Abstract
Traditional antidepressants require many weeks to reveal their therapeutic effects. However, the widely replicated observation that a single subanesthetic dose of the NMDA glutamate receptor antagonist, ketamine, produced meaningful clinical improvement within hours suggested that rapidly acting antidepressants might be possible. The ketamine studies stimulated a new generation of basic antidepressant research that identified new neural signaling mechanisms in antidepressant response and provided a conceptual framework linking a group of novel antidepressant mechanisms. This article presents the path that led to the testing of ketamine, considers its promise as an antidepressant, and reviews novel treatment mechanisms that are emerging from this line of research.
Keywords: glutamate, antidepressants, rapid-acting, ketamine, review, treatment mechanisms
The field of antidepressant research has had little success in developing fundamentally novel antidepressant mechanisms, leaving psychiatrists with relatively few pharmacologic options for the treatment of patients with depression. Available treatments primarily block monoamine transporters, monoamine receptors, or the activity of monoamine oxidase (1). The emergence of serotonin reuptake inhibitors (SRI) revolutionized the treatment of depression by providing physicians with safe and easily prescribed medications. The SRIs were well-suited to prescription by primary care physicians, bringing effective pharmacotherapy for depression to patients who obtained psychiatric treatment in this way (2). Despite becoming a “Prozac Nation”, the limited effectiveness of pharmacotherapies for depression has been discouraging (3, 4). Major depression remains a leading cause of disability in the world (5). Further, the National Institute of Mental Health STAR*D study found that only 27% of depressed patients achieve remission within 12 weeks, 33% of patients did not achieve remission despite trials of four different antidepressant medications, and that the primary or adjunctive medication selected for treatment made very little difference (6). In addition, the average time to reach remission for the treatment-responsive subgroup of patients was 7 weeks. Thus, there is a tremendous need for new antidepressant treatment mechanisms and, particularly, for mechanisms that might reduce the onset of antidepressant efficacy.
This review will describe the path that led to the testing of ketamine as a rapid acting antidepressant, alterations in glutamate neurotransmission in depression that might be targeted by ketamine, and recent insights into the mechanisms underlying the antidepressant effects of ketamine and other novel treatment strategies. This evolving story has been summarized in prior reviews, and the reader is referred to these reports for additional detail (7-9).
NMDA receptor antagonists as rapid-acting antidepressants
The testing of glutamatergic drugs for the treatment of depression has a surprisingly long history. In 1959, Dr. George Crane reported on the effects of a tuberculosis antibiotic that was known to cause seizures and exacerbate psychosis, D-cycloserine. In his first case series, he found that D-cycloserine produced mood improvement in 30 out of 37 tuberculosis patients suffering from depression, predominately within 2 weeks (10). When he replicated these findings in another case series, he concluded “It is difficult to explain why psychiatric benefits should have occurred almost immediately following drug administration” (11). More than fifty years later, the first placebo-controlled replication of this finding reported progressive improvement with D-cycloserine over 6 weeks of treatment (12). D-cycloserine is a partial agonist at the glycineB coagonist site of N-methyl-D-aspartate (NMDA) glutamate receptors bearing the GluN2A and GluN2B subunits (previously NR2A and NR2B subunits) and a full agonist of NMDA receptors containing the GluN2C and GluN2D subunits (13, 14). In light of the antidepressant efficacy of NMDA receptor antagonists, D-cycloserine may have reduced symptoms of depression by attenuating the function of NMDA receptors bearing GluN2A or GluN2B subunits. However, it is not yet clear whether the agonist or antagonist features of D-cycloserine account for its antidepressant efficacy.
The next glutamatergic agent studied was amantadine, which was studied for depression before its mechanism of action was identified. By the late 1960s, amantadine was deemed helpful for treating Parkinson disease, a condition commonly associated with depression. For this reason, it was tested in a pilot study that produced encouraging beneficial effects (15). Amantadine and memantine were demonstrated subsequently to be low- and moderate-affinity NMDA receptor antagonists, respectively (16). However, when administered at 20 mg/day memantine failed in its initial placebo-controlled trial (17). Subsequent memantine studies have not yielded definitive results, but suggest adjunctive memantine merits further study in bipolar depression (18, 19), perhaps at doses up to 40 mg/day (20). More recently, another low affinity NMDA receptor antagonist, AZD6765 showed promising antidepressant effects in humans at doses that do not produce psychosis (21).
Following the early clinical studies, preclinical models revealed the antidepressant potential of glutamatergic agents (22, 23) (see also the review by Pilc, et al. this issue). Building on the observation that inescapable stress exposure in animals attenuated long-term potentiation, an NMDA receptor-dependent process, investigators found that many types of drugs that reduce NMDA receptor function had antidepressant-like effects in several animal models of depression including a glycineB site partial agonist, a competitive NMDA receptor antagonist, an uncompetitive NMDA receptor antagonist, and a GluN2B-selective uncompetitive NMDA receptor antagonist (24-26). Reduced NMDA receptor function emerged as a correlate of long-term antidepressant administration (27, 28). Thus, by the mid-1990s there was a compelling pre-clinical literature that supported the earlier clinical findings suggesting that NMDA receptor antagonists had antidepressant properties, but this hypothesis had not yet had an unambiguous test in humans.
Although we were aware of the earlier literature, when we studied ketamine effects in depressed patients, we did not expect the rapid and robust antidepressant effects that emerged. In the late 1980's we began studying ketamine effects in healthy subjects (29) in order to link glutamate synaptic dysfunction to schizophrenia (30). We also employed ketamine to provide the first clinical evidence of enhanced NMDA receptor function associated with alcoholism risk and alcohol dependence (31). When studying ketamine effects in depressed patients, we employed the ketamine dose and infusion paradigm adopted from these earlier ketamine studies to facilitate comparison of responses to ketamine in depressed patients with healthy subjects and recovering alcohol dependent patients with the objective of characterizing alterations in NMDA receptor function related to depression.
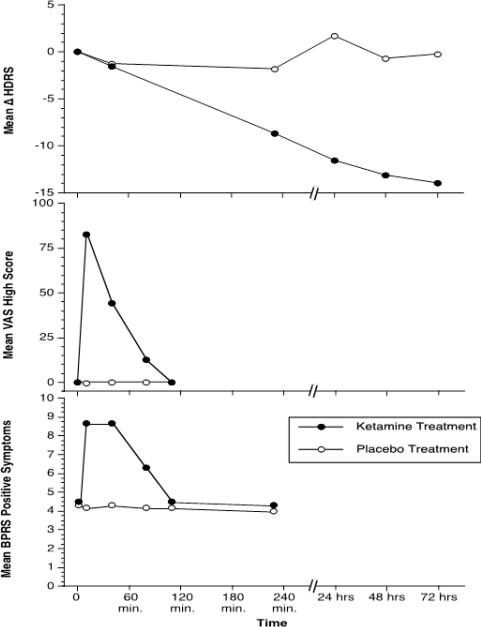
Using this approach, we and other groups were impressed by the rapid-onset of the robust antidepressant effects produced by a single dose of ketamine, producing complete remission within 24 hours in some patients (32-36). As shown in figure 1, the antidepressant effects of ketamine were not present until after the psychotigenic and euphoric effects of ketamine had disappeared. This temporal distinction first suggested that the antidepressant effects arose as a rapid neuroadaptation to the acute effects of ketamine in the brain. Across studies reported to date (figure 2), antidepressant effects emerge by 2-4 hours (36). By 24 hours, studies report substantial improvement and response of depression symptoms in approximately 50%-80% of patients (36, 37). All symptoms of depression improve, including suicidal ideation (38, 39). The clinical benefits after a single ketamine dose may last as briefly as 1-2 days and may last longer than 2 weeks (36).
Figure 1.
The effects of ketamine 0.5 mg/kg, i.v. and a saline placebo in patients with major depression (n=7). In this figure, the antidepressant effects of ketamine emerge after the psychotigenic and euphoric effects of ketamine abate. The top figure presents the reduction in Hamilton Depression Scale (HDRS) scores in patients administered ketamine, but not placebo. The middle figure presents the production of euphoria, as measured by a visual analog scale of “high”, following ketamine, but not saline. The bottom figure presents the production of psychosis, as measured by the Brief Psychiatric Rating Scale (BPRS) positive symptom subscale. The repeated measures ANOVA performed on each of these outcomes revealed highly significant ketamine by time interaction effects (p<.005). This figure is adapted from Berman, et al. (32).
