Abstract
Objective
To compare glycemic outcomes in hospitalized patients with or without type 2 diabetes mellitus receiving neutral protamine Hagedorn insulin (NPH) vs glargine as basal insulin for management of glucocorticoid-associated hyperglycemia.
Methods
We conducted a retrospective review of electronic medical records in prednisone-treated adult patients with hyperglycemia in a university hospital. Consecutive patients were selected in both the NPH and glargine cohorts using inclusion and exclusion criteria. Baseline characteristics were assessed in each cohort. Glycemic outcomes were analyzed by comparing fasting blood glucose, mean daily blood glucose concentration, median daily blood glucose concentration, and the number of hypoglycemic episodes on a prespecified index day.
Results
One hundred twenty patients were included: 60 patients in the NPH cohort and 60 patients in the glargine cohort. The weight-based insulin requirement was lower in the NPH cohort than in the glargine cohort (0.27 ± 0.2 units/kg vs 0.34 ± 0.2 units/kg [P = .04] for basal insulin and 0.26 ± 0.2 units/kg vs 0.36 ± 0.2 units/kg [P = .03] for bolus insulin). NPH and glargine cohorts were similar regarding age, sex, race, body mass index, hemoglobin A1c, serum creatinine, and prednisone dosage. Glycemic outcomes in the NPH cohort compared with outcomes in the glargine cohort were similar regarding mean fasting blood glucose concentration (134 ± 49 mg/dL vs 139 ± 54 mg/dL [P = .63]), mean daily blood glucose (167 ± 46 mg/dL vs 165 ± 52 mg/dL [P = .79]), median blood glucose (160 ± 49 mg/dL vs 159 ± 57 mg/dL [P = .90]), and number of hypoglycemic episodes per day (0.12 ± 0.3 vs 0.10 ± 0.3 [P = .77]).
Conclusions
NPH and glargine appear to be equally effective as basal insulin in the management of hyperglycemia in hospitalized patients receiving prednisone. However, the total daily insulin doses used were lower in the NPH cohort.
INTRODUCTION
The use of glucocorticoids is known to exacerbate or induce hyperglycemia in persons with or without pre-existing diabetes mellitus (1). An increase in markers of insulin resistance was demonstrated in healthy persons after a single dose of dexamethasone (2). In a large-scale population study using an outpatient prescription database, the odds ratio for diabetes was found to be 1.36 with 3 or more prescriptions of oral glucocorticoids (3). In the same study, up to 2% of the incident cases of diabetes in the primary care population were estimated to be associated with glucocorticoid use. Findings from a retrospective study of hospitalized patients on a general medicine service in a university hospital indicated that more than half of the patients without previous diabetes and 64% of all patients who received high-dose glucocorticoid therapy experienced hyperglycemia (4). In patients receiving transplants, in whom glucocorticoids are used commonly as immunosuppressants, new-onset diabetes mellitus was documented in 15% to 20% of renal transplant recipients and 15% of liver transplant recipients (5).
In hospitalized patients, hyperglycemia is associated with an increased risk of infections after surgical procedures (6), increased mortality in patients with acute myocardial infarctions (7), increased risk of complications in transplant recipients (8), and worsened mortality in patients in the medical intensive care unit and patients who have had a stroke (9). Hospitalized patients with diabetes have significantly higher hospitalization costs and higher rates of diabetes-related hospital use (10). In one observational study, newly discovered hyperglycemia was associated with a higher in-hospital mortality rate than normoglycemia and known diabetes (11). These observations emphasize the importance of hyperglycemia in the hospital settings.
There are limited studies on outcomes and management of glucocorticoid-associated diabetes in the hospital settings. An observational study revealed a high prevalence of hyperglycemia with a longer duration of hospital stay in patients with glucocorticoid-associated hyperglycemia (4). Data supporting the benefits of glycemic control in the setting of glucocorticoid use are also limited (12). There are no randomized controlled trials evaluating different treatment regimens for glucocorticoid-associated hyperglycemia (1). Published literature and method of practice are largely based on extrapolation of the knowledge of the nature of hyperglycemia in glucocorticoid-induced diabetes and the pharmacodynamics of the agents used (1). However, the approaches used to treat these patients vary. In a survey of Chicago-area academic internists and subspecialists, opinions varied regarding the choice of agents for new-onset diabetes due to glucocorticoid use (13) and 78% perceived that there is a paucity of objective information about the management of hyperglycemia in this setting.
Pharmacodynamic properties might suggest that neutral protamine Hagedorn (NPH) insulin is a good choice for treatment of hyperglycemia due to oral prednisone and prednisolone, with peak hyperglycemic effects at 4 to 8 hours and duration of action of approximately 12 to 16 hours, mirroring the activity of NPH (1). Insulin glargine is another commonly used basal insulin with a different pharmacokinetic and pharmacodynamic profile; no peak effect and up to 32 hours of action (14). The pharmacokinetics of insulin, however, are affected by multiple variables (15), suggesting that in clinical practice there might be significant interpatient and intrapatient variability. Therefore, it is important to directly compare different insulin regimens in a clinical setting.
