Abstract
Garcinia mangostana, often referred to as “mangosteen,” is a fruit grown in Southeast Asia, and has been used for centuries as a local beverage and natural medicine. Its bioactive compounds, xanthones (i.e. gartanin, α-mangostin, etc), have reported effects on ailments ranging from skin infections and inflammation, to urinary tract infections. We demonstrate that mangosteen xanthones (i.e. gartanin and α-mangostin) at pharmacologically achievable concentrations inhibit the growth of cancer cell lines from different stages of human urinary bladder cancer. The growth inhibitory effects of gartanin in mouse embryonic fibroblasts (MEFs) are at least in part dependent on the existence of p53 or TSC1. Indeed, further studies have shown that gartanin treatment of bladder cancer cell lines T24 and RT4 resulted in a marked suppression of p70S6 and 4E-BP1 expression and induction of autophagy, suggesting the inhibition of the mTOR pathway. In addition, gartanin down-regulated the expression of Bcl-2 and activated the p53 pathway leading to apoptosis induction. Together, these results suggested that gartanin is a multiple targeting agent that is suitable for further study into its chemopreventive properties for human urinary bladder cancer.
Keywords: Gartanin, human urinary bladder cancer, the mTOR pathway, autophagy
Background
Bladder cancer is the fourth most common cancer in men and eighth most common in women in the United States. There will be an estimated 73,510 new cases of bladder cancer and 14,990 related deaths during 2012 (1). In addition, the estimated prevalence is over 500,000 with recurrence rate up to 85% (2). The psychological and economic burdens of human bladder cancer management on the health care system are substantial because of the high frequency of tumor recurrence, the lifetime need for surveillance and the treatment of recurrent tumors, and the high cost of complications associated with treatments. (3). Therefore, a significant reduction of bladder cancer occurrence and recurrence would be a definitive way to easing the burden of bladder cancer.
Chemoprevention is an approach to impede, arrest, or reverse carcinogenesis by administration of natural or synthetic agents before a complex series of genetic and epigenetic events leads to invasive and metastatic malignancy (4). Although proof of principle for chemoprevention approach in reduction of cancer occurrence and recurrence has been clearly demonstrated in both animal and clinical studies (5–7), there is insufficient evidence to make a routine recommendation of any chemopreventive agents for the population at risk to prevent cancer yet. This is because existing chemopreventive agents are not ideal. Either they lack efficacy and potency or have toxic side effects that preclude widespread, long-term use. Therefore, development of new chemopreventive agents is one of the top priorities in the field of cancer chemoprevention. Ideal chemopreventive agents should contain several important features, including high potency, low or no toxicity, known mechanisms of action, low cost, and human acceptability.
Most epidemiological studies report an inverse relationship between fruit and vegetable intake and bladder cancer risk (8–10), suggesting dietary components are rich sources for new chemopreventive agents. Garcinia mangostana (mangosteen), “The Queen of Fruits”, has been consumed as food and medicine by Southeast Asians for centuries (11). As a folk medicine, mangosteen has been used for treatment of variety of conditions, including skin infections, wounds, dysentery and urinary tract infections (11). Recently, mangosteen products have become popular in the United States and have been sold as a health drink or dietary supplements in capsule and tablet form (12, 13). Studies from cell cultures and animals have demonstrated that extracts of mangosteen have antioxidant, antitumoral, antiallergic and anti-microbial activities (12–22). Although currently there are no clinical studies, mangosteen products are marketed to cancer patients as anti-cancer agents (13). Xanthones are major bioactive compounds in mangosteen. Studies have shown that mangosteen xanthones (i.e. α-mangostin, gartanin, etc) inhibited the growth of breast, prostate and colon cancer, sarcoma, glioma, melanoma and leukemia cell lines via cell cycle arrest and apoptosis induction (14–22). Molecular mechanisms of mangosteen xanthones’ anti-cancer effects were involved in inhibition of AKT, MAPK, and NF-κB pathways (16, 19, 22, 23). However, naturally occurring compounds, including mangosteen xanthones, are often limited by their poor bioavailability for in vivo potency (24, 25). Although studies have shown that mangosteen xanthones are concentrated in urine after consumption of mangosteen products (25), there are no reports regarding the effect of mangosteen xanthones on bladder cancer. In addition, mangosteen xanthones have shown potent α-glucosidase inhibitory activity and decreased glucose levels in streptozotocin-induced hypoglycemia rats (26), which suggested their potential involvement in energy metabolism (27). The mTOR pathway is a central pathway for energy metabolism and is one of the most commonly deregulated pathways in human urinary bladder cancer through Ras oncogene activation or via gene mutations in PIK3CA, TSC1/2, and PTEN (28–34). Taken together, these evidences have prompted us to investigate the effect of mangosteen xanthones on bladder cancer cells and the potential mechanisms of their action associated with the mTOR pathway.
In this study, we have shown that mangosteen xanthones (i.e. gartanin and α-mangostin) inhibited the growth of cancer cell lines derived from different stages of human urinary bladder cancer via induction of autophagy and apoptosis. The growth inhibitory effect of gartanin on mouse embryonic fibroblasts (MEFs) is at least in part dependent on the existence of p53 and TSC1. Further studies demonstrated that gartanin reduced the phosphorylation levels of 4E-BP1 and p70S6, suggesting inhibition of the mTOR pathway. In addition, gartanin induced the expression of p53 and Bax protein, leading to activation of caspase cascade and apoptosis in RT4 cells.
