Abstract
The transient receptor potential (TRP) channel TRPM2 is an ion channel that modulates cell survival. We report here that full-length (TRPM2-L) and short (TRPM2-S) isoform expression was significantly increased in human neuroblastoma compared with adrenal gland. To differentiate the roles of TRPM2-L and TRPM2-S in cell proliferation and survival, we established neuroblastoma SH-SY5Y cell lines stably expressing either TRPM2 isoform or empty vector. Cells expressing TRPM2-S showed significantly enhanced proliferation, downregulation of phosphatase and tensin homolog (PTEN), and increased protein kinase B (Akt) phosphorylation and cell surface glucose transporter 1 (Glut1) compared with cells expressing TRPM2-L or empty vector. ERK phosphorylation was increased, and forkhead box O 3a (FOXO3a) levels were decreased. Inhibitor studies demonstrated that enhanced proliferation was dependent on phosphatidylinositol 3-kinase/Akt, ERK, and NADPH oxidase activation. On the other hand, TRPM2-S-expressing cells were significantly more susceptible to cell death induced by low H2O2 concentrations (50–100 μM), whereas TRPM2-L-expressing cells were protected. This was associated with a significant increase in FOXO3a, MnSOD (SOD2), and membrane Glut1 in TRPM2-L-expressing cells compared with TRPM2-S expressing cells. We conclude that TRPM2 channels occupy a key role in cell proliferation and survival following oxidative stress in neuroblastoma. Our results suggest that overexpression of TRPM2-S results in increased proliferation through phosphatidylinositol 3-kinase/Akt and ERK pathways, while overexpression of TRPM2-L confers protection against oxidative stress-induced cell death through FOXO3a and SOD. TRPM2 channels may represent a novel future therapeutic target in diseases involving oxidative stress.
Keywords: transient receptor potential channels, Akt, ERK, FOXO3, MnSOD
the transient receptor potential (TRP) protein superfamily is a diverse group of cation-permeable channels expressed on mammalian cells (4, 8, 44, 74). Mammalian TRP channels have been organized into six subfamilies on the basis of sequence similarities designated C (canonical), V (vanilloid receptor), M (melastatin), A (ANKTM), P (polycystin), and ML (mucolipin). Different subfamilies mediate a broad range of physiological processes (12, 43, 54, 62) and are recognized to be involved in a growing number of diseases (5, 45). Monomeric TRP proteins have six putative transmembrane domains and intracellular NH2 and COOH termini. The functional TRP channel consists of homotetramers or heterotetramers, with the putative pore formed by loops between the fifth and sixth transmembrane domains. Channel regulation includes roles for extracellular signals, second messengers, channel subunit assembly, and macromolecular complex formation. Splice variants have been described for a number of TRP channels. Some of these consist of only NH2-terminal, COOH-terminal, or NH2-terminal and truncated transmembrane domains, and many of these inhibit full-length channel function (47, 80, 87).
The TRPM subfamily of TRP channels has been found to have roles in cell proliferation and survival (61). This subfamily was named after the first described member, TRPM1 (or melastatin), a putative tumor suppressor protein (15). TRPM1 is expressed on melanocytes, and its expression level inversely correlates with melanoma aggressiveness and metastatic potential, suggesting that it functions as a tumor suppressor (10, 15, 17). Other TRPM members, including TRPM2 (23, 43), TRPM4 (65), TRPM5 (51), TRPM7 (1, 21), and TRPM8 (35, 70), have also been demonstrated to have important roles in cell proliferation and survival. TRPM2 was the second member of the TRPM subfamily to be cloned and is expressed in many cell types (43). Extracellular signals that activate TRPM2 include oxidative stress, TNFα, amyloid β-peptide, and concanavalin A (18, 19, 23, 76). Stimulation with these extracellular signals results in production of ADP-ribose (ADPR), which activates TRPM2 by binding to the TRPM2 COOH-terminal NUDT9-H domain, an ADPR hydrolase (19, 31, 43, 50). Cyclic ADPR (cADPR) can gate or potentiate the effects of ADPR on TRPM2. TRPM2 currents are also dependent on and positively regulated by Ca2+ (13, 41, 69). TRPM2 has been reported to be temperature-sensitive (68, 79); TRPM2 is maximally sensitive to activation by cADPR in pancreatic islets at 37°C, and TRPM2 temperature sensitivity has been reported to affect macrophage function (30).
Oxidative stress results from a disturbance of the balance between oxidants and antioxidants and, depending on severity and duration, leads to tissue injury (43). Oxidative stress plays an important role in tissue damage in a large number of physiological and pathophysiological processes, including aging, cancer, neurodegenerative disorders, diabetes mellitus, atherosclerosis, ischemia-reperfusion injury, and autoimmune diseases (14, 24, 34, 75, 81, 84, 88). In oxidative stress, increased reactive oxygen species (ROS) can enhance ADPR production, which activates full-length TRPM2 (TRPM2-L) (23, 29, 33, 58, 66, 67, 82, 88). Elucidating the role of TRPM2 in mediating cell death and survival could have significant implications for a number of human diseases. Among them, targeting TRPM2 could have implications for treatment of malignant disease, because TRPM2 and its isoforms are expressed in a number of types of cancer, and at least one isoform may function as a tumor enhancer (47).
We previously identified a physiological TRPM2 splice variant (TRPM2-S) that lacks four of the six predicted COOH-terminal transmembrane domains and the putative Ca2+ pore (87). Using tumor samples obtained from patients with neuroblastoma, we determined that TRPM2-L and TRPM2-S expression is significantly higher in neuroblastoma than normal adrenal tissues. In the present study, we used neuroblastoma SH-SY5Y cell lines stably expressing TRPM2-L, TRPM2-S, or empty vector to determine the functional significance of TRPM2 isoform expression in cell proliferation.
EXPERIMENTAL PROCEDURES
Reagents.
Wortmannin, LY294002, H2O2, clotrimazole, diphenyleneiodonium (DPI), and anti-actin antibody were purchased from Sigma Chemical (St. Louis, MO); U0126 from Calbiochem Chemicals (San Diego, CA); antibodies to phosphorylated (S473) Akt (pAkt), Akt, phosphatase and tensin homolog (PTEN), Na+-K+-ATPase, GAPDH, caspase-3, poly(ADP-ribose) polymerase (PARP), phosphorylated ERK (pERK1/2), ERK (ERK1/2), and forkhead O 3a (FOXO3a) from Cell Signaling Technology (Boston, MA); antibodies to MnSOD from Abcam (Cambridge, MA); anti-TRPM2-C and anti-TRPM2-N antibodies, targeted to the TRPM2 COOH and NH2 termini, from Bethyl Laboratories (Montgomery, TX) (87); anti-V5 antibody from Invitrogen (Carlsbad, CA); and anti-glucose transporter 1 (Glut1) antibody from Millipore (Temecula, CA).
Tissues and cell lines.
Human adrenal gland and neuroblastoma tissues were obtained from the Pediatric Division of the National Cancer Institute Cooperative Human Tissue Network and the Research Institute at Nationwide Children's Hospital (Columbus, OH) under protocols approved by the Institutional Review Board of the Pennsylvania State University College of Medicine. The neuroblastoma cell line SH-SY5Y was purchased from American Type Culture Collection (Manassas, VA). SH-SY5Y cells were cultured in 50% DMEM-50% Ham's F-12 medium supplemented with 10% heat-inactivated FBS.
Transient transfection.
For overexpression of FOXO3a, human green fluorescent protein-FOXO3a construct was obtained from Origene Technologies (Rockville, MD). For depletion of FOXO3a, short-hairpin RNAs (shRNAs) targeted to FOXO3a were purchased from Origene. All were transfected into SH-SY5Y cells using the Neon transfection system (Invitrogen) following the manufacturer's instructions. Successful optimization of loss or gain of expression was determined with Western blotting.
Stable transfection.
SH-SY5Y cells at 90% confluence were transfected with TRPM2-L or TRPM2-S expression vector (87) or empty vector (pcDNA3.1/V5-His TOPO) for 48 h using Lipofectamine 2000 (Invitrogen) following the manufacturer's instructions. Stably transfected cell lines were selected using 600 μg/ml G418 (Geneticin, an analog of neomycin; Gemini Bio-Products, West Sacramento, CA), and cell cultures were maintained in the presence of 250 μg/ml G418 for ≥2 mo before cells were used for experiments. For Western blotting of proliferating cells, stably transfected cells were harvested when plates were 70–80% confluent. For oxidative stress studies, plates were treated when cells were 70–80% confluent and harvested at the time points noted.
Cell proliferation assay.
The same number of cells from stably or transiently (FOXO3a and shRNAs) transfected cell lines were seeded on 96-well plates and cultured in medium with 250 μg/ml G418 for 96 h. Cell proliferation was measured as ratio of optical density at 490 nm to optical density at 690 nm using 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT) cell proliferation assay (Trevigen, Gaithersburg, MD) according to the manufacturer's instructions (56). Alternatively, the same numbers of cells were seeded on 24-well plates and cultured in medium with 250 μg/ml G418 for 96 h, and cell proliferation was determined by counting cell numbers using the trypan blue exclusion method (Invitrogen). In some experiments, cells were treated with wortmannin (250 or 1,000 nM), LY294002 (2 or 10 μM), U0126 (10 or 20 μM), DPI (0.3 to 1 μM), H2O2 (50 to 100 μM), or clotrimazole (10 μM) during cell culture.
Immunoblot analysis.
Pieces of neuroblastoma and adrenal gland tissues were homogenized by a hand-held homogenizer in Triton lysis buffer (50 mM Tris, pH 7.4, 150 mM NaCl, 1% Triton X-100, 1 mM EDTA, 10 mM NaF, protease inhibitor, and phosphatase inhibitor) and then centrifuged for 10 min at 10,000 rpm at 4°C. The supernatants were collected and subjected to 8% or 10% SDS-PAGE, as previously described. For cell cultures, after appropriate treatment, whole cell lysates from stably transfected cells were isolated with Triton lysis buffer, and supernatants were collected and subjected to SDS-PAGE. All gels were then transblotted onto nitrocellulose membranes. Blots were probed with anti-TRPM2-N (1:250–1:500 dilution), anti-TRPM2-C (1:300 dilution), anti-V5-horseradish peroxidase (1:2,000 dilution), anti-actin (1:10,000 dilution), anti-GAPDH (1:5,000 dilution), anti-Na+-K+-ATPase (1:500 dilution), anti-pAkt (1:750 dilution), anti-Akt (1:2,000 dilution), anti-PTEN (1:1,500 dilution), anti-caspase-3 (1:500 dilution), anti-PARP (1:750 dilution), anti-Glut1 (1:3,000 dilution), anti-FOXO3a (1:400 dilution), anti-MnSOD (1:2,500 dilution), anti-pERK1/2 (1:1,000 dilution), or anti-ERK1/2 (1:1,500 dilution) antibodies. Blots were washed and incubated with the appropriate horseradish peroxidase-conjugated antibodies (1:2,000 dilution). Enhanced chemiluminescence was used for detection of signal. Oxidized PTEN was identified using lysis buffers with 50 mM N-ethylmaleimide on nonreducing SDS-polyacrylamide gels, as previously described (60). Oxidized PTEN has two less cysteines available for alkylation, resulting in a lower molecular weight.
Cell fractionation.
Cell membrane and cytosol fractions were isolated from cells using the Qproteome Cell Compartment kit (Qiagen, Valencia, CA). Both fractions were concentrated by acetone precipitation or with the Nanostep 10K Omega centrifugal device (Pall Life Sciences, Ann Arbor, MI) and then subjected to Western blot analysis. Anti-Na+-K+-ATPase and anti-GAPDH antibodies were used as quality and loading controls for membrane and cytosol fractions, respectively.
Measurement of intracellular Ca2+ concentration and ROS with digital video imaging.