In this study, we retrospectively analyzed glycemic outcomes in hospitalized patients with glucocorticoid-associated hyperglycemia taking prednisone, comparing the use of NPH insulin with insulin glargine as the basal insulin.
RESEARCH DESIGN AND METHODS
Study Design and Patient Selection
A retrospective review of electronic medical records was conducted in patients admitted to the University of Wisconsin Hospital between January 1, 2010, and December 31, 2011. Patients were selected in both the NPH and glargine cohorts using the inclusion and exclusion criteria listed in Box 1. Because type 1 diabetes mellitus, which has absolute β-cell deficiency, may respond differently to glucocorticoids than other forms of diabetes characterized primarily by insulin resistance, we excluded patients with type 1 diabetes mellitus. After the required 60 patients were selected sequentially in each cohort during the given time frame, patient selection was truncated. The baseline characteristics of each cohort were collected. The most recent glycated hemoglobin A1c measurement available within the preceding 3 months was recorded. We defined the index day as the day before hospital discharge in patients taking prednisone at discharge, or as the last day of prednisone use in those who discontinued prednisone before hospital discharge. We selected this date for gathering outcomes because we anticipated that insulin dose titration and overall clinical stability would be at its best before discharge. Two days before the index day was selected as a reference point to record the serum creatinine concentration and the prednisone dosage to minimize the possible effects that fluctuation in renal function or marked changes in prednisone dosage in the preceding days might have on the glycemic control on the index day and to allow a uniform time for comparison between the 2 cohorts (Fig. 1). The amounts of NPH insulin, insulin glargine, and short-acting insulin (aspart and regular) used on the index day were recorded as units/kg body weight. Glycemic outcomes analyzed were fasting blood glucose, mean daily blood glucose, median blood glucose, and number of hypoglycemic episodes per day.
Box 1. Study Inclusion and Exclusion Criteria.
| Inclusion criteria |
| Age >18 years |
| Oral prednisone use during the index day and 2 days preceding it |
| Discharge diagnosis of diabetes or hyperglycemia (ICD-9 codes 249, 250, or 790.29) |
| Use of NPH or glargine |
| Exclusion criteria |
| Blood glucose readings <2 per day |
| Duration of prednisone use <3 days |
| Prednisone dosage <10 mg daily |
| Type 1 diabetes mellitus (identified by discharge ICD-9 codes and medical records review) |
| Duration of hospital stay <3 days |
| Serum creatinine ≥3 mg/dL |
| Clinically relevant hepatic disease |
Abbreviations: ICD-9, International Classification of Diseases, Ninth Revision; NPH, neutral protamine Hagedorn.
Fig. 1.
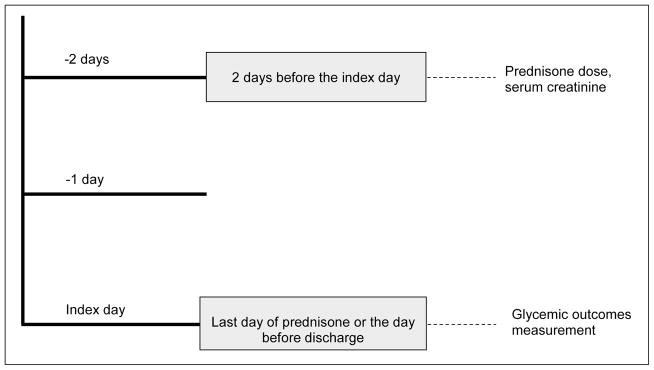
Definition of index day for data collection.
Glycemic Outcomes
All serum and capillary blood glucose readings obtained and recorded in the electronic medical record between midnight and 11:59 PM on the index day were used to analyze glycemic outcomes. Fasting blood glucose was identified as the first morning blood glucose of the day in patients without continuous tube feeding or parenteral nutrition. Hypoglycemia was defined as any blood glucose measurement less than 70 mg/dL and was reported as the number of hypoglycemic episodes on the index day. Mean and median daily blood glucose levels were calculated on the index day.