Materials and Methods
Cells, Compounds, and Reagents
T24, RT4, UMUC3, 5637, TCCSUP, HT1376 and J82 cell lines were obtained from American Type Culture Collection (Manassas, VA). RT4 and T24 cells were cultured in McCoy’s 5A growth medium, with 10% fetal bovine serum (FBS) added. 5637 and J82 cells were maintained in RPMI 1640 with 10% FBS. J82, UMUC3, TCCSUP, and HT1376 cell lines were cultured in EMEM medium containing 10% FBS. p53+/+, p53−/−, p53−/−TSC2−/−, p53−/−TSC2+/+, TSC1−/−, and TSC1+/+ MEFs were gifts from David Kwiatowski (Brigham Women’s hospital). All MEFs were spontaneously immortalized (35) and cultured in DMEM medium with 10% FBS. The gartanin (MW=396.44 g/mol) and α-mangostin (MW=410.46 g/mol) compounds were obtained from ChromaDex (Irvine, CA). To prevent unintended anti-proliferation of cells, the concentration of DMSO in culture was kept below 0.1% (v/v) at all times; this concentration is known to have no appreciable effect on cell proliferation. The pEGFP-LC3 plasmid was purchased from Addgene (Cambridge, MA). Antibodies for phospho-AMP-activated protein kinase α (AMPKα), AMPKα, phospho-4E-BP1, 4E-BP1, phospho-rpS6, phospho-ACC, ACC, Caspase 3, PARP, phospho-mTOR, mTOR, TSC1, TSC2, p21, p70S6, phospho-p70S6, and p62 were purchased from Cell Signaling Technologies (Beverly, MA). Bcl-2, Bax, p53, and α-Tubulin antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Thymidine, 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased from Sigma (St. Louis, MO).
MTT Assay
Cells were plated in 24-well culture plates at 2×104 cells per well into growth medium with 10% FBS. After 24 hours, the growth medium was replenished, and the appropriate amounts of gartanin or α-mangostin were added to each well to treat cells for 3 days, as per the appended figures or tables. After treatments, MTT was added to each well at a final concentration of 1 mg/mL; the cells were then incubated at 37°C for 3 hours. The absorbance of each sample was determined at 570 nm. IC50s were estimated using the best fit regression curve method in Excel.
Western Blot Analysis
Cells were treated with gartanin under appropriate conditions, defined in appended tables or figures. Clarified protein lysates (30–100 μg) were prepared from the treated cells and denatured at 95°C for 5 minutes. Denatured protein samples were then resolved using 6–16% SDS-PAGE, then transferred to nitrocellulose membranes, probed with appropriate antibodies, and visualized with a chemiluminescence system on x-ray film (36).
Stable Transfection and Fluorescence Microscopy (37)
T24 cells were plated to 100 mm culture plates. Once plates reached 60% confluency, the cells were transfected with pEGFP-LC3 using FuGENE 6 from Roche Applied Scientific (Indianapolis, IN). Transfected cells were then selected using the antibiotic G418 (800 μg/mL) beginning 48 hours after initial transfection. All stable transfectants were then pooled as a means of avoiding artifact cloning. T24 cells stably expressing pEGFP-LC3 were cultured in chamber slides. The cells were treated with chloroquine and gartanin, and then fixed in 4% paraformaldehyde solution for 20 minutes, followed by methanol for 2 minutes. The microscopy slides were mounted in Vector shield medium (Vector Laboratories, Inc., Burlingame, CA). A Nikon Eclipse TE2000-S (200x magnification) fluorescent microscope was used to analyze the immunostained samples using 488-excitation wavelength. Autophagic cells were quantified by counting cells with >10 GFP-LC3 punctuate dots as positive. Triplicates of 100 cells were counted and averaged (mean ± SD).
DAPI Nuclear Staining
T24 cells were grown in culture slides and treated with gartanin for 48 hours. After treatment, cells were then rinsed in PBS, and fixed in 4% paraformaldehyde. Fixed cells were mounted in Vector shield medium containing DAPI and visualized with a Nikon Eclipse TE2000-S microscope under UV light. Apoptotic cells were identified by the condensation and fragmentation of their nuclei.
Electron Microscopy (37)
After treatment with chloroquine and gartanin, cells were pelletized, fixed in 1.6% glutaraldehyde, post-fixed in 1% OsO4, and then dehydrated in alcohol series. The resultant samples were then embedded in epoxy resin. Sections of the sample were contrasted with uranyl acetate and lead citrate. Observations were conducted with either a Jeol 1400 with mounted CCD cameras (Morada, Olympus SIS, Münster, Germany), or with a Philips CM12 electron microscope operating at 80 kV (FEI)
Statistics
Microsoft Excel software was used to compute mean and standard deviations of all quantitative data. Cell viability comparisons between treated and untreated (control) cells were accomplished using either analysis of variance (ANOVA) or Student’s t-test. All statistical measures were two-sided, and P-values <0.05 were considered to be statistically significant.0
Results
Mangosteen xanthones (i.e. gartanin and α-mangostin) inhibits the growth of bladder cancer cell lines in a dose-dependent manner
Cancer cell lines derived from different stages (Ta to T4) of human urinary bladder cancer were treated with variable concentrations of gartanin and α-mangostin to examine their anti-bladder cancer efficacy in vitro. After treatments, cell viability was determined quantitatively via MTT assay; all viability percentages are presented relative to their respective vehicle controls. RT4 cells harbor wild-type p53 but do not express TSC1 or TSC2 at any appreciable level (37, 38). T24, UMUC3, HT1376, 5637, and J82 cell lines were derived from different stages (T2-T4) of muscle-invasive urinary bladder tumor samples (37, 38). TCCSUP is a bone metastatic bladder cancer cell line (38). T24, UMUC3, HT1376, 5637, and TCCSUP cell lines all have mutant p53 and express TSC1 and TSC2. J82 cells have mutant p53 but don’t express TSC1/2 (38). Figure 1B shows that both gartanin and α-mangostin inhibit the growth of bladder cancer cell lines in a dose-dependent fashion. The IC50s of gartanin on the growth of these cell lines range from 4.1 to 18.1 μM. For α-mangostin, the range is from 7.0 to 9.8 μM. It appears that there is little difference in the growth inhibitory effects of these two compounds on different bladder cancer cell lines, although the genetic backgrounds of these cell lines are quite different (Figure 1C). These results suggest that mangosteen xanthones (i.e. gartanin and α-mangostin) may either target multiple pathways for their growth inhibitory effects or have a non-specific cell damage effect.
