Abstract
Background
Asthma is a chronic disease associated with airway hyperresponsiveness (AHR), airway obstruction, and airway remodeling. NF-κB is a transcriptional factor that regulates and co-ordinates the expression of various inflammatory genes. The NF-κB subunits, p50 and Rel-A, are translocated to the nucleus by importin α3 and importin α4. There is growing evidence that vitamin D is a potent immunomodulator. However, the evidence for beneficial or adverse effects of vitamin D in asthma is still unclear.
Objective
In this study, we examined the effect of vitamin D status on AHR, airway inflammation, and cytokines in the bronchoalveolar lavage fluid (BALF) in a murine model of allergic asthma.
Methods
Female BALB/c mice were fed with special vitamin D-deficient or vitamin D-sufficient (2,000 IU/kg) or vitamin D-supplemented (10,000 IU/kg) diet for 13 weeks. Mice were sensitized and challenged with ovalbumin. The effect of vitamin D on lung histology, AHR, T-regulatory cells and BALF cytokines was examined. The expression of importin-α3 and Rel-A in the lung of OVA-sensitized mice was analyzed using immunofluorescence.
Results
Vitamin D deficiency was associated with higher AHR in OVA-sensitized and challenged mice than those in vitamin D-sufficient mice. This was accompanied with marked signs of airway remodeling, high BALF eosinophilia, increased BALF pro-inflammatory cytokines, reduced BALF IL-10 levels, reduced blood T-regulatory cells, increased expression of importin-α3 and Rel-A in the lung tissue. Vitamin D supplementation attenuated the pro-inflammatory effects, but did not completely reverse the features of allergic airway inflammation.
Conclusion and Clinical Relevance
Vitamin D could be beneficial as an adjunct therapy in the treatment of allergic asthma.
Introduction
Asthma is a chronic disease associated with airway hyperresponsiveness (AHR), airway obstruction, airway remodeling [1, 2]. It is a leading cause of activity limitation and costs about $56 billion in health care expenses annually in the United States [3]. AHR is an increased ability of the airways to narrow after exposure to bronchoconstrictors, one of the hallmarks of functional abnormalities in asthmatic subjects [4, 5].
In chronic asthmatics, the airway epithelium produces increased amount of pro-inflammatory cytokines and growth factors [6]. These include TNF-α, IL-1, IL-4, IL-6, IL-8, IL-13, and TGF-β [7]. The resultant effect is myofibroblast differentiation, fibroblast proliferation and production of collagen [8]. Similarly, the bronchial smooth muscle cells (BSMCs) in asthmatics behave in an autocrine and paracrine manner by producing and responding to pro-inflammatory and broncho-protective mediators [9]. Some of the main pro-inflammatory cytokines and chemokines secreted by the smooth muscle cells in asthma are TNF-α, IL-1β, IL-5, IL-6, IL-8, IL-17, GM-CSF, TGF-β, RANTES, Eotaxin, MCP-1,2,3[10, 11]. The secretion of these cytokines is regulated by numerous transcriptional factors that play an important role in the pathogenesis of asthma [12].
NF-κB is a transcriptional factor that regulates and co-ordinates the expression of various inflammatory genes and inflammatory mediators, including cytokines, chemokines, adhesion molecules, immunoreceptors and growth factors. Therefore, it is often termed as a “central mediator of human immune response” [13, 14]. Since the size of NF-κB subunits, p50 and RelA, is larger than what nuclear membrane allows to passively diffuse, its translocation to the nucleus is mediated by cytoplasmic molecules called importins [15]. Importin α3 and Importin α4 are the main importin-α isoforms for the nuclear translocation of NF-κB p50-RelA heterodimer upon stimulation with TNF-α or other inflammatory mediators [15–17].
Vitamin D has a significant role in both direct and indirect regulation of proliferation, differentiation, and function of immune cells [18]. It binds to its receptor, VDR, which is a member of the super family of steroid nuclear receptors [19]. VDR is a transcription factor that interacts with its co-regulators and alters the transcription of target gene involved in a wide spectrum of biological responses [20]. Recent studies provide a strong evidence that vitamin D has a role in innate as well as adaptive immune system [21].
There is growing evidence that vitamin D has a role in allergic airway diseases [22]. Vitamin D deficiency has been associated with obesity, African American race (particularly in urban, inner-city settings), and recent immigrants to westernized countries, thus reflecting the same epidemiologic patterns as observed in the asthma epidemic [23].
In a recent study, children with severe, therapy-resistant asthma having lower vitamin D levels had increased airway smooth muscle (ASM) mass and worse asthma control and lung function [24]. Vitamin D deficiency leads to decreased lung volume, decreased lung function and altered lung structure [25]. Supplementing pregnant women with high doses of vitamin D reduces asthma risk by 40% in their children at the age of 3–5 years [23]. These studies emphasize a potential role of vitamin D in modulating the immune response in allergic airway diseases.
We have recently reported decreased expression of importin-α3 leading to decreased translocation and activation of RelA in calcitriol-treated human bronchial smooth muscle cells [26]. Based on these findings, this study was designed to expand our in vitro findings to an in vivo model of vitamin D-deficient, vitamin D-sufficient and vitamin D-supplemental mouse model of allergic airway inflammation. We also examined the effect of vitamin D on lung histology, AHR, T-regulatory cells and BALF cytokines.
Materials and Methods
Animals and diets
Female BALB/c mice were purchased from Harlan Laboratories (Indianapolis, IN). Mice were maintained in a pathogen-free environment at the Animal Resource Facilty of Creighton University. The research protocol of this study was approved by the Institutional Animal Care and Use Committee of Creighton University. Food and water were provided ad libitum. Female mice were divided in three groups. They were fed on vitamin D-deficient (TD 110272), vitamin D-sufficient containing 2,000 IU/kg (TD 110273) and vitamin D-supplemental diets 10,000 IU/kg (TD110742) (Harlan Laboratories, Madison, WI).
In the first group, vitamin D-deficient diet was given to breeding male and female mice. These were fed on a special diet containing 1.2% calcium to prevent them from hypocalcemic tetany. The diet was also fortified with vitamins A, E and K. Female offsprings were weaned on the same diet. The second and third groups of mice were fed with purified diet containing 2000 IU/kg or 10,000 IU/kg of vitamin D3, respectively, starting at day 21.
Induction of allergic airway inflammation
The sensitization protocol was started at six weeks of age, as established in our laboratory [21]. Briefly, mice were sensitized with 20 μg i.p. injections of OVA (Sigma-Aldrich, St. Louis, MO) emulsified in 2.25 mg of Inject alum (Pierce Biotechnology, Rockford, IL) on days 0 and 14. Animals were challenged with 1% OVA for three consecutive days from day 28 to day 30 and with 5% OVA on day 32. On day 33, using whole-body plethysmography (Buxco Electronics, Troy, NY) the response to aerosolized acetyl β-methylcholine (Sigma-Aldrich, St. Louis, MO) was measured in all mice to examine airway hyperresponsiveness (AHR). The mice with established AHR were then challenged with 5% OVA by aerosol on day 44. On day 45, AHR and enhanced pause in response to aerosolized acetyl β-methylcholine were measured in all mice. On day 46, AHR to methacholine was measured with the invasive tracheotomy method to measure specific airway resistance.
Measurement of serum 25(OH) D levels and serum calcium levels
Blood (~800 μL) was collected from the left ventricle of mice. Out of the total blood, 100μl was used for Tregs analysis and the rest was kept at room temperature for 3 hours and centrifuged at 5,000 rpm for 15 minutes. Serum was separated and the samples were sent to Creighton Medical Laboratories for measurements of serum 25(OH)D and calcium.
