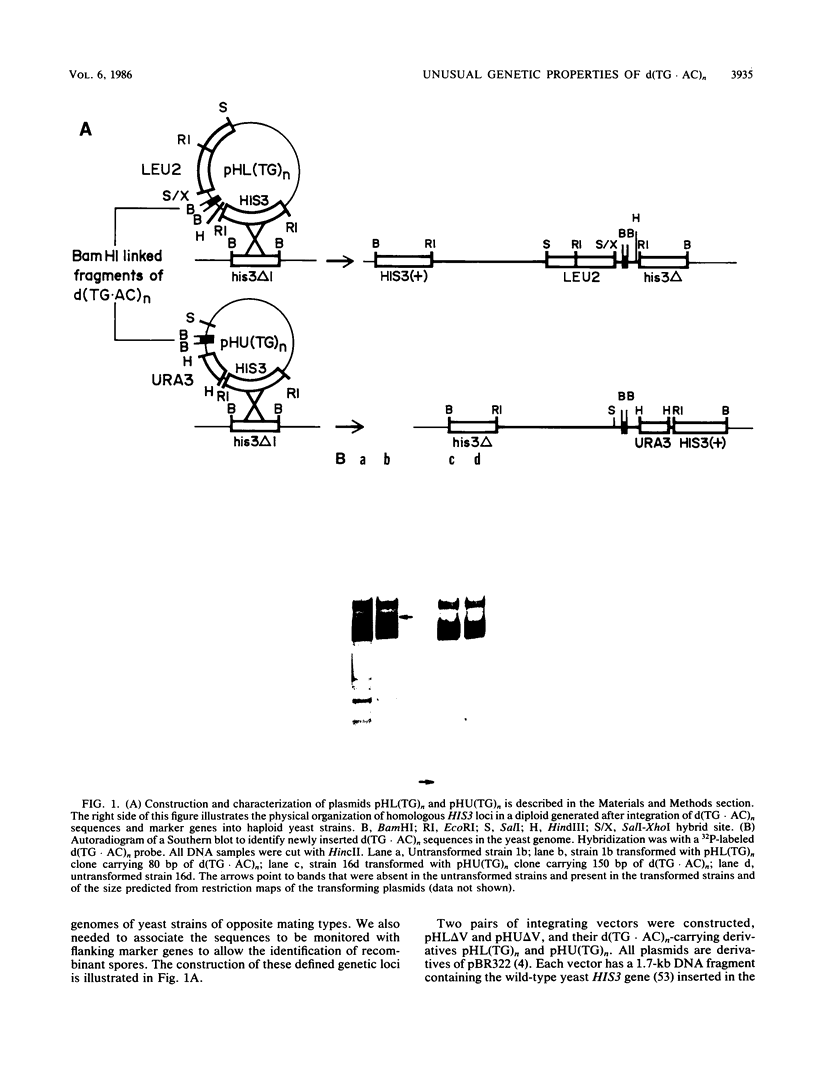
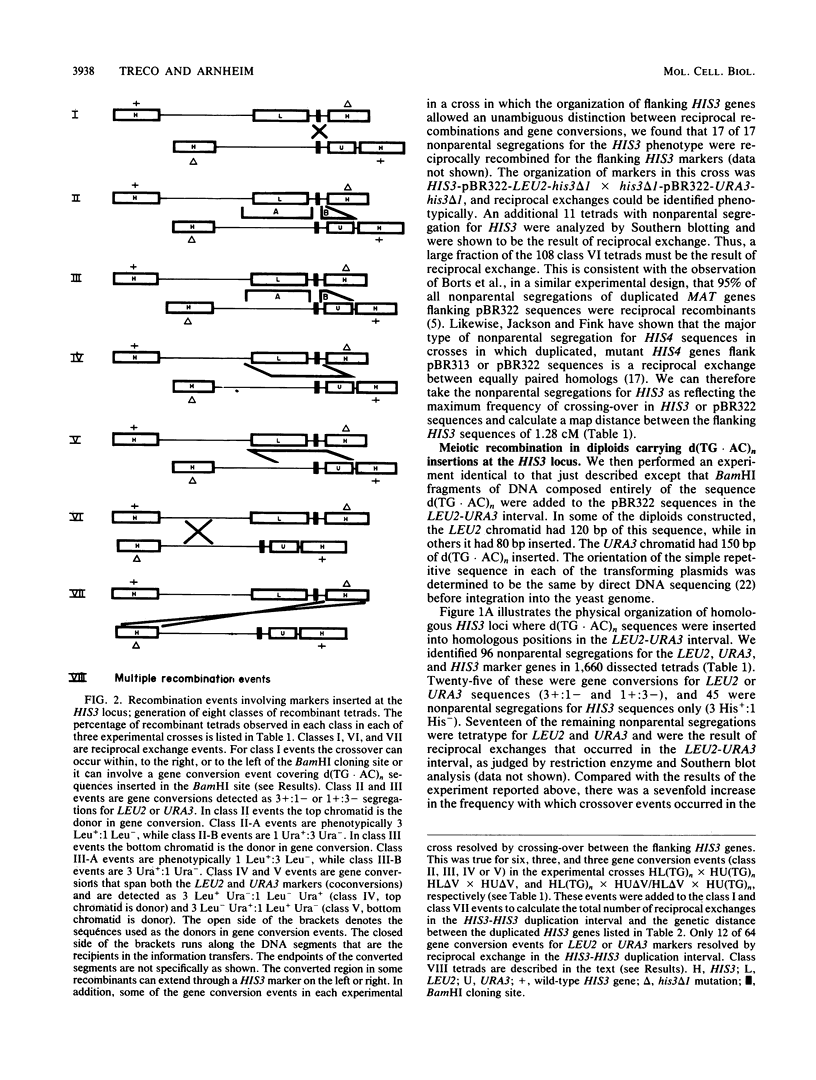
Abstract
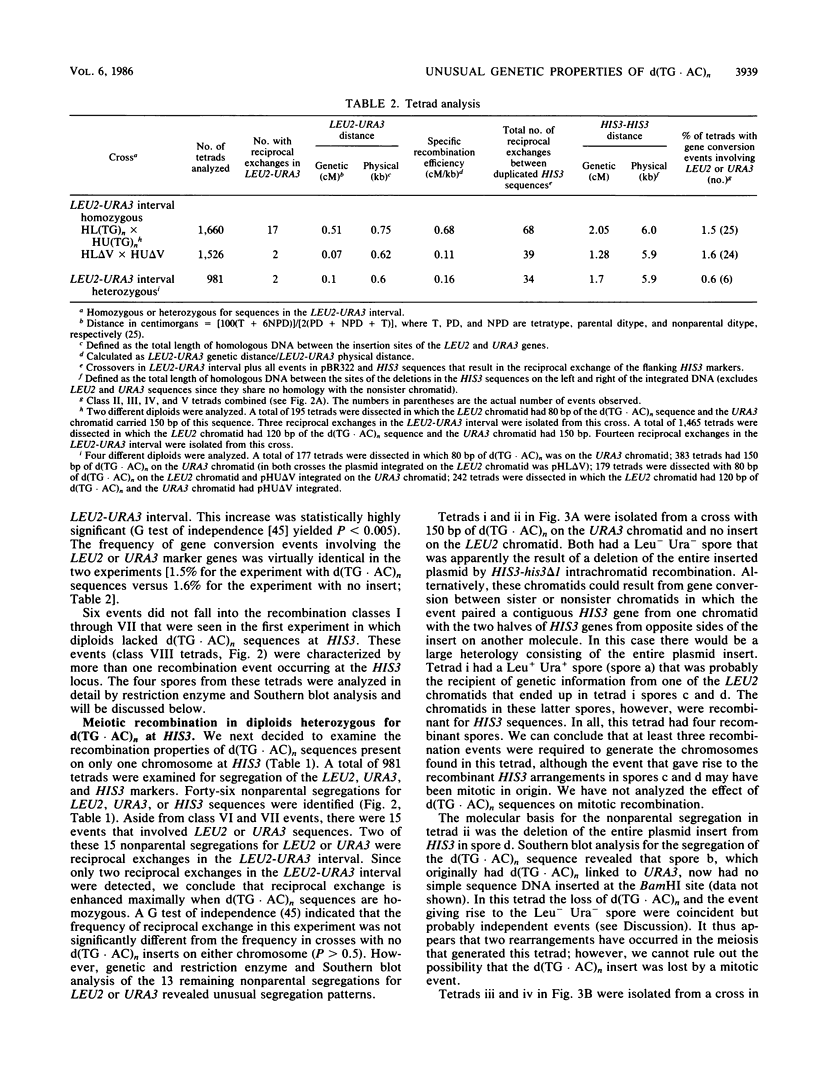
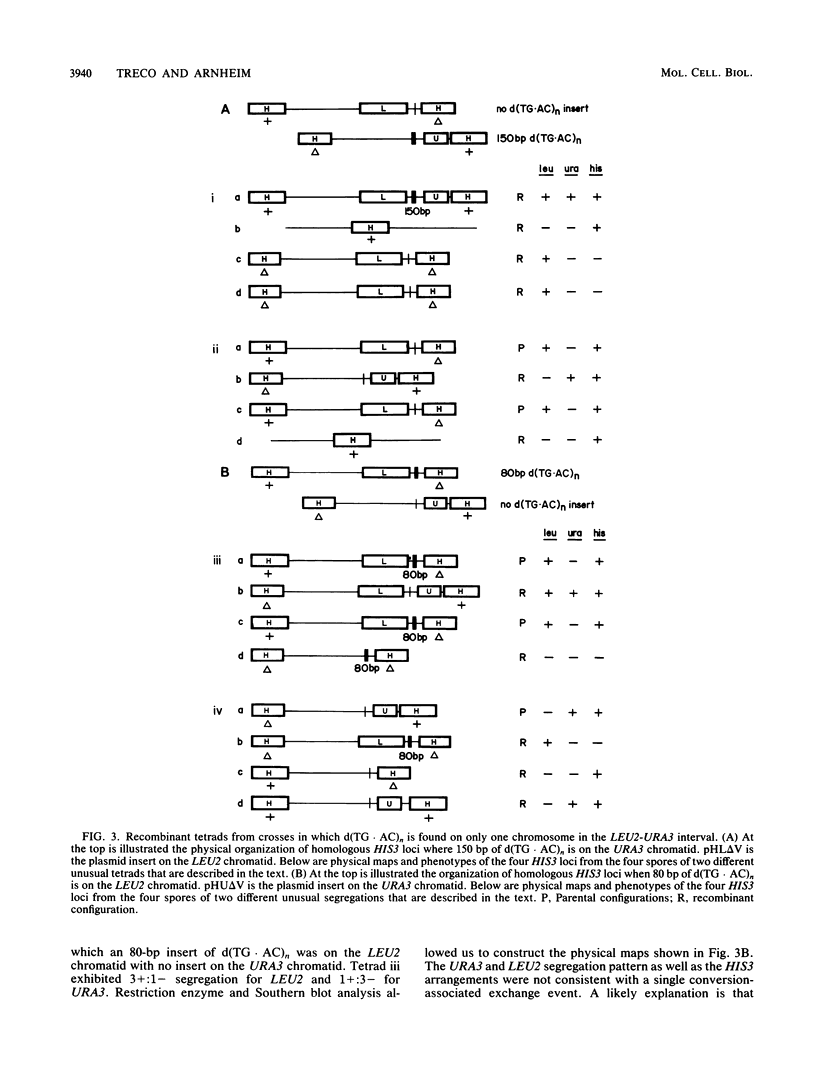
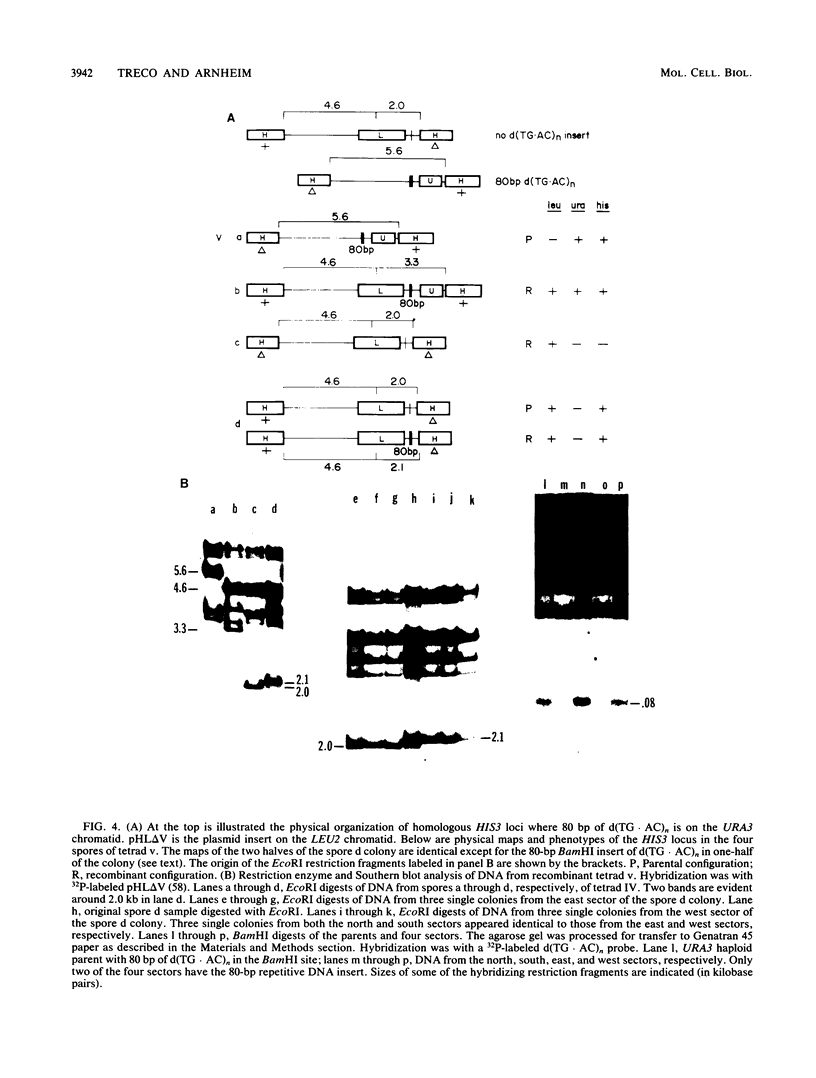
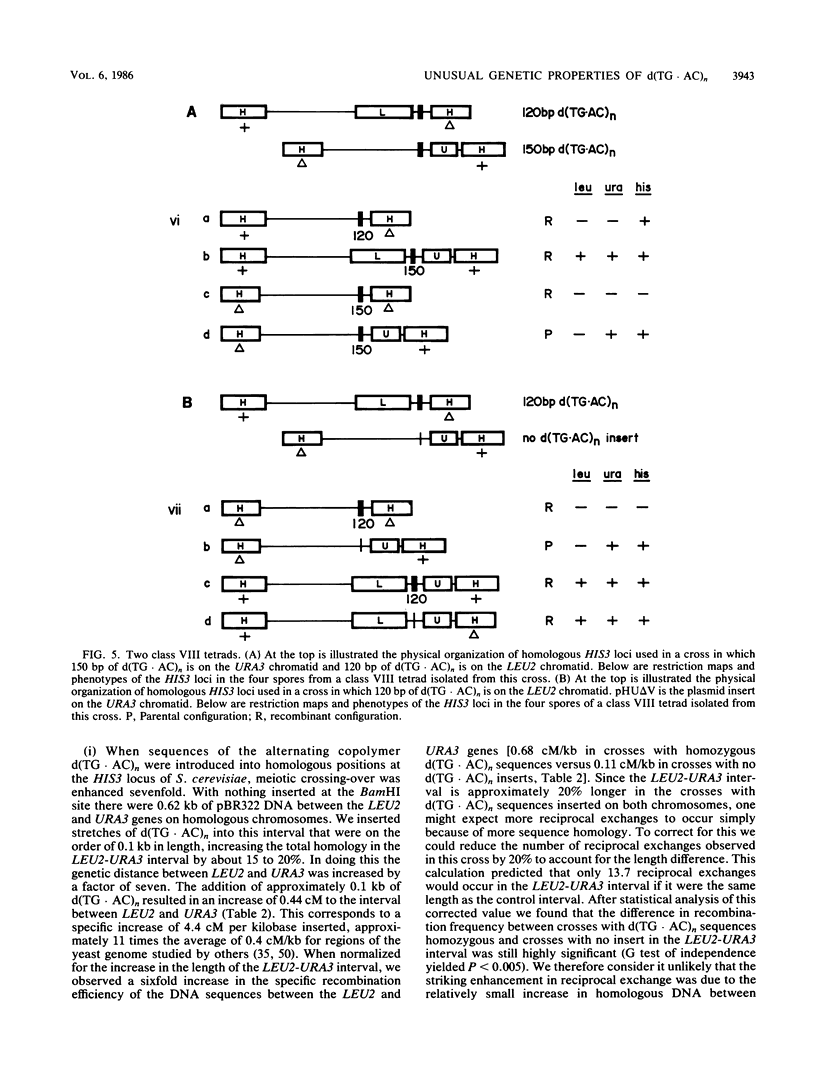
We have studied the genetic behavior of the alternating copolymer d(TG.AC)n inserted into a defined position in the genome of the yeast Saccharomyces cerevisiae. When d(TG.AC)n sequences were present at the HIS3 locus on homologous chromosomes, diploid cells undergoing meiosis generated an excess of tetrads containing reciprocally recombined products with crossover points close to the repetitive DNA insert. Most of these tetrads exhibited gene conversion of a d(TG.AC)n insert. However, the insertion of d(TG.AC)n sequences had no effect on the frequency of gene conversion of closely linked marker genes. Surprisingly, when d(TG.AC)n sequences were present on only one homolog at the HIS3 locus, one-half of the tetrads exhibiting nonparental segregation for marker genes that flanked the repetitive DNA insert were very unusual and appeared to have arisen by multiple recombination events in the vicinity of the d(TG.AC)n insert. Similar multiply recombinant tetrads were seen in crosses in which d(TG.AC)n sequences were present on both homologs. Combined, the data strongly suggest that d(TG.AC)n sequences significantly enhance reciprocal meiotic recombination and may be important in causing multiple recombination events to occur within a relatively small region of the yeast chromosome. Molecular evidence is presented that clearly documents the postmeiotic segregation of an 80-base stretch of d(TG.AC)n.