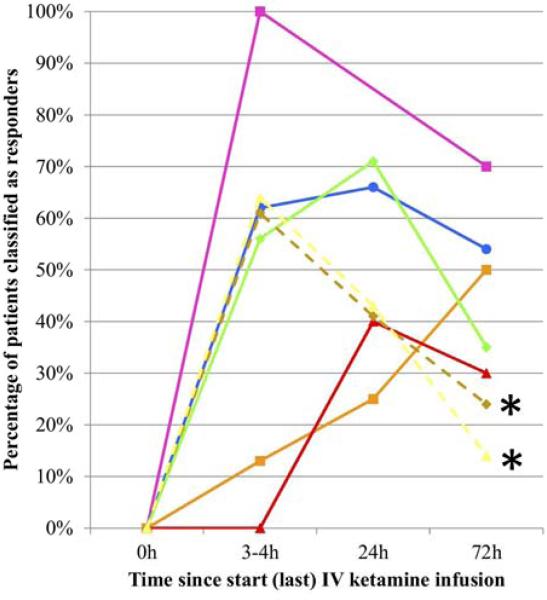
Figure 2.
A summary of the antidepressant effects of ketamine from seven published studies (5 in unipolar major depression and 2 in bipolar depression*). The response rates are both impressive and consistent across studies. The data are collected from a total of 130 patients. This figure is adapted from aan het Rot et al. (36).
Even at this early research stage, investigators began to explore whether subgroups of depressed patients might respond particularly well to ketamine. For example, ketamine appears effective for “treatment resistant” patients (40) (even patients who did not respond to electroconvulsive therapy (35)), depressed individuals with a family history of alcoholism (41, 42), and depressed patients with comorbid alcohol dependence (43). Also, because ketamine is an effective analgesic, it has been used to treat depression emerging in the context of chronic pain (44, 45).
Ketamine, thusfar, has shown evidence that it is safe for the treatment of depression. Ketamine has a long track record of safety when administered as a surgical anesthetic at doses far higher than needed for antidepressant treatment when administered with appropriate levels of medical screening, monitoring, and clinical follow-up (46). It is not yet clear whether this safety profile extends to settings where patients are less well prepared and less intensively monitored and followed. A principal safety concern is whether inducing psychosis via ketamine administration is safe for depressed patients. Prospective follow-up data in healthy human subjects (47) and psychiatric patient populations including schizophrenia patients (48, 49) suggest that psychotigenic doses of ketamine in research settings do not pose a clinical risk to patients. In the limited collected reports of the antidepressant effects of ketamine, the safety profile in depressed patients appears to resemble that of healthy subjects (for example see (32-35, 50)). A particular concern for rapid-acting antidepressants, like ketamine, is whether there is an abrupt worsening or rebound of suicidal ideation or impulses as the antidepressant effects of ketamine dissipate. So far, in the modest number of patients studied to date, this problem has not been reported. However, this important issue will need careful prospective study in larger samples. An additional concern for depressed patients who might have bipolar disorder is whether ketamine accelerates the transition to mania. This has not been a problem in the larger trials to date (34, 51) or in two cases of extended treatment with ketamine (52). One case of mania attributed to ketamine infusion has been reported, however attributions of causality related to switches in mood state in bipolar disorder are extremely complicated and so this important issue awaits further rigorous study (53).
The intravenous infusion paradigm employed in the published antidepressant trials provides excellent control of drug exposure. This level of control facilitates the exploration of the lowest effective dose of ketamine for the treatment of depression and the margin of safety between the antidepressant and perception-altering doses of ketamine. Further it is important to evaluate rigorously whether its antidepressant effects are dose-related, i.e., if maximal antidepressant effects emerge in the perceptual dose range. Nonetheless, unpleasant ketamine effects generally abate within an hour after the termination of drug administration (29, 32). This property, combined with the ability to terminate infusions if unpleasant effects emerge to limit drug exposure, enables clinicians to minimize risks to patients (54). Restricting ketamine administration to clinical settings also provides a way to minimize the impact of its abuse liability (55). We are aware of one unpublished case of a person with a substance abuse history who abused ketamine after receiving it intravenously for treatment-refractory depression, highlighting the importance of addressing its abuse liability within the context of treatment.
Alternatives to intravenous ketamine administration may increase access to ketamine. Case reports describe antidepressant effects associated with ketamine administered via intramuscular and oral routes (52, 56, 57). The antidepressant effects of intranasal ketamine are being studied (NCT01304147). If ketamine were effective in doses below the threshold for perceptual effects, then the availability of oral and intranasal ketamine could provide convenient modes of administration for treatment in ambulatory settings. The risks may be analogous to the NMDA receptor antagonist dextromethorphan. Dextromethorphan is dispensed widely as a cough suppressant despite concerns related to its abuse liability (58) and psychotigenic potential (59).
A critical obstacle to the broader study and implementation of ketamine treatment for depression is the lack of clarity as to how to sustain its antidepressant effects. Pilot studies suggest that ketamine may be sustained by repeated intermittent administration (50, 60). Although the data are extremely preliminary and not placebo-controlled, with repeated ketamine dosing the antidepressant effects may persist for longer periods in some patients. This observation may be consistent with sensitization to some ketamine effects with weekly administration in animals (61). The possible sensitization to the therapeutic effects of ketamine may contrast with the psychotigenic effects of ketamine, which do not appear to sensitize with a limited number of exposures in the laboratory (62). Also, non-response to initial dosing may predict poor response to repeated dosing (50, 60). Thus one advantage of the use of ketamine as an antidepressant may be the ability to predict whether a longer course of ketamine treatment is indicated after a dose or two of the drug. The maximal duration of the beneficial effects of ketamine are unknown. There are now reports of cases where repeated dosing of ketamine has treated symptoms of unipolar or bipolar depression for six months or more (52, 63, 64). But, 6 months of treatment is still far shorter than the duration of long-term antidepressant treatment for chronic major affective disorder.
One question raised by the prospect of long-term repeated dosing of NMDA receptor antagonists is whether, at some point, adverse effects that have been observed in animals or associated with ketamine abuse might emerge. In animals, repeated daily administration of NMDA receptor antagonists produce glutamatergic synaptic deficits including reduced ligand binding to NMDA receptors in frontal cortex (65), reduced GABA neuronal function (66), increased striatal dopamine release (67) depletion of prefrontal cortex dopamine (68), impaired cognitive function, and an amotivational state (69). Repeated ketamine administration also causes oxidative stress that contributes to loss of parvalbumin positive GABAergic interneurons (70). At doses higher than the current antidepressant dose of ketamine, it produces reversible neural injuries (71), including the expression of heat shock proteins and vacuolization (72, 73).
In humans, ketamine abuse has been associated with cognitive impairments, and altered thought content (74, 75). Ketamine abusers also show cortical white matter deficits in a study employing diffusion tensor imaging (76), cortical gray matter deficits on MRI (77), and alterations in cortical functional connectivity (78). In addition, ketamine abuse may be associated with cystitis (79) and biliary dilatation (80). The findings in chronic ketamine abusers may overestimate the clinical risks of long-term treatment. Ketamine abusers often take multiple substances, administer higher ketamine doses than needed to treat depression, and they administer these doses more frequently than would be needed for treatment (75). Nonetheless, the development of long-term ketamine treatment for depression would need to be accompanied by careful studies of its safety.
An alternative strategy would be to employ ketamine to induce remission and then to prevent depressive relapse using other medications or psychotherapeutic approaches. Two inadequately powered studies tested this hypothesis, failed to demonstrate that riluzole extended ketamine effects (81, 82). However, further study of the protective effects of psychotherapy, antidepressants and lithium, given their capacity to protect against relapse of depression is justified (83-86). As ketamine may enhance neuroplasticity (87), it is conceivable that it would promote response to psychotherapy; perhaps in a manner analogous to D-cycloserine facilitation of psychotherapies (88). Similarly, as both ketamine and lithium inhibit glycogen synthase kinase-3β (89, 90), the possibility of synergism of these treatments should be explored.
Glutamate and the neurobiology of depression
Glutamatergic neurotransmission emerged over a decade ago as an extremely important area for depression research (91). This progress was inevitable as glutamate neurons are important targets for monoamine systems previously implicated in depression neurobiology and treatment, cortical neuroimaging studies in depression describe alterations in glutamatergic connectivity, and glutamatergic functional connectivity is targeted by both psychotherapeutic and neurostimulation treatments for depression (92).
In order to set the stage for the subsequent consideration of novel treatment mechanisms, this review will briefly consider the downstream consequences of the loss of glia. A more detailed consideration of these issues is presented in another paper in this issue (Sanacora et al. Biol Psychiatry this issue). Glia are key regulators of glutamatergic neurotransmission because of their role in the inactivation of glutamate (93). There is robust evidence that astrocyte and satellite oligodendrocyte populations are reduced in prefrontal cortex and other cortical regions in post-mortem tissue from individuals who had suffered from major depression (94) and bipolar disorder (95), consistent with the efficacy of ketamine in both unipolar and bipolar depression, as noted earlier. The glial loss in depression may reflect the convergence of many features of stress response in animal models that are also associated with episodes of major depression in humans. These factors include immunologic attack on glial integrity as a consequence of elevated brain cytokine and cortisol levels, increased levels of reactive oxygen species as a consequence of metabolic activation and reduced levels of free radical scavengers including glutathione, and stress-induced glutamate release(96, 97).