Figure 1.
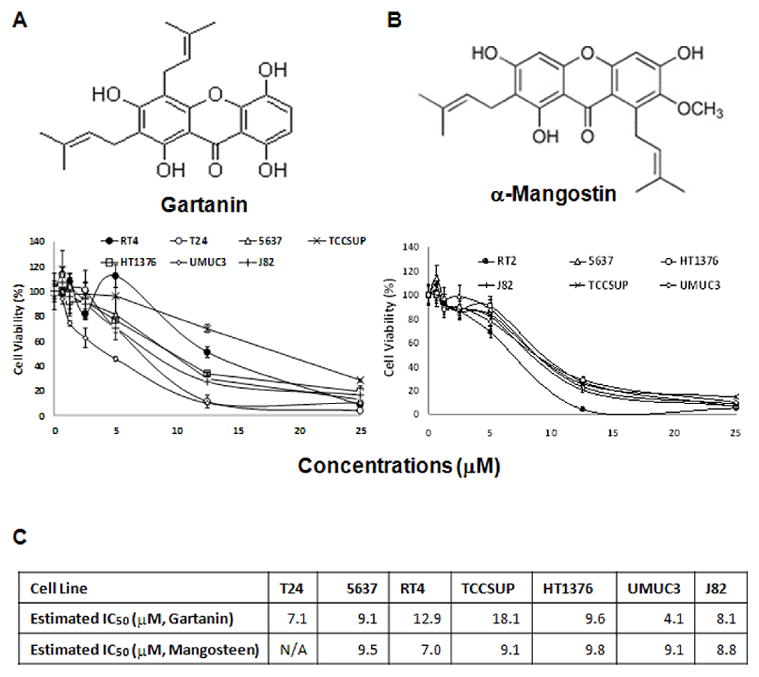
The effect of mangosteen xanthones (i.e. gartanin and α-mangostin) on the growth of human urinary bladder cancer cell lines (RT4, T24, 5637, UMUC3, HT1376, J82 and TCCSUP). A, chemical structures of gartanin or α-mangostin. B, cells in 24-well culture plates were treated with 0.1% DMSO, gartanin or α-mangostin at the indicated concentrations. After 72 hours of treatment, cell densities were measured by MTT assay. Each point is the mean of values from four independent plates; bars, SD. Each sample was counted in duplicate. C, IC50s were estimated by the best fit regression curves in excel. Abbreviations: WT, wild-type; MT, mutant allele.
The growth inhibitory effect of gartanin is at least in part dependent on the existence of TSC1 and p53 in MEFs
Since cancer cell lines have multiple genetic alterations, it is difficult to identify the potential mechanisms of a compound’s action using a multiple pathway converging endpoint assay (i.e. cell growth inhibition) if the tested compound has multiple targeting effects. Therefore, to execute experimental rigor, primary MEFs from p53, TSC1 or TSC2 knockout mice (35) were used to examine the requirements of these molecules for the growth inhibitory effect of gartanin. Figures 2A and B show that p53 and TSC1 knockout MEFs are significantly less sensitive to the growth inhibitory effect of gartanin (The IC50 for wild-type MEFs is about 12.3μM; whereas the IC50s for p53 and TSC1 knockout MEFs are about 18.5 and 31.2 μM, respectively; Student t test, Ps<0.05). Under the p53 deficient condition, loss of TSC2 in MEFs is more sensitive to the growth inhibitor effect of gartanin (Figure 2C). These results suggest that the growth inhibitory effect of gartanin in MEFs at least in part requires the existence of p53 and TSC1.
Figure 2.
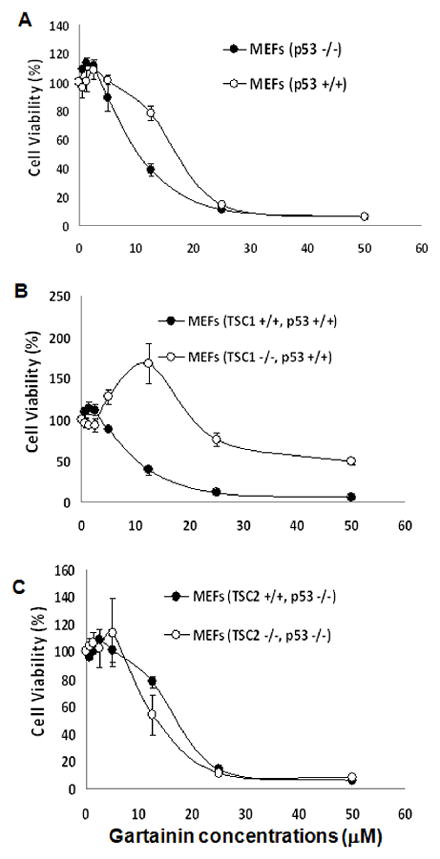
The effect of gartanin on the growth of MEFs (p53+/+, p53−/−, p53−/−TSC2−/−, p53−/−TSC2+/+, TSC1−/−, and TSC1+/+). Cells in 24-well culture plates were treated with 0.1% DMSO, gartanin at the indicated concentrations. After 72 hours of treatment, cell densities were measured by MTT assay. Each point is the mean of values from four independent plates; bars, SD. Each sample was counted in duplicate. A, p53+/+ versus p53−/− MEFs; B, TSC1−/− versus TSC1+/+ MEFs; C, p53−/−TSC2−/− versus p53−/−TSC2+/+ MEFs.