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonarakis S. E., Boehm C. D., Giardina P. J., Kazazian H. H., Jr Nonrandom association of polymorphic restriction sites in the beta-globin gene cluster. Proc Natl Acad Sci U S A. 1982 Jan;79(1):137–141. doi: 10.1073/pnas.79.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach M. L., Lacroute F., Botstein D. Evidence for transcriptional regulation of orotidine-5'-phosphate decarboxylase in yeast by hybridization of mRNA to the yeast structural gene cloned in Escherichia coli. Proc Natl Acad Sci U S A. 1979 Jan;76(1):386–390. doi: 10.1073/pnas.76.1.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behe M., Felsenfeld G. Effects of methylation on a synthetic polynucleotide: the B--Z transition in poly(dG-m5dC).poly(dG-m5dC). Proc Natl Acad Sci U S A. 1981 Mar;78(3):1619–1623. doi: 10.1073/pnas.78.3.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Borts R. H., Lichten M., Hearn M., Davidow L. S., Haber J. E. Physical monitoring of meiotic recombination in Saccharomyces cerevisiae. Cold Spring Harb Symp Quant Biol. 1984;49:67–76. doi: 10.1101/sqb.1984.049.01.010. [DOI] [PubMed] [Google Scholar]
- Bullock P., Forrester W., Botchan M. DNA sequence studies of simian virus 40 chromosomal excision and integration in rat cells. J Mol Biol. 1984 Mar 25;174(1):55–84. doi: 10.1016/0022-2836(84)90365-6. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Dunn B., Szauter P., Pardue M. L., Szostak J. W. Transfer of yeast telomeres to linear plasmids by recombination. Cell. 1984 Nov;39(1):191–201. doi: 10.1016/0092-8674(84)90205-8. [DOI] [PubMed] [Google Scholar]