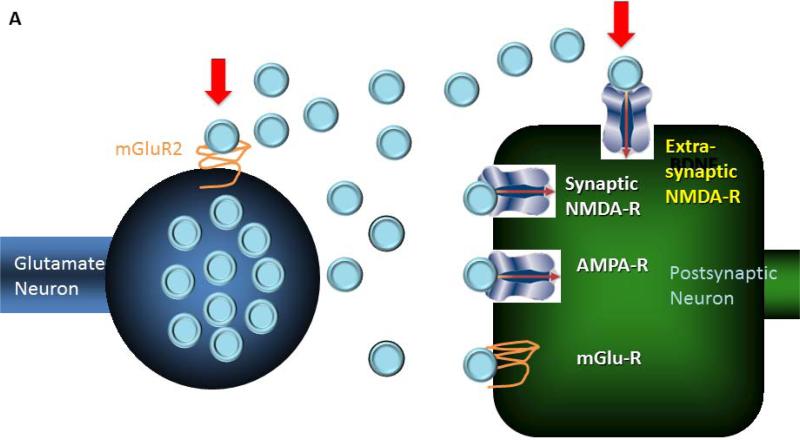
In preclinical stress models, glial loss has a number of downstream consequences that may carryover into the pathophysiology of major depression. Glial loss, produced by specific glial toxins, produces biochemical and behavioral signs of depression in animal models (98). As glia are centrally involved in glutamate inactivation, glial loss may elevate glutamate levels in both synaptic and extrasynaptic spaces. Increased extrasynaptic glutamate levels would be expected to have additional consequences for glutamate synaptic transmission (see figure 3A). First, one might see excessive stimulation of perisynaptic and extrasynaptic metabotropic glutamate receptors (mGluRs). Overstimulation of presynaptic mGluR2 receptors has been shown to depress glutamate neurotransmission and compromise synaptic connectivity (99), consistent with the association of elevated anterior cingulate glutamate levels and reduced cingulate functional connectivity in major depression (100). Stress induced glutamate release also may enhance an NMDA/AMPA bias in signaling as stimulation of postsynaptic group I mGluRs enhances NMDA receptor signaling promotes long-term depression and internalization of AMPA receptors (101). A second consequence of elevated extrasynaptic glutamate levels is overstimulation of extrasynaptic NMDA receptors, particularly those bearing the GluN2B/NR2B subunit. Overstimulation of these receptors has a number of downstream consequences including phosphorylation of elongation factor 2 (ElF2), suppression of CREB levels, reduction in BDNF levels, culminating in dendritic regression and activation of apoptotic signaling pathways (102, 103). These processes contribute to loss of dendritic spines and dendritic atrophy consistently reported in animal models of stress and may contribute to neuronal atrophy and synapse loss in major depression (104). Thus, glial deficits in depression may contribute to abnormalities in cortical functional connectivity by compromising structural connectivity and dysregulating glutamate synaptic transmission.
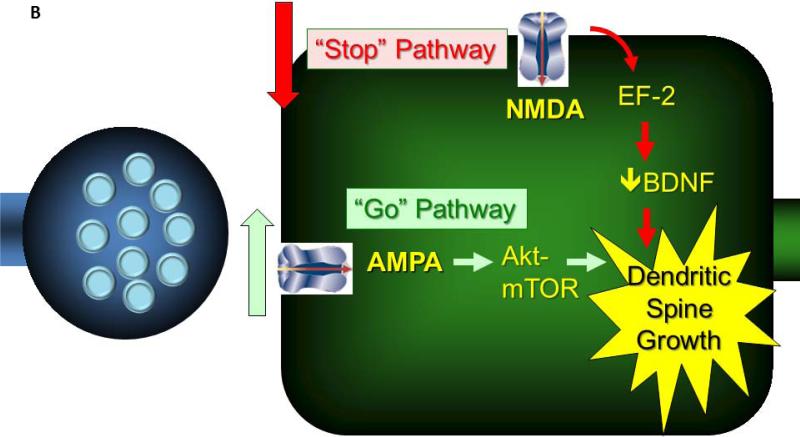
Figure 3.
A figure illustrating a link between the neurobiology of depression and the mechanisms through which ketamine produces its antidepressant effects.
A. Two consequences of glial deficits or dysfunction on glutamate neurotransmission are illustrated. It is hypothesized that glial loss elevates glutamate levels (blue circles) in the extracellular space. On the left side, this figure depicts how glutamate overflow may stimulate inhibitory presynaptic mGluR2 receptors, located at the periphery of these glutamate synapses. Through this mechanism, glial loss may depress glutamate neurotransmission, compromising functional connectivity. The right side of this figure shows elevated extracellular glutamate causing overstimulation of extrasynaptic NMDA receptors, particularly those containing the GluN2B subunit. Through this mechanism, glial loss may activate a signaling cascade responsible for loss of dendritic spines and dendritic regression, contributing to impaired functional connectivity.
B. Ketamine is thought to produce its antidepressant effects by disinhibiting the release of glutamate by the presynaptic neuron, increasing the stimulation of the “go” pathway, i.e., postsynaptic AMPA receptors and signaling via the Akt/mTOR pathway (87). In addition, it blocks extrasynaptic NMDA receptors, reducing signaling via ElF2, which suppresses BDNF levels (103). Although other ketamine effects may contribute in important ways to its antidepressant effects, these converging effects of ketamine may contribute to its capacity to rapidly increase the number of dendritic spines and to restore aspects of functional connectivity.
From glutamate pathophysiology to novel therapeutics
This model of glutamate synaptic dysfunction in depression yields a number of testable hypotheses regarding the treatment of this disorder. Studies support the hypothesis that immunologic activation, perhaps mediated by glial dysfunction, contributes to the emergence of depression-like states that are responsive to the effects of NMDA receptor antagonists. For example, NMDA receptor antagonists appear to treat fatigue and dysphoric mood associated with multiple sclerosis and interferon treatment for hepatitis C infection (105, 106). A component of this response to NMDA receptor antagonists may be related to their anti-inflammatory effects (107).
New pathways to antidepressant development are suggested by the two novel mechanisms underlying the antidepressant efficacy of ketamine, i.e., that ketamine overcomes glutamate synaptic depression by 1) stimulating synaptic glutamate release and enhancing synaptic AMPA receptor signaling and 2) blocks extrasynaptic NMDA receptors (see figure 3B). The hypothesis that ketamine might increase synaptic release is supported by a number of observations related to the effects of NMDA receptor antagonists: 1) they reduce the activation of GABA neurons in hippocampus and prefrontal cortex, 2) they increase neuronal firing and raise extracellular glutamate levels in PFC, and 3) they increase cortical glutamate levels and activation (30, 108-110). The increase in glutamate release produced by ketamine seems to be essential for its antidepressant effects as AMPA receptor antagonists block its efficacy in animal models (111). Also, reduced glutamatergic activation appears to predict the magnitude of the antidepressant response to ketamine in humans (112). Once stimulation of AMPA receptors is restored, antidepressant effects are mediated by stimulation of signaling in the Akt/mTOR pathway, resulting in a rapid sprouting of dendritic spines (87). These effects are dependent upon the induction of brain-derived neurotrophic factor (BDNF), as the antidepressant effects of ketamine in both animals and humans are attenuated in association with the low activity Met allele of the of the BDNF gene (113, 114). This property may render BDNF genotyping an important biomarker for the clinical response to ketamine in humans. The blockade of extrasynaptic NMDA receptors also appears to be critical to the antidepressant effects of ketamine. By preventing the phosphorylation of ElF2 and relieving inhibition of BDNF synthesis, NMDA receptor antagonists enable the regrowth of dendritic spines and contribute to antidepressant behavioral effects (103).
The first alternatives to ketamine suggested by this model are drugs that selectively block NMDA receptors bearing the GluN2B subunit. This subunit is an important component of the extrasynaptic NMDA receptors (102). Perhaps for this reason, there might be greater separation of the dose thresholds for the antidepressant and psychotigenic effects of GluN2B selective and non-selective NMDA receptor antagonists. To date, the data do not adequately test this hypothesis. For example, the GluN2B-preferring NMDA receptor antagonist, CP 101,606 (115) had rapidly emerging antidepressant effects, but it also produced dissociative effects in 6 of 15 patients. In another study, no reports of psychosis emerged during 12 days of oral dosing of a GluN2B-preferring NMDA receptor antagonist, MK-0657, but antidepressant effects during this study were quite limited (116).