The effect of gartanin on the mTOR pathway in T24 and RT4 cells
We next examined whether gartanin treatment resulted in a change in expression levels of molecular components along the mTOR pathway. In T24 cells, Figure 3 shows that gartanin significantly increases phosphorylation levels of AMPKα and slightly reduces phosphorylation levels of the mTOR. It appears that the change in AMPKα and the mTOR is not due to the expression of their total proteins. In RT4 cells, gartanin did not significantly change phosphorylation levels of the mTOR and AMPKα (Figure 3), while gartanin treatment resulted in a dose-dependent decrease of both phospho-AKT and total AKT levels. However, protein expression levels of total 4E-BP1 and p70S6 in both RT4 and T24 cells are highly or completely inhibited by gartanin treatment at concentrations of 15 and 20 μM (Figure 3). These results indicate that gartanin inhibits the down-stream events (p70S6 and 4E-BP1) of the mTOR pathway via two different mechanisms in T24 and RT4 cells: activation of AMPKα and inactivation of AKT, respectively.
Figure 3.
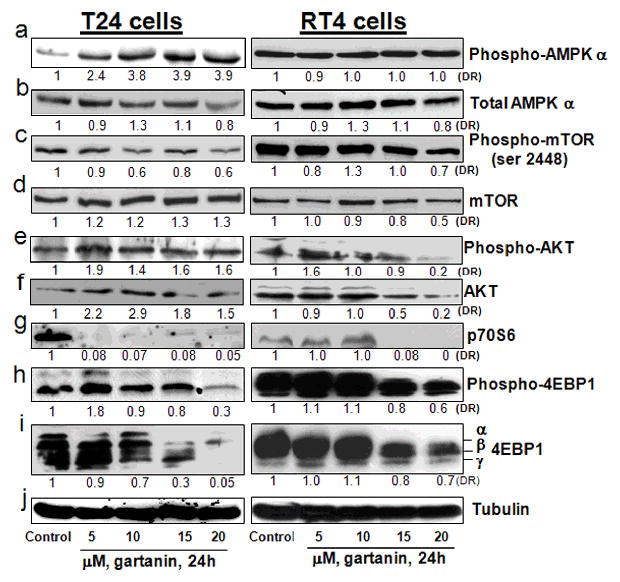
The effect of gartanin on the mTOR pathway in RT4 and T24 cells. T24 and RT4 cells were treated with 0.1%DMSO or gartanin at indicated concentrations for 24 hours. After treatments, cell lysates were prepared and analyzed by Western blot. α-Tubulin was detected as a loading control. A representative blot was shown from three independent experiments. The density of each protein band was determined using NIH J imaging. The density of each band was adjusted by corresponding α-Tubulin. The density ratio (DR) for each treatment was calculated by dividing by the adjusted density of corresponding control bands. All density ratios were placed below their western blot bands.
Gartanin induces autophagy in T24 cells
The mTOR pathway critically regulates the energy balance and autophagy as a survival pathway (33). We therefore examined whether inhibition of the mTOR pathway by gartanin led to induction of autophagy. T24 cells were stably transfected with pEGFP-LC3 to observe fluorescent LC3 protein. During autophagocytosis LC3 protein migrates from the intracellular cytoplasm to autophagic vesicles, thus concentrated vesicles of fluorescent LC3 indicate autophagy (38). Figure 4A shows that chloroquine (an autophagy inhibitor that is known to induce defective autophagosomes by blocking the fusion of autophagic vesicles with lysosomes) and gartanin treatment at a concentration of 50 μM and 10μM for 8 hours, respectively, increased the presence of LC3-GFP puncta in T24 cells stably expressing pEGFP-LC3. Transmission electron microscopy of chloroquine- and gartanin- treated T24 cells reveals a visible increase in the number and size of intracellular autophagic vesicles and vacuoles (Figure 4B). Quantitative analysis of the percentage of T24 cells with autophagosome formation under microscopy indicates that 50 μM chloroquine and 10 and 25 μM gartanin treatments for 8 hours resulted in a marked increase in the number of T24 cells with autophagosome formation by about 71%, 66% and 86%, respectively, whereas control treatment only have about 11% of cells with autophagosome formation (Figure 4C, ANOVA, Ps < 0.01). The induction of autophagy by gartanin was further confirmed by Western blotting analysis, showing that gartanin decreased the expression levels of phospho-Acetyl CoA Carboxylase (ACC) and induced cleavage of LC-3II. These results demonstrated that gartanin is a potent autophagy inducer.
Figure 4.
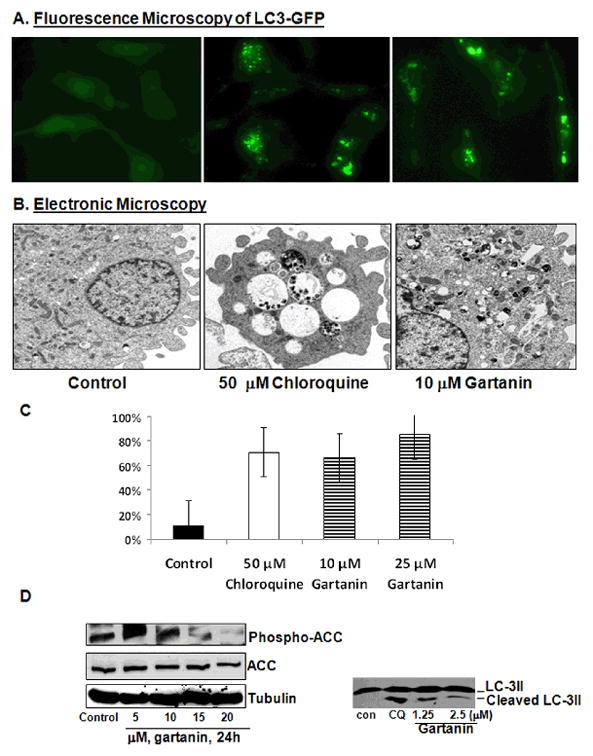
Gartanin induces autophagy in T24 cells. A, RT4 cells stably expressing pEGFP-LC3 were treated with vehicle control, 50 μM chloroquine, or 10 or 25 μM gartanin for 8 h. After treatments, cells were examined by a fluorescence microscopy. B, T24 cells were treated with vehicle control, 50 μM chloroquine, and 10 and 25 μM gartanin for 8 h. After treatments, cells are fixed and examined by an electronic microscopy for the presences of autophagosomes. C, Quantitative analysis of percentage of T24 cells with LC3-puncta. Bar: mean ± SE. D, Western blotting analysis of phosphor-ACC, total ACC and LC3-II cleavage.
