Abstract
Purpose
To compare two-dimensional (2D) echocardiography, the current method of screening for treatment-related cardiomyopathy recommended by the Children's Oncology Group Guidelines, to cardiac magnetic resonance (CMR) imaging, the reference standard for left ventricular (LV) function.
Patients and Methods
Cross-sectional, contemporaneous evaluation of LV structure and function by 2D and three-dimensional (3D) echocardiography and CMR imaging in 114 adult survivors of childhood cancer currently median age 39 years (range, 22 to 53 years) exposed to anthracycline chemotherapy and/or chest-directed radiation therapy.
Results
In this survivor population, 14% (n = 16) had an ejection fraction (EF) less than 50% by CMR. Survivors previously undiagnosed with cardiotoxicity (n = 108) had a high prevalence of EF (32%) and cardiac mass (48%) that were more than two standard deviations below the mean of normative CMR data. 2D echocardiography overestimated the mean EF of this population by 5%. Compared with CMR, 2D echocardiography (biplane method) had a sensitivity of 25% and a false-negative rate of 75% for detection of EF less than 50%, although 3D echocardiography had 53% and 47%, respectively. Twelve survivors (11%) had an EF less than 50% by CMR but were misclassified as ≥ 50% (range, 50% to 68%) by 2D echocardiography (biplane method). Detection of cardiomyopathy was improved (sensitivity, 75%) by using a higher 2D echocardiography cutoff (EF < 60%) to detect an EF less than 50% by the reference standard CMR.
Conclusion
CMR identified a high prevalence of cardiomyopathy among adult survivors previously undiagnosed with cardiac disease. 2D echocardiography demonstrated limited screening performance. In this high-risk population, survivors with an EF 50% to 59% by 2D echocardiography should be considered for comprehensive cardiac assessment, which may include CMR.
INTRODUCTION
More than 80% of children diagnosed with a malignancy will become 5-year survivors of their cancer,1 the majority of whom will survive into adulthood.2 As a result, the National Cancer Institute's Office of Cancer Survivorship projected that, as of 2005, there were 328,600 survivors of childhood cancer in the United States.3 Thus, improved survival has led to a new and growing population of adult survivors of childhood cancer that did not exist just a few decades ago. However, treatment for childhood cancer may include chemotherapeutic agents, such as the anthracycline class of drugs, and/or chest-directed radiation therapy (RT). Both have been documented to have an adverse impact on cardiac function in the immediate treatment period and to increase the risk for reduced left ventricular (LV) function later on in adolescence and young adulthood.4–8
Guidelines for screening and early detection of cardiomyopathy, developed by the Children's Oncology Group, recommend transthoracic two-dimensional (2D) echocardiography because it is a noninvasive and widely available technique.9 However, 2D echocardiography is dependent on the quality of the acoustic windows obtained and on geometric assumptions that may not be valid in patients with dilated or remodeled ventricles.10 Three-dimensional (3D) echocardiography may improve on some of the limitations imposed by 2D echocardiography. Cardiac magnetic resonance (CMR) imaging is considered the reference standard to which alternative cardiac imaging techniques are compared for measurement of cardiac structure and function.11–13 CMR imaging has demonstrated superior intraobserver and interobserver reproducibility and accuracy compared with echocardiography in both normal and remodeled ventricles as a result of its large field of view with data acquisition that encompasses the entire heart, lack of limitation by acoustic windows, and absence of geometric assumption bias through its use of the disc-summation volumetric calculation technique.10,14 We evaluated the ability of transthoracic 2D and 3D echocardiography to identify cancer survivors with decreased ejection fraction (EF) compared with CMR imaging.
PATIENTS AND METHODS
Study Participants
The St. Jude Lifetime Cohort Study (SJLIFE) is a longitudinal cohort of adult survivors of cancer diagnosed before reaching age 21 years, treated at St. Jude Children's Research Hospital (SJCRH), now 10 or more years from their original cancer diagnosis, and age ≥ 18 years.15 Participation involved completion of questionnaires and a risk-based medical evaluation as recommended by the Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers9 developed by the Children's Oncology Group. Details of eligibility, recruitment methods, and study design were published previously.15
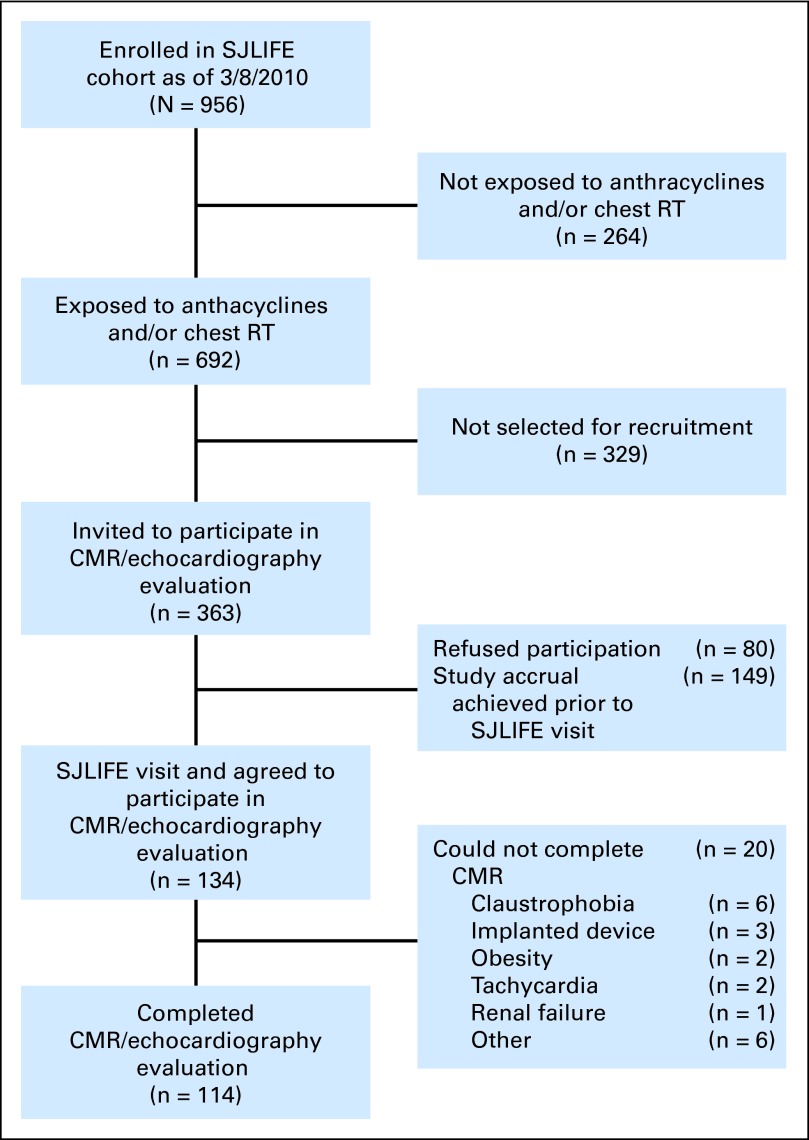