The fluorescence microscopy-coupled digital video imaging system used to measure changes in intracellular Ca2+ concentration ([Ca2+]i) is described elsewhere (7, 42). Stably transfected SH-SY5Y cells were adhered to fibronectin-coated glass coverslips and loaded for 20 min with 0.1 μM fura 2-AM (Molecular Probes, Eugene, OR). Fura 2-loaded cells were excited alternately at 360 and 380 nm, and fluorescence emissions (510 nm) were captured. The ratio of fluorescence at 360 nm to fluorescence at 380 nm (F360/F380) was measured at baseline and over a 20-min interval following H2O2 treatment.
To measure ROS, cells were loaded with 5-(and 6-)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate acetyl ester (1.25 μM, 5 min at 37°C; Molecular Probes). Fluorescent cell images were obtained at 490-nm excitation and 535-nm emission. The gain of the intensified charge-coupled device camera was adjusted to obtain the optimal dynamic range and remained fixed for the duration of the experiments. Fluorescence in images was quantified by digitizing to eight-bit (0–255) resolution. Autofluorescence of unloaded cells was not detectable with our settings.
Statistical analysis.
Student's t-test or one-way analysis of variance was performed to verify significant differences among the experimental groups.
RESULTS
Expression of TRPM2 isoforms in adrenal gland, neuroblastoma, and neuroblastoma SH-SY5Y cells.
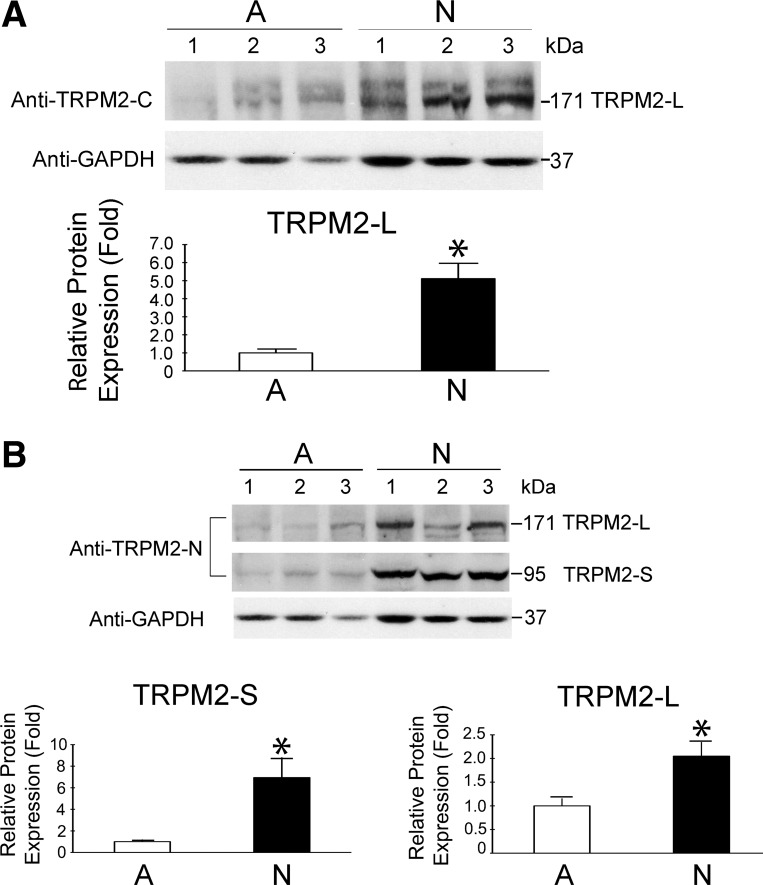
Since TRPM2 is highly expressed in neuronal tissue (18), we examined expression of TRPM2-L and TRPM2-S in neuroblastoma. Lysates from 13 adrenal glands and 17 neuroblastoma tissues were probed with anti-TRPM2-C antibody, which recognizes only TRPM2-L (171 kDa), or with anti-TRPM2-N antibody, which recognizes TRPM2-L and TRPM2-S (95 kDa). TRPM2-L expression was higher in neuroblastoma than normal adrenal gland (Fig. 1; P ≤ 0.01). TRPM2-S expression was also increased in neuroblastoma compared with adrenal gland (Fig. 1B; P ≤ 0.008). The identity of TRPM2-L was confirmed by immunoprecipitation from primary neuroblastoma tissue with anti-TRPM2-C antibody followed by mass spectrometry (Nextgen Sciences, Ann Arbor, MI). These results demonstrate that the endogenous TRPM2 isoforms TRPM2-L and TRPM2-S are expressed in normal adrenal gland and neuroblastoma. Greater expression in neuroblastoma suggests that TRPM2 may have a physiological function in tumor cells that has not been defined.
Fig. 1.
Western blots of endogenous transient receptor potential (TRP) M2 isoforms expressed in adrenal glands and neuroblastoma. Whole cell lysates from adrenal gland tissues and neuroblastoma were isolated, and 200 μg of protein were loaded in each lane. Western blots were probed with anti-TRPM2-C antibody (A) to detect TRPM2-L (171 kDa) and then with anti-TRPM2-N antibody (B) to detect TRPM2-L and TRPM2-S (95 kDa). GAPDH was probed to determine equivalent loading. Representative blots with 3 adrenal (A) and 3 neuroblastoma (N) samples probed with anti-TRPM2-C (A), anti-TRPM2-N (B), and GAPDH (A and B) are shown. GAPDH loading control was the same for A and B. Bands were quantified by densitometry, and relative expression of TRPM2/GAPDH for neuroblastoma, normalized to mean adrenal gland/GAPDH on the same blot, was determined. Statistical differences between adrenal gland tissue (13 samples) and neuroblastoma (17 tumor samples in A and 16 in B) were analyzed using Student's t-test: *P ≤ 0.01.
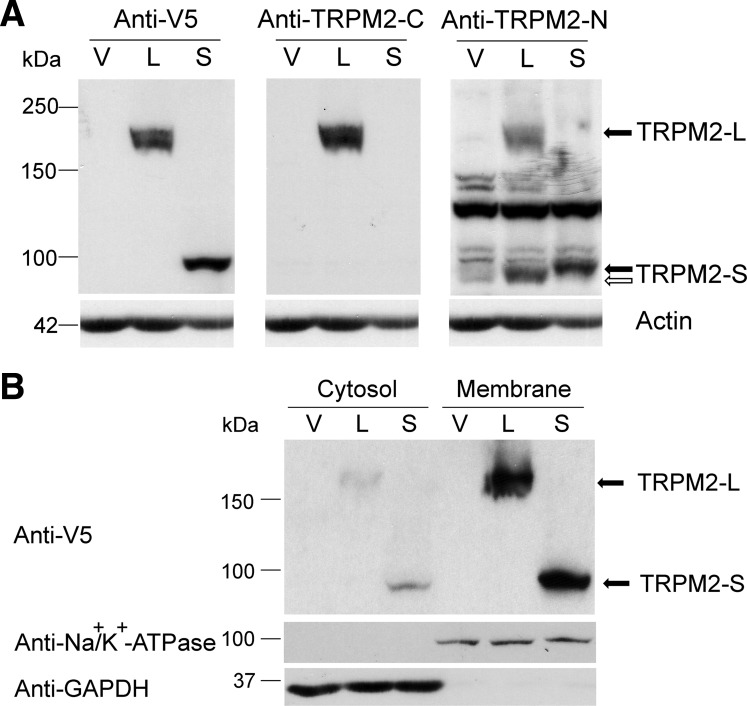
To study the role of TRPM2 in cell proliferation, we generated neuroblastoma SH-SY5Y cells stably expressing TRPM2-L or TRPM2-S. Expression of TRPM2-L or TRPM2-S was confirmed by Western blotting of lysates (Fig. 2A). Subcellular fractionation verified that TRPM2-L and TRPM2-S were predominantly expressed on the cell membrane (Fig. 2B). Endogenous TRPM2-L and TRPM2-S proteins were present in lysates of SH-SY5Y cells, but long exposure times were required to detect endogenous channels (data not shown).
Fig. 2.
TRPM2 isoforms are expressed on the membrane of SH-SY5Y neuroblastoma cells. Western blots were prepared with whole cell lysates from SH-SY5Y cells stably transfected with empty vector (V, pcDNA3.1/V5-His TOPO), TRPM2-L (L), or TRPM2-S (S) (A) or cytosol (100 μg/lane) or membrane (60 μg/lane) proteins (B) following subcellular fractionation of these cells. A: the same blots were probed with anti-V5-horseradish peroxidase (HRP), anti-TRPM2-C, or anti-TRPM2-N antibodies. Blots were also probed with anti-actin antibody as a control for equivalent loading, and actin loading control was the same for A and B. B: anti-GAPDH and anti-Na+-K+-ATPase antibodies were used as controls for quality of fractionation. Representative results of 3 experiments are shown. Open arrows, endogenous TRPM2-S; solid arrows, V5-tagged TRPM2.
TRPM2-S expression enhances cell proliferation.
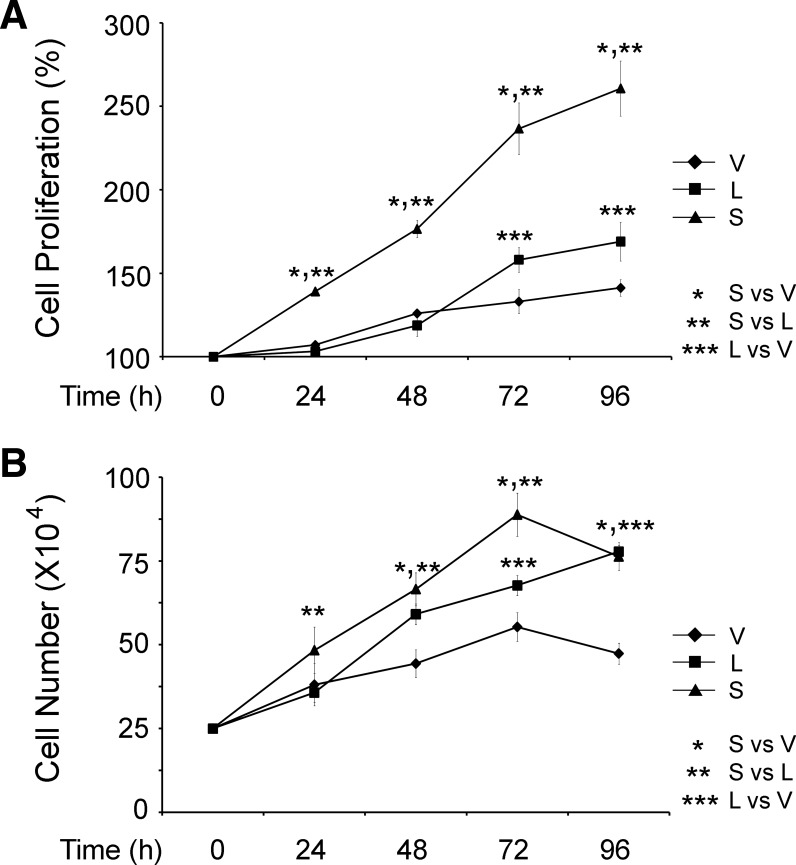
SH-SY5Y cells stably expressing TRPM2-S proliferated significantly faster than cells expressing TRPM2-L or empty vector (Fig. 3A). Proliferation was quantified with the XTT assay, a measure of metabolic activity. Cells expressing TRPM2-L and vector proliferated at a similar rate at 24 and 48 h, although TRPM2-L-expressing cells showed significantly faster cell proliferation than empty vector-transfected cells at 72 and 96 h. Trypan blue exclusion showed significantly greater increases in number of cells expressing TRPM2-S at 24, 48, and 72 h (Fig. 3B). TRPM2-L-expressing cells proliferated significantly faster than vector-expressing cells after 48 h. These data demonstrate that TRPM2-S expression promotes cell proliferation in neuroblastoma, although TRPM2-L expression also moderately enhanced proliferation compared with empty vector.
Fig. 3.
TRPM2-S expression significantly enhances SH-SY5Y proliferation. SH-SY5Y cells stably transfected with TRPM2-L, TRPM2-S, or empty vector were studied at 24-h intervals for 96 h after replating. Cell proliferation was measured by 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT) assay (A) or trypan blue exclusion (B). Results in A are expressed as percentage of time 0; results in B are expressed as live cell number. Values are means ± SE of 4 (A) or 2 (B) experiments, each counted in triplicate. Significance at P ≤ 0.05 (*, **, and ***).
