treated with statins. Am J Cardiol. 2007;99:1409–12. doi: 10.1016/j.amjcard.2006.12.063. [DOI] [PubMed] [Google Scholar]
- 8.Young JM, Florkowski CM, Molyneux SL, McEwan RG, Frampton CM, George PM, Scott RS. Effect of coenzyme Q(10) supplementation on simvastatin-induced myalgia. Am J Cardiol. 2007;100:1400–3. doi: 10.1016/j.amjcard.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 9.Bruckert E, Hayem G, Dejager S, Yau C, Begaud B. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients--the PRIMO study. Cardiovasc Drugs Ther. 2005;19:403–14. doi: 10.1007/s10557-005-5686-z. [DOI] [PubMed] [Google Scholar]
- 10.Tan G, Jensen MP, Thornby JI, Shanti BF. Validation of the Brief Pain Inventory for chronic nonmalignant pain. J Pain. 2004;5:133–7. doi: 10.1016/j.jpain.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 11.Thompson PD, Parker BA, Clarkson PM, Pescatello LS, White CM, Grimaldi AS, Levine BD, Haller RG, Hoffman EP. A randomized clinical trial to assess the effect of statins on skeletal muscle function and performance: rationale and study design. Prev Cardiol. 2010;13:104–11. doi: 10.1111/j.1751-7141.2009.00063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]