This study is an analysis of data from five pilot studies, convenience sampled from the larger SJLIFE cohort (Fig 1), that used contemporaneous evaluation by echocardiography and CMR. Recruitment was discontinued for each study when the accrual target was met. Survivors were treated with chest-directed RT and/or anthracycline chemotherapy. Patients with an implanted medical device or a history of congenital heart disease were excluded. Of 134 who agreed to participate in the study, 114 completed the evaluation. Of those, 108 were previously undiagnosed with cardiac dysfunction (ie, myocardial infarction, acute coronary syndrome, coronary artery bypass grafting, or percutaneous coronary intervention, or they were previously told by a physician that they had cardiac disease or LV dysfunction). This investigation was approved by the institutional review board at SJCRH.
Fig 1.
Study recruitment flow diagram. CMR, cardiac magnetic resonance imaging; RT, radiotherapy; SJLIFE, St Jude Lifetime Cohort Study.
Outcome Measures
Participants were evaluated with 2D and 3D echocardiography and CMR within a 48-hour period. Noninvasive assessment of cardiac function by CMR was performed on a commercially available 3.0 Tesla GE TwinSpeed system (General Electric, Milwaukee, WI) equipped with parallel imaging methods, electrocardiographic gating, and an 8-channel cardiac phased-array coil. LVEF, volumes, and mass measurements were obtained by using breath hold ECG-gated 2D cine steady-state free-precession sequences in the 2-chamber, 3-chamber, 4-chamber, and contiguous short-axis orientations. Imaging parameters were repetition time, 3.4 ms; echo time, 1.5 ms; flip angle of 30 degrees; slice thickness, 10 mm; field of view, 240 to 320 mm; matrix, 256 × 192; and acquired typical in-plane spatial resolution, 1.5 × 1.2 mm. Computation of end-diastolic volumes, end-systolic volumes, stroke volume, cardiac output, and LV mass were performed in standard fashion.16 CMR analysis was supervised and/or performed by a single investigator (S.D.F.) at a cardiovascular magnetic resonance core laboratory by using the commercially available software package cmr42 (Circle Cardiovascular Imaging, Calgary, Ontario, Canada).
All echocardiograms were performed by using a General Electric Vivid-7 echocardiography machine (General Electric) and a standardized imaging protocol. A complete 3D as well as a 2D echocardiogram with Doppler and time-motion mode (M-mode) were performed and reported by following the Intersocietal Commission for the Accreditation of Echocardiography Laboratories (ICAEL) guidelines. LV volumes, function, and mass were calculated by using the recommendations of the American Society of Echocardiography.17 For 3D echocardiographic assessment, a full-volume acquisition of the left ventricle was obtained. The end-diastolic and end-systolic volumes, as well as the EF were calculated by using the 4D left ventricular quantification algorithm on a commercially available EchoPAC Workstation (General Electric). Echocardiographic analysis was performed by a single investigator (J.C.P.) at an echocardiography core laboratory.
Demographic and Exposure Variables
Cumulative dose of anthracycline exposure and maximum dose of chest-directed RT exposure were quantified by medical record abstraction. Patients included in this analysis completed the SJLIFE questionnaire and a physical examination.
Statistical Analyses
For the 114 survivors evaluated, descriptive statistics quantified cardiac volumes, function, and demographic and diagnosis-related variables. Paired t tests compared cardiac parameters measured by echocardiography with those measured by CMR. Comparisons of EF, stroke volume, and cardiac output were based on the raw scores, whereas raw scores indexed for body-surface area were used for comparisons of LV end-diastolic volumes, end-systolic volumes, and cardiac mass. Pearson's correlation was calculated between echocardiography and CMR measurements. Sensitivity, specificity, false-negative rate, and false-positive rate for echocardiography were calculated to assess the validity of echocardiography for detection of abnormal EF compared with CMR. Because reduced LV function on screening should result in referral for further evaluation, misclassification of LV function by echocardiography compared with CMR was assessed at a 50% cut point. Bland-Altman plots evaluated agreement (bias) between echocardiography and CMR assessment of EF. To determine the prevalence of cardiomyopathy among the 108 survivors previously undiagnosed with cardiomyopathy, detailed descriptive statistics for cardiac parameters measured by CMR were computed by stratifying the population by their radiation and anthracycline exposure status. Expected means for the survivor population were calculated by using normative values corresponding to each survivor's age and sex group from published normative data.16
RESULTS
Of 134 patients recruited onto the study, 114 patients (85%) completed evaluations. Twenty were unable to complete the CMR (Fig 1). The median age at evaluation was 39 years (range, 22 to 53 years; Table 1), and survivors were a median of 28 years (range, 18 to 38 years) from cancer diagnosis. Acute lymphoblastic leukemia (n = 44) and Hodgkin's lymphoma (n = 37) were the most common diagnoses. The prevalence of EF less than 50% was 14% (n = 16; CMR), 19% (n = 22; 3D echocardiography), 5% (n = 6; 2D biplane echocardiography), 7% (n = 8; 2D apical 4-chamber echocardiography), and 21% (n = 24; Teichholz method).