Several treatment mechanisms predicted by their ability to reduce the consequences of excessive extrasynaptic glutamate levels show efficacy in animal models of depression. Harkening back to Crane's early work, a number of groups have studied partial agonists at the glycineB site of the NMDA glutamate receptor including ACPC (24). Glyx-13 is another promising NMDA receptor partial agonist that was initially thought to act via the glycineB site (117), although the specific site at which it acts is not settled. Other treatment mechanisms implicated by this model include mGluR2 and mGluR5 receptor antagonists. Like ketamine, mGluR2 antagonists appear to produce rapid antidepressant effects in animals models (118) that are mediated by increased glutamate release and stimulation of AMPA receptors (119). mGluR2 agonists have anxiolytic activity in animals and humans (120). Thus, if mGluR2 antagonists are developed for humans, it will be important to determine whether they have anxiogenic effects. mGluR5 receptor antagonists, known to potentiate psychotigenic effects of NMDA receptor antagonists (120), also have anxiolytic and antidepressant effects in animal models (121, 122). Thus, tests of the efficacy of novel mGluR antidepressants will need to address safety and tolerability issues. AMPA receptor potentiators or AMPAkines also would be predicted to rapid-onset antidepressant effects, consistent with their rapid “antidepressant’ effects in a submission model (123), via their capacity to compensate for deficits in synaptic AMPA receptor function.
Lastly, one might develop strategies for promoting glial viability and function. To the extent that glial function is compromised as a consequence of hypercortisolemia and elevated cytokine levels (Sanacora, this issue), one might attempt to protect glia using glucocorticoid receptor antagonists (124) or cytokine receptor antagonists (125). Another strategy would be to try to protect glia from free radical injury by raising glial glutathione levels. For example, raising glutathione levels using N-acetylcysteine may be helpful as an adjunctive treatment strategy for bipolar depression (126). Lastly, riluzole and beta-lactam antibiotics increase the expression of glutamate transporters and show efficacy in animal models (98) (Sanacora, this issue). To date, there are preliminary open label data supporting the efficacy of riluzole in the treatment of depression (127), but larger and more rigorous studies are needed.
Discussion
Research on rapid-acting antidepressants is shaping expectations regarding what might be achieved through antidepressant treatment. Ketamine research has proven to unlock new insights into the neurobiology of depression and to point to new and otherwise unexpected classes of antidepressant medications. Fifty years of antidepressant research suggested that antidepressants needed to act on monoamine systems and that treatment required weeks to months to produce benefits in responding patients. It is now clear that significant clinical improvement in depression symptoms may occur within hours of drug administration. Further, ketamine and the putative antidepressants that have followed it have clearly demonstrated that antidepressants need not produce their therapeutic effects via direct effects on monoamine receptors. Ketamine administration remains a research procedure with significant potential risks. Despite growing enthusiasm for rapid implementation, further research will be needed before it is appropriate to consider it a routine treatment for depression. Until that time, ketamine should be limited predominately to research contexts to insure that adequate review of risks and benefits has occurred prior to ketamine infusion, enhance the informed consent process prior to ketamine infusion, protect individuals patients undergoing ketamine treatment, and increase the likelihood that the growing experience with ketamine infusion informs the field of depression research. Ketamine has proven to be a useful prototype for rapid-acting antidepressants, but there may be better alternatives to ketamine for this purpose. Nonetheless, it is a moment of tremendous excitement. Important new treatments may emerge from the research directions outlined in this paper that may help to address the tremendous need for depression treatments that are more effective and that relieve depression rapidly.
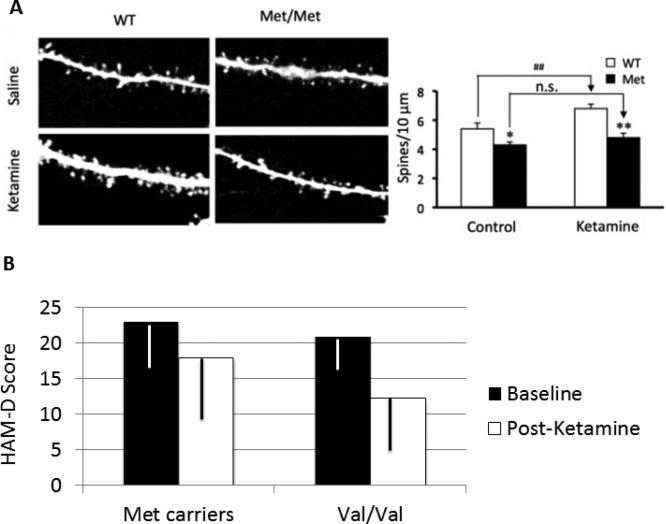
Figure 4.
This figure depicts the impact of a Val66Met polymorphism in the BDNF gene on the effects of ketamine upon dendritic spine growth in rodents (figure A) and on improvement in depression (Hamilton Depression Scale Score: HamD Score).
A. The left slide of this figure presents the results of two-photon microscopy of labeled layer 5 pyramidal neurons in slices from the prefrontal cortex. A robust proliferation of large and long dendritic spines is observed following ketamine administration in Wild Type (WT) animals. However, mice that have had the Met allele inserted into the BDNF gene (Met/Met), rendering that gene less effective, show 1) reduced dendritic spine density compared with the WT animals at baseline, and 2) markedly blunted increases in dendritic spine density following administration. *:p<0.05, **/##:p<0.01, n.s.=not significant. This figure is reprinted from Liu, et al. (113).
B. This figure presents the association of the rs6265 SNP in the BDNF gene with clinical response to ketamine in patients with major depression (data from (114)). In this group, 41 patients possessed the Val/Val genotype, 19 patients were Val/Met, and 2 patients were Met/Met. Fifty-eight patients were of European ancestry and 4 patients were African-American. The interaction of genotype and ketamine effects was highly significant (F = 5.59, df = 4, p = .0007).
Acknowledgements
The authors gratefully acknowledge the support of the State of Connecticut Department of Mental Health and Addiction Services for its support of the Abraham Ribicoff Research Facilities of the Connecticut Mental Health Center, the Department of Veterans Affairs, via its funding of the VA National Center for PTSD, the National Institute of Mental Health (MH17871, MH093897), and the National Center for Advancing Translational Science (CTSA Grant Number UL1 RR024139). Dr. Krystal has served as a scientific consultant to the following companies (the Individual Consultant Agreements listed are < $10,000/year): Aisling Capital, Astellas Pharma Global Development, AstraZeneca Pharmaceuticals, Biocortech, Brintnall & Nicolini, Easton Associates, Gilead Sciences, GlaxoSmithKline, Janssen Pharmaceuticals, Lundbeck Research USA, Medivation, Merz Pharmaceuticals, MK Medical Communications, F. Hoffmann-La Roche, SK Holdings, Sunovion Pharmaceuticals, Takeda Industries, and Teva Pharmaceutical Industries. He is on the Scientific Advisory Board for the following companies: Abbott Laboratories, Bristol-Myers-Squibb, Eisai, Eli Lilly, Forest Laboratories, Lohocla Research Corporation, Mnemosyne Pharmaceuticals, Naurex, Pfizer Pharmaceuticals, and Shire Pharmaceuticals. He holds < $150 in exercisable warrant options with Tetragenex Pharmaceuticals. He is on the Board of Directors of the Coalition for Translational Research in Alcohol and Substance Use Disorders. He was the principal investigator of a multicenter study in which Janssen Research Foundation provided drug and some support to the Department of Veterans Affairs. He is Editor of Biological Psychiatry (Income > $10,000). He has patents and inventions: 1) Seibyl JP, Krystal JH, Charney DS. Dopamine and noradrenergic reuptake inhibitors in treatment of schizophrenia. Patent number: 5447948, 5 September 1995; 2) he is a co-inventor with Dr. Gerard Sanacora on a filed patent application by Yale University related to targeting the glutamatergic system for the treatment of neuropsychiatric disorders (PCTWO06108055A1); 3) Intranasal Administration of Ketamine to Treat Depression (pending). Dr. Sanacora has received consulting fees from Abbott Laboratories, AstraZeneca, Avanier Pharmaceuticals, Bristol-Myers Squibb, Evotec, Eli Lilly & Co., Hoffman La-Roche, Novartis, and Novum Pharmaceuticals over the last 24 months. He has also received additional grant support from AstraZeneca, Bristol-Myers Squibb, Hoffman La-Roche, Merck & Co., and Sunovion Inc over the past 24 months. In addition, he is a coinventor on a filed patent application by Yale University (PCTWO06108055A1). Dr. Duman reports having received honorarium fees from Lilly, Pfizer, Bristol Myers Squibb, Johnson & Johnson, Forest, and Lundbeck; consulting fees from Taisho; and research support rom Lilly, Lundbeck, Johnson & Johnson, and Forest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lopez-Munoz F, Alamo C. Monoaminergic neurotransmission: the history of the discovery of antidepressants from 1950s until today. Curr Pharm Des. 2009;15(14):1563–86. doi: 10.2174/138161209788168001. [DOI] [PubMed] [Google Scholar]