Gartanin induces apoptotic cell death in T24 and RT4 bladder cancer cell lines
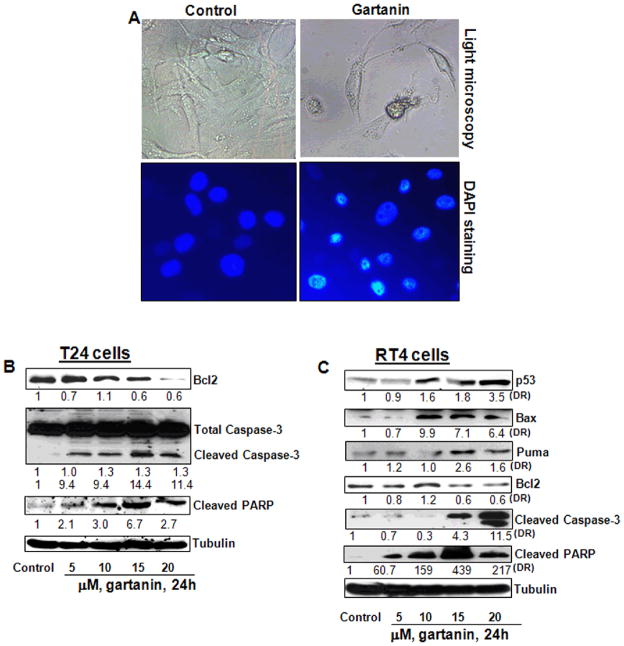
We next determined whether gartanin also induced apoptotic cell death in human urinary bladder cancer cell lines T24 and RT4. As shown in Figure 5A, gartanin treatment at a concentration of 20 μM for 48 hours significantly increased the number of T24 cells with apoptotic morphology (i.e. cell shrinkage and rounding up, as well as nuclear fragmentation and condensation) by about 40.8%, compared to 6.1% in control (Student t test, P<0.01). Western blotting analysis revealed that gartanin decreased the expression of Bcl-2 and increased the cleavage of both Caspase 3 and PARP (Figure 5B).
Figure 5.
Gartanin induces apoptosis in RT4 and T24 cells. A, T24 cells were treated with vehicle control or 20 μM gartanin for 8 h. After treatments, cells were fixed, and stained with DAPI and then examined by a light microscopy and a fluorescence microscopy. Representative photographs are shown. B, T24 cells were treated with vehicle control or gartanin at indicated concentrations for 24 hours. Bcl-2 expression and caspase-3 and PARP cleavages were analyzed by Western blotting analysis. C, RT4 cells were treated with vehicle control or gartanin at indicated concentrations for 24 hours. p53, PUMA, Bax and Bcl-2 expression, as well as caspase-3 and PARP cleavages were analyzed by Western blotting analysis.
Gartanin treatment of RT4 cells with wild-type p53 resulted in up-regulation of p53 and its target gene (i.e. Bax and PUMA) expression and down-regulation of Bcl-2 expression, leading to the cleavage of both Caspase 3 and PARP (Figure 5C). Taken together, gartanin induces apoptosis in both p53 wild-type and mutant bladder cancer cell lines.
Discussion
Nutraceutical juice beverages, including mangosteen, pomegranate, acai and noni juices, have recently become a rapid growing section of the US beverage market (13) and have gained considerable attention as cancer chemopreventive agents. The extract of mangosteen and its active components (i.e. xanthones) have demonstrated their activities against the growth of prostate, breast, and colon cancer, sarcoma, glioma and leukemia cells (14–23). In addition, a pharmacokinetics study of healthy adults who drank 100% mangosteen juice have shown that mangosteen xanthones were excreted through the urinary tract and concentrated in urine with a concentration of up to 11.1 μmol/L, while the maximum concentration of xanthones in plasma was only about 113 nmol/L (25). In this study, we have shown that mangosteen xanthones at pharmacologically achievable concentrations effectively inhibited the growth of cancer cell lines from different stages of human urinary bladder cancer. Our results in combination with available pharmacokinetic data and human acceptance of mangosteen xanthones suggested that mangosteen juice and/or mangosteen xanthones deserve further investigation as a novel nutraceutical product for bladder cancer chemoprevention in particular.
Previous studies have shown that mangosteen xanthones affected multiple pathways, including AKT, MAPK, and NF-κB pathways, for their effect on induction of apoptosis in cancer cells (16, 19, 22, 23). To our best knowledge, we are the first to report that gartanin, one of major xanthones in mangosteen, induces autophagy in bladder cancer cell lines by targeting the mTOR pathway. Mangosteen xanthones (i.e. gartanin and α-mangostin) appear to inhibit the growth of bladder cancer cell lines with different genetic backgrounds with equal potency. In addition, using p53, TSC1 and TSC2 knockout MEFs, we demonstrated that the growth inhibitory effect of gartanin requires existence of both p53 or TSC1. Taken together, these results suggested that gartanin is a multi-targeting agent that affects p53, mTOR, AKT, MAPK, and NF-κB pathways and has anti-oxidant properties. Recently, Dr. Wu and his associates (40) demonstrated in mouse transgenic models of urothelial carcinoma of the bladder (UCB) that activated Ha-ras or a Simian virus 40 T antigen (SV40T) alone at low gene dosages were insufficient to initiate early-onset UCB and that deregulation of multiple signaling pathways via disabling p53 and pRb family proteins and expressing mutant H-Ras together is required for initiating high-grade papillary UCB. This high-grade papillary UCB in this mouse model strongly recapitulated pTaG3 human urinary bladder cancer that is a major challenge in clinical management of human urinary bladder cancer (41, 42). Dr. Wu and his associates (40) also have shown that inhibition of AKT-mTOR, MAPK and STAT3 altogether resulted in much greater tumor reduction and longer survival than did inhibition of AKT-mTOR pathway alone in the mutant-Ha-ras/SV40T transgenic UCB model. These results indicated that agents inhibiting multiple signaling targets had a better therapeutic effect than inhibiting a single target at least in animal models. Therefore, mangosteen xanthones deserve further testing in this model for its usefulness in prevention of high-grade papillary UCB.