- Fink G. R., Styles C. A. Gene conversion of deletions in the his4 region of yeast. Genetics. 1974 Jun;77(2):231–244. doi: 10.1093/genetics/77.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan J. G., Lefranc M. P., Rabbitts T. H. Mechanisms of divergence and convergence of the human immunoglobulin alpha 1 and alpha 2 constant region gene sequences. Cell. 1984 Mar;36(3):681–688. doi: 10.1016/0092-8674(84)90348-9. [DOI] [PubMed] [Google Scholar]
- Greene P. J., Heyneker H. L., Bolivar F., Rodriguez R. L., Betlach M. C., Covarrubias A. A., Backman K., Russel D. J., Tait R., Boyer H. W. A general method for the purification of restriction enzymes. Nucleic Acids Res. 1978 Jul;5(7):2373–2380. doi: 10.1093/nar/5.7.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada H., Petrino M. G., Kakunaga T. A novel repeated element with Z-DNA-forming potential is widely found in evolutionarily diverse eukaryotic genomes. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6465–6469. doi: 10.1073/pnas.79.21.6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haniford D. B., Pulleyblank D. E. Facile transition of poly[d(TG) x d(CA)] into a left-handed helix in physiological conditions. Nature. 1983 Apr 14;302(5909):632–634. doi: 10.1038/302632a0. [DOI] [PubMed] [Google Scholar]
- Hentschel C. C. Homocopolymer sequences in the spacer of a sea urchin histone gene repeat are sensitive to S1 nuclease. Nature. 1982 Feb 25;295(5851):714–716. doi: 10.1038/295714a0. [DOI] [PubMed] [Google Scholar]
- Hinnen A., Hicks J. B., Fink G. R. Transformation of yeast. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1929–1933. doi: 10.1073/pnas.75.4.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson J. A., Fink G. R. Meiotic recombination between duplicated genetic elements in Saccharomyces cerevisiae. Genetics. 1985 Feb;109(2):303–332. doi: 10.1093/genetics/109.