Akt and ERK activation are enhanced in TRPM2-S-expressing cells.
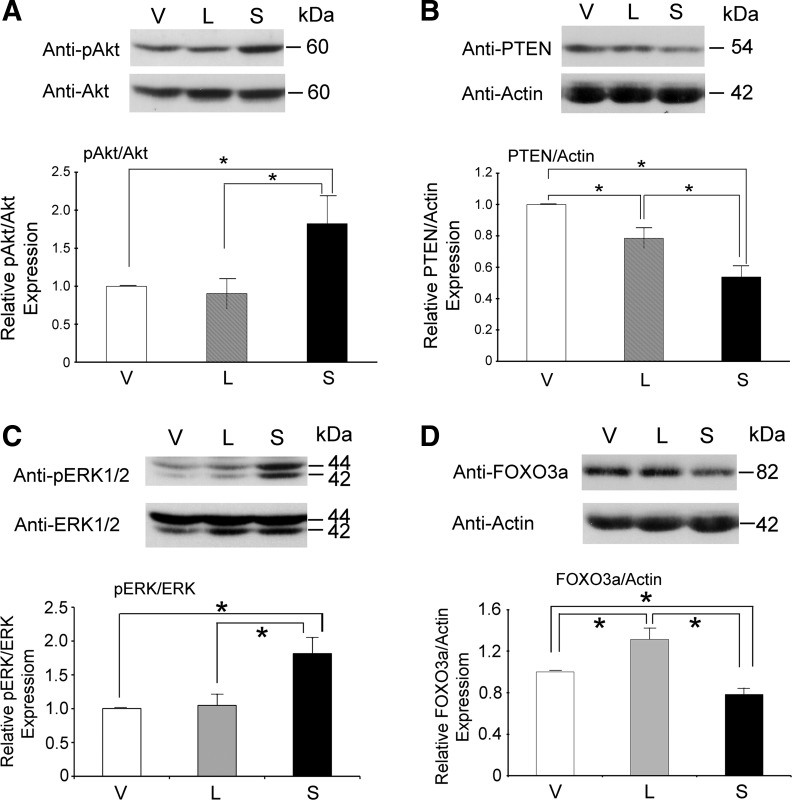
A number of kinase signaling pathways, including Akt and ERK pathways, are involved in neuroblastoma proliferation (49, 59). Phosphorylation of Akt was significantly greater in SH-SY5Y cells stably expressing TRPM2-S than TRPM2-L- or vector-expressing cells (Fig. 4A). Expression of PTEN, an endogenous inhibitor of phosphorylation and activation of Akt (57), was significantly less in SH-SY5Y cells expressing TRPM2-S than cells expressing empty vector or TRPM2-L (Fig. 4B). ERK phosphorylation was also significantly greater in TRPM2-S- than empty vector- or TRPM2-L-expressing cells (Fig. 4C). These data suggest that the increased phosphorylation and activation of Akt and ERK pathways may be involved in faster proliferation of TRPM2-S-expressing cells. Since Akt and ERK mediate FOXO3a phosphorylation and degradation (83, 85), we measured FOXO3a expression. In TRPM2-S-expressing cells, FOXO3a levels were significantly reduced compared with empty vector- or TRPM2-L-expressing cells and were significantly higher in TRPM2-L-expressing cells (Fig. 4D).
Fig. 4.
Akt and ERK phosphorylation is increased and phosphatase and tensin homolog (PTEN) and forkhead box O 3a (FOXO3a) are downregulated in TRPM2-S-expressing cells. Whole cell lysates from SH-SY5Y cells stably expressing empty vector, TRPM2-L, or TRPM2-S were used for Western blotting for phosphorylated Akt (pAkt) and Akt (A), PTEN (B), phosphorylated ERK (pERK1/2) and ERK1/2 (C), and FOXO3a (D). Representative Western blots are shown. Expression was quantitated by densitometry. Values are means ± SE from 10 (A), 13 (B), 11 (C), and 15 (D) experiments. *P ≤ 0.05.
Inhibitors of phosphatidylinositol 3-kinase, ERK, and NADPH oxidase block enhanced proliferation of TRPM2-S-expressing cells.
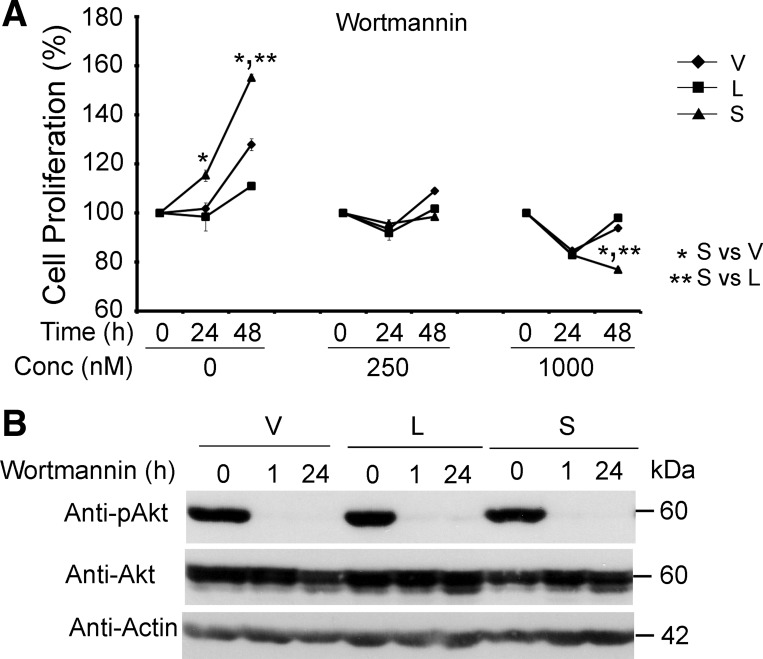
To determine whether Akt has a functional role in the enhanced proliferation in TRPM2-S-expressing cells, inhibitors of phosphatidylinositol 3-kinase (PI3K), wortmannin and LY294002, were utilized. The faster proliferation of TRPM2-S- than TRPM2-L- or empty vector-expressing cells was completely abolished by treatment with wortmannin (Fig. 5A). Phosphorylation of Akt was effectively inhibited by wortmannin in all three cell lines at 1 and 24 h (Fig. 5B). Results were similar when SH-SY5Y cells expressing TRPM2-S were incubated with LY294002 (data from 3 experiments not shown).
Fig. 5.
Phosphatidylinositol 3-kinase (PI3K) inhibitors block enhanced proliferation of TRPM2-S-expressing SH-SY5Y cells. A: SH-SY5Y cells stably transfected with TRPM2-L, TRPM2-S, or empty vector were treated with 250 or 1,000 nM wortmannin twice daily for 24 or 48 h, and cell proliferation was measured by XTT assay. Results are expressed as percentage of time 0. Values are means ± SE of 1 of 3 experiments performed in triplicate. Significance at P ≤ 0.05 (* and **). B: Western blots of whole cell lysates from cells treated with 1,000 nM wortmannin for 1 or 24 h. Akt phosphorylation was assessed by probing with anti-pAkt, anti-Akt, and anti-actin antibodies to confirm effectiveness of inhibitor concentration used in blocking enzyme activation. Representative results of 3 experiments are shown.
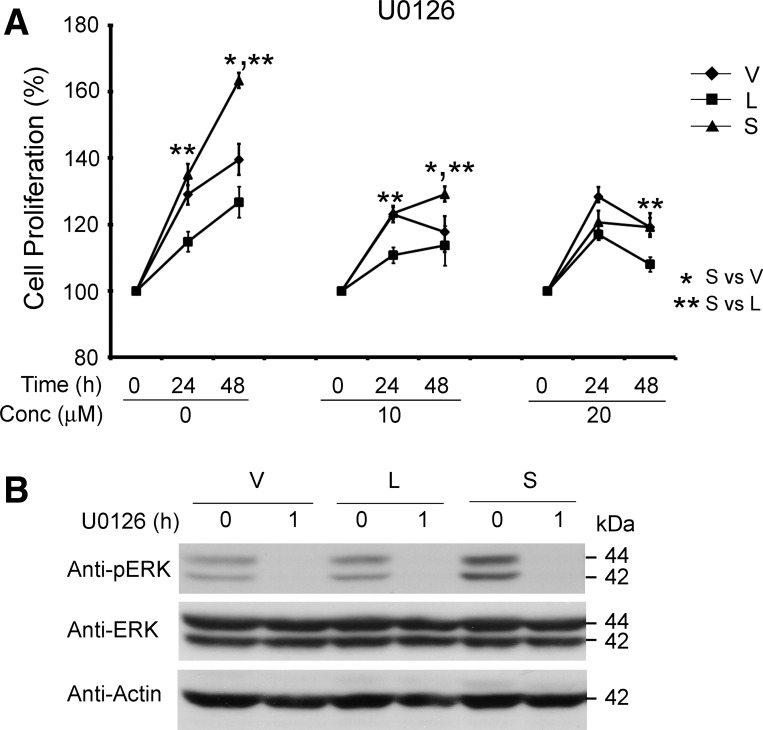
Similarly, the ERK kinase inhibitor U0126 partially reduced the enhanced proliferation of TRPM2-S-expressing cells (Fig. 6A). The ability of U0126 to inhibit ERK phosphorylation is shown in Fig. 6B. These data support the hypothesis that activation of the PI3K/Akt and ERK pathways is required for the enhanced proliferation of TRPM2-S-expressing cells.
Fig. 6.
ERK kinase inhibitors partially block enhanced proliferation of TRPM2-S-expressing SH-SY5Y cells. A: SH-SY5Y cells stably transfected with TRPM2-L, TRPM2-S, or empty vector were treated with 10 or 20 μM U0126 for 24 or 48 h, and cell proliferation was measured by XTT assay. Results are expressed as percentage of time 0. Values are means ± SE of combined results of 3 experiments performed in triplicate. Significance at P ≤ 0.05 (* and **). B: Western blots of whole cell lysates from cells treated with 20 μM U0126 for 1 h. ERK phosphorylation was assessed by probing with anti-pERK, anti-ERK, and anti-actin antibodies to confirm effectiveness of inhibitor concentrations used in blocking enzyme activation. Representative results of 3 experiments are shown.