- 2.Donoghue J. Selective serotonin reuptake inhibitor use in primary care: a 5-year naturalistic study. Clin Drug Investig. 1998;16(6):453–62. doi: 10.2165/00044011-199816060-00005. [DOI] [PubMed] [Google Scholar]
- 3.Turner EH, Matthews AM, Linardatos E, Tell RA, Rosenthal R. Selective publication of antidepressant trials and its influence on apparent efficacy. N Engl J Med. 2008;358(3):252–60. doi: 10.1056/NEJMsa065779. [DOI] [PubMed] [Google Scholar]
- 4.Kirsch I, Deacon BJ, Huedo-Medina TB, Scoboria A, Moore TJ, Johnson BT. Initial severity and antidepressant benefits: a meta-analysis of data submitted to the Food and Drug Administration. PLoS Med. 2008;5(2):e45. doi: 10.1371/journal.pmed.0050045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Organization WH. The global burden of diseasse: 2004 update. WHO Press; Geneva, Switzerland: 2008. [Google Scholar]
- 6.Gaynes BN, Warden D, Trivedi MH, Wisniewski SR, Fava M, Rush AJ. What did STAR*D teach us? Results from a large-scale, practical, clinical trial for patients with depression. Psychiatr Serv. 2009;60(11):1439–45. doi: 10.1176/ps.2009.60.11.1439. [DOI] [PubMed] [Google Scholar]
- 7.Krystal JH, G. S, Blumberg H, Anand A, Charney DS, Marek G, et al. Glutamate and GABA systems as targets for novel antidepressant and mood stabilizing treatments. Molecular Psychiatry. 2002;7:S71–S80. doi: 10.1038/sj.mp.4001021. [DOI] [PubMed] [Google Scholar]
- 8.Sanacora G, Zarate CA, Krystal JH, Manji HK. Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nat Rev Drug Discov. 2008;7(5):426–37. doi: 10.1038/nrd2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duman RS, Li N, Liu RJ, Duric V, Aghajanian G. Signaling pathways underlying the rapid antidepressant actions of ketamine. Neuropharmacology. 2012;62(1):35–41. doi: 10.1016/j.neuropharm.2011.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crane GE. Cycloserine as an antidepressant agent. American Journal of Psychiatry. 1959;115:1025–6. doi: 10.1176/ajp.115.11.1025. [DOI] [PubMed] [Google Scholar]
- 11.Crane GE. The psychotropic effect of cycloserine: a new use of an antibiotic. Comprehenisve Psychiatry. 1961;2:51–9. [Google Scholar]
- 12.Heresco-Levy U, Gelfin G, Bloch B, Levin R, Edelman S, Javitt DC, et al. A randomized add-on trial of high-dose d-cycloserine for treatment-resistant depression. Int J Neuropsychopharmacol. 2013;16(3):501–6. doi: 10.1017/S1461145712000910. [DOI] [PubMed] [Google Scholar]
- 13.Sheinin A, Shavit S, Benveniste M. Subunit specificity and mechanism of action of NMDA partial agonist D-cycloserine. Neuropharmacology. 2001;41(2):151–8. doi: 10.1016/s0028-3908(01)00073-9. [DOI] [PubMed] [Google Scholar]
- 14.Dravid SM, Burger PB, Prakash A, Geballe MT, Yadav R, Le P, et al. Structural determinants of D-cycloserine efficacy at the NR1/NR2C NMDA receptors. J Neurosci. 2010;30(7):2741–54. doi: 10.1523/JNEUROSCI.5390-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vale S, Espejel MA, Dominguez JC. Amantadine in depression. Lancet. 1971;2(7721):437. doi: 10.1016/s0140-6736(71)90153-x. [DOI] [PubMed] [Google Scholar]
- 16.Danysz W, Parsons CG, Kornhuber J, Schmidt WJ, Quack G. Aminoadamantanes as NMDA receptor antagonists and antiparkinsonian agents--preclinical studies. Neuroscience & Biobehavioral Reviews. 1997;21(4):455–68. doi: 10.1016/s0149-7634(96)00037-1. [DOI] [PubMed] [Google Scholar]
- 17.Zarate CA, Jr., Singh JB, Quiroz JA, De Jesus G, Denicoff KK, Luckenbaugh DA, et al. A double-blind, placebo-controlled study of memantine in the treatment of major depression. Am J Psychiatry. 2006;163(1):153–5. doi: 10.1176/appi.ajp.163.1.153. [DOI] [PubMed] [Google Scholar]
- 18.Anand A, Gunn AD, Barkay G, Karne HS, Nurnberger JI, Mathew SJ, et al. Early antidepressant effect of memantine during augmentation of lamotrigine inadequate response in bipolar depression: a double-blind, randomized, placebo-controlled trial. Bipolar Disord. 2012;14(1):64–70. doi: 10.1111/j.1399-5618.2011.00971.x. [DOI] [PubMed] [Google Scholar]
- 19.Stevens J, Bies RR, Shekhar A, Anand A. Bayesian Model of HDRS with Memantine Augmentation in Bipolar Depression. Br J Clin Pharmacol. 2012 doi: 10.1111/j.1365-2125.2012.04398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferguson JM, Shingleton RN. An open-label, flexible-dose study of memantine in major depressive disorder. Clin Neuropharmacol. 2007;30(3):136–44. doi: 10.1097/WNF.0b013e3180314ae7. [DOI] [PubMed] [Google Scholar]
- 21.Zarate CA, Jr., Mathews D, Ibrahim L, Chaves JF, Marquardt C, Ukoh I, et al. A Randomized Trial of a Low-Trapping Nonselective N-Methyl-D-Aspartate Channel Blocker in Major Depression. Biol Psychiatry. 2012 doi: 10.1016/j.biopsych.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skolnick P, Popik P, Trullas R. Glutamate-based antidepressants: 20 years on. Trends Pharmacol Sci. 2009;30(11):563–9. doi: 10.1016/j.tips.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 23.Sofia RD, Harakal JJ. Evaluation of ketamine HCl for anti-depressant activity. Arch Int Pharmacodyn Ther. 1975;214(1):68–74. [PubMed] [Google Scholar]
- 24.Trullas R, Skolnick P. Functional antagonists at the NMDA receptor complex exhibit antidepressant actions. European Journal of Pharmacology. 1990;185(1):1–10. doi: 10.1016/0014-2999(90)90204-j. [DOI] [PubMed] [Google Scholar]
- 25.Trullas R, Folio T, Young A, Miller R, Boje K, Skolnick P. 1-aminocyclopropanecarboxylates exhibit antidepressant and anxiolytic actions in animal models. European Journal of Pharmacology. 1991;203(3):379–85. doi: 10.1016/0014-2999(91)90894-v. [DOI] [PubMed] [Google Scholar]
- 26.Layer RT, Popik P, Olds T, Skolnick P. Antidepressant-like actions of the polyamine site NMDA antagonist, eliprodil (SL-82.0715). Pharmacology, Biochemistry & Behavior. 1995;52(3):621–7. doi: 10.1016/0091-3057(95)00155-p. [DOI] [PubMed] [Google Scholar]
- 27.Nowak G, Trullas R, Layer RT, Skolnick P, Paul IA. Adaptive changes in the N-methyl-D-aspartate receptor complex after chronic treatment with imipramine and 1-aminocyclopropanecarboxylic acid. Journal of Pharmacology & Experimental Therapeutics. 1993;265(3):1380–6. [PubMed] [Google Scholar]
- 28.Paul IA, Layer RT, Skolnick P, Nowak G. Adaptation of the NMDA receptor in rat cortex following chronic electroconvulsive shock or imipramine. European Journal of Pharmacology. 1993;247(3):305–11. doi: 10.1016/0922-4106(93)90199-j. [DOI] [PubMed] [Google Scholar]
- 29.Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51(3):199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- 30.Krystal JH, D'Souza DC, Mathalon D, Perry E, Belger A, Hoffman R. NMDA receptor antagonist effects, cortical glutamatergic function, and schizophrenia: toward a paradigm shift in medication development. Psychopharmacology (Berl) 2003;169(3-4):215–33. doi: 10.1007/s00213-003-1582-z. [DOI] [PubMed] [Google Scholar]
- 31.Krystal JH, Petrakis IL, Krupitsky E, Schutz C, Trevisan L, D'Souza DC. NMDA receptor antagonism and the ethanol intoxication signal: from alcoholism risk to pharmacotherapy. Ann N Y Acad Sci. 2003;1003:176–84. doi: 10.1196/annals.1300.010. [DOI] [PubMed] [Google Scholar]