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazazian H. H., Jr, Orkin S. H., Antonarakis S. E., Sexton J. P., Boehm C. D., Goff S. C., Waber P. G. Molecular characterization of seven beta-thalassemia mutations in Asian Indians. EMBO J. 1984 Mar;3(3):593–596. doi: 10.1002/j.1460-2075.1984.tb01853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kmiec E. B., Holloman W. K. Synapsis promoted by Ustilago rec1 protein. Cell. 1984 Mar;36(3):593–598. doi: 10.1016/0092-8674(84)90338-6. [DOI] [PubMed] [Google Scholar]
- Kmiec E., Kroeger P., Holliday R., Holloman W. Homologous pairing promoted by Ustilago RecI protein. Cold Spring Harb Symp Quant Biol. 1984;49:675–682. doi: 10.1101/sqb.1984.049.01.076. [DOI] [PubMed] [Google Scholar]
- Maeda N., Yang F., Barnett D. R., Bowman B. H., Smithies O. Duplication within the haptoglobin Hp2 gene. Nature. 1984 May 10;309(5964):131–135. doi: 10.1038/309131a0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Meselson M. S., Radding C. M. A general model for genetic recombination. Proc Natl Acad Sci U S A. 1975 Jan;72(1):358–361. doi: 10.1073/pnas.72.1.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miesfeld R., Krystal M., Arnheim N. A member of a new repeated sequence family which is conserved throughout eucaryotic evolution is found between the human delta and beta globin genes. Nucleic Acids Res. 1981 Nov 25;9(22):5931–5947. doi: 10.1093/nar/9.22.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mroczka D. L., Cassidy B., Busch H., Rothblum L. I. Characterization of rat ribosomal DNA. The highly repetitive sequences that flank the ribosomal RNA transcription unit are homologous and contain RNA polymerase III transcription initiation sites. J Mol Biol. 1984 Mar 25;174(1):141–162. doi: 10.1016/0022-2836(84)90369-3. [DOI] [PubMed] [Google Scholar]