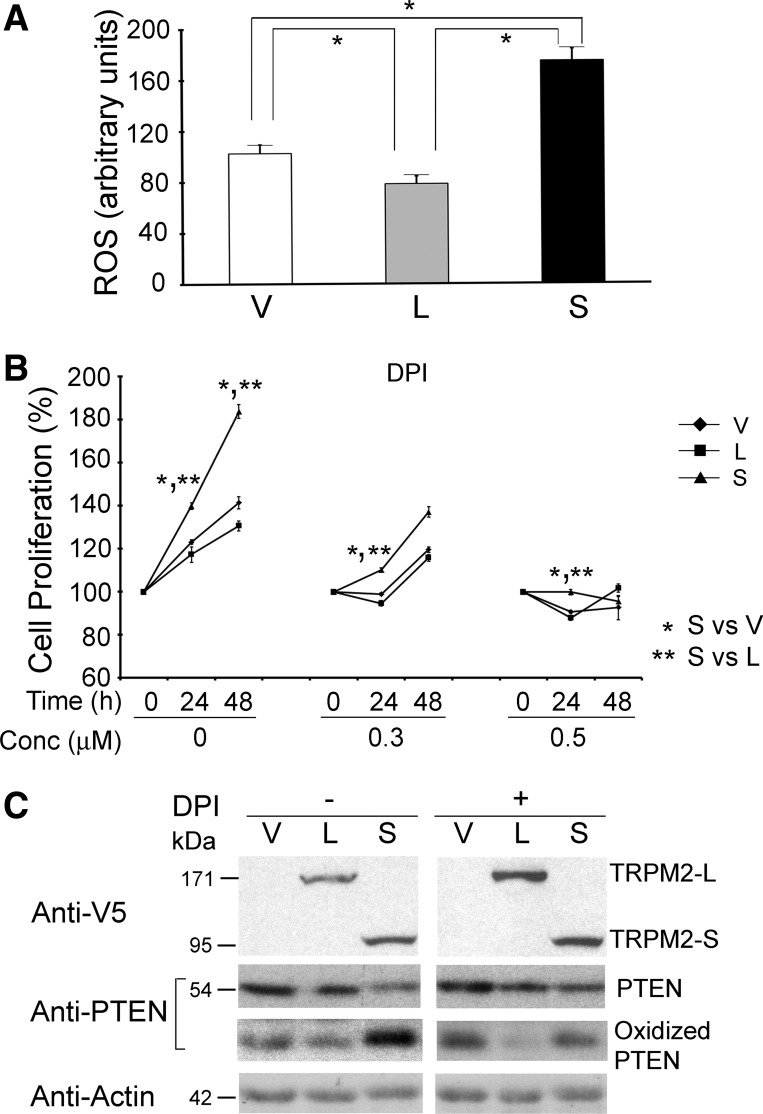
Phagocytes from TRPM2-expressing mice demonstrate reduced ROS production through inhibition of NADPH oxidase activity compared with the TRPM2 knockout mouse, in which greater ROS production was observed (11). We demonstrated significantly greater ROS levels in proliferating SH-SH5Y cells expressing TRPM2-S than in cells expressing TRPM2-L or empty vector (Fig. 7A). Because TRPM2-S functions as a dominant-negative and ROS can enhance cell proliferation, we examined whether NADPH oxidase is involved in the increased proliferation of TRPM2-S-expressing cells. TRPM2-L-, TRPM2-S-, and empty vector-expressing cells were treated with the NADPH oxidase inhibitor DPI (0.3, 0.5 μM), and proliferation was measured with the XTT assay. DPI blocked the enhanced proliferation of TRPM2-S-expressing cells (Fig. 7B), demonstrating that ROS production has a role. TRPM2-S-expressing cells demonstrated enhanced oxidation of PTEN and reduced PTEN levels compared with cells expressing empty vector or TRPM2-L (Fig. 7C). After treatment with DPI, oxidation of PTEN was reduced in TRPM2-S-expressing cells, and PTEN levels increased. These data suggest that enhanced NADPH oxidase activation and ROS production in TRPM2-S-expressing cells result in increased PTEN oxidation, reduced PTEN levels, augmented Akt phosphorylation and activation, and increased proliferation.
Fig. 7.
NADPH inhibitor diphenyleneiodonium (DPI) blocks enhanced proliferation of TRPM2-S expressing cells. A: reactive oxygen species (ROS) in untreated single SH-SY5Y cells expressing empty vector (n = 30 cells), TRPM2-L (n = 37), or TRPM2-S (n = 40). *P ≤ 0.05. B: SH-SY5Y cells stably transfected with TRPM2-L, TRPM2-S, or empty vector were treated with 0.3 or 0.5 μM DPI for 24 or 48 h, and cell proliferation was measured by XTT assay. Results are expressed as percentage of time 0. Values are means ± SE from 4 experiments each performed in 6 replicates. Significance at P ≤ 0.05 (* and **). C: Western blots of whole cell lysates from untreated cells or cells treated with 1 μM DPI for 4 h were prepared with reducing (V5, PTEN, and actin antibodies) or nonreducing (oxidized PTEN) lysis buffer. TRPM2 isoform expression was assessed by probing with anti-V5 antibody and PTEN and oxidized PTEN with anti-PTEN antibody. Representative results of 2 experiments are shown.
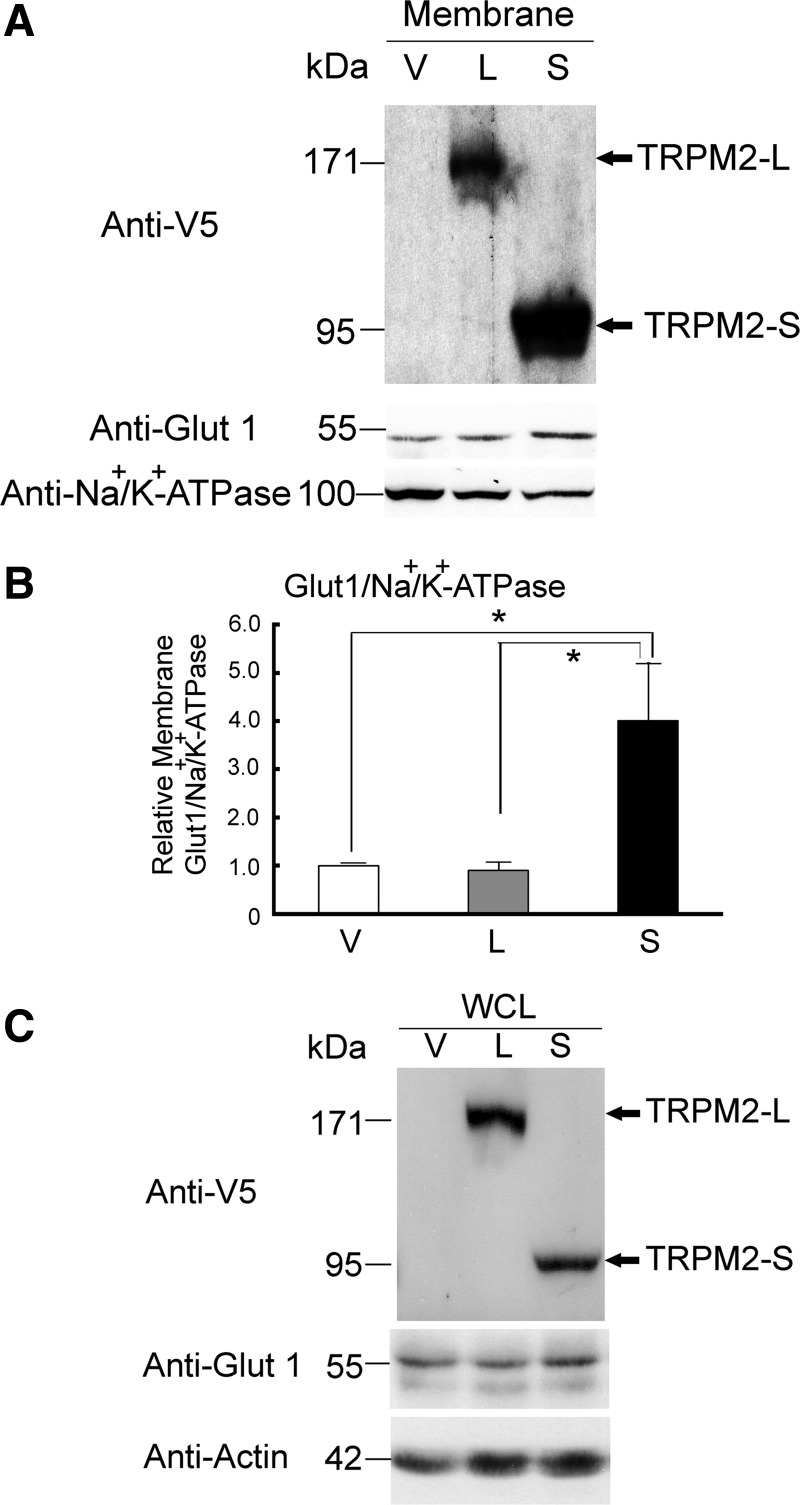
Membrane expression of Glut1 is significantly elevated in TRPM2-S-expressing cells.
Glut1 has been widely implicated in enhanced proliferation in cancer through its role in glucose uptake, and membrane expression of Glut1 is regulated by Akt (72, 78, 89). In SH-SY5Y cells stably expressing TRPM2 isoforms, Glut1 was significantly elevated in the membrane fraction of cells expressing TRPM2-S (Fig. 8, A and B) compared with cells expressing TRPM2-L or empty vector. Glut1 expression was not significantly enhanced in the whole cell lysates of TRPM2-L- or TRPM2-S-expressing cells compared with cells expressing empty vector (Fig. 8C).
Fig. 8.
Membrane glucose transporter (Glut1) expression is significantly increased in TRPM2-S-expressing cells. A and C: cell membrane fractions or whole cell lysates (WCL) from SH-SY5Y cells stably transfected with empty vector, TRPM2-L, or TRPM2-S were prepared from culture dishes when cells were 50–70% confluent. Western blots were probed with anti-V5-HRP, anti-Glut1, or anti-Na+-K+-ATPase antibody (to confirm quality of fractionation) or anti-actin antibody. Representative blots from 6 experiments are shown. B: intensity of bands was quantitated with denstitometry, and relative expression levels were standardized by comparing Glut1/Na+-K+-ATPase in membrane fractions (n = 6). *P ≤ 0.05.
TRPM2-S-expressing cells are highly susceptible to oxidative stress-induced cell death.
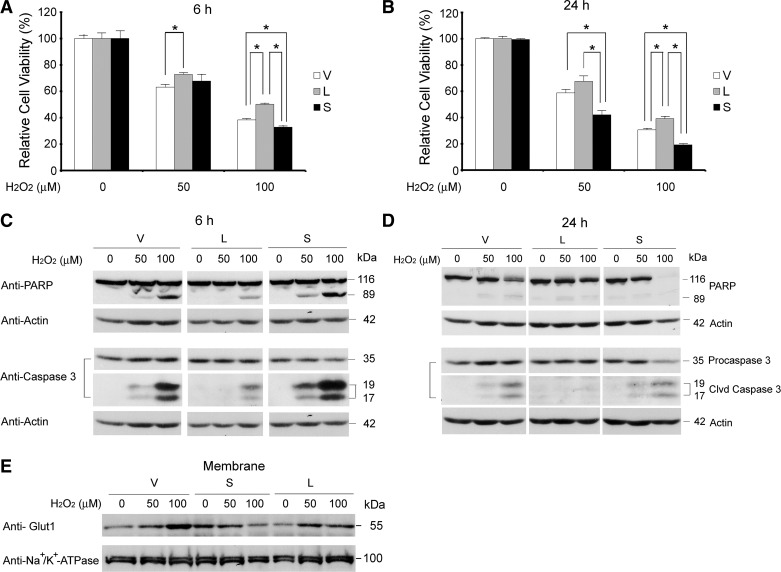
Under basal conditions, SH-SY5Y cells stably expressing TRPM2-L, TRPM2-S, or empty vector showed no difference in the percentage of apoptotic cells. When these stably transfected cells were treated with low concentrations of H2O2 (50 and 100 μM) for 6 or 24 h (27), cell viability was reduced in all three cell lines in a dose- and time-dependent manner (Fig. 9, A and B). However, the largest decrease in viability was observed in cells expressing TRPM2-S, whereas cells expressing TRPM2-L showed the greatest viability. In agreement with the cell viability assay, TRPM2-S-expressing cells showed greater cleavage of PARP and caspase-3 than cells transfected with empty vector (Fig. 9, C and D) at 6 and 24 h after treatment. The least cleavage of PARP and caspase-3 was found in TRPM2-L-expressing cells. These results demonstrate that TRPM2-S-expressing cells are much more sensitive to low-dose oxidative stress-induced cell death than are TRPM2-L-expressing cells.
Fig. 9.
TRPM2-S-expressing cells are more susceptible to H2O2-induced cell death. SH-SY5Y cells stably transfected with empty vector, TRPM2-L, or TRPM2-S were treated with 50 or 100 μM H2O2 for 6 h (A and C) or 24 h (B and D). A and B: cell viability determined by XTT assay. Values are means ± SE of 2 experiments done in triplicate at 6 h and 4 experiments at 24 h. *P ≤ 0.05. C and D: cell death confirmed by examining cleavage (Clvd) of poly(ADP-ribose) polymerase (PARP) and caspase-3 on Western blots. V, L, and S in C and D were probed at the same time, and spaces indicate where treatment with 1,000 μM H2O2 was removed because of poor viability. Western blotting was done on all experiments in A and B, and representative Western blots are shown in C and D. In D, light exposure shows reduced uncleaved PARP in S compared with V and L. E: Western blots from membrane fractionation of lysates of cells treated with H2O2 for 24 h were probed with anti-Glut1 or anti-Na+-K+-ATPase. Representative results of 2 experiments are shown. After exposure to H2O2, membrane Glut1 was decreased in TRPM2-S-expressing cells but increased in TRPM2-L-expressing cells.