Table 1.
Demographic and Treatment Characteristics (n = 114)
| Characteristic | No. | % | Mean | SD |
|---|---|---|---|---|
| Current age, years | 38.3 | 6.3 | ||
| Median | 38.5 | |||
| Range | 22.7-53.7 | |||
| Age at diagnosis, years | 10.5 | 5.8 | ||
| Median | 11.3 | |||
| Range | 0.02-19.0 | |||
| Time since diagnosis, years | 27.74 | 4.6 | ||
| Median | 27.8 | |||
| Range | 18.4-38.3 | |||
| Sex | ||||
| Male | 47 | 41 | ||
| Female | 67 | 59 | ||
| Race | ||||
| White | 103 | 90 | ||
| Black | 11 | 10 | ||
| Diagnosis | ||||
| Acute lymphoblastic leukemia | 44 | 39 | ||
| Hodgkin's lymphoma | 37 | 32 | ||
| Osteosarcoma | 11 | 9 | ||
| Non-Hodgkin's lymphoma | 8 | 7 | ||
| Acute myeloid leukemia | 6 | 5 | ||
| Neuroblastoma | 3 | 3 | ||
| Ewing sarcoma | 2 | 2 | ||
| Wilms tumor | 2 | 2 | ||
| Soft tissue sarcomas | 1 | 1 | ||
| Anthracycline cumulative dose exposure, mg/m2 | 186 | 173 | ||
| Median | 117 | |||
| Range | 0-803 | |||
| > 350 | 29 | 25 | ||
| > 150-350 | 16 | 14 | ||
| 1-150 | 52 | 46 | ||
| None | 17 | 15 | ||
| RT dose to chest, Gy | ||||
| None | 77 | 68 | ||
| 1-30 | 16 | 14 | ||
| > 30 | 21 | 18 | ||
| Cardiovascular risk factors | ||||
| Hypertension | 29 | 25.4 | ||
| Diabetes | 8 | 7.0 | ||
| Dyslipidemia | 60 | 52.6 | ||
| Chronic renal insufficiency | 0 | 0.0 | ||
| Overweight (BMI 25-< 30) | 35 | 30.7 | ||
| Obese (BMI ≥ 30) | 38 | 33.3 | ||
Abbreviations: BMI, body mass index; RT, radiotherapy; SD, standard deviation.
The mean EF by 3D echocardiography (54.7%) did not differ from that by CMR (55.9%; P = .08). The mean EF on all 2D echocardiographic methods (area-length using biplane or apical 4-chamber and Teichholz M-mode) were 5% higher than with CMR (P ≤ .01; Table 2). In addition, 2D echocardiographic methods had wider ranges and standard deviations (SD) than CMR. Measures of volume were consistently lower with both 2D and 3D echocardiography than with CMR, but cardiac mass was overestimated by 2D echocardiography compared with CMR (P < .01).
Table 2.
Comparison of Measures of Cardiac Structure and Function Obtained by CMR and Echocardiogram
| Variable | No. of Patients* | Mean | Range | SD | P† |
|---|---|---|---|---|---|
| Ejection fraction (%) | |||||
| CMR | 114 | 55.9 | 38.4-68.5 | 5.8 | — |
| 3D echocardiography | 113 | 54.7 | 42.3-73.0 | 5.9 | .08 |
| 2D echocardiography | |||||
| Biplane | 113 | 61.0 | 45.0-83.2 | 6.9 | < .001 |
| Apical 4-chamber | 114 | 61.2 | 26.2-80.2 | 8.6 | < .001 |
| Teichholz | 108 | 59.3 | 13.4-89.0 | 13.4 | .01 |
| Left ventricular end-diastolic volume/body-surface area (mL/m2) | |||||
| CMR | 114 | 70.5 | 34.7-112.8 | 14.2 | — |
| 3D echocardiography | 113 | 58.1 | 29.9-95.6 | 11.9 | < .001 |
| 2D echocardiography | |||||
| Biplane | 97 | 48.6 | 22.9-100.2 | 13.4 | < .001 |
| Apical 4-chamber | 114 | 46.9 | 22.8-104.4 | 13.1 | < .001 |
| Left ventricular end-systolic volume/body-surface area (mL/m2) | |||||
| CMR | 114 | 31.5 | 16.7-60.2 | 9.1 | — |
| 3D echocardiography | 113 | 26.2 | 12.6-45.5 | 6.4 | < .001 |
| 2D echocardiography | |||||
| Biplane | 97 | 18.9 | 6.4-49.7 | 7.0 | < .001 |
| Apical 4-chamber | 114 | 18.3 | 6.3-49.2 | 7.1 | < .001 |
| Stroke volume (mL) | |||||
| CMR | 114 | 75.4 | 32.6-121.1 | 17.9 | — |
| 3D echocardiography | 113 | 61.1 | 22.1-128.5 | 17.6 | < .001 |
| 2D echocardiography | |||||
| Biplane | 97 | 56.9 | 20.3-115.8 | 17.6 | < .001 |
| Apical 4-chamber | 98 | 55.4 | 20.3-139.4 | 20.2 | < .001 |
| Cardiac output (mL/min) | |||||
| CMR | 114 | 5.5 | 3-10 | 1.3 | — |
| 3D echocardiography | 113 | 4.3 | 1.9-8.2 | 1.2 | < .001 |
| Cardiac mass/body-surface area (g/m2) | |||||
| CMR | 114 | 44.9 | 29.3-73.3 | 8.8 | — |
| 2D echocardiography (Teichholz) | 74 | 61.1 | 26.3-138.6 | 18.1 | < .001 |
| Fractional shortening (%) | |||||
| 2D echocardiography | 97 | 33.8 | 17.0-57.3 | 7.4 | — |
Abbreviations: 2D, two-dimensional; 3D, three-dimensional; CMR, cardiac magnetic resonance [imaging]; SD, standard deviation.