T-regulatory cell identification
The anti-mouse CD4+ (FITC) (eBioscience, San Diego, CA) and anti-mouse CD25+ (PE) (Milteny-biotech, Germany) were added to the whole blood. Flow cytometric analysis was performed to gate CD4+CD25+ cells by using FACS Aria Flow Cytometry System (BD Biosciences, San Jose, CA). FITC and PE-labeled IgG (BD Pharmingen, USA) served as the isotype control.
Multiplex cytokine analysis
After sacrificing the mice, lungs were gently lavaged with 1 ml of warm saline (37° C) via a tracheal cannula. All samples were centrifuged and stored in −80°C freezer until analyzed. The levels of IL-4, IL-5, IL-6, IL-10, IL-13, IL-17,RANTES, IP-10 and TNF-α TNF-α in the BAL fluid supernatants were measured by the Milliplex mouse cytokine/chemokine kit (Millipore, Billerica, MA) on a Luminex 200 analyzer (Luminex Corp, Austin, TX, USA) following Manufacturer's recommendations The analysis was done in duplicate, and the cytokine concentrations were calculated against the standards using Beadview® software (ver. 1.03, Upstate).
Staining Lung Tissue sections
Lung lobes were fixed in 4% formalin and paraffin embedded. These lung sections were stained with hematoxylin and eosin following manufacturer's recommendation (Newcomer Supply, Middleton, WI). Mucus production was identified by periodic acid-Schiff (PAS) reaction according to the manufacturer's recommendation (Sigma-Aldrich St. Louis, MO). Trichrome staining was used to identify collagen following the standard protocol recommended by the manufacturer (IMEB Inc., San Marcos, CA).
Immunofluorescence
Paraffin embedded lung sections were stained for immunofluorescence using specific antibodies to importin-α3 [(ab6039) goat, Abcam MA] and NF-κB p65 [(sc-109) (rabbit, SantaCruz Biotechnology, CA)]. The sections were stained by the methods described previously [21]. Negative controls of tissue sections were run without incubation with the primary antibody.
Statistical Analysis
Values of all measurements are reported as mean ± SEM. Unpaired Student's t test was used to determine differences between two groups. Multiple group comparison was made using one-way ANOVA with the Bonferroni correction. Values of all measurements are reported as mean ± SEM. The P< 0.05 was considered significant. (*P < 0.05, **P < 0.01, ***P < 0.001)
Results
Effect of vitamin D on AHR and Infiltratory Cells in OVA-sensitized and challenged Mice
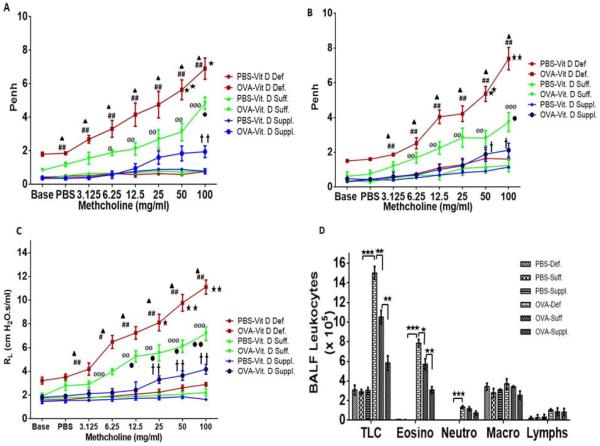
The AHR to methacholine was examined and established on days 33 and 45 as measured by non-invasive whole-body plethysmography (Figure 1A–B). The OVA-sensitized and -challenged mice demonstrated elevated AHR to methacholine compared to PBS control in all vitamin D groups of mice. Vitamin D-deficient OVA-sensitized and challenged mice exhibited a significantly higher AHR to methacholine compared to vitamin D-sufficient OVA-sensitized and challenged mice. Interestingly, OVA-sensitized mice fed on vitamin D-supplemented diet exhibited significant reduction in AHR compared to vitamin D-sufficient OVA-sensitized mice. However, the AHR exhibited by vitamin D- supplemented OVA-sensitized and challenged mice were significantly higher than PBS control mice of the same group (Figure 1A–B). These results were confirmed with a more rigorous invasive method using tracheostomy in anesthetized mice on day 46 to measure specific airway resistance (Figure 1C) and a similar pattern was observed. There was no significant difference in AHR among PBS control vitamin D-Deficient, PBS control vitamin D-sufficient, and PBS control vitamin D-supplemented groups.
Figure 1.
AHR and Specific Airway Resistance to Methacholine. A. Day 33 AHR to methacholine (Mch) was established (N = 7 in each experimental group). B. Day 45 AHR to methacholine (Mch) was measured again (N = 7 in each experimental group). C. F0ur randomly selected animals from each group of PBS and OVA-sensitized and challenged groups were subjected to invasive method of tracheostomy and specific airway resistance (RL) to Mch was recorded.  - Vitamin D-deficient OVA-sensitized and challenged mice vs Vitamin D-sufficient OVA-sensitized and challenged mice - (*p <0.05; **p <0.01; ***p <0.001); # Vitamin D-deficient OVA-sensitized and challenged mice vs Vitamin D-supplemented OVA-sensitized and challenged mice; (#p <0.05; ##p <0.001);
- Vitamin D-deficient OVA-sensitized and challenged mice vs Vitamin D-sufficient OVA-sensitized and challenged mice - (*p <0.05; **p <0.01; ***p <0.001); # Vitamin D-deficient OVA-sensitized and challenged mice vs Vitamin D-supplemented OVA-sensitized and challenged mice; (#p <0.05; ##p <0.001);  Vitamin D-sufficient OVA-sensitized and challenged mice vs Vitamin D-supplemented OVA-sensitized and challenged mice (
Vitamin D-sufficient OVA-sensitized and challenged mice vs Vitamin D-supplemented OVA-sensitized and challenged mice ( p <0.05;
p <0.05;  p <0.01);
p <0.01);  Vitamin D-deficient PBS control mice vs Vitamin D-deficient OVA-sensitized and challenged mice;(
Vitamin D-deficient PBS control mice vs Vitamin D-deficient OVA-sensitized and challenged mice;( p <0.001); ø Vitamin D-sufficient PBS control mice vs Vitamin D-sufficient OVA-sensitized and challenged mice (øp <0.05; øøp <0.01; øøøp <0.001);
p <0.001); ø Vitamin D-sufficient PBS control mice vs Vitamin D-sufficient OVA-sensitized and challenged mice (øp <0.05; øøp <0.01; øøøp <0.001);  Vitamin D-supplemented PBS control mice vs Vitamin D-supplemented OVA-sensitized and challenged mice - (
Vitamin D-supplemented PBS control mice vs Vitamin D-supplemented OVA-sensitized and challenged mice - ( p <0.05;
p <0.05;  p <0.01;
p <0.01;  p <0.001);
p <0.001);
Effect of vitamin D on total and differential leukocytes in bronchoalveolar lavage fluid of PBS-control and OVA-sensitized mice (D). Total cells in the BALF were counted using coulter counter and differential analysis was performed using standard morphological criteria. A total of 300 cells were examined per cytospin slide and absolute cell numbers were calculated per milliliter of the BALF based on the percentage of individual cell in a slide. Data is shown as mean ± SEM for seven animals in each group (*p < 0.05; **p < 0.01; ***p < 0.001)
Aerosolized administration of 100 mg/ml methacholine at day 45 exhibited the following Penh values: 7.38 ± 0.64 in vitamin D-deficient OVA-sensitized and challenged mice; 3.73 ± 0.55 in vitamin D-sufficient OVA-sensitized and challenged mice; and 2.12 ± 0.39 in vitamin D-supplemented OVA-sensitized and challenged mice (n = 7 in each group) (Figure 1 B). Specific airway resistance induced by 100 mg/ml methacholine exhibited mean values of 11.11 ± 0.59 cm H2O.s/ml in vitamin D-deficient OVA-sensitized and challenged mice; 7.18 ± 0.58 cm H2O.s/ml in vitamin D-sufficient OVA-sensitized and challenged mice; 4.18 ± 0.41 cm H2O.s/ml in vitamin D-supplemented OVA-sensitized and challenged mice (Figure 1C).