Because membrane localization of Glut1 was increased in TRPM2-S-expressing cells, we examined the effect of exposure to low-dose oxidative stress. After treatment with 50 or 100 μM H2O2, TRPM2-S-expressing cells demonstrated lower levels of membrane Glut1 than TRPM2-L- or empty vector-expressing cells, in which membrane Glut1 increased after treatment (Fig. 9E).
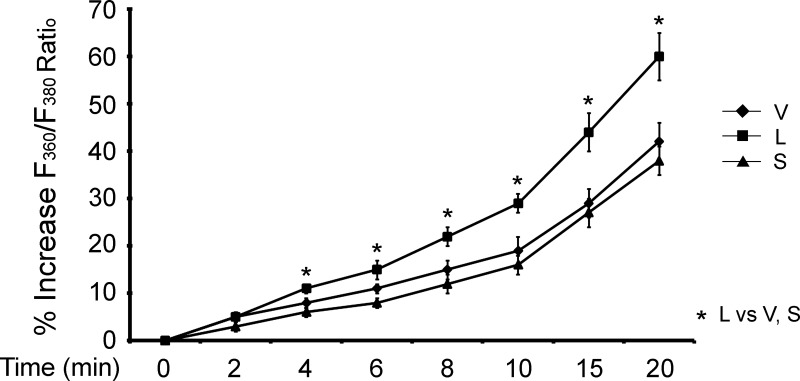
TRPM2-S suppresses Ca2+ influx in SH-SY5Y neuroblastoma cells.
In untreated cells, baseline F360/F380 values were not significantly different between cells expressing empty vector, TRPM2-L, or TRPM2-S. Single SH-SY5Y cells stably expressing TRPM2-L, TRPM2-S, or empty vector were treated for 20 min with 100 μM H2O2. The increase in F360/F380 was significantly greater in TRPM2-L- than empty vector- or TRPM2-S-expressing cells (Fig. 10; P < 0.05). Our data from SH-SY5Y cells are consistent with previous studies using HEK-293T cells (87), in that TRPM2-L promotes significantly greater Ca2+ entry with H2O2 stimulation. These data demonstrate that enhanced Ca2+ entry in TRPM2-L-expressing cells after exposure to low doses of H2O2 does not necessarily enhance susceptibility to death.
Fig. 10.
Modulation of Ca2+ influx by TRPM2 isoforms in SH-SY5Y cells. SH-SY5Y cells stably transfected with empty vector or vector expressing TRPM2-L or TRPM2-S were loaded with fura 2-AM. Cells were treated with 100 μM H2O2 for 20 min, and ratio of fluorescence at 360 nm to fluorescence at 380 nm (F360/F380) was measured in single cells with digital video imaging at baseline and at 2- to 5-min intervals after treatment. Values are means ± SE from 2 experiments and a total of 41 (V), 39 (S), and 34 (L) single cells in each group. Significance at P ≤ 0.05 (*).
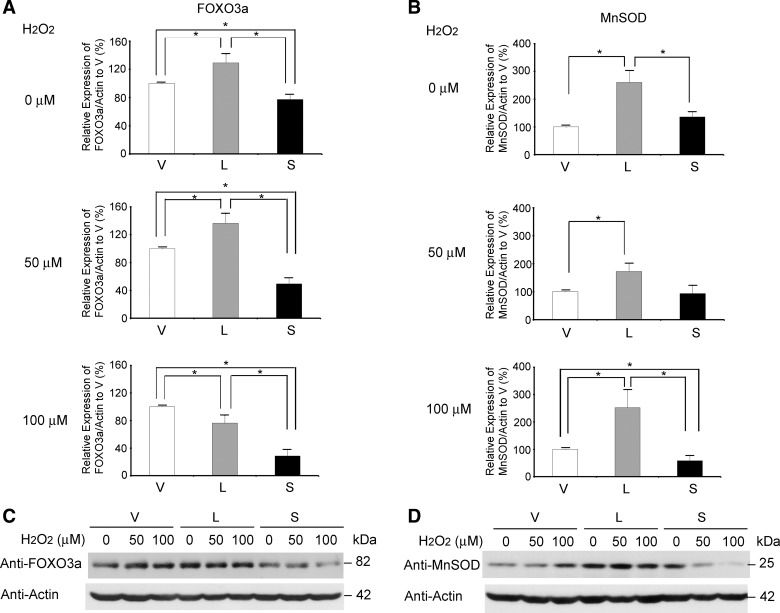
FOXO3a and MnSOD expression is increased in TRPM2-L-expressing cells and decreased in TRPM2-S-expressing cells.
To explore the pathways involved in the susceptibility of cells expressing different TRPM2 isoforms to low-dose oxidative stress, PI3K/Akt and ERK activation was examined. After treatment with 50 or 100 μM H2O2 for 24 h, phosphorylation of Akt and ERK1/2 increased in TRPM2-S-, TRPM2-L-, and empty vector-expressing cells. Although phosphorylation of Akt and ERK was increased in TRPM2-S-expressing cells compared with the other groups, differences did not reach statistical significance (data not shown).
FOXO3a is phosphorylated by a number of regulators, in addition to Akt and ERK, resulting in its sequestration in the cytoplasm and its degradation by a ubiquitin-proteasome-dependent mechanism (83, 85). FOXO3a can protect cells from oxidative stress through its involvement in regulation of transcription of catalase and SODs (26, 32, 37, 40). Increased FOXO3a can modulate increased MnSOD production and reduce ROS (32). Here, in untreated cells, FOXO3a levels were significantly increased in TRPM2-L-expressing cells and reduced in TRPM2-S-expressing cells compared with cells transfected with vector alone (Fig. 11A). After treatment with 50 μM H2O2 for 24 h, cellular levels of FOXO3a remained the highest in TRPM2-L-expressing cells, although after exposure to 100 μM H2O2, FOXO3a levels decreased (Fig. 11A). MnSOD levels were significantly increased in TRPM2-L-expressing cells compared with empty vector-expressing cells (Fig. 11B) before and at 24 h after treatment, which may contribute to the enhanced viability of these cells following exposure to oxidative stress. In contrast, TRPM2-S-expressing cells showed a significant and dose-dependent reduction in FOXO3a levels compared with TRPM2-L- or empty vector-expressing cells after treatment. This was associated with a dose-dependent decrease in MnSOD levels, which may enhance susceptibility to oxidative stress. Although FOXO3a levels were decreased at 24 h after treatment of TRPM2-L-expressing cells with 100 μM H2O2, MnSOD levels remained elevated. This may be because MnSOD is transcriptionally regulated by FOXO3a, and upregulation occurred prior to the treatment-induced decrease in FOXO3a.
Fig. 11.
FOXO3a and MnSOD levels are increased in TRPM2-L-expressing cells and decreased in TRPM2-S-expressing cells after exposure to oxidative stress. SH-SY5Y cells stably transfected with empty vector, TRPM2-L, or TRPM2-S were treated with 50 or 100 μM H2O2 for 24 h. Western blots of whole cell lysates were probed with anti-FOXO3a (A and C) or anti-MnSOD (B and D) and anti-actin antibodies. Expression of FOXO3a/actin (A) and MnSOD/actin (B) in Western blots (C and D) was quantitated by densitometry. Values are means ± SE from 13 (FOXO3a, untreated) or 6 (other groups in A and B) experiments. *P ≤ 0.05.
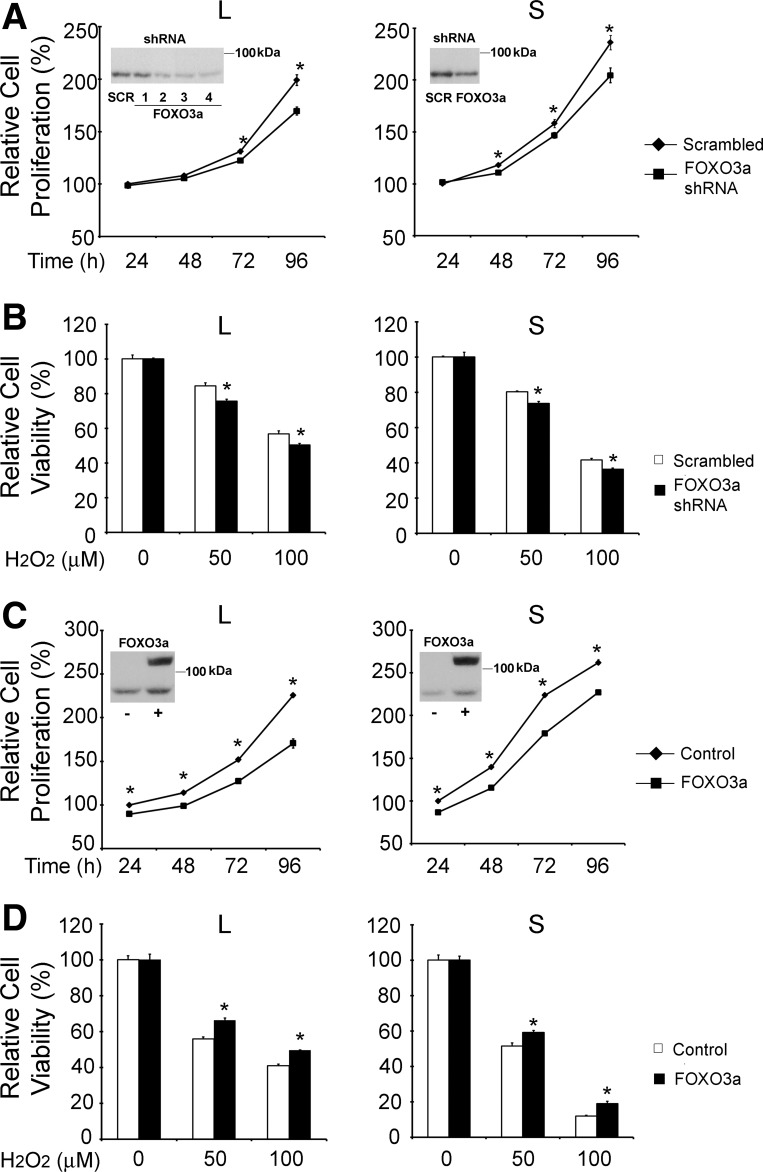
To determine whether FOXO3a has a role in regulating susceptibility of cells expressing different TRPM2 isoforms to oxidative stress, we modulated FOXO3a expression. Western blotting demonstrated that FOXO3a levels were reduced with shRNAs (Fig. 12A, insets). Proliferation of SH-SY5Y cells expressing TRPM2-L or TRPM2-S was modestly, but significantly, reduced following FOXO3a depletion (Fig. 12A). When these cells were treated with 50 or 100 μM H2O2, cell survival in both groups was decreased, and protection of cell viability by TRPM2-L was reduced (Fig. 12B). SH-SY5Y cells expressing TRPM2 isoforms were also transfected to overexpress FOXO3a, confirmed by Western blotting (Fig. 12C, insets). Proliferation was modestly reduced in TRPM2-L- and TRPM2-S-expressing cells (Fig. 12C), suggesting that optimal FOXO3a levels may play a minor role in regulating cell proliferation. In contrast to cells with reduced FOXO3a, overexpression of FOXO3a increased cell viability after exposure to 50 and 100 μM H2O2 in both groups, and viability of TRPM2-S-expressing cells was significantly enhanced (Fig. 12D). Similar results were obtained in wild-type SH-SY5Y cells and in cells expressing empty vector (data not shown). These data demonstrate the important role of FOXO3a in modulating susceptibility of cells expressing different TRPM2 isoforms to oxidative stress.
Fig. 12.