- Nishioka Y., Leder P. Organization and complete sequence of identical embryonic and plasmacytoma kappa V-region genes. J Biol Chem. 1980 Apr 25;255(8):3691–3694. [PubMed] [Google Scholar]
- Nordheim A., Rich A. The sequence (dC-dA)n X (dG-dT)n forms left-handed Z-DNA in negatively supercoiled plasmids. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1821–1825. doi: 10.1073/pnas.80.7.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkin S. H., Antonarakis S. E., Kazazian H. H., Jr Polymorphism and molecular pathology of the human beta-globin gene. Prog Hematol. 1983;13:49–73. [PubMed] [Google Scholar]
- Peck L. J., Wang J. C. Energetics of B-to-Z transition in DNA. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6206–6210. doi: 10.1073/pnas.80.20.6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot N. J., Maniatis T. The structure of a human alpha-globin pseudogene and its relationship to alpha-globin gene duplication. Cell. 1980 Sep;21(2):537–544. doi: 10.1016/0092-8674(80)90491-2. [DOI] [PubMed] [Google Scholar]
- Ratzkin B., Carbon J. Functional expression of cloned yeast DNA in Escherichia coli. Proc Natl Acad Sci U S A. 1977 Feb;74(2):487–491. doi: 10.1073/pnas.74.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich A. Right-handed and left-handed DNA: conformational information in genetic material. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):1–12. doi: 10.1101/sqb.1983.047.01.003. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Scherer S., Davis R. W. Replacement of chromosome segments with altered DNA sequences constructed in vitro. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4951–4955. doi: 10.1073/pnas.76.10.4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shampay J., Szostak J. W., Blackburn E. H. DNA sequences of telomeres maintained in yeast. Nature. 1984 Jul 12;310(5973):154–157. doi: 10.1038/310154a0. [DOI] [PubMed] [Google Scholar]