Sensitization and allergen challenge of mice with OVA significantly increased the total number of cells in the BALF with predominant increase in eosinophils, neutrophils and lymphocytes in all vitamin D OVA-sensitized and challenged group of mice compared to PBS. Vitamin D-deficient OVA-sensitized and challenged mice had increased number of eosinophils compared to vitamin D-sufficient OVA-sensitized and challenged mice and vitamin D-supplemented OVA-sensitized and challenged mice. A decrease in the number of eosinophils was observed in the BALF of vitamin D-supplemented OVA-sensitized and challenged mice compared to vitamin D-sufficient OVA-sensitized and challenged mice (Figure 1D). However, no significant difference in the density of macrophages, neutrophils and lymphocytes was observed among OVA-sensitized vitamin D groups.
Differences in the degree of Airway Remodeling after OVA Sensitization and Challenge in different vitamin D groups
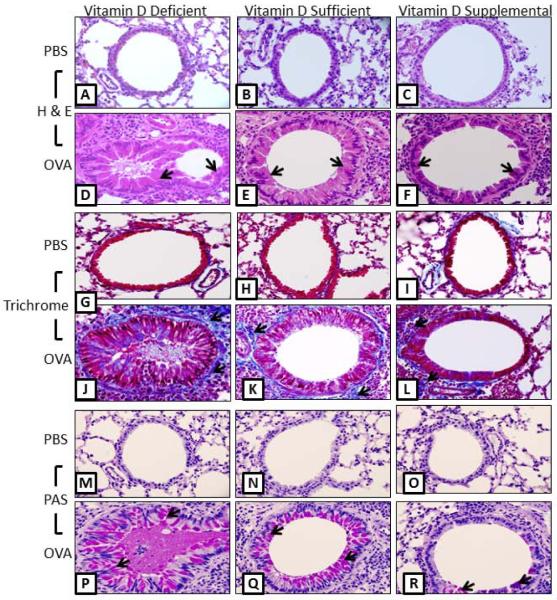
After tracheostomy animals were sacrificed; lungs were harvested and sectioned, followed by staining with H&E, Trichrome and PAS stain to examine histological hallmarks of asthmatic airways. Airways of the PBS control mice in all vitamin D groups exhibited normal parenchyma with no mucus staining and little collagen staining around the respiratory epithelium.
Vitamin D-deficient OVA-sensitized and challenged mice exhibited exaggerated features of severe airway remodeling compared to vitamin D-sufficient OVA-sensitized and challenged mice (Figure 2). The airway had marked epithelial cell hypertrophy indicated by an increase in epithelial cell height, airway occlusion, and more mucus staining and collagen deposition. The lungs of vitamin D-supplemented OVA-sensitized and challenged mice exhibited the signs of mild airway remodeling, showing less airway epithelial cell hypertrophy, mild mucus staining, and less collagen deposition (Figure 2 A–Q).
Figure 2.
Lung histological examination: Hematoxylin and eosin (H&E) showing differences in the airway remodeling (A–F), Trichrome staining showing collagen deposition in blue color (G–L) and, periodic acid-Schiff (PAS) showing mucous staining in pink color (M–Q), in the airway of PBS-control and OVA-sensitized and challenged vitamin D deficient, sufficient and supplemental mice. Note the airway thickening, smooth muscle cell thickening, collagen deposition, epithelial hyperplasia with abundant cytoplasmic as well as airway lumen filled with mucin in vitamin D-deficient group compared to vitamin D-sufficient and Vitamin D-supplemental groups (arrowhead). (N = 7 in each experimental group).
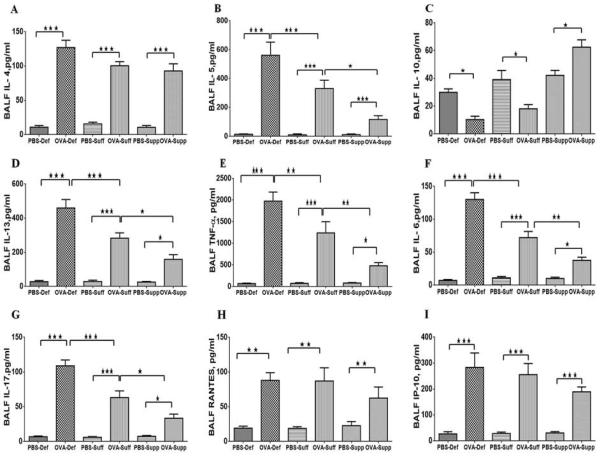
Effect of vitamin D on cytokines and chemokines in the BALF of OVA-sensitized and challenged mice
A significant increase was found in the levels of Th2 cytokines, IL-4 (Fig. 3A), IL-5 (Fig. 3B), and IL-13 (Fig. 3D), in the BALF of all OVA-sensitized and challenged groups of mice compared to the PBS control. However, the levels of IL-5 and IL-13 were more pronounced in vitamin D-deficient OVA-sensitized and challenged mice. Vitamin D-supplemented OVA-sensitized and challenged mice had substantially reduced levels of these cytokines compared to vitamin D-sufficient OVA-sensitized and challenged mice (Fig. 3). There was no significant effect of vitamin D supplementation on the level of BALF IL-4 among the OVA-sensitized groups. However, there was a significant reduction in the BALF levels of IL-10 in vitamin D-deficient OVA-sensitized and challenged mice and vitamin D-sufficient OVA-sensitized and challenged mice with no significant change in PBS control groups (Fig. 3C). The vitamin D-supplemented OVA-sensitized and challenged mice had significantly higher BALF levels ofIL-10 compared to vitamin D-deficient OVA-sensitized and challenged mice and vitamin D-sufficient OVA-sensitized and challenged mice (Figure 3A–D).
Figure 3.
The levels of cytokines and chemokines in the BALF of PBS and OVA-sensitized and -challenged mice. After euthanizing the mice BALF was immediately collected and centrifuged. The supernatant was collected and frozen. The levels of cytokines and chemokines in the BALF of OVA-sensitized and -challenged mice compared to PBS control mice with different vitamin D groups. (A) IL-4; (B) IL-5; (C) IL-10 and (D) IL-13, TNF-α,(E), IL-6 (F), and IL-17(G), RANTES(H) and IP-10 (I) in BALF of OVA-sensitized and -challenged mice compared to PBS control mice in all vitamin D groups. Data are shown as means (±SEM) for six animals in each experimental group. *P <0.05, **P < 0.01, ***P < 0.001.