Loss or gain of FOXO3a function modulates survival of TRPM2-L- and TRPM2-S-expressing cells. SH-SY5Y cells stably transfected with TRPM2-L or TRPM2-S were transfected with short-hairpin RNA (shRNA) targeted to FOXO3a or scrambled shRNAs. A: cell proliferation measured by XTT assay. Results are expressed as percentage of XTT at 24 h after shRNA transfection; 3 experiments were performed with 6 replicates each. Values are means ± SE of 1 representative experiment. *P < 0.05. In TRPM2-L, Western blot for cells transfected with scrambled shRNA (SCR) or 4 different shRNAs (1, 2, 3, and 4) targeted to FOXO3a; shRNA 4 was used in proliferation and viability experiments. In TRPM2-S, Western blot (inset) demonstrates reduced expression of FOXO3a after transfection with FOXO3a shRNA 4 compared with scrambled shRNA. B: XTT assay of viability of cells treated with 50 or 100 μM H2O2 for 24 h. Values are means ± SE of 1 of 2 experiments with 4 replicates each. *P < 0.05. C: SH-SY5Y cells stably transfected with TRPM2-L or TRPM2-S were also transfected with the human green fluorescent protein (GFP)-FOXO3a construct. Cell proliferation was measured by XTT assay, and results are expressed as percentage of XTT at 24 h after FOXO3a or sham control transfection. Values are means ± SE of 1 of 2 experiments with 6 replicates each. *P < 0.05. Representative Western blots (insets) demonstrate increased expression of FOXO3a in cells transfected with GFP-FOXO3a (top band) compared with transfection control; bottom band represents endogenous FOXO3a. D: XTT assay of viability of cells treated with 50 or 100 μM H2O2 for 24 h. Values are means ± SE of 1 of 2 experiments performed with 4 replicates. *P < 0.05.
Inhibition of TRPM2-L enhances susceptibility to low-dose oxidative stress.
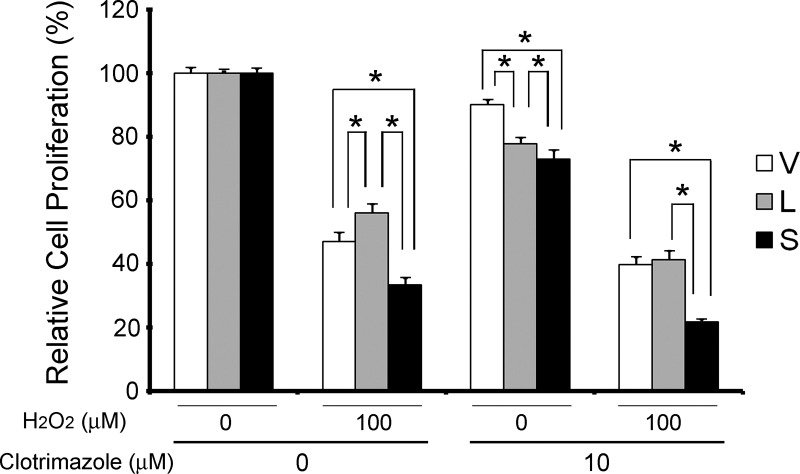
To confirm that TRPM2-L activation modulates protection from low-dose oxidative stress, SH-SY5Y cells stably expressing TRPM2-L, TRPM2-S, or empty vector were pretreated for 10 min with clotrimazole, an inhibitor of TRPM2-L (25). In four experiments, pretreatment with clotrimazole blocked the protection of TRPM2-L-expressing cells from 100 μM H2O2 (Fig. 13). No effect was observed on the reduced viability of TRPM2-S-expressing cells, suggesting that the mechanism through which TRPM2-S enhances susceptibility to cell death is not inhibited by short exposure to clotrimazole. These data support the hypothesis that TRPM2-L protects SH-SY5Y cells from low-dose oxidative stress.
Fig. 13.
Inhibition of TRPM2-L with clotrimazole blocks protection from low-dose H2O2-induced cell death. SH-SY5Y cells stably expressing empty vector, TRPM2-L, or TRPM2-S were pretreated with or without 10 μM clotrimazole for 10 min and then with 100 μM H2O2 or vehicle. Cell viability was assessed with the XTT assay after 24 h. Four experiments were performed, and combined results were standardized to viability with no treatment. *Significant differences in viability between groups (P ≤ 0.05).
DISCUSSION
TRPM2 is widely recognized as an ion channel that is intimately involved in cell survival (61). Our first major finding is that TRPM2-L and TRPM2-S are increased in neuroblastoma compared with adrenal gland. Different tumor samples had different ratios of TRPM2-S to TRPM2-L, and whether this ratio influences tumor aggressiveness or chemotherapy responsiveness remains to be elucidated.
To evaluate the individual roles of TRPM2-L and TRPM2-S in cell proliferation and survival, we engineered neuroblastoma cell lines stably expressing either TRPM2 isoform or empty vector. Our second major finding is that neuroblastoma cells expressing TRPM2-S proliferate faster than cells expressing TRPM2-L or empty vector. The increased proliferation of TRPM2-S-expressing cells was associated with increased ROS, increased PTEN oxidation, decreased levels of PTEN phosphatase, increased pAkt, increased levels of membrane Glut1, and increased pERK. Decreased levels of PTEN have been associated with increased cell proliferation and have been observed in a number of cancers (6, 48, 52, 86). PTEN downregulation promotes cell proliferation by enhancing Akt activation (57), as observed here in TRPM2-S-expressing cells. In wild-type mice, TRPM2 downregulated NADPH oxidase-mediated ROS production in phagocytes through cation influx-dependent depolarization of the plasma membrane potential, whereas in knockout mice, ROS production was enhanced (11). ROS can inactivate PTEN through oxidation (38, 53, 63), resulting in increased Akt phosphorylation. ROS can also increase phosphorylation of ERK, which is inhibited by antioxidants (28). Our experiments with the NADPH oxidase inhibitor DPI resulted in reduced proliferation of TRPM2-S-expressing cells, strongly suggesting that increased NADPH oxidase activity and increased ROS production are involved in increased proliferation of TRPM2-S-expressing cells. Our data support the conclusion that enhanced neuroblastoma proliferation by TRPM2-S is mediated through increased PTEN oxidation, reduced PTEN levels, and increased phosphorylation and activation of Akt and ERK. The importance of PI3K/Akt and ERK activation in mediating increased proliferation in cells expressing TRPM2-S was confirmed by treatment with PI3K and ERK inhibitors, which abolished the increase in cell proliferation. PTEN deficiency has also been reported to enhance cell proliferation by increasing phosphorylation of cAMP-responsive element-binding protein (CREB), a target of its phosphatase, resulting in activation and CREB-mediated transcription (20).
Akt enhances cell proliferation through a number of pathways, including regulation of energy metabolism. Akt activation modulates expression and membrane translocation of glucose transporters (55), increasing cellular uptake and utilization of glucose (9, 16). Cancer cells generate energy primarily through glycolysis, rather than mitochondrial oxidative phosphorylation, known as the “Warburg effect” (36, 55, 71, 72), and many cancer cells have increased glucose uptake through increased Glut1 expression, which forms the basis of the PET-CT scan (16). Indeed, membrane expression of Glut1, a downstream effector of Akt, was increased in TRPM2-S-expressing neuroblastoma cells and may be an additional mechanism through which pAkt mediates enhanced cell proliferation.
Our third major finding is that neuroblastoma cells expressing TRPM2-L are relatively protected from cell death in response to low concentrations (≤100 μM) of H2O2, whereas TRPM2-S-expressing cells have increased susceptibility. This is different from previous observations, in which TRPM2-L expression increased susceptibility of hematopoietic cells to death induced by high doses (1 mM) of H2O2 and susceptibility of HEK-293T cells to death induced by high and low doses of H2O2, associated with sustained large increases in [Ca2+]i (87, 88). In those experiments, TRPM2-S protected cells from death by inhibiting Ca2+ influx. Different concentrations of H2O2 have been shown to affect different p53-regulated gene expression patterns and levels of antioxidants or prooxidants (73). The lower concentrations of H2O2 used here may contribute to the different responses (87). Exposure of TRPM2-L-expressing cells to low levels of H2O2 results in lower levels of cation influx and activation of signaling pathways, including increased MnSOD, that protect viability. Exposure of TRPM2-L-expressing cells to high levels of H2O2 results in significant increases in [Ca2+]i and cell death. In the former case, TRPM2-S inhibition of TRPM2-L function may be detrimental; in the later case, it may preserve viability. Different results may also be secondary to the use of different cell types, which have different endogenous oxidant production and antioxidant defenses (22). We hypothesize that the different levels of oxidative stress (H2O2 dose) and molecular differences between cell types may account for the different findings. For example, in neuroendocrine cells, L-type Ca2+ channels are expressed and provide another pathway for Ca2+ entry, which may enhance Ca2+ entry through TRPM2 (2, 64). Treatment with low concentrations of H2O2 may also be more physiologically relevant; the peak increase in striatal H2O2 above baseline after exposure to ischemia-reperfusion was 100 μM (27).
FOXO3a plays an important role in the mechanisms by which TRPM2-L protects cells from oxidative stress-induced cell death. FOXO3a is regulated by a number of signaling pathways, including ERK, Akt, IκB kinase, and serum glucocorticoid-related kinases (83, 85). FOXO3a regulates levels of antioxidant enzymes, including MnSOD (26, 32, 37, 40). In TRPM2-L-expressing cells, FOXO3a and its downstream transcriptional target MnSOD are increased. After exposure to low-level oxidant stress, viability of TRPM2-L-expressing cells is preserved by increased capacity to degrade ROS. When FOXO3a levels are depleted by shRNA, protection of TRPM2-L-expressing cells from low-dose oxidative stress is reduced. Our data also suggest a mechanism for enhanced death of TRPM2-S-expressing cells. In proliferating TRPM2-S-expressing cells, ROS levels are enhanced, leading to increased oxidized PTEN, reduced total PTEN, and activation of Akt, sensitizing these cells to oxidative stress. ROS levels are further enhanced through increased metabolism. Akt and ERK activation in these cells contributes to reduced FOXO3a levels through its phosphorylation and degradation (46, 83, 85), resulting in reduced expression of MnSOD. After exposure to low doses of H2O2, decreased MnSOD in TRPM2-S-expressing cells increases susceptibility to ROS (46, 83).
The cellular insult in TRPM2-S-expressing cells is compounded by reduced membrane Glut1 after exposure to H2O2, further compromising cells metabolically (77, 78). A role for [Ca2+]i in regulation of Glut1 and Glut4 translocation to the plasma membrane has been demonstrated (39, 77). We hypothesize that the reduced [Ca2+]i in TRPM2-S-expressing cells compared with TRPM2-L- or empty vector-expressing cells after H2O2 exposure (87, 88) may contribute to the reduced membrane Glut1 in those cells.
While early reports showed that TRPM2-L expression can be associated with enhanced Ca2+ influx and reduced cell viability (43), recent publications support a new paradigm in which TRPM2 activation can lead to reduced tissue injury. Di et al. (11) showed that the presence of TRPM2 in phagocytes resulted in reduced inflammation and preserved lung viability compared with its absence in TRPM2 knockout mice through modulation of NADPH oxidase activation and reduced ROS production. Bai and Lipski (3) reported that inhibition of TRPM2 increased damage of pyramidal neurons caused by some oxidants. Our current data confirm that, in cells exposed to mild-to-moderate oxidative stress, TRPM2-L expression reduces susceptibility to oxidant-induced neuroendocrine cell death.
In summary, TRPM2-S mediates neuroendocrine cell proliferation through modulation of PI3K/PTEN/Akt and ERK pathways and increased susceptibility to oxidative stress-induced cell death through reduction of FOXO3a and MnSOD. By contrast, TRPM2-L affords protection against mild-to-moderate oxidative stress via increases in FOXO3a and MnSOD. Increased Ca2+ entry via TRPM2 channels in response to H2O2 stress does not invariably lead to increased cell death in 6–24 h. Our results indicate that the role of TRPM2 in cell proliferation and survival after oxidative stress is complex. These studies have relevance to a broad range of experimental models, including the study of ischemia-reperfusion injury in neuronal, cardiac, or renal tissues, to examine the function of TRPM2 in oxidative stress. A number of chemotherapy agents, including doxorubicin, mediate their cytotoxic effects partially through oxidative stress. TRPM2 isoforms may have important roles in chemotherapy susceptibility. Efforts to target TRPM2 as rational therapy against oncologic and other diseases will require detailed understanding of the functions and mechanism of action of TRPM2 isoforms under different conditions and in different cell types.