Statistical Analyses
To calculate required sample size, we predicted a difference in glycemic outcomes of 27 mg/dL based on the difference seen between 4-times-a-day regular insulin supplemental scale and basal-bolus regimen in the RABBIT-2 trial (16) that was done in an inpatient setting. Because no reliable randomized controlled trial has been conducted comparing NPH with glargine in hospitalized patients, we resorted to this study with different insulin groups. However, the outcome difference is comparable to a net difference of 37 mg/dL in fasting plasma glucose observed between NPH and glargine in a meta-analysis of randomized controlled trials involving patients with type 2 diabetes in outpatient settings (17), supporting the reliability of this choice. To detect this difference between the NPH and the glargine cohorts, with a power of 90%, we required a sample size of 59 in each group. The outcomes are expressed as mean ± standard deviation or as the median value on the index day. The cohorts were compared using an unpaired t test (with the Welch correction when variances were unequal). A P value less than .05 was considered significant. All statistical analyses were performed using GraphPad Prism, version 5.04 for Windows (GraphPad Software, La Jolla, California).
The University of Wisconsin-Madison Health Sciences Institutional Review Board approved this study.
RESULTS
Overall, we observed minimal differences between the baseline characteristics of the patients in the 2 cohorts. Patients in the NPH and glargine cohorts were similar regarding the demographic characteristics of age, sex, race, and body mass index (Table 1). Median time to index day from the date of admission was 7 days (range, 3 to 59 days) for the NPH cohort vs 7.5 days (range, 3 to 42 days) for the glargine cohort. Overall baseline glycemic control was similar as indicated by a mean hemoglobin A1c value of 7.2 ± 1.5% vs 6.8 ± 1.1% (P = .15). There was no difference in serum creatinine recorded 2 days before the index day (1.5 ± 0.6 mg/dL vs 1.5 ± 0.6 mg/dL; P = .94). Both of the cohorts were taking high-dosage prednisone: 31 ± 26 mg daily in the NPH cohort compared with 31 ± 23 mg daily in the glargine cohort (P = .89) with pronounced but similar variability as indicated by high standard deviations (Fig. 2). Most of the patients had been taking glucocorticoids before hospital admission, with no difference between the 2 cohorts (65% vs 60%; P = .57).
Table 1.
Baseline Characteristics of Patients in the Neutral Protamine Hagedorn Cohort and the Insulin Glargine Cohort
| Characteristics | NPH cohort (n = 60) | Insulin glargine cohort (n = 60) | P valuea |
|---|---|---|---|
| Age, mean (±SD), y | 58 (±15) | 58 (±13) | .84 |
| Sex, No. (%) | >.99 | ||
| Male | 36 (60) | 36 (60) | … |
| Female | 24 (40) | 24 (40) | … |
| Race, No. (%) | .79 | ||
| White | 51 (85) | 52 (87) | … |
| Other | 9 (15) | 8 (13) | … |
| Type of diabetes, No. (%) | |||
| Type 2 diabetes | 30 (50) | 31 (52) | .86 |
| Glucocorticoid-induced diabetes | 29 (48) | 21 (35) | .14 |
| Other | 1 (2) | 8 (13) | .02 |
| Body mass index, mean (±SD), kg/m2 | 29.8 (±7) | 30.9 (±9) | .46 |
| Hemoglobin A1c, mean (±SD), % | 7.2 (±1.5) | 6.8 (±1.1) | .15 |
| Serum creatinine, mean (±SD), mg/dL | 1.5 (±0.6) | 1.5 (±0.6) | .94 |
| Prednisone dosage, mean (±SD), mg daily | 31 (±26) | 31 (±23) | .89 |
| Indications for glucocorticoid use, No. (%) | |||
| Renal transplant | 26 (43) | 22 (37) | .46 |
| Pulmonary disease | 15 (25) | 24 (40) | .08 |
| Other | 19 (32) | 14 (23) | .31 |
| On glucocorticoids before admission, No. (%) | 39 (65) | 36 (60) | .57 |
| No. of fingerstick blood glucose readings per day, mean (±SD) | 4.5 (±0.9) | 4.4 (±1.0) | .71 |
| Managed by Diabetes Management Service, No. (%) | 43 (72) | 43 (72) | >.99 |
Abbreviations: NPH, neutral protamine Hagedorn; SD, standard deviation.
P values were determined by unpaired t test (with the Welch correction when variances were unequal).
Fig. 2.
Total daily prednisone dose in patients receiving neutral protamine Hagedorn (NPH) insulin (n = 60) or insulin glargine (n = 60) as basal insulin in a retrospective study of glucocorticoid-associated hyperglycemia.
There were some differences in the underlying disease states between the groups. The glargine cohort had significantly more patients with other types of diabetes mellitus than the NPH cohort (P = .02). These were cystic fibrosis – or pancreatitis-associated diabetes mellitus. Disease states for which prednisone was indicated were similar in the 2 cohorts.
The weight-based dosage of basal insulin was significantly different between the 2 cohorts. The NPH cohort received 0.27 ± 0.2 units/kg of NPH insulin, while the glargine cohort received 0.34 ± 0.2 units/kg of insulin glargine. The rapid- or short-acting insulin dosage was also lower in the NPH cohort than in the glargine cohort (0.26 ± 0.2 units/kg vs 0.36 ± 0.2 units/kg). The proportion of basal to bolus insulin was approximately 1:1 in each of the cohorts (Table 2).