OVA-sensitization and challenge significantly increased the BALF levels of TNF-α (Fig. 3E), IL-6 (Fig. 3F) and IL-17 (Fig. 3G) compared to PBS control mice. The levels of these cytokines were higher in vitamin D-deficient OVA-sensitized and challenged mice compared to vitamin D-sufficient OVA-sensitized and challenged mice. In contrast, vitamin D-supplemented OVA-sensitized and challenged mice had significantly decreased BALF levels of TNF-α, IL-6 and IL-17, but, significantly higher than the levels in PBS controls (Figure 3E–G).
The BALF levels of chemokines, RANTES and IP-10, were higher in all OVA-sensitization and challenged mice than PBS control mice (Fig. 3H–I). However, there was no significant difference in the levels of these cytokines among the OVA-sensitized and challenged mice in any of the vitamin D groups. (Figure 3H–I).
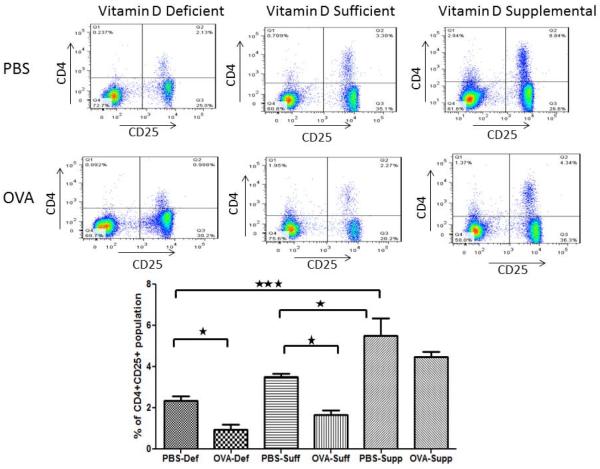
Effect of vitamin D on CD4+CD25+ T-cells in the blood
We evaluated the percentage of CD4+CD25+ T-cells in the mouse peripheral blood and compared between different groups of vitamin D- PBS and OVA-sensitized and challenged groups. The percentage of T-regulatory cells in mice blood is shown in the upper right hand Quadrant 2 (Figure 4). A substantial decrease in the percentage of CD4+CD25+ T-cells was observed in vitamin D-deficient and vitamin D-sufficient OVA sensitized and challenged mice compared to PBS control mice. Interestingly, vitamin D supplemented group of mice had significantly higher percentage of CD4+CD25+T-cells indicating a positive correlation between serum 25(OH)D levels and T-regulatory cells.
Figure 4.
Flow cytometry data showing percentage of CD4+ CD25+ lymphocytes in mice peripheral blood. Quadrant 2 shows CD4+ CD25+ cells (regulatory T cell markers). FITC conjugated monoclonal anti-mouse CD4 and PE-labeled anti-mouse CD25 was used. Flow cytometric analysis was performed by using FACS Aria Flow Cytometry System. FITC, PE-labeled IgG served as the isotype control. Data is shown as mean ± SEM for seven animals in each group. *P <0.05, **P < 0.01, ***P < 0.001.
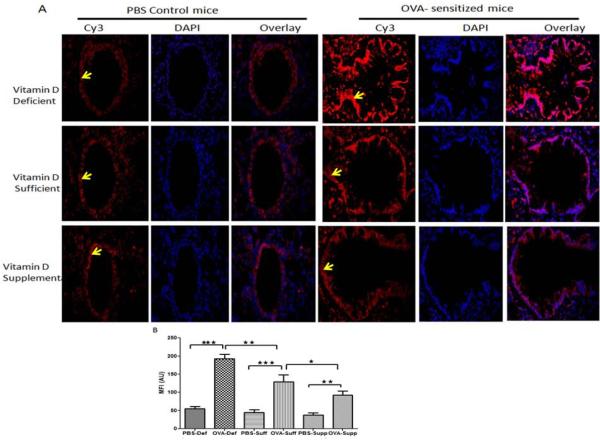
Effect of vitamin D on the expression of importin-α3 in the lung of OVA– sensitized and challenged mice
A moderate expression of importin-α3 was observed in the lung tissue of PBS control mice in all vitamin D groups. The lungs of OVA- sensitized and challenged mice exhibited a significantly increased expression of importin α3 in all vitamin D groups (Figure 5 A, B). However, the expression of importin-α3 in the lung was higher in vitamin D-deficient OVA-sensitized and challenged mice compared to vitamin D-sufficient OVA-sensitized and challenged mice and vitamin D-supplemented OVA-sensitized and challenged mice. Additionally, vitamin D-supplemented OVA-sensitized and challenged mice demonstrated a lower expression than vitamin D-sufficient OVA-sensitized and challenged mice, but higher than control PBS mice (Figure 5 A, B).
Figure 5.
Protein expression of importin α3 (arrowhead) in the lung tissue: Immunofluorescence showing protein expression of importin α3 in lung of PBS control vitamin D deficient, vitamin D sufficient and vitamin D supplemental mice compared to OVA sensitized and challenged vitamin D deficient, vitamin D sufficient and vitamin D supplemental mice at 600 × magnification (A). Sections were stained using goat anti- importin α3 antibody and Donkey anti-goat as secondary antibody. DAPI was used to stain the nuclei. The lungs of seven mice were analyzed per group. (B) Mean fluorescent intensity (MFI) of importin α3 immunostaining in lung tissue measured in arbitrary units (AU) (using NIH Image J software). Data is shown as mean ± SEM from ten measurements per mouse *p <0.05, **p<0.01, ***p <0.001.
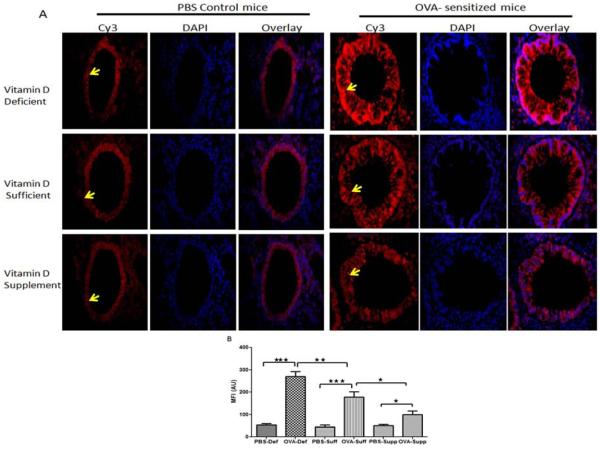
Effect of vitamin D on the expression of RelA in the lungs of OVA –sensitized and challenged mice
A mild expression of RelA was observed in the lung tissue of PBS control mice in all vitamin D groups. OVA-sensitized and challenged mice exhibited a significantly increased expression of RelA in all vitamin D groups (Figure 6A, B). However, the expression of RelA was higher in vitamin D-deficient OVA-sensitized and challenged mice compared to vitamin D-sufficient OVA-sensitized and challenged mice and vitamin D-supplemented OVA-sensitized and challenged mice. Additionally, the lungs of vitamin D-supplemented OVA-sensitized and challenged mice demonstrated a lower expression than vitamin D-sufficient OVA-sensitized and challenged mice, but higher than control PBS mice (Figure 6A, B). These results confirm our earlier studies in HBSMCs that in an inflammatory environment vitamin D decreases the expression of importin-α3 and RelA leading to decrease in the release of cytokines regulated by NF-κB [26].
Figure 6.