The PI3K-AKT-mTOR pathway has been shown to be over-activated in a large portion of high-grade and late-stage human urinary bladder tumors (34). Therefore, targeting the mTOR pathway may have major impact in bladder cancer prevention. In this study, we have shown that gartanin markedly down-regulated expression of p70S6 and 4E-BP1 in both T24 and RT4 cells. The genetic backgrounds in these two cell lines are very different. T24 cells harbor mutant p53 and wild-type TSC1/2 (a upstream component of the mTOR pathway), whereas RT4 cells have wild-type p53 and lack of TSC1/2 due to gene deletion. In T24 cells, gartanin activated AMPKα and then caused the inhibition of the mTOR pathway to down-regulate the expression of p70S6 and 4E-BP1. In RT4 cells, it appears that the decreased expression of p70S6 and 4E-BP1 by gartanin may be due to the inhibition of AKT activation. The underlying mechanism for these differences remains unclear. Further studies are still in progress to investigate the difference of the gartanin’s effect on the mTOR pathway in these two cell lines.
Members of the Bcl-2 family of proteins are central regulators of the apoptotic pathway (43). Bcl-2 is a potent suppressor of apoptosis by interacting with Bax and preventing the opening of the mitochondrial pore to release cytochrome C and to activate the caspase-3 cascade (43). Bcl-2 was found to be silenced in human urinary bladder tumors compared with nonmalignant adjacent tissue (44). Bcl-2 expression predicted a poor response to neo adjuvant chemotherapyand synchronous chemoradiotherapy (45). We have shown that gartanin decreased the expression of Bcl-2 in both T24 and RT4 cells. The mechanism of gartanin-regulated Bcl-2 expression remains unclear and needs to be further investigated.
In summary, gartanin inhibits the growth of bladder cancer cell lines with different genetic backgrounds via targeting multiple pathways: 1) inhibits the mTOR pathway leading to reduction of p70S6 and 4E-BP1 and induction of autophagy, and 2) down-regulates the expression of Bcl-2 and up-regulates expression of p53, PUMA and Bax resulting in apoptosis (Figure 6). Since gartanin has apoptotic effects on bladder cancer cells regardless of p53 expression, it could prove to be a useful broad-spectrum preventive agent for bladder cancer. Further experiments are needed to investigate the potential of gartanin in chemoprevention of human urinary bladder cancer.
Figure 6.
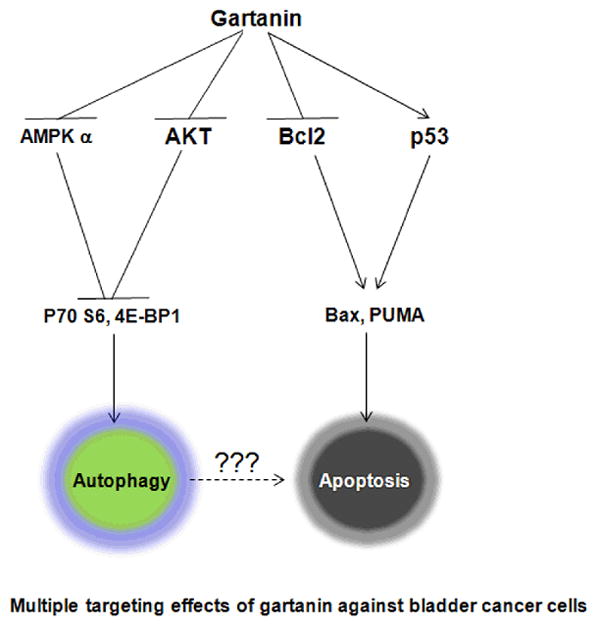
Schematic presentation of mechanisms of gartanin’s action in induction of autophagy and apoptosis. (1) Gartanin activates AMPK α and inhibits AKT activation, which causes down-regulation of p70S6 and 4E-BP1 leading to autophagy; (2) Gartanin down-regulates Bcl-2 and up-regulates p53, PUMA and Bax, which results in activation of Caspase-3 and PARP cleavages to induce apoptosis.
Acknowledgments
This work was in part supported by NIH award 5R01CA122558-05 and 1R21CA152804-01A1 (to X. Z.). We thank Dr. David J. Kwiatkowski for the TSC1 and TSC2 null MEFs.
Footnotes
Conflict of Interest: Authors declare no conflict of interests.
References
- 1. [Accessed October 15, 2010];Bladder cancer key statistics. at http://www.cancer.org/Cancer/BladderCancer/DetailedGuide/bladder-cancer-key-statistics.