- 32.Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47(4):351–4. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 33.Zarate CA, Jr., Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63(8):856–64. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 34.Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, et al. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. 2010;67(8):793–802. doi: 10.1001/archgenpsychiatry.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ibrahim L, Diazgranados N, Luckenbaugh DA, Machado-Vieira R, Baumann J, Mallinger AG, et al. Rapid decrease in depressive symptoms with an N-methyl-d-aspartate antagonist in ECT-resistant major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011 doi: 10.1016/j.pnpbp.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aan Het Rot M, Zarate CA, Jr., Charney DS, Mathew SJ. Ketamine for Depression: Where Do We Go from Here? Biol Psychiatry. 2012 doi: 10.1016/j.biopsych.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murrough JW. Ketamine as a novel antidepressant: from synapse to behavior. Clin Pharmacol Ther. 2012;91(2):303–9. doi: 10.1038/clpt.2011.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Diazgranados N, Ibrahim LA, Brutsche NE, Ameli R, Henter ID, Luckenbaugh DA, et al. Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment-resistant major depressive disorder. J Clin Psychiatry. 2010 doi: 10.4088/JCP.09m05327blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Price RB, Nock MK, Charney DS, Mathew SJ. Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol Psychiatry. 2009;66(5):522–6. doi: 10.1016/j.biopsych.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, et al. A randomized add-on trial of an N-methyl-D-aspartate (NMDA) antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. 2010 doi: 10.1001/archgenpsychiatry.2010.90. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Phelps LE, Brutsche N, Moral JR, Luckenbaugh DA, Manji HK, Zarate CA., Jr. Family History of Alcohol Dependence and Initial Antidepressant Response to an N-methyl-D-aspartate Antagonist. Biol Psychiatry. 2009;65(2):181–4. doi: 10.1016/j.biopsych.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luckenbaugh DA, Ibrahim L, Brutsche N, Franco-Chaves J, Mathews D, Marquardt CA, et al. Family history of alcohol dependence and antidepressant response to an N-methyl-d-aspartate antagonist in bipolar depression. Bipolar Disord. 2012 doi: 10.1111/bdi.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muhonen LH, Lonnqvist J, Juva K, Alho H. Double-blind, randomized comparison of memantine and escitalopram for the treatment of major depressive disorder comorbid with alcohol dependence. J Clin Psychiatry. 2008;69(3):392–9. doi: 10.4088/jcp.v69n0308. [DOI] [PubMed] [Google Scholar]
- 44.Thangathurai D, Roby J, Roffey P. Treatment of resistant depression in patients with cancer with low doses of ketamine and desipramine. J Palliat Med. 2010;13(3):235. doi: 10.1089/jpm.2009.0312. [DOI] [PubMed] [Google Scholar]
- 45.Zanicotti CG, Perez D, Glue P. Mood and pain responses to repeat dose intramuscular ketamine in a depressed patient with advanced cancer. J Palliat Med. 2012;15(4):400–3. doi: 10.1089/jpm.2011.0314. [DOI] [PubMed] [Google Scholar]
- 46.Reich DL, Silvay G. Ketamine: an update on the first twenty-five years of clinical experience. Canadian Journal of Anaesthesiology. 1989;36(2):186–97. doi: 10.1007/BF03011442. [DOI] [PubMed] [Google Scholar]
- 47.Perry EB, Jr., Cramer JA, Cho HS, Petrakis IL, Karper LP, Genovese A, et al. Psychiatric safety of ketamine in psychopharmacology research. Psychopharmacology (Berl) 2007;192(2):253–60. doi: 10.1007/s00213-007-0706-2. [DOI] [PubMed] [Google Scholar]
- 48.Carpenter WT., Jr. The schizophrenia ketamine challenge study debate. Biol Psychiatry. 1999;46(8):1081–91. doi: 10.1016/s0006-3223(99)00194-8. [DOI] [PubMed] [Google Scholar]
- 49.Lahti AC, Warfel D, Michaelidis T, Weiler MA, Frey K, Tamminga CA. Long-term outcome of patients who receive ketamine during research. Biol Psychiatry. 2001;49(10):869–75. doi: 10.1016/s0006-3223(00)01037-4. [DOI] [PubMed] [Google Scholar]
- 50.aan het Rot M, Collins KA, Murrough JW, Perez AM, Reich DL, Charney DS, et al. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry. 2010;67(2):139–45. doi: 10.1016/j.biopsych.2009.08.038. [DOI] [PubMed] [Google Scholar]
- 51.Zarate CA, Jr., Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, Cravchik A, et al. Replication of ketamine's antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry. 2012;71(11):939–46. doi: 10.1016/j.biopsych.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cusin C, Hilton GQ, Nierenberg AA, Fava M. Long-Term Maintenance With Intramuscular Ketamine for Treatment-Resistant Bipolar II Depression. Am J Psychiatry. 2012;169(8):868–9. doi: 10.1176/appi.ajp.2012.12020219. [DOI] [PubMed] [Google Scholar]
- 53.Ricke AK, Snook RJ, Anand A. Induction of prolonged mania during ketamine therapy for reflex sympathetic dystrophy. Biol Psychiatry. 2011;70(4):e13–4. doi: 10.1016/j.biopsych.2011.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Perry EB, Cramer J, Cho H-S, Petrakis I, Karper L, Krystal JH, et al. Psychiatric safety of ketamine in clinical psychopharmacology research. Psychopharmacology. 2007 doi: 10.1007/s00213-007-0706-2. epublication. [DOI] [PubMed] [Google Scholar]
- 55.Ng SH, Tse ML, Ng HW, Lau FL. Emergency department presentation of ketamine abusers in Hong Kong: a review of 233 cases. Hong Kong Med J. 2010;16(1):6–11. [PubMed] [Google Scholar]
- 56.Irwin SA, Iglewicz A. Oral ketamine for the rapid treatment of depression and anxiety in patients receiving hospice care. J Palliat Med. 2010;13(7):903–8. doi: 10.1089/jpm.2010.9808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Paslakis G, Gilles M, Meyer-Lindenberg A, Deuschle M. Oral administration of the NMDA receptor antagonist S-ketamine as add-on therapy of depression: a case series. Pharmacopsychiatry. 2010;43(1):33–5. doi: 10.1055/s-0029-1237375. [DOI] [PubMed] [Google Scholar]
- 58.Wilson MD, Ferguson RW, Mazer ME, Litovitz TL. Monitoring trends in dextromethorphan abuse using the National Poison Data System: 2000-2010. Clin Toxicol (Phila) 2011;49(5):409–15. doi: 10.3109/15563650.2011.585429. [DOI] [PubMed] [Google Scholar]
- 59.Reissig CJ, Carter LP, Johnson MW, Mintzer MZ, Klinedinst MA, Griffiths RR. High doses of dextromethorphan, an NMDA antagonist, produce effects similar to classic hallucinogens. Psychopharmacology (Berl) 2012;223(1):1–15. doi: 10.1007/s00213-012-2680-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Murrough JW, Perez AM, Pillemer S, Stern J, Parides MK, Aan Het Rot M, et al. Rapid and Longer-Term Antidepressant Effects of Repeated Ketamine Infusions in Treatment-Resistant Major Depression. Biol Psychiatry. 2012 doi: 10.1016/j.biopsych.2012.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Trujillo KA, Zamora JJ, Warmoth KP. Increased response to ketamine following treatment at long intervals: implications for intermittent use. Biol Psychiatry. 2008;63(2):178–83. doi: 10.1016/j.biopsych.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 62.Cho HS, D'Souza DC, Gueorguieva R, Perry EB, Madonick S, Karper LP, et al. Absence of behavioral sensitization in healthy human subjects following repeated exposure to ketamine. Psychopharmacology (Berl) 2005;179(1):136–43. doi: 10.1007/s00213-004-2066-5. [DOI] [PubMed] [Google Scholar]
- 63.Blier P, Zigman D, Blier J. On the Safety and Benefits of Repeated Intravenous Injections of Ketamine For Depression. Biol Psychiatry. 2012 doi: 10.1016/j.biopsych.2012.02.039. [DOI] [PubMed] [Google Scholar]
- 64.Murrough JW, Perez AM, Mathew SJ, Charney DS. A case of sustained remission following an acute course of ketamine in treatment-resistant depression. J Clin Psychiatry. 2011;72(3):414–5. doi: 10.4088/JCP.10l06447blu. [DOI] [PubMed] [Google Scholar]
- 65.Manallack DT, Lodge D, Beart PM. Subchronic administration of MK-801 in the rat decreases cortical binding of [3H]D-AP5, suggesting down-regulation of the cortical N-methyl-D-aspartate receptors. Neuroscience. 1989;30(1):87–94. doi: 10.1016/0306-4522(89)90355-2. [DOI] [PubMed] [Google Scholar]