Protein expression of RelA (arrowhead) in lung tissue: Immunofluorescence showing protein expression of RelA in lung of PBS control vitamin D deficient, vitamin D sufficient and vitamin D supplemental mice compared to OVA sensitized and challenged vitamin D deficient, vitamin D sufficient and vitamin D supplemental mice at 600 × magnification (A). Sections were stained using rabbit anti- NF-κB p65 antibody and goat anti-rabbit as secondary antibody. DAPI was used to stain the nuclei. Seven mice were analyzed per group. (B) Mean fluorescent intensity (MFI) of RelA immunostaining in lung tissue measured in arbitrary units (AU) (using NIH Image J software). Data is shown as mean ± SEM from ten measurements per mouse *p <0.05, **p<0.01, ***p <0.001.
Discussion
Immunomodulatory potential of vitamin D has been implicated in various inflammatory diseases [27]. Calcitriol, the biologically active form of vitamin D, exerts its effects through VDR [28]. Vitamin D deficiency predisposes to many chronic diseases, including type 1 diabetes, rheumatoid arthritis, Crohn's disease, infectious diseases, and cancers [29]. Vitamin D affects the inflammatory pathways and cells involved in the development and course of allergic asthma [30].
We report findings in OVA-sensitized and challenged mice that vitamin D deficiency is associated with higher AHR to methacholine, which is decreased upon vitamin D supplementation. The histological changes in the lungs and level of airway inflammation were comparable to the changes in the AHR to methacholine. Vitamin D status had no effect on AHR or histological changes in PBS control mice. In OVA-sensitized and challenged mice, vitamin D deficiency was associated with exaggerated response to OVA-antigen and exhibited marked signs of airway remodeling than vitamin D sufficient mice. Although, high serum vitamin D levels were associated with low airway inflammation and reduced hallmarks of asthma, it was not associated with complete reversal of asthma.
Our results are in agreement with the findings of Sutherland and colleagues [31] that reduced levels of vitamin D are associated with impaired lung function and increased AHR. In addition, vitamin D deficiency was associated with high BALF eosinophilia and the density of eosinophils in the BALF was significantly reduced in the vitamin D-supplemented group. This could be supported by the findings of Brehm and colleagues [32] in children where vitamin D levels were significantly and inversely associated with peripheral blood eosinophil count.
The effect of vitamin D on allergic asthma is still under debate. A previous study by Gorman and colleagues [33] reported no change in the inflammatory cells and cytokines in the lungs and OVA-specific IgE levels in serum of vitamin D3-replete and vitamin D3-deficient BALB/c mice. However, this study did report that the vitamin D deficiency significantly enhanced the ability of airways-draining lymph node cells to proliferate and secrete cytokines in response to OVA ex vivo, suggesting that the ability of lymphocytes to responds to allergens is modulated by vitamin D deficiency [33]. However, the timing and doses of experimental protocols for OVA sensitization to induce allergic airways inflammatory response were different in the experimental design of the reports by Gorman et al. [33] and our own study. In another study, Wittke et al. [34] reported that lung inflammation was not modified by oral supplementation of 1, 25(OH)2D3 in vitamin D3-deficient mice. However, the experimental protocol of the study by Wittke and colleagues [34] had major differences including the strain of mice (C57Bl/6 vs BALB/c), type of vitamin D used for the supplementation (1,25(OH)2D3 vs vitamin D3 and the treatment plan for vitamin D diets. Such differences in the experimental protocols could explain the discrepant results.
The pro-inflammatory cytokines, TNF-α a n d I L-6, are released from activated macrophages [35, 36]. Macrophages rather than Th17 cells are the primary producer of IL-17 in allergic inflammation [37]. The active form of vitamin D, 1α,25-dihydroxyvitamin D3, is a potent suppressor of interferon-γ–mediated macrophage activation [38]. We did not observe any difference in the macrophage number in the BALF in any of the groups. However, there was a significant decrease in the levels of pro-inflammatory cytokines, TNF-α, IL-6 and IL-17, released from activated macrophages in the OVA-sensitized vitamin D supplemented group. Our data support the findings of a recent study that vitamin D treatment inhibits IL-17 production [39, 40]. In another study in a mouse model, decreased levels of vitamin D were associated with elevated levels of IL-17 in the distal and proximal colon [41]. Vitamin D inhibits the synthesis and release of IL-23 and IL-6 that are the cytokines important for the development of Th17 cells [39]. These results suggest that vitamin D might be inhibiting macrophage activation leading to the inhibition of cytokines release. Vitamin D diminishes the production of pro-inflammatory cytokines (IL-1 and TNF-α) by modulating T-cell/DC interaction leading to decreased production of IL-2 and IFN-γ and increased production of IL-10 and TGF-β [42, 43].
The reduction in the levels of Th2 cytokines (IL-4, IL-5, and IL-13) has been shown to be a potential therapeutic approach to suppress airway inflammation and AHR [44]. In the present study, sensitization with OVA antigen in vitamin D-deficient mice caused a substantial increase in cytokines, including IL-5 and IL-13, in the BALF. Conversely, vitamin D supplementation decreased the BALF levels of these cytokines in OVA-sensitized and challenged mice but not to the levels in the control PBS mice. However, vitamin D status was independent of the BALF levels of IL-4, RANTES and IP-10.
Calcitriol decreases the proliferation of Th1 cells and also inhibits the production of IL-2, IFN-γ, and TNF-α from Th1 cells [45]. Despite many reports on the role of vitamin D in inhibiting Th1 immune response, the effect of vitamin D on Th2 response is controversial [23]. Most reports simply state that vitamin D induces a shift in the balance between Th1- and Th2-type cytokines toward Th2 dominance or vitamin D promotes Th2 responses [46, 47]. However, a closer evaluation of the findings in the literature reveals both the inhibition and enhancement of Th2 responses [48]. In addition, the effect of vitamin D on the release and activity of IL-4 is under debate. Some studies show an increase in the levels of IL-4 with vitamin D treatment [49, 50]. On the contrary, in human cord blood T cells, vitamin D not only decreased Th1 cytokine production but also suppressed IL-4 content and IL-4–induced expression of IL-13 [51]. In a murine model of asthma, the administration of 1,25-dihydroxyvitamin D was found to inhibit airway inflammation with decreased levels of IL-4 in BALF and decreased T-cell migration, and the inhibition of the inflammatory response [45]. We observed no change in the levels of BALF IL-4 of mice supplemented with vitamin D. The reasons for the underlying discrepancy between our results and others are unclear, but could be related to the time and dose of vitamin D supplementation. Nevertheless, additional studies are warranted to further clarify this issue.
Vitamin D promotes the stimulation of CD4+CD25+ regulatory T cells (Tregs) by dendritic cells [52]. Calcitriol increases the expression of Foxp3 and IL-10, two crucial factors for Treg induction [39]. Our results show that OVA-sensitization and challenge is associated with reduction in the levels of IL-10 and the number of Tregs, and this was more pronounced in vitamin D-deficient mice. Conversely, vitamin D supplementation resulted in significant increase in IL-10 levels and the density of circulating Tregs.
In the presence of vitamin D, Tregs develop and function normally, by suppressing inappropriate Th1 and Th2 responses thereby, leading to a more balanced immune response [23]. Low levels of vitamin D affects normal development and function of Tregs, and under inappropriate environmental conditions, Th1 or Th2 responses are allowed to proceed unabated, leading to disease [23]. We find that a decrease in Tregs is associated with increase in pro-inflammatory cytokines and decrease in IL-10, supporting published studies. Vitamin D deficiency resulted in marked decrease in Tregs and further increase in pro-inflammatory cytokines and decrease in IL-10.