GRANTS
This work was supported in part by National Institutes of Health Grants R01 DK-46778 (B. A. Miller) and R01 HL-58672 and R01 HL-74854 (J. Y. Cheung) and by the Four Diamonds Fund of the Pennsylvania State University College of Medicine.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.C., W.Z., I.H.-L., M.B., J.K.K., J.Y.C., and B.A.M. are responsible for conception and design of the research; S.C., Q.T., and K.C. performed the experiments; S.C., Q.T., K.C., and B.A.M. analyzed the data; S.C., Q.T., J.Y.C., and B.A.M. interpreted the results of the experiments; S.C. prepared the figures; S.C. drafted the manuscript; S.C., J.Y.C., and B.A.M. edited and revised the manuscript; S.C., W.Z., Q.T., K.C., I.H.-L., M.B., J.K.K., J.Y.C., and B.A.M. approved the final version of the manuscript.
ACKNOWLEDGMENTS
We appreciate the assistance of Tina Brissette in preparation of the manuscript and the assistance of Dr. Hui Li in optimization of Neon transfection.
REFERENCES
- 1. Aarts M, Iihara K, Wei WL, Xiong ZG, Arundine M, Cerwinski W, MacDonald JF, Tymianski M. A key role for TRPM7 channels in anoxic neuronal death. Cell 115: 863–877, 2003. [DOI] [PubMed] [Google Scholar]
- 2. An Haack K, Narayan SB, Li H, Warnock A, Tan L, Bennett MJ. Screening for calcium channel modulators in CLN3 siRNA knock down SH-SY5Y neuroblastoma cells reveals a significant decrease of intracellular calcium levels by selected L-type calcium channel blockers. Biochim Biophys Acta 1810: 186–191, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bai JZ, Lipski J. Differential expression of TRPM2 and TRPV4 channels and their potential role in oxidative stress-induced cell death in organotypic hippocampal culture. Neurotoxicology 31: 204–214, 2010. [DOI] [PubMed] [Google Scholar]
- 4. Birnbaumer L. The TRPC class of ion channels: a critical review of their roles in slow, sustained increases in intracellular Ca2+ concentrations. Annu Rev Pharmacol Toxicol 49: 395–426, 2009. [DOI] [PubMed] [Google Scholar]
- 5. Bodding M. TRP proteins and cancer. Cell Signal 19: 617–624, 2007. [DOI] [PubMed] [Google Scholar]
- 6. Carracedo A, Alimonti A, Pandolfi PP. PTEN level in tumor suppression: how much is too little? Cancer Res 71: 629–633, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cheung JY, Zhang XQ, Bokvist K, Tillotson DL, Miller BA. Modulation of calcium channels in human erythroblasts by erythropoietin. Blood 89: 92–100, 1997. [PubMed] [Google Scholar]
- 8. Clapham DE. TRP channels as cellular sensors. Nature 426: 517–524, 2003. [DOI] [PubMed] [Google Scholar]
- 9. DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab 7: 11–20, 2008. [DOI] [PubMed] [Google Scholar]
- 10. Deeds J, Cronin F, Duncan LM. Patterns of melastatin mRNA expression in melanocytic tumors. Hum Pathol 31: 1346–1356, 2000. [PubMed] [Google Scholar]
- 11. Di A, Gao XP, Qian F, Kawamura T, Han J, Hecquet C, Ye RD, Vogel SM, Malik AB. The redox-sensitive cation channel TRPM2 modulates phagocyte ROS production and inflammation. Nat Immunol 13: 29–35, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Di A, Malik AB. TRP channels and the control of vascular function. Curr Opin Pharmacol 10: 127–132, 2010. [DOI] [PubMed] [Google Scholar]
- 13. Du J, Xie J, Yue L. Intracellular calcium activates TRPM2 and its alternative spliced isoforms. Proc Natl Acad Sci USA 106: 7239–7244, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Duchen MR, Verkhratsky A, Muallem S. Mitochondria and calcium in health and disease. Cell Calcium 44: 1–5, 2008. [DOI] [PubMed] [Google Scholar]
- 15. Duncan LM, Deeds J, Hunter J, Shao J, Holmgren LM, Woolf EA, Tepper RI, Shyjan AW. Down-regulation of the novel gene melastatin correlates with potential for melanoma metastasis. Cancer Res 58: 1515–1520, 1998. [PubMed] [Google Scholar]
- 16. Elstrom RL, Bauer DE, Buzzai M, Karnauskas R, Harris MH, Plas DR, Zhuang H, Cinalli RM, Alavi A, Rudin CM, Thompson CB. Akt stimulates aerobic glycolysis in cancer cells. Cancer Res 64: 3892–3899, 2004. [DOI] [PubMed] [Google Scholar]
- 17. Fang D, Setaluri V. Expression and up-regulation of alternatively spliced transcripts of melastatin, a melanoma metastasis-related gene, in human melanoma cells. Biochem Biophys Res Commun 279: 53–61, 2000. [DOI] [PubMed] [Google Scholar]
- 18. Fonfria E, Marshall IC, Boyfield I, Skaper SD, Hughes JP, Owen DE, Zhang W, Miller BA, Benham CD, McNulty S. Amyloid β-peptide(1–42)- and hydrogen peroxide-induced toxicity are mediated by TRPM2 in rat primary striatal cultures. J Neurochem 95: 715–723, 2005. [DOI] [PubMed] [Google Scholar]
- 19. Gasser A, Glassmeier G, Fliegert R, Langhorst MF, Meinke S, Hein D, Kruger S, Weber K, Heiner I, Oppenheimer N, Schwarz JR, Guse AH. Activation of T cell calcium influx by the second messenger ADP-ribose. J Biol Chem 281: 2489–2496, 2006. [DOI] [PubMed] [Google Scholar]
- 20. Gu T, Zhang Z, Wang J, Guo J, Shen WH, Yin Y. CREB is a novel nuclear target of PTEN phosphatase. Cancer Res 71: 2821–2825, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guilbert A, Gautier M, Dhennin-Duthille I, Haren N, Sevestre H, Ouadid-Ahidouch H. Evidence that TRPM7 is required for breast cancer cell proliferation. Am J Physiol Cell Physiol 297: C493–C502, 2009. [DOI] [PubMed] [Google Scholar]
- 22. Halliwell B. Oxidative stress and cancer: have we moved forward? Biochem J 401: 1–11, 2007. [DOI] [PubMed] [Google Scholar]
- 23. Hara Y, Wakamori M, Ishii M, Maeno E, Nishida M, Yoshida T, Yamada H, Shimizu S, Mori E, Kudoh J, Shimizu N, Kurose H, Okada Y, Imoto K, Mori Y. LTRPC2 Ca2+-permeable channel activated by changes in redox status confers susceptibility to cell death. Mol Cell 9: 163–173, 2002. [DOI] [PubMed] [Google Scholar]
- 24. Hecquet CM, Malik AB. Role of H2O2-activated TRPM2 calcium channel in oxidant-induced endothelial injury. Thromb Haemost 101: 619–625, 2009. [PMC free article] [PubMed] [Google Scholar]
- 25. Hill K, McNulty S, Randall AD. Inhibition of TRPM2 channels by the antifungal agents clotrimazole and econazole. Naunyn Schmiedebergs Arch Pharmacol 370: 227–237, 2004. [DOI] [PubMed] [Google Scholar]
- 26. Huang H, Tindall DJ. Dynamic FoxO transcription factors. J Cell Sci 120: 2479–2487, 2007. [DOI] [PubMed] [Google Scholar]
- 27. Hyslop PA, Zhang Z, Pearson DV, Phebus LA. Measurement of striatal H2O2 by microdialysis following global forebrain ischemia and reperfusion in the rat: correlation with the cytotoxic potential of H2O2 in vitro. Brain Res 671: 181–186, 1995. [DOI] [PubMed] [Google Scholar]
- 28. Iiyama M, Kakihana K, Kurosu T, Miura O. Reactive oxygen species generated by hematopoietic cytokines play roles in activation of receptor-mediated signaling and in cell cycle progression. Cell Signal 18: 174–182, 2006. [DOI] [PubMed] [Google Scholar]
- 29. Kaneko S, Kawakami S, Hara Y, Wakamori M, Itoh E, Minami T, Takada Y, Kume T, Katsuki H, Mori Y, Akaike A. A critical role of TRPM2 in neuronal cell death by hydrogen peroxide. J Pharm Sci 101: 66–76, 2006. [DOI] [PubMed] [Google Scholar]
- 30. Kashio M, Sokabe T, Shintaku K, Uematsu T, Fukuta N, Kobayashi N, Mori Y, Tominaga M. Redox signal-mediated sensitization of transient receptor potential melastatin 2 (TRPM2) to temperature affects macrophage functions. Proc Natl Acad Sci USA 109: 6745–6750, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kolisek M, Beck A, Fleig A, Penner R. Cyclic ADP-ribose and hydrogen peroxide synergize with ADP-ribose in the activation of TRPM2 channels. Mol Cell 18: 61–69, 2005. [DOI] [PubMed] [Google Scholar]
- 32. Kops GJ, Dansen TB, Polderman PE, Saarloos I, Wirtz KW, Coffer PJ, Huang TT, Bos JL, Medema RH, Burgering BM. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature 419: 316–321, 2002. [DOI] [PubMed] [Google Scholar]
- 33. Kuhn FJ, Heiner I, Luckhoff A. TRPM2: a calcium influx pathway regulated by oxidative stress and the novel second messenger ADP-ribose. Pflügers Arch 451: 212–219, 2005. [DOI] [PubMed] [Google Scholar]
- 34. Langley B, Ratan RR. Oxidative stress-induced death in the nervous system: cell cycle dependent or independent? J Neurosci Res 77: 621–629, 2004. [DOI] [PubMed] [Google Scholar]
- 35. Lehen'kyi V, Prevarskaya N. Oncogenic TRP channels. Adv Exp Med Biol 704: 929–945, 2011. [DOI] [PubMed] [Google Scholar]
- 36. Levine AJ, Puzio-Kuter AM. The control of the metabolic switch in cancers by oncogenes and tumor suppressor genes. Science 330: 1340–1344, 2010. [DOI] [PubMed] [Google Scholar]
- 37. Li M, Chiu JF, Mossman BT, Fukagawa NK. Down-regulation of manganese-superoxide dismutase through phosphorylation of FOXO3a by Akt in explanted vascular smooth muscle cells from old rats. J Biol Chem 281: 40429–40439, 2006. [DOI] [PubMed] [Google Scholar]
- 38. Li ZY, Yang Y, Ming M, Liu B. Mitochondrial ROS generation for regulation of autophagic pathways in cancer. Biochem Biophys Res Commun 414: 5–8, 2011. [DOI] [PubMed] [Google Scholar]
- 39. Maraldi T, Rugolo M, Fiorentini D, Landi L, Hakim G. Glucose transport activation in human hematopoietic cells M07e is modulated by cytosolic calcium and calmodulin. Cell Calcium 40: 373–381, 2006. [DOI] [PubMed] [Google Scholar]
- 40. Marinkovic D, Zhang X, Yalcin S, Luciano JP, Brugnara C, Huber T, Ghaffari S. Foxo3 is required for the regulation of oxidative stress in erythropoiesis. J Clin Invest 117: 2133–2144, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. McHugh D, Flemming R, Xu SZ, Perraud AL, Beech DJ. Critical intracellular Ca2+ dependence of transient receptor potential melastatin 2 (TRPM2) cation channel activation. J Biol Chem 278: 11002–11006, 2003. [DOI] [PubMed] [Google Scholar]
- 42. Miller BA, Barber DL, Bell LL, Beattie BK, Zhang MY, Neel BG, Yoakim M, Rothblum LI, Cheung JY. Identification of the erythropoietin receptor domain required for calcium channel activation. J Biol Chem 274: 20465–20472, 1999. [DOI] [PubMed] [Google Scholar]
- 43. Miller BA, Zhang W. TRP channels as mediators of oxidative stress. Adv Exp Med Biol 704: 531–544, 2011. [DOI] [PubMed] [Google Scholar]
- 44. Montell C. The TRP superfamily of cation channels. Sci STKE 2005: re3, 2005. [DOI] [PubMed] [Google Scholar]