No. of patients with sufficient image quality for evaluation.
P for comparison of specific echocardiography modality with CMR.
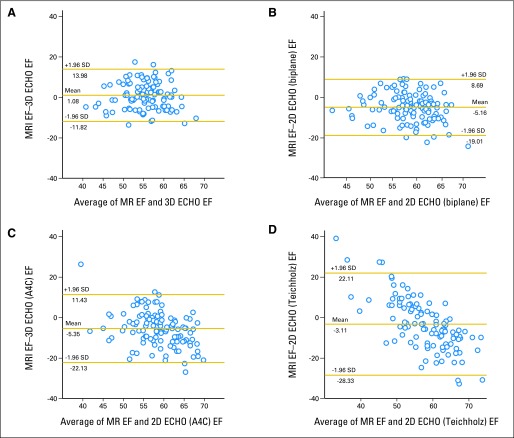
All echocardiographic methods correlated poorly with CMR (Teichholz, r = .29; apical 4-chamber view, r = .34; biplane echocardiography, r = .39; 3D echocardiography, r = 0.37; Appendix Table A1, online only). Bland-Altman measures of agreement with CMR (Fig 2) demonstrated wide ranges of agreement for both 2D and 3D echocardiography, although a smaller bias (mean difference) was noted for 3D echocardiography (bias, 1%; Bland-Altman limits of agreement [± 1.96 standard deviation], −11.8% to 14.0%); for CMR and 2D echocardiography: 2D biplane (bias, −5.2%; −19.0% to 8.69%), 2D apical 4-chamber (bias, −5.4%; −22.1% to 11.4%), and Teichholz M-mode (bias, −3.1%; −28.3% to 22.1%).
Fig 2.
Bland-Altman plots for agreement of cardiac magnetic resonance imaging (MRI) with (A) three-dimensional (3D) echocardiography (ECHO), (B) two-dimensional (2D) biplane echocardiography, (C) 2D apical 4-chamber (A4C), and (D) Teichholz method for assessment of ejection fraction (EF). SD, standard deviation.
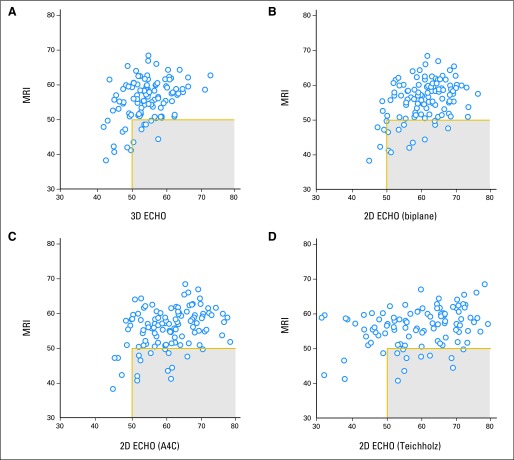
Compared with a CMR-determined cutoff of less than 50% for EF, 2D echocardiographic methods demonstrated reduced sensitivity (25% to 29%; Table 3) with improvement by using 3D echocardiography (sensitivity 53%). High false-negative rates were observed with echocardiography (3D, 47%; 2D, 71% to 75%). Survivors with an EF less than 50% by CMR (n = 16; Fig 3) but identified as ≥ 50% by echocardiography were identified (3D echocardiography: seven patients, 6%; 2D biplane echocardiography: 12 patients, 11%).
Table 3.
Screening Performance of Echocardiogram Compared With CMR for Detection of an EF < 50%
| Variable | 2D Echocardiography |
|||||||
|---|---|---|---|---|---|---|---|---|
| 3D Echocardiography |
Biplane |
Apical 4-Chamber |
Teichholz |
|||||
| No. | % | No. | % | No. | % | No. | % | |
| Patients with EF < 50%* | 22 | 6 | 8 | 24 | ||||
| Sensitivity | 53 | 25 | 25 | 29 | ||||
| Specificity | 86 | 98 | 96 | 79 | ||||
| False-negative rate | 47 | 75 | 75 | 71 | ||||
| False-positive rate | 14 | 2 | 4 | 21 | ||||
| Positive predictive value | 36 | 67 | 50 | 17 | ||||
| Negative predictive value | 92 | 89 | 89 | 88 | ||||
Abbreviations: 2D, two-dimensional; 3D, three-dimensional; CMR, cardiac magnetic resonance [imaging]; EF, ejection fraction.
Frequency of patients for a given modality with an EF < 50% (n = 16 for CMR).
Fig 3.
Scatter plots identifying survivors with an ejection fraction by cardiac magnetic resonance imaging (MRI) of less than 50% but ≥ 50% (gray box) by echocardiography (ECHO) for (A) three-dimensional (3D) echocardiography, (B) two-dimensional (2D) biplane, (C) 2D apical 4-chamber (A4C), and (D) 2D Teichholz method.
Among 108 participants previously undiagnosed with cardiomyopathy, 32% had an EF by CMR more than 2 SD below established age- and sex-stratified normal values (Table 4),16 with the highest prevalence among those who received both anthracyclines and chest-directed RT (42%). The mean EF by CMR was 56% (expected mean, 64%). Few survivors had evidence of dilated cardiomyopathy (prevalence, 1%), yet almost half (48%) had reduced cardiac mass (> 2 SD below normal). Notably, even patients who received less than 150 mg/m2 of anthracyclines had a high prevalence of reduced (> 2 SD below normal) EF (27%), stroke volume (29%), or cardiac mass (56%).
Table 4.