- 66.Braun I, Genius J, Grunze H, Bender A, Moller HJ, Rujescu D. Alterations of hippocampal and prefrontal GABAergic interneurons in an animal model of psychosis induced by NMDA receptor antagonism. Schizophr Res. 2007;97(1-3):254–63. doi: 10.1016/j.schres.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 67.Jentsch JD, Taylor JR, Roth RH. Subchronic phencyclidine administration increases mesolimbic dopaminergic system responsivity and augments stress- and psychostimulant-induced hyperlocomotion. Neuropsychopharmacology. 1998;19(2):105–13. doi: 10.1016/S0893-133X(98)00004-9. [DOI] [PubMed] [Google Scholar]
- 68.Jentsch JD, Redmond DE, Jr., Elsworth JD, Taylor JR, Youngren KD, Roth RH. Enduring cognitive deficits and cortical dopamine dysfunction in monkeys after long-term administration of phencyclidine. Science. 1997;277(5328):953–5. doi: 10.1126/science.277.5328.953. [DOI] [PubMed] [Google Scholar]
- 69.Neill JC, Barnes S, Cook S, Grayson B, Idris NF, McLean SL, et al. Animal models of cognitive dysfunction and negative symptoms of schizophrenia: Focus on NMDA receptor antagonism. Pharmacol Ther. 2010 doi: 10.1016/j.pharmthera.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 70.Behrens MM, Sejnowski TJ. Does schizophrenia arise from oxidative dysregulation of parvalbumin-interneurons in the developing cortex? Neuropharmacology. 2009;57(3):193–200. doi: 10.1016/j.neuropharm.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sharp FR, Butman M, Koistinaho J, Aardalen K, Nakki R, Massa SM, et al. Phencyclidine induction of the hsp 70 stress gene in injured pyramidal neurons is mediated via multiple receptors and voltage gated calcium channels. Neuroscience. 1994;62(4):1079–92. doi: 10.1016/0306-4522(94)90345-x. [DOI] [PubMed] [Google Scholar]
- 72.Olney JW, Labruyere J, Price MT. Pathological changes induced in cerebrocortical neurons by phencyclidine and related drugs. Science. 1989;244(4910):1360–2. doi: 10.1126/science.2660263. [DOI] [PubMed] [Google Scholar]
- 73.Nakki R, Nickolenko J, Chang J, Sagar SM, Sharp FR. Haloperidol prevents ketamine- and phencyclidine-induced HSP70 protein expression but not microglial activation. Exp Neurol. 1996;137(2):234–41. doi: 10.1006/exnr.1996.0022. [DOI] [PubMed] [Google Scholar]
- 74.Morgan CJ, Curran HV. Acute and chronic effects of ketamine upon human memory: a review. Psychopharmacology (Berl) 2006;188(4):408–24. doi: 10.1007/s00213-006-0572-3. [DOI] [PubMed] [Google Scholar]
- 75.Morgan CJ, Curran HV. Ketamine use: a review. Addiction. 2012;107(1):27–38. doi: 10.1111/j.1360-0443.2011.03576.x. [DOI] [PubMed] [Google Scholar]
- 76.Liao Y, Tang J, Ma M, Wu Z, Yang M, Wang X, et al. Frontal white matter abnormalities following chronic ketamine use: a diffusion tensor imaging study. Brain. 2010;133(Pt 7):2115–22. doi: 10.1093/brain/awq131. [DOI] [PubMed] [Google Scholar]
- 77.Liao Y, Tang J, Corlett PR, Wang X, Yang M, Chen H, et al. Reduced dorsal prefrontal gray matter after chronic ketamine use. Biol Psychiatry. 2011;69(1):42–8. doi: 10.1016/j.biopsych.2010.08.030. [DOI] [PubMed] [Google Scholar]
- 78.Liao Y, Tang J, Fornito A, Liu T, Chen X, Chen H, et al. Alterations in regional homogeneity of resting-state brain activity in ketamine addicts. Neurosci Lett. 2012;522(1):36–40. doi: 10.1016/j.neulet.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 79.Chen LY, Chen KP, Huang MC. Cystitis associated with chronic ketamine abuse. Psychiatry Clin Neurosci. 2009;63(4):591. doi: 10.1111/j.1440-1819.2009.01972.x. [DOI] [PubMed] [Google Scholar]
- 80.Wong SW, Lee KF, Wong J, Ng WW, Cheung YS, Lai PB. Dilated common bile ducts mimicking choledochal cysts in ketamine abusers. Hong Kong Med J. 2009;15(1):53–6. [PubMed] [Google Scholar]
- 81.Mathew SJ, Murrough JW, aan het Rot M, Collins KA, Reich DL, Charney DS. Riluzole for relapse prevention following intravenous ketamine in treatment-resistant depression: a pilot randomized, placebo-controlled continuation trial. Int J Neuropsychopharmacol. 2010;13(1):71–82. doi: 10.1017/S1461145709000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ibrahim L, Diazgranados N, Franco-Chaves J, Brutsche N, Henter ID, Kronstein P, et al. Course of improvement in depressive symptoms to a single intravenous infusion of ketamine vs add-on riluzole: results from a 4-week, double-blind, placebo-controlled study. Neuropsychopharmacology. 2012;37(6):1526–33. doi: 10.1038/npp.2011.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Geddes JR, Goodwin GM, Rendell J, Azorin JM, Cipriani A, Ostacher MJ, et al. Lithium plus valproate combination therapy versus monotherapy for relapse prevention in bipolar I disorder (BALANCE): a randomised open-label trial. Lancet. 375(9712):385–95. doi: 10.1016/S0140-6736(09)61828-6. [DOI] [PubMed] [Google Scholar]
- 84.Baethge C, Gruschka P, Smolka MN, Berghofer A, Bschor T, Muller-Oerlinghausen B, et al. Effectiveness and outcome predictors of long-term lithium prophylaxis in unipolar major depressive disorder. J Psychiatry Neurosci. 2003;28(5):355–61. [PMC free article] [PubMed] [Google Scholar]
- 85.Prien RF, Kupfer DJ, Mansky PA, Small JG, Tuason VB, Voss CB, et al. Drug therapy in the prevention of recurrences in unipolar and bipolar affective disorders. Report of the NIMH Collaborative Study Group comparing lithium carbonate, imipramine, and a lithium carbonate-imipramine combination. Arch Gen Psychiatry. 1984;41(11):1096–104. doi: 10.1001/archpsyc.1983.01790220086014. [DOI] [PubMed] [Google Scholar]
- 86.Spanier C, Frank E, McEachran AB, Grochocinski VJ, Kupfer DJ. The prophylaxis of depressive episodes in recurrent depression following discontinuation of drug therapy: integrating psychological and biological factors. Psychol Med. 1996;26(3):461–75. doi: 10.1017/s0033291700035546. [DOI] [PubMed] [Google Scholar]
- 87.Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329(5994):959–64. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Krystal JH, Tolin DF, Sanacora G, Castner SA, Williams GV, Aikins DE, et al. Neuroplasticity as a target for the pharmacotherapy of anxiety disorders, mood disorders, and schizophrenia. Drug Discov Today. 2009;14(13-14):690–7. doi: 10.1016/j.drudis.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Beurel E, Song L, Jope RS. Inhibition of glycogen synthase kinase-3 is necessary for the rapid antidepressant effect of ketamine in mice. Mol Psychiatry. 2011;16(11):1068–70. doi: 10.1038/mp.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Manji HK, Moore GJ, Chen G. Lithium at 50: have the neuroprotective effects of this unique cation been overlooked? Biological Psychiatry. 1999;46(7):929–40. doi: 10.1016/s0006-3223(99)00165-1. [DOI] [PubMed] [Google Scholar]
- 91.Krystal JH, Sanacora G, Blumberg H, Anand A, Charney DS, Marek G, et al. Glutamate and GABA systems as targets for novel antidepressant and mood-stabilizing treatments. Mol Psychiatry. 2002;7(Suppl 1):S71–80. doi: 10.1038/sj.mp.4001021. [DOI] [PubMed] [Google Scholar]
- 92.Holtzheimer PE, Mayberg HS. Stuck in a rut: rethinking depression and its treatment. Trends Neurosci. 2011;34(1):1–9. doi: 10.1016/j.tins.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Magistretti PJ, Pellerin L, Rothman DL, Shulman RG. Energy on demand. Science. 1999;283(5401):496–7. doi: 10.1126/science.283.5401.496. [DOI] [PubMed] [Google Scholar]
- 94.Rajkowska G, Miguel-Hidalgo JJ. Gliogenesis and glial pathology in depression. CNS Neurol Disord Drug Targets. 2007;6(3):219–33. doi: 10.2174/187152707780619326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rajkowska G. Postmortem studies in mood disorders indicate altered numbers of neurons and glial cells. Biol Psychiatry. 2000;48(8):766–77. doi: 10.1016/s0006-3223(00)00950-1. [DOI] [PubMed] [Google Scholar]
- 96.Banasr M, Dwyer JM, Duman RS. Cell atrophy and loss in depression: reversal by antidepressant treatment. Curr Opin Cell Biol. 2011;23(6):730–7. doi: 10.1016/j.ceb.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Duman RS, Voleti B. Signaling pathways underlying the pathophysiology and treatment of depression: novel mechanisms for rapid-acting agents. Trends Neurosci. 2012;35(1):47–56. doi: 10.1016/j.tins.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Banasr M, Chowdhury GM, Terwilliger R, Newton SS, Duman RS, Behar KL, et al. Glial pathology in an animal model of depression: reversal of stress-induced cellular, metabolic and behavioral deficits by the glutamate-modulating drug riluzole. Mol Psychiatry. 2008 doi: 10.1038/mp.2008.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bonansco C, Couve A, Perea G, Ferradas CA, Roncagliolo M, Fuenzalida M. Glutamate released spontaneously from astrocytes sets the threshold for synaptic plasticity. Eur J Neurosci. 2011;33(8):1483–92. doi: 10.1111/j.1460-9568.2011.07631.x. [DOI] [PubMed] [Google Scholar]