- 2.Patton SE, Hall MC, Ozen H. Bladder cancer. Curr Opin Oncol. 2002;14:265–72. doi: 10.1097/00001622-200205000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Noyes K, Singer EA, Messing EM. Healthcare economics of bladder cancer: cost-enhancing and cost-reducing factors. Curr Opin Urol. 2008;18:533–9. doi: 10.1097/MOU.0b013e32830b8910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sporn MB, Suh N. Chemoprevention: an essential approach to controlling cancer. Nat Rev Cancer. 2002;2:537–43. doi: 10.1038/nrc844. [DOI] [PubMed] [Google Scholar]
- 5.Thompson IM, Goodman PJ, Tangen CM. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003;349:215–24. doi: 10.1056/NEJMoa030660. [DOI] [PubMed] [Google Scholar]
- 6.Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, Vogel V, Robidoux A, Dimitrov N, Atkins J, Daly M, Wieand S, Tan-Chiu E, Ford L, Wolmark N. Tamoxifen for prevention of breast cancer: report of the national surgical adjuvant breast and bowel project P-1 study. J Natl Cancer Inst. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 7.Fischer SM, Hawk ET, Lubet RA. Coxibs and other nonsteroidal anti-inflammatory drugs in animal models of cancer chemoprevention. Cancer Prev Res (Phila) 2011;4:1728–35. doi: 10.1158/1940-6207.CAPR-11-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reddy BS. Micronutrients as chemopreventive agents. IARC Sci Publ. 1996;139:221–35. [PubMed] [Google Scholar]
- 9.Riboli E, Norat T. Epidemiologic evidence of the protective effect of fruit and vegetables on cancer risk. Am J Clin Nutr. 2003;78(3 Suppl):559S–569S. doi: 10.1093/ajcn/78.3.559S. [DOI] [PubMed] [Google Scholar]
- 10.Steinmaus CM, Nunez S, Smith AH. Diet and bladder cancer: a meta-analysis of six dietary variables. Am J Epidemiol. 2000;151:693–702. doi: 10.1093/oxfordjournals.aje.a010264. [DOI] [PubMed] [Google Scholar]
- 11.Pedraza-Chaverri J, Cárdenas-Rodríguez N, Orozco-Ibarra M, Pérez-Rojas JM. Medicinal properties of mangosteen (Garcinia mangostana) Food Chem Toxicol. 2008;46:3227–39. doi: 10.1016/j.fct.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 12.Yeung S. Mangosteen for the cancer patient: facts and myths. J Soc Integr Oncol. 2006;4:130–4. doi: 10.2310/7200.2006.022. [DOI] [PubMed] [Google Scholar]
- 13.Lobb AL. Science in liquid dietary supplement promotion: the misleading case of mangosteen juice. Hawaii J Med Public Health. 2012;71:46–8. [PMC free article] [PubMed] [Google Scholar]
- 14.Aisha AF, Abu-Salah KM, Ismail Z, Abdul Majid AM. In vitro and in vivo anti-colon cancer effects of Garcinia mangostana xanthones extract. BMC Complement Altern Med. 2012;12:104. doi: 10.1186/1472-6882-12-104. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Wang JJ, Shi QH, Zhang W, Sanderson BJ. Anti-skin cancer properties of phenolic-rich extract from the pericarp of mangosteen (Garcinia mangostana Linn.) Food Chem Toxicol. 2012;50:3004–3013. doi: 10.1016/j.fct.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Johnson JJ, Petiwala SM, Syed DN, Rasmussen JT, Adhami VM, Siddiqui IA, Kohl AM, Mukhtar H. α-Mangostin, a xanthone from mangosteen fruit, promotes cell cycle arrest in prostate cancer and decreases xenograft tumor growth. Carcinogenesis. 2012;33:413–9. doi: 10.1093/carcin/bgr291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang JJ, Sanderson BJ, Zhang W. Cytotoxic effect of xanthones from pericarp of the tropical fruit mangosteen (Garcinia mangostana Linn.) on human melanoma cells. Food Chem Toxicol. 2011;49:2385–91. doi: 10.1016/j.fct.2011.06.051. [DOI] [PubMed] [Google Scholar]
- 18.Shibata MA, Iinuma M, Morimoto J, Kurose H, Akamatsu K, Okuno Y, Akao Y, Otsuki Y. α-Mangostin extracted from the pericarp of the mangosteen (Garcinia mangostana Linn) reduces tumor growth and lymph node metastasis in an immunocompetent xenograft model of metastatic mammary cancer carrying a p53 mutation. BMC Med. 2011;9:69. doi: 10.1186/1741-7015-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krajarng A, Nakamura Y, Suksamrarn S, Watanapokasin R. α-Mangostin induces apoptosis in human chondrosarcoma cells through downregulation of ERK/JNK and Akt signaling pathway. J Agric Food Chem. 2011;59:5746–54. doi: 10.1021/jf200620n. [DOI] [PubMed] [Google Scholar]
- 20.Chang HF, Huang WT, Chen HJ, Yang LL. Apoptotic effects of γ-mangostin from the fruit hull of Garcinia mangostana on human malignant glioma cells. Molecules. 2010;15:8953–66. doi: 10.3390/molecules15128953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watanapokasin R, Jarinthanan F, Jerusalmi A, Suksamrarn S, Nakamura Y, Sukseree S, Uthaisang-Tanethpongtamb W, Ratananukul P, Sano T. Potential of xanthones from tropical fruit mangosteen as anti-cancer agents: caspase-dependent apoptosis induction in vitro and in mice. Appl Biochem Biotechnol. 2010;162:1080–94. doi: 10.1007/s12010-009-8903-6. [DOI] [PubMed] [Google Scholar]
- 22.Matsumoto K, Akao Y, Kobayashi E, Ohguchi K, Ito T, Tanaka T, Iinuma M, Nozawa Y. Induction of apoptosis by xanthones from mangosteen in human leukemia cell lines. J Nat Prod. 2003;66:1124–7. doi: 10.1021/np020546u. [DOI] [PubMed] [Google Scholar]
- 23.Nakatani K, Yamakuni T, Kondo N, Arakawa T, Oosawa K, Shimura S, Inoue H, Ohizumi Y. gamma-Mangostin inhibits inhibitor-kappaB kinase activity and decreases lipopolysaccharide-induced cyclooxygenase-2 gene expression in C6 rat glioma cells. Mol Pharmacol. 2004;66:667–74. doi: 10.1124/mol.104.002626. [DOI] [PubMed] [Google Scholar]