CMR Results in Survivors Who Received Low-Dose (< 150 mg/m2) or High-Dose (> 350 mg/m2) Anthracyclines, RT Only, or RT Plus Anthracyclines Among Patients Previously Undiagnosed With Cardiotoxicity
| Variable | No. of Evaluable Patients | Median | Range | Mean | SD | Expected Mean* | Prevalence Beyond 2 SD of Normal |
|
|---|---|---|---|---|---|---|---|---|
| No. | % | |||||||
| Ejection fraction (%) | ||||||||
| ≤ 150 mg/m2 anthracycline, no RT | 45 | 58.5 | 50.6-67.0 | 58.1 | 4.1 | 63.9 | 12 | 27 |
| > 350 mg/m2 anthracycline, no RT | 26 | 55.9 | 42.4-66.1 | 55.5 | 5.7 | 64.1 | 9 | 35 |
| RT only | 18 | 57.2 | 40.8-68.5 | 55.3 | 7.1 | 64.1 | 6 | 33 |
| RT plus anthracycline | 19 | 55.1 | 41.3-65.5 | 54.5 | 5.5 | 64.1 | 8 | 42 |
| Total | 108 | 57.2 | 40.8-68.5 | 56.3 | 5.5 | 64.0 | 35 | 32 |
| End-diastolic volume/body-surface area (mL/m2) | ||||||||
| ≤ 150 mg/m2 anthracycline, no RT | 45 | 67.9 | 34.7-100.7 | 66.0 | 14.3 | 83.0 | 0 | 0 |
| > 350 mg/m2 anthracycline, no RT | 26 | 80.4 | 53.5-102.5 | 77.6 | 13.5 | 79.9 | 1 | 4 |
| RT only | 18 | 62.8 | 43.9-88.0 | 63.7 | 11.3 | 78.8 | 0 | 0 |
| RT plus anthracycline | 19 | 70.4 | 52.6-86.9 | 68.7 | 8.74 | 80.7 | 0 | 0 |
| Total | 108 | 70.2 | 34.7-102.5 | 69.7 | 13.5 | 81.2 | 1 | 1 |
| Stroke volume, mL | ||||||||
| ≤ 150 mg/m2 anthracycline, no RT | 45 | 75.1 | 40.5-121.1 | 77.5 | 19.2 | 96.6 | 13 | 29 |
| > 350 mg/m2 anthracycline, no RT | 26 | 85.1 | 45.6-116.7 | 81.5 | 16.8 | 95.3 | 4 | 15 |
| RT only | 18 | 69.0 | 32.6-105.4 | 67.8 | 16.1 | 96.6 | 6 | 33 |
| RT plus anthracycline | 19 | 70.6 | 44.4-113.1 | 73.1 | 16.1 | 95.3 | 6 | 32 |
| Total | 108 | 74.1 | 32.6-121.1 | 76.06 | 18.0 | 96.1 | 29 | 27 |
| Cardiac mass/body-surface area (g/m2) | ||||||||
| ≤ 150 mg/m2 anthracycline, no RT | 45 | 41.2 | 29.3-61.9 | 42.6 | 8.1 | 55.5 | 25 | 56 |
| > 350 mg/m2 anthracycline, no RT | 26 | 45.7 | 32.8-73.3 | 46.2 | 9.1 | 59.8 | 11 | 43 |
| RT only | 18 | 44.6 | 32.4-68.9 | 45.0 | 10.3 | 61.4 | 12 | 67 |
| RT plus anthracycline | 19 | 43.4 | 34.4-57.6 | 45.5 | 6.5 | 58.6 | 4 | 21 |
| Total | 108 | 43.5 | 29.3-73.3 | 44.4 | 8.5 | 58.0 | 52 | 48 |
Abbreviations: CMR, cardiac magnetic resonance [imaging]; RT, radiotherapy; SD, standard deviation.
Based on normative, age- and sex-stratified CMR data.
DISCUSSION
Echocardiography is the recommended instrument for noninvasive screening of childhood cancer survivors for detecting reduced LV function.9 The American Society of Echocardiography specifically identifies 2D echocardiography assessment using the biplane method of disks (modified Simpson's rule) as the echocardiographic method of choice by consensus for both screening and monitoring of function.17 However, the validity of 2D echocardiogram to detect abnormal LV function in this population has not been tested. More recently, 3D echocardiography based on volumetric measurement rather than estimation may better approximate true LV function, but it is not routinely available for clinical use.
CMR is the reference standard measure of cardiac structure (volume, mass) and function (EF).11,12,18 Unlike 2D echocardiographic techniques, CMR does not rely on geometric assumptions or calculations based on incomplete sampling of cardiac volumes.19 It is noninvasive and does not require exposure to ionizing radiation as does radionuclide ventriculography or cardiac computed tomography. Despite these advantages, CMR is not currently the recommended screening tool for evaluation of cardiac function among adult survivors of childhood cancer exposed to cardiotoxic therapies, primarily because of the higher cost of CMR as well as limited availability. On the basis of exposure rates from the Childhood Cancer Survivor Study,20 we estimate that 47% of all survivors were exposed to anthracyclines and/or chest-directed RT. When estimates of numbers of survivors in the population are carried forward to 2011,3 it is likely that there are more than 172,000 survivors in the United States previously exposed to cardiotoxic therapy. Thus, understanding the strengths and limitations of echocardiography compared with CMR as a screening tool for detection of reduced cardiac function is essential.
Although an EF between 50% and 55% is considered to be in the mildly abnormal range,17 adult survivors exposed to cardiotoxic therapy who develop an EF less than 50% should certainly be referred for further cardiology assessment and for consideration of medical intervention (angiotensin-converting enzyme inhibitors or beta blockade). The ideal screening test for detection of treatment-induced cardiomyopathy should be readily available at reasonably low cost. It should also have a high sensitivity such that patients with an EF less than 50% would be correctly identified and referred to a cardiologist for additional evaluation. Further, this screening test should have a low false-negative rate so that, among survivors screened and identified to have an EF ≥ 50%, the number of patients with true cardiac dysfunction would be minimized. Although our findings are limited by the small number of patients with an EF less than 50%, they suggest that 2D echocardiographic screening (using the biplane method) may not achieve the desired level of accuracy (sensitivity of 25% and false-negative rate of 75%) required for an ideal screening test. Use of 3D echocardiography may improve sensitivity (53%) as well as the false-negative rate (47%), yet it fails to reproduce the performance of CMR in individual patients.