NF-κB induces expression of many pro-inflammatory cytokines, including IL-12 [53] and IFN-γ [54] released from Th1 cells, IL-4 [55], IL-5 [55], IL-8 [53] and IL-13 [55, 56] released from Th2 cells, TNF-α and IL-6 [57]released from macrophages [57], and IL-17 released from macrophages as well as Th17 cells [37]. Thus, NF-κB can induce the release of Th1, Th2 and Th17 cytokines. Therefore, NF-κB signaling has been a focus of extensive research for the treatment of chronic inflammatory diseases such as asthma, inventing molecules that can decrease the activation or nuclear translocation of NF-κB [58]. On stimulation, the nuclear translocation of NF-κB p50-RelA subunits is mediated by importin-α3 and importin-α4 [15–17]. We recently reported that calcitriol decreases expression of importin-α3 leading to a decreases in NF-κB p65 (RelA) expression [26], suggesting that vitamin D might decrease the cytokines released from Th1, Th2 and Th17 cells by decreasing NF-κB translocation through importin-α3. We have also reported decreased VDR and increased TNF-α expression under inflammatory conditions in the lungs of OVA sensitized mice [21]. Reduced serum level of vitamin D was associated with further decrease in VDR and increase TNF-α [21]. Dysfunction of VDR and vitamin D3 deficiency can cause poor bone development and health, as well as increase the risk of many chronic inflammatory diseases and cancers [21].
Vitamin D has an inhibitory action on NF-κB activation [26]. We recently reported decreased expression of importin-α3 in calcitriol-treated HBSMCs, leading to decreased nuclear expression of NF-κB and thus resulting in decreased secretion of pro-inflammatory cytokines [26]. Based on our previous in-vitro studies, we analyzed the expression of importin-α3 and NF-κB in the lungs of OVA- sensitized and challenged mice. An increased expression of importin-α3 was observed in colonic mucosal biopsies of moderately-to-severely inflamed Crohn's disease patients [16], suggesting that inflammation is associated with increase in importin-α3 expression. Our results demonstrating increased expression of importin-α3 and NF-κB in the lung of OVA-sensitized and challenged mice support the findings in inflamed Crohn's disease. In addition, our in vivo findings are consistent with our previous in vitro studies in HBSMCs that treatment with TNF-α increased the expression of importin-α3 and NF-κB, confirming that inflammation results in increased expression of importin-α3 [26]. Vitamin D deficiency was also associated with higher expression of importin-α3 and NF-κB, and vitamin D supplemental group had a significantly reduced lung expression of these two genes, suggesting that vitamin D deficiency is associated with marked inflammatory changes. As per the Institute of Medicine guidelines vitamin D deficiency is defined as serum 25(OH) D levels <20ng/dl. The findings in our study suggest that the allergic airway inflammation and asthma symptoms were less severe in vitamin D-supplemented group compared to vitamin D-deficient and vitamin D-sufficient groups. Although the level of vitamin D is influenced by many factors including the melanin content of the skin, age, factors affecting sun exposure (latitude, season, time outdoors, and clothing), body fat, and sunscreen use [48], earlier studies have shown that in general each additional 100 IU of vitamin D per day raises serum 25(OH)D concentration by approximately 1 ng/mL [59] Therefore, it is possible that the use of vitamin D supplementation as add-on treatment might help reducing the symptoms in vitamin D-deficient asthmatic patients.
Conclusion
In summary, in allergic inflammatory environment in the lungs there is increase in the expression of NF-κB and importin-α3. Vitamin D deficiency causes further increase in NF-κB and importin-α3 that might be responsible for increased inflammation and higher AHR. Supplementation with vitamin D reduces the expression and activity of NF-κB and importin-α3. Additionally, higher serum levels of vitamin D is associated with increased number of circulating T-regulatory cells which might be stimulating the secretion of anti-inflammatory cytokine, IL-10, resulting in a significant reduction in the levels of many pro-inflammatory cytokines involved in the pathogenesis of asthma. However, the precise underlying mechanism(s) for these effects of vitamin D need to be elucidated. The alleviation but not the complete reversal of allergic airway inflammation and AHR by vitamin D supplementation suggests the potential role of vitamin D as an adjunct in the treatment of asthma.
Table-I.
| Experimental Group | Serum 25(OH)D levels (ng/ml) | Serum Calcium (mg/dl) | |
|---|---|---|---|
| 6 weeks | 13 weeks | 13 weeks | |
|
Mice-0 IU/kg
Vitamin D |
6.00 ±0.63 | 5.0 ± 0.25 | 9.06 ± 0.06 |
|
Mice-2000 IU/kg
Vitamin D |
30.75 ± 0.45 | 31.00 ± 0.75 | 9.16 ± 0.02 |
|
Mice-10,000 IU/kg
Vitamin D |
62.15 ± 1.5 | 67.12 ± 0.5 | 9.18 ± 0.27 |
Acknowledgments
This work was supported by National Institutes of Health awards number R01AI75315 and R01HL116042 to DKA. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- AHR
Airway Hyperresponsiveness
- BALF
Bronchoalveolar Lavage Fluid
- HBSMCs
Human Bronchial Smooth Muscle Cells
- T regs
T-regulatory cells
- VDR
Vitamin D Receptor
Footnotes
Conflict of interests The authors declare no conflict of interests.
References
- 1.Agrawal DK, Shao Z. Pathogenesis of allergic airway inflammation. Current allergy and asthma reports. 2010;10:39–48. doi: 10.1007/s11882-009-0081-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGee HS, Stallworth AL, Agrawal T, Shao Z, Lorence L, Agrawal DK. Fms-like tyrosine kinase 3 ligand decreases T helper type 17 cells and suppressors of cytokine signaling proteins in the lung of house dust mite-sensitized and -challenged mice. American journal of respiratory cell and molecular biology. 2010;43:520–9. doi: 10.1165/rcmb.2009-0241OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnett SB, Nurmagambetov TA. Costs of asthma in the United States: 2002–2007. The Journal of allergy and clinical immunology. 2011;127:145–52. doi: 10.1016/j.jaci.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 4.O'Byrne PM, Inman MD. Airway hyperresponsiveness. Chest. 2003;123:411S–6S. doi: 10.1378/chest.123.3_suppl.411s. [DOI] [PubMed] [Google Scholar]
- 5.Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FitzGerald M, Gibson P, Ohta K, O'Byrne P, Pedersen SE, Pizzichini E, Sullivan SD, Wenzel SE, Zar HJ. Global strategy for asthma management and prevention: GINA executive summary. The European respiratory journal : official journal of the European Society for Clinical Respiratory Physiology. 2008;31:143–78. doi: 10.1183/09031936.00138707. [DOI] [PubMed] [Google Scholar]
- 6.Holgate ST. The airway epithelium is central to the pathogenesis of asthma. Allergology international : official journal of the Japanese Society of Allergology. 2008;57:1–10. doi: 10.2332/allergolint.R-07-154. [DOI] [PubMed] [Google Scholar]