- Shen L. P., Rutter W. J. Sequence of the human somatostatin I gene. Science. 1984 Apr 13;224(4645):168–171. doi: 10.1126/science.6142531. [DOI] [PubMed] [Google Scholar]
- Shen S. H., Slightom J. L., Smithies O. A history of the human fetal globin gene duplication. Cell. 1981 Oct;26(2 Pt 2):191–203. doi: 10.1016/0092-8674(81)90302-0. [DOI] [PubMed] [Google Scholar]
- Singleton C. K., Kilpatrick M. W., Wells R. D. S1 nuclease recognizes DNA conformational junctions between left-handed helical (dT-dG n. dC-dA)n and contiguous right-handed sequences. J Biol Chem. 1984 Feb 10;259(3):1963–1967. [PubMed] [Google Scholar]
- Singleton C. K., Klysik J., Stirdivant S. M., Wells R. D. Left-handed Z-DNA is induced by supercoiling in physiological ionic conditions. Nature. 1982 Sep 23;299(5881):312–316. doi: 10.1038/299312a0. [DOI] [PubMed] [Google Scholar]
- Slightom J. L., Blechl A. E., Smithies O. Human fetal G gamma- and A gamma-globin genes: complete nucleotide sequences suggest that DNA can be exchanged between these duplicated genes. Cell. 1980 Oct;21(3):627–638. doi: 10.1016/0092-8674(80)90426-2. [DOI] [PubMed] [Google Scholar]
- Smith G. P. Evolution of repeated DNA sequences by unequal crossover. Science. 1976 Feb 13;191(4227):528–535. doi: 10.1126/science.1251186. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Long range periodicities in mouse satellite DNA. J Mol Biol. 1975 May 5;94(1):51–69. doi: 10.1016/0022-2836(75)90404-0. [DOI] [PubMed] [Google Scholar]