Despite these poor screening characteristics, we identified no difference in the mean EF between CMR and 3D echocardiography. This suggests that despite variability between these two modalities on any given individual, 3D echocardiography may provide meaningful estimates of EF across a large population that approach those obtained by CMR. By contrast, 2D echocardiographic methods report a mean EF 5% higher than CMR. In addition, 2D echocardiographic methods have a wider range of values (increased variability) compared with CMR as seen in the increased SD, the low correlation with CMR values, and the wide Bland-Altman limits of agreement. Although this analysis focused on EF as the primary screening end point, it is worth noting that both end-systolic and end-diastolic ventricular volumes were generally underestimated by 2D echocardiography, a finding documented in previous studies that have compared these modalities.10
Perhaps the most clinically relevant approach to assessing echocardiography as a screening device is to identify the number of patients within our study population who would have been misclassified by routine 2D echocardiography screening (ie, identified to have an abnormal EF by CMR but a normal EF by echocardiography). Fortunately, this number is relatively low (11%) when using an EF cutoff of 50% (2D biplane method), primarily because only 16 survivors had an EF less than 50% by CMR.
To the best of our knowledge, we present the first CMR assessment of prevalence of cardiotoxicity in a population of survivors previously undiagnosed with cardiac disease. We identified a mean EF of 56% and also that one third of these previously undiagnosed survivors had a CMR EF more than 2 SD below the expected mean when using age- and sex-stratified normal CMR data. Notably, despite previous reports that anthracycline-mediated injury results in a dilated cardiomyopathy,21 few adult survivors have increased ventricular volumes. Nearly half of all survivors in our study have reduced cardiac mass indicative of therapy-related injury. Of greatest concern, among patients who received doses of anthracyclines lower than 150 mg/m2 (traditionally thought to be a safe dose range) and who are currently healthy (ie, not previously diagnosed with cardiac compromise), 27% were found to have reduced EF and 56% had reduced cardiac mass.
Limitations of this analysis should be considered in the interpretation of the results. Despite being the largest direct comparison of CMR and echocardiography to date, the analysis of the screening parameters of echocardiography is limited by the low absolute number (n = 16) of patients with an EF less than 50% by CMR. Thus, although we report the direct comparison by assessing sensitivity, false-negative rate, and misclassification, these findings should be interpreted in light of this study's limited sample size. In addition, since the primary aim of this analysis was comparison of multiple screening modalities, the sample was selected by convenience from the larger SJLIFE population. Thus, even among survivors previously undiagnosed with cardiotoxicity, there is the possibility that our prevalence rates of abnormalities in structure and function may not be representative of the entire SJLIFE population. However, we clearly describe key demographic variables (current age, cumulative dose of anthracyclines, and chest RT exposure), allowing clinicians to assess the generalizability of these results relative to their specific population.
Two factors—the cost differential and the reduced availability of CMR compared with echocardiography—have previously resulted in use of echocardiography as the front-line imaging modality. National Medicare figures22 for global reimbursement rates for CMR ($449) and echocardiography ($232) suggest an absolute differential of only $217 per examination. Although a full accounting of downstream and other costs is not included in this study, considering a misclassification of 11% for 2D echocardiography, the additional cost of a CMR-only screening strategy per case of cardiotoxicity identified is $1,973 ($217/11%), a cost low enough to warrant further consideration and investigation. At present, however, the limited availability of CMR compared with echocardiography is real and may prevent use of CMR in the broad population of cancer survivors at risk for cardiomyopathy. Finally, 15% of our population was unable to complete CMR evaluation for a variety of reasons. On the basis of the limited ability of echocardiography to identify survivors with an EF less than 50%, future studies of larger cohorts are needed to conduct cost-benefit analyses that take into account the high rate of patients unable to complete a CMR evaluation.
Considering that 2D echocardiography remains the screening modality of choice, the most important contribution of this analysis is to better define how to most effectively use echocardiography as a screening evaluation. On the basis of our data, survivors with 2D echocardiography (biplane method) EF values greater than 60% can be reasonably certain to have normal cardiac function. In addition, use of a 2D echocardiography cutoff for referral to less than 60% EF (as opposed to < 50% with the biplane method) improved sensitivity (75% sensitive) for detection of a CMR EF of less than 50%. Thus, for this high-risk population, previously exposed to cardiotoxic therapy, consideration should be given to referring survivors with an EF of 50% to 59% by 2D echocardiography for comprehensive cardiology assessment that includes cardiac history, symptom index and examination, biomarker assessment, consideration of CMR, functional assessment by treadmill testing, and possibly medical therapy to prevent progression of disease. Future studies should consider the use of intravenous contrast with echocardiography, a technique that may provide better definition of ventricular volumes, perhaps improving sensitivity as well, although there would be additional cost and the need for intravascular access. Evidence from the general population indicating that intervention with angiotensin-converting enzyme inhibitors significantly decreases the progression of heart failure (Studies of Left Ventricular Dysfunction [SOLVD] trial,23 risk reduction 29%; P < .001) among patients with asymptomatic ventricular dysfunction suggests that early identification of asymptomatic cardiotoxicity in survivors may be important. However, randomized trials of medical interventions among survivors of childhood cancer are needed.
In conclusion, we showed that 2D echocardiography demonstrates limited performance for detection of reduced EF among survivors of childhood cancer treated with chemotherapy or RT. CMR identifies that even previously undiagnosed survivors who received low-dose anthracycline exposure have significant rates of subclinical dysfunction. These findings should inform clinical use of echocardiography for screening by suggesting a lower threshold for additional cardiology referral in this population exposed to cardiotoxic therapy. In addition, future evaluation of this study population is needed to assess for cardiac events, hospitalizations, and cardiac mortality that will identify whether survivors within this low normal range for EF by 2D echocardiography may eventually develop heart failure. The design of the SJLIFE study, to provide lifetime follow-up, including screening evaluations, for these aging survivors will allow future identification of the development of cardiotoxicity in this population.