- 100.Horn DI, Yu C, Steiner J, Buchmann J, Kaufmann J, Osoba A, et al. Glutamatergic and resting-state functional connectivity correlates of severity in major depression - the role of pregenual anterior cingulate cortex and anterior insula. Front Syst Neurosci. 2010;4 doi: 10.3389/fnsys.2010.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chaouloff F, Hemar A, Manzoni O. Acute stress facilitates hippocampal CA1 metabotropic glutamate receptor-dependent long-term depression. J Neurosci. 2007;27(27):7130–5. doi: 10.1523/JNEUROSCI.1150-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hardingham GE, Bading H. Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat Rev Neurosci. 2010;11(10):682–96. doi: 10.1038/nrn2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475(7354):91–5. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kang HJ, Voleti B, Hajszan T, Rajkowska G, Stockmeier CA, Licznerski P, et al. Decreased expression of synapse-related genes and loss of synapses in major depressive disorder. Nat Med. 2012 doi: 10.1038/nm.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kronenberger B, Berg T, Herrmann E, Hinrichsen H, Gerlach T, Buggisch P, et al. Efficacy of amantadine on quality of life in patients with chronic hepatitis C treated with interferon-alpha and ribavirin: results from a randomized, placebo-controlled, double-blind trial. Eur J Gastroenterol Hepatol. 2007;19(8):639–46. doi: 10.1097/MEG.0b013e3281ac20ca. [DOI] [PubMed] [Google Scholar]
- 106.Krupp LB, Coyle PK, Doscher C, Miller A, Cross AH, Jandorf L, et al. Fatigue therapy in multiple sclerosis: results of a double-blind, randomized, parallel trial of amantadine, pemoline, and placebo. Neurology. 1995;45(11):1956–61. doi: 10.1212/wnl.45.11.1956. [DOI] [PubMed] [Google Scholar]
- 107.Kubera M, Maes M, Budziszewska B, Basta-Kaim A, Leskiewicz M, Grygier B, et al. Inhibitory effects of amantadine on the production of pro-inflammatory cytokines by stimulated in vitro human blood. Pharmacol Rep. 2009;61(6):1105–12. doi: 10.1016/s1734-1140(09)70173-2. [DOI] [PubMed] [Google Scholar]
- 108.Stone JM, Dietrich C, Edden R, Mehta MA, De Simoni S, Reed LJ, et al. Ketamine effects on brain GABA and glutamate levels with 1H-MRS: relationship to ketamine-induced psychopathology. Mol Psychiatry. 2012;17(7):664–5. doi: 10.1038/mp.2011.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chowdhury GM, Behar KL, Cho W, Thomas MA, Rothman DL, Sanacora G. (1)H-[(1)(3)C]-nuclear magnetic resonance spectroscopy measures of ketamine's effect on amino acid neurotransmitter metabolism. Biol Psychiatry. 2012;71(11):1022–5. doi: 10.1016/j.biopsych.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17(8):2921–7. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Maeng S, Zarate CA, Jr., Du J, Schloesser RJ, McCammon J, Chen G, et al. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63(4):349–52. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 112.Salvadore G, van der Veen JW, Zhang Y, Marenco S, Machado-Vieira R, Baumann J, et al. An investigation of amino-acid neurotransmitters as potential predictors of clinical improvement to ketamine in depression. Int J Neuropsychopharmacol. 2012;15(8):1063–72. doi: 10.1017/S1461145711001593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu RJ, Lee FS, Li XY, Bambico F, Duman RS, Aghajanian GK. Brain-derived neurotrophic factor Val66Met allele impairs basal and ketamine-stimulated synaptogenesis in prefrontal cortex. Biol Psychiatry. 2012;71(11):996–1005. doi: 10.1016/j.biopsych.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Laje G, Lally N, Mathews D, Brutsche N, Chemerinski A, Akula N, et al. Brain-Derived Neurotrophic Factor Val66Met Polymorphism and Antidepressant Efficacy of Ketamine in Depressed Patients. Biol Psychiatry. 2012 doi: 10.1016/j.biopsych.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Preskorn SH, Baker B, Kolluri S, Menniti FS, Krams M, Landen JW. An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-D-aspartate antagonist, CP-101,606, in patients with treatment-refractory major depressive disorder. J Clin Psychopharmacol. 2008;28(6):631–7. doi: 10.1097/JCP.0b013e31818a6cea. [DOI] [PubMed] [Google Scholar]
- 116.Ibrahim L, Diazgranados N, Jolkovsky L, Brutsche N, Luckenbaugh DA, Herring WJ, et al. A Randomized, Placebo-Controlled, Crossover Pilot Trial of the Oral Selective NR2B Antagonist MK-0657 in Patients With Treatment-Resistant Major Depressive Disorder. J Clin Psychopharmacol. 2012 doi: 10.1097/JCP.0b013e31825d70d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Burgdorf J, Kroes RA, Weiss C, Oh MM, Disterhoft JF, Brudzynski SM, et al. Positive emotional learning is regulated in the medial prefrontal cortex by GluN2B-containing NMDA receptors. Neuroscience. 2011;192:515–23. doi: 10.1016/j.neuroscience.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chaki S, Yoshikawa R, Hirota S, Shimazaki T, Maeda M, Kawashima N, et al. MGS0039: a potent and selective group II metabotropic glutamate receptor antagonist with antidepressant-like activity. Neuropharmacology. 2004;46(4):457–67. doi: 10.1016/j.neuropharm.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 119.Dwyer JM, Lepack AE, Duman RS. mTOR activation is required for the antidepressant effects of mGluR(2)/(3) blockade. Int J Neuropsychopharmacol. 2012;15(4):429–34. doi: 10.1017/S1461145711001702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Krystal JH, Mathew SJ, D'Souza DC, Garakani A, Gunduz-Bruce H, Charney DS. Potential psychiatric applications of metabotropic glutamate receptor agonists and antagonists. CNS Drugs. 2010;24(8):669–93. doi: 10.2165/11533230-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 121.Palucha A, Branski P, Szewczyk B, Wieronska JM, Klak K, Pilc A. Potential antidepressant-like effect of MTEP, a potent and highly selective mGluR5 antagonist. Pharmacol Biochem Behav. 2005;81(4):901–6. doi: 10.1016/j.pbb.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 122.Belozertseva IV, Kos T, Popik P, Danysz W, Bespalov AY. Antidepressant-like effects of mGluR1 and mGluR5 antagonists in the rat forced swim and the mouse tail suspension tests. Eur Neuropsychopharmacol. 2007;17(3):172–9. doi: 10.1016/j.euroneuro.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 123.Knapp RJ, Goldenberg R, Shuck C, Cecil A, Watkins J, Miller C, et al. Antidepressant activity of memory-enhancing drugs in the reduction of submissive behavior model. European Journal of Pharmacology. 2002;440(1):27–35. doi: 10.1016/s0014-2999(02)01338-9. [DOI] [PubMed] [Google Scholar]
- 124.Gallagher P, Malik N, Newham J, Young AH, Ferrier IN, Mackin P. Antiglucocorticoid treatments for mood disorders. Cochrane Database Syst Rev. 2008;(1):CD005168. doi: 10.1002/14651858.CD005168.pub2. [DOI] [PubMed] [Google Scholar]
- 125.Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, et al. A Randomized Controlled Trial of the Tumor Necrosis Factor Antagonist Infliximab for Treatment-Resistant Depression: The Role of Baseline Inflammatory Biomarkers. Arch Gen Psychiatry. 2012:1–11. doi: 10.1001/2013.jamapsychiatry.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Berk M, Copolov DL, Dean O, Lu K, Jeavons S, Schapkaitz I, et al. N-acetyl cysteine for depressive symptoms in bipolar disorder--a double-blind randomized placebo-controlled trial. Biol Psychiatry. 2008;64(6):468–75. doi: 10.1016/j.biopsych.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 127.Sanacora G, Kendell SF, Fenton L, Coric V, Krystal JH. Riluzole augmentation for treatment-resistant depression. Am J Psychiatry. 2004;161(11):2132. doi: 10.1176/appi.ajp.161.11.2132. [DOI] [PubMed] [Google Scholar]