- 7.Holgate ST. Epithelium dysfunction in asthma. The Journal of allergy and clinical immunology. 2007;120:1233–44. doi: 10.1016/j.jaci.2007.10.025. quiz 45–6. [DOI] [PubMed] [Google Scholar]
- 8.Knight DA, Holgate ST. The airway epithelium: structural and functional properties in health and disease. Respirology. 2003;8:432–46. doi: 10.1046/j.1440-1843.2003.00493.x. [DOI] [PubMed] [Google Scholar]
- 9.Bosse Y, Maghni K, Hudson TJ. 1alpha,25-dihydroxy-vitamin D3 stimulation of bronchial smooth muscle cells induces autocrine, contractility, and remodeling processes. Physiological genomics. 2007;29:161–8. doi: 10.1152/physiolgenomics.00134.2006. [DOI] [PubMed] [Google Scholar]
- 10.Gerthoffer WT, Singer CA. Secretory functions of smooth muscle: cytokines and growth factors. Molecular interventions. 2002;2:447–56. doi: 10.1124/mi.2.7.447. [DOI] [PubMed] [Google Scholar]
- 11.Tliba O, Panettieri RA., Jr. Noncontractile functions of airway smooth muscle cells in asthma. Annual review of physiology. 2009;71:509–35. doi: 10.1146/annurev.physiol.010908.163227. [DOI] [PubMed] [Google Scholar]
- 12.Hershenson MB, Brown M, Camoretti-Mercado B, Solway J. Airway smooth muscle in asthma. Annual review of pathology. 2008;3:523–55. doi: 10.1146/annurev.pathmechdis.1.110304.100213. [DOI] [PubMed] [Google Scholar]
- 13.Clarke D, Damera G, Sukkar MB, Tliba O. Transcriptional regulation of cytokine function in airway smooth muscle cells. Pulmonary pharmacology & therapeutics. 2009;22:436–45. doi: 10.1016/j.pupt.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wertz IE, Dixit VM. Signaling to NF-kappaB: regulation by ubiquitination. Cold Spring Harbor perspectives in biology. 2010;2:a003350. doi: 10.1101/cshperspect.a003350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fagerlund R, Kinnunen L, Kohler M, Julkunen I, Melen K. NF-{kappa}B is transported into the nucleus by importin {alpha}3 and importin {alpha}4. The Journal of biological chemistry. 2005;280:15942–51. doi: 10.1074/jbc.M500814200. [DOI] [PubMed] [Google Scholar]
- 16.Theiss AL, Jenkins AK, Okoro NI, Klapproth JM, Merlin D, Sitaraman SV. Prohibitin inhibits tumor necrosis factor alpha-induced nuclear factor-kappa B nuclear translocation via the novel mechanism of decreasing importin alpha3 expression. Molecular biology of the cell. 2009;20:4412–23. doi: 10.1091/mbc.E09-05-0361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okada N, Ishigami Y, Suzuki T, Kaneko A, Yasui K, Fukutomi R, Isemura M. Importins and exportins in cellular differentiation. Journal of cellular and molecular medicine. 2008;12:1863–71. doi: 10.1111/j.1582-4934.2008.00437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guillot X, Semerano L, Saidenberg-Kermanac'h N, Falgarone G, Boissier MC. Vitamin D and inflammation. Joint, bone, spine : revue du rhumatisme. 2010;77:552–7. doi: 10.1016/j.jbspin.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 19.Damera G, Fogle HW, Lim P, Goncharova EA, Zhao H, Banerjee A, Tliba O, Krymskaya VP, Panettieri RA., Jr. Vitamin D inhibits growth of human airway smooth muscle cells through growth factor-induced phosphorylation of retinoblastoma protein and checkpoint kinase 1. British journal of pharmacology. 2009;158:1429–41. doi: 10.1111/j.1476-5381.2009.00428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bosse Y, Lemire M, Poon AH, Daley D, He JQ, Sandford A, White JH, James AL, Musk AW, Palmer LJ, Raby BA, Weiss ST, Kozyrskyj AL, Becker A, Hudson TJ, Laprise C. Asthma and genes encoding components of the vitamin D pathway. Respiratory research. 2009;10:98. doi: 10.1186/1465-9921-10-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agrawal T, Gupta GK, Agrawal DK. Vitamin D deficiency decreases the expression of VDR and prohibitin in the lungs of mice with allergic airway inflammation. Experimental and molecular pathology. 2012;93:74–81. doi: 10.1016/j.yexmp.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sandhu MS, Casale TB. The role of vitamin D in asthma. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2010;105:191–9. doi: 10.1016/j.anai.2010.01.013. quiz 200–2, 17. [DOI] [PubMed] [Google Scholar]
- 23.Litonjua AA, Weiss ST. Is vitamin D deficiency to blame for the asthma epidemic? The Journal of allergy and clinical immunology. 2007;120:1031–5. doi: 10.1016/j.jaci.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 24.Gupta A, Sjoukes A, Richards D, Banya W, Hawrylowicz C, Bush A, Saglani S. Relationship between serum vitamin D, disease severity, and airway remodeling in children with asthma. American journal of respiratory and critical care medicine. 2011;184:1342–9. doi: 10.1164/rccm.201107-1239OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zosky GR, Berry LJ, Elliot JG, James AL, Gorman S, Hart PH. Vitamin D deficiency causes deficits in lung function and alters lung structure. American journal of respiratory and critical care medicine. 2011;183:1336–43. doi: 10.1164/rccm.201010-1596OC. [DOI] [PubMed] [Google Scholar]
- 26.Agrawal T, Gupta GK, Agrawal DK. Calcitriol Decreases Expression of Importin alpha3 and Attenuates RelA Translocation in Human Bronchial Smooth Muscle Cells. Journal of clinical immunology. 2012;32:1093–103. doi: 10.1007/s10875-012-9696-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mathieu C, Adorini L. The coming of age of 1,25-dihydroxyvitamin D(3) analogs as immunomodulatory agents. Trends in molecular medicine. 2002;8:174–9. doi: 10.1016/s1471-4914(02)02294-3. [DOI] [PubMed] [Google Scholar]
- 28.Carlberg C, Seuter S. The vitamin D receptor. Dermatologic clinics. 2007;25:515–23. viii. doi: 10.1016/j.det.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 29.Wu S, Sun J. Vitamin D, vitamin D receptor, and macroautophagy in inflammation and infection. Discovery medicine. 2011;11:325–35. [PMC free article] [PubMed] [Google Scholar]
- 30.Reinholz M, Ruzicka T, Schauber J. Vitamin D and its role in allergic disease. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2012;42:817–26. doi: 10.1111/j.1365-2222.2011.03923.x. [DOI] [PubMed] [Google Scholar]
- 31.Sutherland ER, Goleva E, Jackson LP, Stevens AD, Leung DY. Vitamin D levels, lung function, and steroid response in adult asthma. American journal of respiratory and critical care medicine. 2010;181:699–704. doi: 10.1164/rccm.200911-1710OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brehm JM, Celedon JC, Soto-Quiros ME, Avila L, Hunninghake GM, Forno E, Laskey D, Sylvia JS, Hollis BW, Weiss ST, Litonjua AA. Serum vitamin D levels and markers of severity of childhood asthma in Costa Rica. American journal of respiratory and critical care medicine. 2009;179:765–71. doi: 10.1164/rccm.200808-1361OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gorman S, Tan DH, Lambert MJ, Scott NM, Judge MA, Hart PH. Vitamin D(3) deficiency enhances allergen-induced lymphocyte responses in a mouse model of allergic airway disease. Pediatric allergy and immunology : official publication of the European Society of Pediatric Allergy and Immunology. 2012;23:83–7. doi: 10.1111/j.1399-3038.2011.01146.x. [DOI] [PubMed] [Google Scholar]