- 24.Li L, Brunner I, Han AR, Hamburger M, Kinghorn AD, Frye R, Butterweck V. Pharmacokinetics of α-mangostin in rats after intravenous and oral application. Mol Nutr Food Res. 2011;55 (Suppl 1):S67–74. doi: 10.1002/mnfr.201000511. [DOI] [PubMed] [Google Scholar]
- 25.Chitchumroonchokchai C, Riedl KM, Suksumrarn S, Clinton SK, Kinghorn AD, Failla ML. Xanthones in mangosteen juice are absorbed and partially conjugated by healthy adults. J Nutr. 2012;142:675–80. doi: 10.3945/jn.111.156992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ryu HW, Cho JK, Curtis-Long MJ, Yuk HJ, Kim YS, Jung S, Kim YS, Lee BW, Park KH. α-Glucosidase inhibition and antihyperglycemic activity of prenylated xanthones from Garcinia mangostana. Phytochemistry. 2011;72:2148–54. doi: 10.1016/j.phytochem.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 27.Inoki K, Kim J, Guan KL. AMPK and mTOR in cellular energy homeostasis and drug targets. Annu Rev Pharmacol Toxicol. 2012;52:381–400. doi: 10.1146/annurev-pharmtox-010611-134537. [DOI] [PubMed] [Google Scholar]
- 28.Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–430. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- 29.Mo L, Zheng X, Huang HY, et al. Hyperactivation of Ha-ras oncogene, but not Ink4a/Arf deficiency, triggers bladder tumorigenesis. J Clin Invest. 2007;117:314–325. doi: 10.1172/JCI30062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knowles MA, Habuchi T, Kennedy W, Cuthbert-Heavens D. Mutation spectrum of the 9q34 tuberous sclerosis gene TSC1 in transitional cell carcinoma of the bladder. Cancer Res. 2003;63:7652–7656. [PubMed] [Google Scholar]
- 31.Platt FM, Hurst CD, Taylor CF, Gregory WM, Harnden P, Knowles MA. Spectrum of phosphatidylinositol 3-kinase pathway gene alterations in bladder cancer. Clin Cancer Res. 2009;15:6008–6017. doi: 10.1158/1078-0432.CCR-09-0898. [DOI] [PubMed] [Google Scholar]
- 32.Sekulić A, Hudson CC, Homme JL, et al. A direct linkage between the phosphoinositide 3-kinase-AKT signaling pathway and the mammalian target of rapamycin in mitogen-stimulated and transformed cells. Cancer Res. 2000;60:3504–3513. [PubMed] [Google Scholar]
- 33.Chen M, Gu J, Delclos GL, et al. Genetic variations of the PI3K-AKT-mTOR pathway and clinical outcome in muscle invasive and metastatic bladder cancer patients. Carcinogenesis. 2010;31:1387–1391. doi: 10.1093/carcin/bgq110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hansel DE, Platt E, Orloff M, Harwalker J, Sethu S, Hicks JL, De Marzo A, Steinle RE, Hsi ED, Theodorescu D, Ching CB, Eng C. Mammalian target of rapamycin (mTOR) regulates cellular proliferation and tumor growth in urothelial carcinoma. Am J Pathol. 2010;176:3062–72. doi: 10.2353/ajpath.2010.090872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang H, Cicchetti G, Onda H, Koon HB, Asrican K, Bajraszewski N, Vazquez F, Carpenter CL, Kwiatkowski DJ. Loss of Tsc1/Tsc2 activates mTOR and disrupts PI3K-Akt signaling through down-regulation of PDGFR. J Clin Invest. 2003;112:1223–33. doi: 10.1172/JCI17222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zi X, Simoneau AR. Flavokawain A, a novel chalcone from kava extract, induces apoptosis in bladder cancer cells by involvement of Bax protein-dependent and mitochondria-dependent apoptotic pathway and suppresses tumor growth in mice. Cancer Res. 2005;65:3479–86. doi: 10.1158/0008-5472.CAN-04-3803. [DOI] [PubMed] [Google Scholar]
- 37.Liu Z, Li X, Simoneau AR, Jafari M, Zi X. Rhodiola rosea extracts and salidroside decrease the growth of bladder cancer cell lines via inhibition of the mTOR pathway and induction of autophagy. Mol Carcinog. 2012;51:257–67. doi: 10.1002/mc.20780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang Y, Simoneau AR, Xie J, Shahandeh B, Zi X. Effects of the kava chalcone flavokawain A differ in bladder cancer cells with wild-type versus mutant p53. Cancer Prev Res (PhilaPa) 2008;1:439–451. doi: 10.1158/1940-6207.CAPR-08-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Herman-Antosiewicz A, Johnson DE, Singh SV. Sulforaphane causes autophagy to inhibit release of cytochrome C and apoptosis in human prostate cancer cells. Cancer Res. 2006;66:5828–35. doi: 10.1158/0008-5472.CAN-06-0139. [DOI] [PubMed] [Google Scholar]
- 40.Zhou H, Huang HY, Shapiro E, Lepor H, Huang WC, Mohammadi M, Mohr I, Tang MS, Huang C, Wu XR. Urothelial tumor initiation requires deregulation of multiple signaling pathways: implications in target-based therapies. Carcinogenesis. 2012;33:770–80. doi: 10.1093/carcin/bgs025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goebell PJ, et al. Bladder cancer or bladder cancers? Genetically distinct malignant conditions of the urothelium. Urol Oncol. 2010;28:409–428. doi: 10.1016/j.urolonc.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 42.Knowles MA. Molecular subtypes of bladder cancer: Jekyll and Hyde or chalk and cheese? Carcinogenesis. 2006;27:361–373. doi: 10.1093/carcin/bgi310. [DOI] [PubMed] [Google Scholar]
- 43.Oltersdorf T, Elmore SW, Shoemaker AR, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–81. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 44.Friedrich MG, Weisenberger DJ, Cheng JC, et al. Detection of methylated apoptosis-associated genes in urine sediments of bladder cancer patients. Clin Cancer Res. 2004;10:7457–65. doi: 10.1158/1078-0432.CCR-04-0930. [DOI] [PubMed] [Google Scholar]
- 45.Hussain SA, Ganesan R, Hiller L, et al. BCL2 expression predicts survival in patients receiving synchronous chemoradiotherapy in advanced transitional cell carcinoma of the bladder. Oncol Rep. 2003;10:571–6. [PubMed] [Google Scholar]