Appendix
Summary of Methods for CMR and Echocardiography
This study complied with the Standards for Reporting of Diagnostic Accuracy (STARD) initiative (Bossuyt PM, et al: Ann Intern Med 138:40-44, 2003). In addition to the study research questions and design already detailed within the main manuscript, the following information completes the outlined STARD criteria.
Core Laboratories Readers/Investigators
S.D.F. is fellowship trained in cardiac magnetic resonance (CMR) imaging and has 18 years of experience, 13 years of which have been spent leading CMR laboratories. J.C.P. is fellowship trained in echocardiography and has 10 years of experience in high-volume academic centers. Both investigators were blinded to the results of the other imaging examination, other clinical testing, and patient history and were aware only of patient sex and age during image analysis.
Adverse Events
There were no adverse events associated with performance of CMR or two-dimensional (2D) or three-dimensional (3D) echocardiography.
CMR Imaging
For each patient, a set of multiplanar survey images was acquired and was used for positioning of conventional bright-blood cine steady-state free precession (SSFP) images of the heart in the standard orientations: 2-chamber long-axis view, 3-chamber long-axis view, and 4-chamber long-axis view. A set of short-axis slices covering the entire left ventricle (LV) from base to apex was also acquired for each patient. The cine imaging sequence was an SSFP sequence with the following parameters: TR, 3.4 ms; TE, 1.5 ms; flip angle of 30 degrees; slice thickness, 10 mm; slice gap, 0 mm; field of view, 240 to 320 mm; matrix, 256 × 192; acquired typical in-plane spatial resolution, 1.5 × 1.2 mm; temporal resolution, 38 to 43 ms; and breath-hold duration, 10 to 12 heartbeats per slice.
CMR imaging protocol.
Scout imaging was transaxial, coronal, and sagittal.
2-chamber long-axis cine image.
(1) 2-chamber long-axis prescribed orthogonal to transaxial scouts aligned through the apex and center of the mitral valve; (2) horizontal long axis aligned orthogonal to the vertical long axis, passing through the apex and center of the mitral valve.
SSFP short-axis cine images.
(1) Short-axis cine images were acquired from the mitral valve plane through the apex. The basal most short-axis slice was located immediately on the atrial side of the atrioventricular junction at end diastole prescribed from the previously acquired 2-chamber and horizontal long-axis cines.
SSFP 4-chamber and 3-chamber long-axis cine images.
The 4-chamber long axis is prescribed from the vertical long axis through the left ventricular apex and center of the mitral valve and angled on the basis of basal short-axis cines such that the imaging plane passes through the acute margin of the right ventricular free wall. (2) The 3-chamber long axis similarly is prescribed from the vertical long axis through the left ventricular apex and center of the mitral valve and angled on the basis of basal short-axis cines such that the imaging plane passes through the center of the aortic valve.
Echocardiography
Each patient underwent conventional 2D echocardiography with a commercially available 3.5 MHz handheld surface transducer (General Electric Vivid 7; General Electric Healthcare, Milwaukee, WI). LV ejection fraction was calculated by using three different standardized techniques (biplane, apical 4-chamber, and Teichholz) from the acquired apical 4-chamber and 2-chamber views with a modified Simpson's biplane method, all according to American Society of Echocardiography guidelines.
3D echocardiography assessment of LV ejection fraction was performed by acquiring a full-volume LV data set by using a 3.5 MHz matrix array transducer (General Electric Vivid 7). Offline analysis software (EchoPAC, General Electric) was used to reconstruct this data set deriving conventional 4-chamber, 2-chamber, and short-axis views. After selection of two annular and apical reference points, a 3D endocardial shell was constructed by using semiautomated contour tracing. Following manual adjustment of endocardial contours (as needed) at end diastole and end systole by the expert reader (J.C.P.), resultant end-diastolic and end-systolic volumes were calculated to derive 3D LV ejection fraction.
Table A1.
Correlation of Measures of Cardiac Structure and Function by Echocardiogram With CMR
| Measure of Cardiac Structure or Function | 3D Echocardiography | 2D Echocardiography |
||
|---|---|---|---|---|
| Biplane | Apical 4-Chamber | Teichholz | ||
| Ejection fraction | 0.37 | 0.39 | 0.34 | 0.29 |
| LVEDV/BSA | 0.63 | 0.70 | 0.67 | — |
| LVESV/BSA | 0.62 | 0.77 | 0.72 | — |
| Stroke volume | 0.68 | 0.56 | 0.54 | — |
| Cardiac output | 0.57 | — | — | — |
| Cardiac mass/BSA | — | — | — | 0.66 |
Abbreviations: 2D, two-dimensional; 3D, three-dimensional; BSA, body-surface area; CMR, cardiac magnetic resonance [imaging]; LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume.