- 34.Wittke A, Chang A, Froicu M, Harandi OF, Weaver V, August A, Paulson RF, Cantorna MT. Vitamin D receptor expression by the lung micro-environment is required for maximal induction of lung inflammation. Archives of biochemistry and biophysics. 2007;460:306–13. doi: 10.1016/j.abb.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duffield JS. The inflammatory macrophage: a story of Jekyll and Hyde. Clin Sci (Lond) 2003;104:27–38. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 36.Gordon S. Alternative activation of macrophages. Nature reviews Immunology. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 37.Song C, Luo L, Lei Z, Li B, Liang Z, Liu G, Li D, Zhang G, Huang B, Feng ZH. IL-17-producing alveolar macrophages mediate allergic lung inflammation related to asthma. J Immunol. 2008;181:6117–24. doi: 10.4049/jimmunol.181.9.6117. [DOI] [PubMed] [Google Scholar]
- 38.Helming L, Bose J, Ehrchen J, Schiebe S, Frahm T, Geffers R, Probst-Kepper M, Balling R, Lengeling A. 1alpha,25-Dihydroxyvitamin D3 is a potent suppressor of interferon gamma-mediated macrophage activation. Blood. 2005;106:4351–8. doi: 10.1182/blood-2005-03-1029. [DOI] [PubMed] [Google Scholar]
- 39.Daniel C, Sartory NA, Zahn N, Radeke HH, Stein JM. Immune modulatory treatment of trinitrobenzene sulfonic acid colitis with calcitriol is associated with a change of a T helper (Th) 1/Th17 to a Th2 and regulatory T cell profile. The Journal of pharmacology and experimental therapeutics. 2008;324:23–33. doi: 10.1124/jpet.107.127209. [DOI] [PubMed] [Google Scholar]
- 40.Tang J, Zhou R, Luger D, Zhu W, Silver PB, Grajewski RS, Su SB, Chan CC, Adorini L, Caspi RR. Calcitriol suppresses antiretinal autoimmunity through inhibitory effects on the Th17 effector response. J Immunol. 2009;182:4624–32. doi: 10.4049/jimmunol.0801543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu N, Nguyen L, Chun RF, Lagishetty V, Ren S, Wu S, Hollis B, DeLuca HF, Adams JS, Hewison M. Altered endocrine and autocrine metabolism of vitamin D in a mouse model of gastrointestinal inflammation. Endocrinology. 2008;149:4799–808. doi: 10.1210/en.2008-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gauzzi MC, Purificato C, Donato K, Jin Y, Wang L, Daniel KC, Maghazachi AA, Belardelli F, Adorini L, Gessani S. Suppressive effect of 1alpha,25-dihydroxyvitamin D3 on type I IFN-mediated monocyte differentiation into dendritic cells: impairment of functional activities and chemotaxis. J Immunol. 2005;174:270–6. doi: 10.4049/jimmunol.174.1.270. [DOI] [PubMed] [Google Scholar]
- 43.Almerighi C, Sinistro A, Cavazza A, Ciaprini C, Rocchi G, Bergamini A. 1Alpha,25-dihydroxyvitamin D3 inhibits CD40L-induced pro-inflammatory and immunomodulatory activity in human monocytes. Cytokine. 2009;45:190–7. doi: 10.1016/j.cyto.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 44.Edwan JH, Perry G, Talmadge JE, Agrawal DK. Flt-3 ligand reverses late allergic response and airway hyper-responsiveness in a mouse model of allergic inflammation. J Immunol. 2004;172:5016–23. doi: 10.4049/jimmunol.172.8.5016. [DOI] [PubMed] [Google Scholar]
- 45.Topilski I, Flaishon L, Naveh Y, Harmelin A, Levo Y, Shachar I. The anti-inflammatory effects of 1,25-dihydroxyvitamin D3 on Th2 cells in vivo are due in part to the control of integrin-mediated T lymphocyte homing. European journal of immunology. 2004;34:1068–76. doi: 10.1002/eji.200324532. [DOI] [PubMed] [Google Scholar]
- 46.Cantorna MT, Zhu Y, Froicu M, Wittke A. Vitamin D status, 1,25-dihydroxyvitamin D3, and the immune system. The American journal of clinical nutrition. 2004;80:1717S–20S. doi: 10.1093/ajcn/80.6.1717S. [DOI] [PubMed] [Google Scholar]
- 47.Matheu V, Back O, Mondoc E, Issazadeh-Navikas S. Dual effects of vitamin D-induced alteration of TH1/TH2 cytokine expression: enhancing IgE production and decreasing airway eosinophilia in murine allergic airway disease. The Journal of allergy and clinical immunology. 2003;112:585–92. doi: 10.1016/s0091-6749(03)01855-4. [DOI] [PubMed] [Google Scholar]
- 48.Lange NE, Litonjua A, Hawrylowicz CM, Weiss S. Vitamin D, the immune system and asthma. Expert review of clinical immunology. 2009;5:693–702. doi: 10.1586/eci.09.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boonstra A, Barrat FJ, Crain C, Heath VL, Savelkoul HF, O'Garra A. 1alpha,25-Dihydroxyvitamin d3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J Immunol. 2001;167:4974–80. doi: 10.4049/jimmunol.167.9.4974. [DOI] [PubMed] [Google Scholar]
- 50.Cantorna MT, Woodward WD, Hayes CE, DeLuca HF. 1,25-dihydroxyvitamin D3 is a positive regulator for the two anti-encephalitogenic cytokines TGF-beta 1 and IL-4. J Immunol. 1998;160:5314–9. [PubMed] [Google Scholar]
- 51.Pichler J, Gerstmayr M, Szepfalusi Z, Urbanek R, Peterlik M, Willheim M. 1 alpha,25(OH)2D3 inhibits not only Th1 but also Th2 differentiation in human cord blood T cells. Pediatric research. 2002;52:12–8. doi: 10.1203/00006450-200207000-00005. [DOI] [PubMed] [Google Scholar]
- 52.Boissier MC, Assier E, Biton J, Denys A, Falgarone G, Bessis N. Regulatory T cells (Treg) in rheumatoid arthritis. Joint, bone, spine : revue du rhumatisme. 2009;76:10–4. doi: 10.1016/j.jbspin.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 53.Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene. 1999;18:6853–66. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- 54.Shea LM, Beehler C, Schwartz M, Shenkar R, Tuder R, Abraham E. Hyperoxia activates NF-kappaB and increases TNF-alpha and IFN-gamma gene expression in mouse pulmonary lymphocytes. J Immunol. 1996;157:3902–8. [PubMed] [Google Scholar]
- 55.Edwards MR, Bartlett NW, Clarke D, Birrell M, Belvisi M, Johnston SL. Targeting the NF-kappaB pathway in asthma and chronic obstructive pulmonary disease. Pharmacology & therapeutics. 2009;121:1–13. doi: 10.1016/j.pharmthera.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fichtner-Feigl S, Fuss IJ, Preiss JC, Strober W, Kitani A. Treatment of murine Th1- and Th2-mediated inflammatory bowel disease with NF-kappa B decoy oligonucleotides. The Journal of clinical investigation. 2005;115:3057–71. doi: 10.1172/JCI24792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hetland RB, Cassee FR, Lag M, Refsnes M, Dybing E, Schwarze PE. Cytokine release from alveolar macrophages exposed to ambient particulate matter: heterogeneity in relation to size, city and season. Particle and fibre toxicology. 2005;2:4. doi: 10.1186/1743-8977-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Caramori G, Oates T, Nicholson AG, Casolari P, Ito K, Barnes PJ, Papi A, Adcock IM, Chung KF. Activation of NF-kappaB transcription factor in asthma death. Histopathology. 2009;54:507–9. doi: 10.1111/j.1365-2559.2009.03239.x. [DOI] [PubMed] [Google Scholar]
- 59.Heaney RP, Holick MF. Why the IOM recommendations for vitamin D are deficient. J Bone Miner Res. 2011;26:455–7. doi: 10.1002/jbmr.328. [DOI] [PubMed] [Google Scholar]