- Southern E. Gel electrophoresis of restriction fragments. Methods Enzymol. 1979;68:152–176. doi: 10.1016/0076-6879(79)68011-4. [DOI] [PubMed] [Google Scholar]
- Stanton L. W., Watt R., Marcu K. B. Translocation, breakage and truncated transcripts of c-myc oncogene in murine plasmacytomas. Nature. 1983 Jun 2;303(5916):401–406. doi: 10.1038/303401a0. [DOI] [PubMed] [Google Scholar]
- Strathern J. N., Newlon C. S., Herskowitz I., Hicks J. B. Isolation of a circular derivative of yeast chromosome III: implications for the mechanism of mating type interconversion. Cell. 1979 Oct;18(2):309–319. doi: 10.1016/0092-8674(79)90050-3. [DOI] [PubMed] [Google Scholar]
- Stringer J. R. DNA sequence homology and chromosomal deletion at a site of SV40 DNA integration. Nature. 1982 Mar 25;296(5855):363–366. doi: 10.1038/296363a0. [DOI] [PubMed] [Google Scholar]
- Stringer J. R. Recombination between poly[d(GT).d(CA)] sequences in simian virus 40-infected cultured cells. Mol Cell Biol. 1985 Jun;5(6):1247–1259. doi: 10.1128/mcb.5.6.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl K., Davis R. W. Production of a functional eukaryotic enzyme in Escherichia coli: cloning and expression of the yeast structural gene for imidazole-glycerolphosphate dehydratase (his3). Proc Natl Acad Sci U S A. 1977 Dec;74(12):5255–5259. doi: 10.1073/pnas.74.12.5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J. G. Complete nucleotide sequence of the Escherichia coli plasmid pBR322. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):77–90. doi: 10.1101/sqb.1979.043.01.013. [DOI] [PubMed] [Google Scholar]
- Szostak J. W., Orr-Weaver T. L., Rothstein R. J., Stahl F. W. The double-strand-break repair model for recombination. Cell. 1983 May;33(1):25–35. doi: 10.1016/0092-8674(83)90331-8. [DOI] [PubMed] [Google Scholar]