Footnotes
Supported by American Society of Clinical Oncology Career Development Award (G.T.A.), by Cancer Center Support (CORE) Grant No. CA 21765 to St. Jude Children's Research Hospital, and by the American Lebanese-Syrian Associated Charities.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Juan Carlos Plana, General Electric (C) Stock Ownership: None Honoraria: Scott D. Flamm, Philips Healthcare Research Funding: None Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Gregory T. Armstrong, Juan Carlos Plana, Deokumar Srivastava, F. Daniel Donovan, Alejandro Arevalo, Jean-Bernard Durand, Vijaya Joshi, Melissa M. Hudson, Leslie L. Robison, Scott D. Flamm
Collection and assembly of data: Gregory T. Armstrong, Juan Carlos Plana, F. Daniel Donovan, Vijaya Joshi, Melissa M. Hudson, Scott D. Flamm
Data analysis and interpretation: Gregory T. Armstrong, Juan Carlos Plana, Nan Zhang, Deokumar Srivastava, Daniel M. Green, Kirsten K. Ness, F. Daniel Donovan, Monika L. Metzger, Alejandro Arevalo, Jean-Bernard Durand, Melissa M. Hudson, Leslie L. Robison, Scott D. Flamm
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Ries LA, Melbert D, Krapcho M, et al. Bethesda, MD: National Cancer Institute; 2008. SEER Cancer Statistics Review, 1975-2005. http://seer.cancer.gov/csr/1975_2005/ [Google Scholar]
- 2.Mertens AC, Liu Q, Neglia JP, et al. Cause-specific late mortality among 5-year survivors of childhood cancer: The Childhood Cancer Survivor Study. J Natl Cancer Inst. 2008;100:1368–1379. doi: 10.1093/jnci/djn310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mariotto AB, Rowland JH, Yabroff KR, et al. Long-term survivors of childhood cancers in the United States. Cancer Epidemiol Biomarkers Prev. 2009;18:1033–1040. doi: 10.1158/1055-9965.EPI-08-0988. [DOI] [PubMed] [Google Scholar]
- 4.van der Pal HJ, van Dalen EC, Hauptmann M, et al. Cardiac function in 5-year survivors of childhood cancer: A long-term follow-up study. Arch Intern Med. 2010;170:1247–1255. doi: 10.1001/archinternmed.2010.233. [DOI] [PubMed] [Google Scholar]
- 5.Lipshultz SE, Vlach SA, Lipsitz SR, et al. Cardiac changes associated with growth hormone therapy among children treated with anthracyclines. Pediatrics. 2005;115:1613–1622. doi: 10.1542/peds.2004-1004. [DOI] [PubMed] [Google Scholar]
- 6.Lipshultz SE, Colan SD, Gelber RD, et al. Late cardiac effects of doxorubicin therapy for acute lymphoblastic leukemia in childhood. N Engl J Med. 1991;324:808–815. doi: 10.1056/NEJM199103213241205. [DOI] [PubMed] [Google Scholar]
- 7.Hudson MM, Rai SN, Nunez C, et al. Noninvasive evaluation of late anthracycline cardiac toxicity in childhood cancer survivors. J Clin Oncol. 2007;25:3635–3643. doi: 10.1200/JCO.2006.09.7451. [DOI] [PubMed] [Google Scholar]
- 8.Hull MC, Morris CG, Pepine CJ, et al. Valvular dysfunction and carotid, subclavian, and coronary artery disease in survivors of Hodgkin lymphoma treated with radiation therapy. JAMA. 2003;290:2831–2837. doi: 10.1001/jama.290.21.2831. [DOI] [PubMed] [Google Scholar]
- 9.Landier W, Bhatia S, Eshelman DA, et al. Development of risk-based guidelines for pediatric cancer survivors: The Children's Oncology Group Long-Term Follow-Up Guidelines from the Children's Oncology Group Late Effects Committee and Nursing Discipline. J Clin Oncol. 2004;22:4979–4990. doi: 10.1200/JCO.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 10.Bellenger NG, Burgess MI, Ray SG, et al. Comparison of left ventricular ejection fraction and volumes in heart failure by echocardiography, radionuclide ventriculography and cardiovascular magnetic resonance: Are they interchangeable? Eur Heart J. 2000;21:1387–1396. doi: 10.1053/euhj.2000.2011. [DOI] [PubMed] [Google Scholar]
- 11.American College of Cardiology Foundation Task Force on Expert Consensus Documents, Hundley WG, Bluemke DA, et al. ACCF/ACR/AHA/NASCI/SCMR 2010 expert consensus document on cardiovascular magnetic resonance: A report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. J Am Coll Cardiol. 2010;55:2614–2662. doi: 10.1016/j.jacc.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Constantine G, Shan K, Flamm SD, et al. Role of MRI in clinical cardiology. Lancet. 2004;363:2162–2171. doi: 10.1016/S0140-6736(04)16509-4. [DOI] [PubMed] [Google Scholar]
- 13.Lima JA, Desai MY. Cardiovascular magnetic resonance imaging: Current and emerging applications. J Am Coll Cardiol. 2004;44:1164–1171. doi: 10.1016/j.jacc.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 14.Grothues F, Smith GC, Moon JC, et al. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol. 2002;90:29–34. doi: 10.1016/s0002-9149(02)02381-0. [DOI] [PubMed] [Google Scholar]
- 15.Hudson MM, Ness KK, Nolan VG, et al. Prospective medical assessment of adults surviving childhood cancer: Study design, cohort characteristics, and feasibility of the St. Jude Lifetime Cohort Study. Pediatr Blood Cancer. 2011;56:825–836. doi: 10.1002/pbc.22875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alfakih K, Plein S, Thiele H, et al. Normal human left and right ventricular dimensions for MRI as assessed by turbo gradient echo and steady-state free precession imaging sequences. J Magn Reson Imaging. 2003;17:323–329. doi: 10.1002/jmri.10262. [DOI] [PubMed] [Google Scholar]
- 17.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: A report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Lima JA, Judd RM, Bazille A, et al. Regional heterogeneity of human myocardial infarcts demonstrated by contrast-enhanced MRI: Potential mechanisms. Circulation. 1995;92:1117–1125. doi: 10.1161/01.cir.92.5.1117. [DOI] [PubMed] [Google Scholar]
- 19.Pennell DJ, Sechtem UP, Higgins CB, et al. Clinical indications for cardiovascular magnetic resonance (CMR): Consensus Panel report. Eur Heart J. 2004;25:1940–1965. doi: 10.1016/j.ehj.2004.06.040. [DOI] [PubMed] [Google Scholar]
- 20.Mulrooney DA, Yeazel MW, Kawashima T, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: Retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ. 2009;339:b4606. doi: 10.1136/bmj.b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lipshultz SE, Lipsitz SR, Sallan SE, et al. Chronic progressive cardiac dysfunction years after doxorubicin therapy for childhood acute lymphoblastic leukemia. J Clin Oncol. 2005;23:2629–2636. doi: 10.1200/JCO.2005.12.121. [DOI] [PubMed] [Google Scholar]
- 22.Centers for Medicare and Medicaid Services: Overview. http://www.cms.gov/apps/physician-fee-schedule/
- 23.[No authors listed]: Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions: The SOLVD Investigators. N Engl J Med. 1992;327:685–691. doi: 10.1056/NEJM199209033271003. [DOI] [PubMed] [Google Scholar]