- 45. Nilius B, Owsianik G. Transient receptor potential channelopathies. Pflügers Arch 460: 437–450, 2010. [DOI] [PubMed] [Google Scholar]
- 46. Nogueira V, Park Y, Chen CC, Xu PZ, Chen ML, Tonic I, Unterman T, Hay N. Akt determines replicative senescence and oxidative or oncogenic premature senescence and sensitizes cells to oxidative apoptosis. Cancer Cell 14: 458–470, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Orfanelli U, Wenke AK, Doglioni C, Russo V, Bosserhoff AK, Lavorgna G. Identification of novel sense and antisense transcription at the TRPM2 locus in cancer. Cell Res 18: 1128–1140, 2008. [DOI] [PubMed] [Google Scholar]
- 48. Parsons R. Human cancer, PTEN and the PI-3 kinase pathway. Semin Cell Dev Biol 15: 171–176, 2004. [DOI] [PubMed] [Google Scholar]
- 49. Pellicano F, Thomson RE, Inman GJ, Iwata T. Regulation of cell proliferation and apoptosis in neuroblastoma cells by ccp1, a FGF2 downstream gene. BMC Cancer 10: 657, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Perraud AL, Takanishi CL, Shen B, Kang S, Smith MK, Schmitz C, Knowles HM, Ferraris D, Li W, Zhang J, Stoddard BL, Scharenberg AM. Accumulation of free ADP-ribose from mitochondria mediates oxidative stress-induced gating of TRPM2 cation channels. J Biol Chem 280: 6138–6148, 2005. [DOI] [PubMed] [Google Scholar]
- 51. Prawitt D, Enklaar T, Klemm G, Gartner B, Spangenberg C, Winterpacht A, Higgins M, Pelletier J, Zabel B. Identification and characterization of MTR1, a novel gene with homology to melastatin (MLSN1) and the trp gene family located in the BWS-WT2 critical region on chromosome 11p15.5 and showing allele-specific expression. Hum Mol Genet 9: 203–216, 2000. [DOI] [PubMed] [Google Scholar]
- 52. Ramaswamy S, Nakamura N, Vazquez F, Batt DB, Perera S, Roberts TM, Sellers WR. Regulation of G1 progression by the PTEN tumor suppressor protein is linked to inhibition of the phosphatidylinositol 3-kinase/Akt pathway. Proc Natl Acad Sci USA 96: 2110–2115, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal 24: 981–990, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ribelayga C. Vertebrate vision: TRP channels in the spotlight. Curr Biol 20: R278–280, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Robey RB, Hay N. Is Akt the “Warburg kinase”? Akt-energy metabolism interactions and oncogenesis. Semin Cancer Biol 19: 25–31, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Roehm NW, Rodgers GH, Hatfield SM, Glasebrook AL. An improved colorimetric assay for cell proliferation and viability utilizing the tetrazolium salt XTT. J Immunol Methods 142: 257–265, 1991. [DOI] [PubMed] [Google Scholar]
- 57. Salmena L, Carracedo A, Pandolfi PP. Tenets of PTEN tumor suppression. Cell 133: 403–414, 2008. [DOI] [PubMed] [Google Scholar]
- 58. Sano Y, Inamura K, Miyake A, Mochizuki S, Yokoi H, Matsushime H, Furuichi K. Immunocyte Ca2+ influx system mediated by LTRPC2. Science 293: 1327–1330, 2001. [DOI] [PubMed] [Google Scholar]
- 59. Sartelet H, Oligny LL, Vassal G. AKT pathway in neuroblastoma and its therapeutic implication. Expert Rev Anticancer Ther 8: 757–769, 2008. [DOI] [PubMed] [Google Scholar]
- 60. Seo JH, Ahn Y, Lee SR, Yeol Yeo C, Chung Hur K. The major target of the endogenously generated reactive oxygen species in response to insulin stimulation is phosphatase and tensin homolog and not phosphoinositide-3 kinase (PI-3 kinase) in the PI-3 kinase/Akt pathway. Mol Biol Cell 16: 348–357, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Shapovalov G, Lehen'kyi V, Skryma R, Prevarskaya N. TRP channels in cell survival and cell death in normal and transformed cells. Cell Calcium 50: 295–302, 2011. [DOI] [PubMed] [Google Scholar]
- 62. Sharif-Naeini R, Folgering JH, Bichet D, Duprat F, Delmas P, Patel A, Honore E. Sensing pressure in the cardiovascular system: Gq-coupled mechanoreceptors and TRP channels. J Mol Cell Cardiol 48: 83–89, 2010. [DOI] [PubMed] [Google Scholar]
- 63. Silva A, Yunes JA, Cardoso BA, Martins LR, Jotta PY, Abecasis M, Nowill AE, Leslie NR, Cardoso AA, Barata JT. PTEN posttranslational inactivation and hyperactivation of the PI3K/Akt pathway sustain primary T cell leukemia viability. J Clin Invest 118: 3762–3774, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Soletti RC, del Barrio L, Daffre S, Miranda A, Borges HL, Moura-Neto V, Lopez MG, Gabilan NH. Peptide gomesin triggers cell death through L-type channel calcium influx, MAPK/ERK, PKC and PI3K signaling and generation of reactive oxygen species. Chem Biol Interact 186: 135–143, 2010. [DOI] [PubMed] [Google Scholar]
- 65. Suguro M, Tagawa H, Kagami Y, Okamoto M, Ohshima K, Shiku H, Morishima Y, Nakamura S, Seto M. Expression profiling analysis of the CD5+ diffuse large B-cell lymphoma subgroup: development of a CD5 signature. Cancer Sci 97: 868–874, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sumoza-Toledo A, Penner R. TRPM2: a multifunctional ion channel for calcium signalling. J Physiol 589: 1515–1525, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Takahashi N, Kozai D, Kobayashi R, Ebert M, Mori Y. Roles of TRPM2 in oxidative stress. Cell Calcium 50: 279–287, 2011. [DOI] [PubMed] [Google Scholar]
- 68. Togashi K, Hara Y, Tominaga T, Higashi T, Konishi Y, Mori Y, Tominaga M. TRPM2 activation by cyclic ADP-ribose at body temperature is involved in insulin secretion. EMBO J 25: 1804–1815, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Tong Q, Zhang W, Conrad K, Mostoller K, Cheung JY, Peterson BZ, Miller BA. Regulation of the transient receptor potential channel TRPM2 by the Ca2+ sensor calmodulin. J Biol Chem 281: 9076–9085, 2006. [DOI] [PubMed] [Google Scholar]
- 70. Tsavaler L, Shapero MH, Morkowski S, Laus R. Trp-p8, a novel prostate-specific gene, is up-regulated in prostate cancer and other malignancies and shares high homology with transient receptor potential calcium channel proteins. Cancer Res 61: 3760–3769, 2001. [PubMed] [Google Scholar]
- 71. Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324: 1029–1033, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Vander Heiden MG, Plas DR, Rathmell JC, Fox CJ, Harris MH, Thompson CB. Growth factors can influence cell growth and survival through effects on glucose metabolism. Mol Cell Biol 21: 5899–5912, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Veal EA, Day AM, Morgan BA. Hydrogen peroxide sensing and signaling. Mol Cell 26: 1–14, 2007. [DOI] [PubMed] [Google Scholar]
- 74. Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem 76: 387–417, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Waring P. Redox active calcium ion channels and cell death. Arch Biochem Biophys 434: 33–42, 2005. [DOI] [PubMed] [Google Scholar]
- 76. Wehage E, Eisfeld J, Heiner I, Jungling E, Zitt C, Luckhoff A. Activation of the cation channel long transient receptor potential channel 2 (LTRPC2) by hydrogen peroxide. A splice variant reveals a mode of activation independent of ADP-ribose. J Biol Chem 277: 23150–23156, 2002. [DOI] [PubMed] [Google Scholar]
- 77. Whitehead JP, Molero JC, Clark S, Martin S, Meneilly G, James DE. The role of Ca2+ in insulin-stimulated glucose transport in 3T3-L1 cells. J Biol Chem 276: 27816–27824, 2001. [DOI] [PubMed] [Google Scholar]
- 78. Wieman HL, Wofford JA, Rathmell JC. Cytokine stimulation promotes glucose uptake via phosphatidylinositol-3 kinase/Akt regulation of Glut1 activity and trafficking. Mol Biol Cell 18: 1437–1446, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wilkinson JA, Scragg JL, Boyle JP, Nilius B, Peers C. H2O2-stimulated Ca2+ influx via TRPM2 is not the sole determinant of subsequent cell death. Pflügers Arch 455: 1141–1151, 2008. [DOI] [PubMed] [Google Scholar]
- 80. Xu XZ, Moebius F, Gill DL, Montell C. Regulation of melastatin, a TRP-related protein, through interaction with a cytoplasmic isoform. Proc Natl Acad Sci USA 98: 10692–10697, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Yamamoto S, Shimizu S, Kiyonaka S, Takahashi N, Wajima T, Hara Y, Negoro T, Hiroi T, Kiuchi Y, Okada T, Kaneko S, Lange I, Fleig A, Penner R, Nishi M, Takeshima H, Mori Y. TRPM2-mediated Ca2+ influx induces chemokine production in monocytes that aggravates inflammatory neutrophil infiltration. Nat Med 14: 738–747, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Yamamoto S, Takahashi N, Mori Y. Chemical physiology of oxidative stress-activated TRPM2 and TRPC5 channels. Prog Biophys Mol Biol 103: 18–27, 2010. [DOI] [PubMed] [Google Scholar]
- 83. Yang JY, Zong CS, Xia W, Yamaguchi H, Ding Q, Xie X, Lang JY, Lai CC, Chang CJ, Huang WC, Huang H, Kuo HP, Lee DF, Li LY, Lien HC, Cheng X, Chang KJ, Hsiao CD, Tsai FJ, Tsai CH, Sahin AA, Muller WJ, Mills GB, Yu D, Hortobagyi GN, Hung MC. ERK promotes tumorigenesis by inhibiting FOXO3a via MDM2-mediated degradation. Nat Cell Biol 10: 138–148, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Yang KT, Chang WL, Yang PC, Chien CL, Lai MS, Su MJ, Wu ML. Activation of the transient receptor potential M2 channel and poly(ADP-ribose) polymerase is involved in oxidative stress-induced cardiomyocyte death. Cell Death Differ 13: 1815–1826, 2006. [DOI] [PubMed] [Google Scholar]
- 85. Yang W, Dolloff NG, El-Deiry WS. ERK and MDM2 prey on FOXO3a. Nat Cell Biol 10: 125–126, 2008. [DOI] [PubMed] [Google Scholar]
- 86. Zhang S, Yu D. PI3king apart PTEN's role in cancer. Clin Cancer Res 16: 4325–4330, 2010. [DOI] [PubMed] [Google Scholar]
- 87. Zhang W, Chu X, Tong Q, Cheung JY, Conrad K, Masker K, Miller BA. A novel TRPM2 isoform inhibits calcium influx and susceptibility to cell death. J Biol Chem 278: 16222–16229, 2003. [DOI] [PubMed] [Google Scholar]
- 88. Zhang W, Hirschler-Laszkiewicz I, Tong Q, Conrad K, Sun SC, Penn L, Barber DL, Stahl R, Carey DJ, Cheung JY, Miller BA. TRPM2 is an ion channel that modulates hematopoietic cell death through activation of caspases and PARP cleavage. Am J Physiol Cell Physiol 290: C1146–C1159, 2006. [DOI] [PubMed] [Google Scholar]
- 89. Zhao Y, Altman BJ, Coloff JL, Herman CE, Jacobs SR, Wieman HL, Wofford JA, Dimascio LN, Ilkayeva O, Kelekar A, Reya T, Rathmell JC. Glycogen synthase kinase-3α and -3β mediate a glucose-sensitive antiapoptotic signaling pathway to stabilize Mcl-1. Mol Cell Biol 27: 4328–4339, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]