- Treco D., Thomas B., Arnheim N. Recombination hot spot in the human beta-globin gene cluster: meiotic recombination of human DNA fragments in Saccharomyces cerevisiae. Mol Cell Biol. 1985 Aug;5(8):2029–2038. doi: 10.1128/mcb.5.8.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorlícková M., Sedlácek P., Kypr J., Sponar J. Conformational transitions of poly(dA-dT)poly(dA-dT) in ethanolic solutions. Nucleic Acids Res. 1982 Nov 11;10(21):6969–6979. doi: 10.1093/nar/10.21.6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walmsley R. M., Szostak J. W., Petes T. D. Is there left-handed DNA at the ends of yeast chromosomes? Nature. 1983 Mar 3;302(5903):84–86. doi: 10.1038/302084a0. [DOI] [PubMed] [Google Scholar]
- Walmsley R. W., Chan C. S., Tye B. K., Petes T. D. Unusual DNA sequences associated with the ends of yeast chromosomes. Nature. 1984 Jul 12;310(5973):157–160. doi: 10.1038/310157a0. [DOI] [PubMed] [Google Scholar]
- Wang A. H., Hakoshima T., van der Marel G., van Boom J. H., Rich A. AT base pairs are less stable than GC base pairs in Z-DNA: the crystal structure of d(m5CGTAm5CG). Cell. 1984 May;37(1):321–331. doi: 10.1016/0092-8674(84)90328-3. [DOI] [PubMed] [Google Scholar]
- White J. H., Lusnak K., Fogel S. Mismatch-specific post-meiotic segregation frequency in yeast suggests a heteroduplex recombination intermediate. Nature. 1985 May 23;315(6017):350–352. doi: 10.1038/315350a0. [DOI] [PubMed] [Google Scholar]
- Zimmer C., Tymen S., Marck C., Guschlbauer W. Conformational transitions of poly(dA-dC).poly(dG-dT) induced by high salt or in ethanolic solution. Nucleic Acids Res. 1982 Feb 11;10(3):1081–1091. doi: 10.1093/nar/10.3.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]