Table 2.
Total Daily Insulin Dose in Patients Receiving Neutral Protamine Hagedorn Insulin or Insulin Glarginea
| Categories | NPH cohort (n = 60) | Insulin glargine cohort (n = 60) | P valueb |
|---|---|---|---|
| Basal insulin, units/kg | 0.27 (±0.2) | 0.34 (±0.2) | .04 |
| Bolus insulin, units/kg | 0.26 (±0.2) | 0.36 (±0.2) | .03 |
Data are presented as mean (±standard deviation).
Analysis by unpaired t test (with the Welch correction when variances were unequal).
The overall glycemic outcomes in the NPH cohort vs the glargine cohort were similar (Table 3, PFig. 3). Mean fasting blood glucose was not significantly different between cohorts (134 ± 49 mg/dL vs 139 ± 54 mg/dL, = .63). To have a better picture of overall glycemic control, we also examined all the blood glucose measurements on a single index day. The overall mean blood glucose value on the index day was not different between the 2 cohorts (167 ± 46 mg/dL vs 165 ± 52 mg/dL, P = .79). To avoid effects of outlier blood glucose readings causing shifts in the mean, we also examined the median blood glucose on the index day. There was also no difference in median blood glucose with values of 160 ± 49 mg/dL vs 159 ± 57 mg/dL (P = .90). The number of hypoglycemia episodes on the index day was also not different between the 2 cohorts (0.12 ± 0.3 vs 0.10 ± 0.3; P = .77).
Table 3.
Glycemic Outcomes in Patients Receiving Neutral Protamine Hagedorn Insulin or Insulin Glarginea
| Categories | NPH cohort (n = 60) | Insulin glargine cohort (n = 60) | P valueb |
|---|---|---|---|
| Fasting blood glucose, mg/dL | 134 (±49) | 139 (±54) | .63 |
| Mean daily blood glucose, mg/dL | 167 (±46) | 165 (±52) | .79 |
| Median dialy blood glucose, mg/dL | 160 (±49) | 159 (±57) | .90 |
| No. of hypoglycemic episodes per day | 0.12 (±0.3) | 0.10 (±0.3) | .77 |
Data are presented as mean (±standard deviation).
Analysis by unpaired t test (with the Welch correction when variances were unequal).
Fig. 3.
Glycemic outcomes in patients receiving neutral protamine Hagedorn (NPH) insulin (n = 60) or insulin glargine (n = 60) as basal insulin in a retrospective study of glucocorticoid-associated hyperglycemia. Panel A, Mean fasting blood glucose concentration. Panel B, Mean daily blood glucose concentration. Panel C, Median daily blood glucose concentration. Panel D, Mean number of hypoglycemic episodes per day. Error bars represent standard deviation.
DISCUSSION
There is a paucity of information regarding the proper choice of agents for the management of glucocorticoid-associated hyperglycemia in hospitalized patients. In this retrospective study, 60 patients receiving NPH insulin were compared with 60 patients receiving glargine as basal insulin during prednisone administration.
There are many factors that may influence the decision about what type of insulin to use for treating glucocorticoid-associated hyperglycemia. Most patients in both the cohorts were cared for by an inpatient diabetes management service that tends to have a uniform management style. One typical approach used by this team is to use twice-daily NPH insulin with twice-daily oral prednisone. The fact that the pharmacokinetic profile of prednisone parallels that of NPH is a primary argument for its use in patients with glucocorticoid-associated diabetes on prednisone (1). Patients with preexisting diabetes are often maintained on the same type of insulin used before hospital admission. In this type of retrospective study, we are unable to evaluate the decision-making process that led to the choice of insulin therapy in these patients, but we can analyze the outcomes associated with that choice.
In this study, blood glucose values achieved were within the goal ranges of less than 140 mg/dL for fasting and less than 180 mg/dL for random blood glucose measurements that major guidelines recommend for hospitalized patients in general medical wards (18,19). We found that control of hyperglycemia was similar in the NPH and the glargine cohorts, suggesting that both regimens are able to achieve glycemic targets. A notable difference, however, was the dosage of insulin required. The weight-based dose of glargine was comparable to doses used in patients with type 2 diabetes during “treat-to-target” studies (20,21), but the dose of NPH was significantly lower. Our results suggest that to achieve similar glycemic control in prednisone-treated patients, a higher dose of glargine and rapid-acting insulin is required than in NPH and rapid- or short-acting insulin regimens. The fact that the bolus insulin dosing is also different is not really surprising, since we would predict that NPH is also giving some “meal coverage” while glargine is not. This makes the difference in NPH dosing even more striking. The proportion of basal to bolus requirements were nearly 1:1 as recommended for glycemic management in other settings. The number of hypoglycemic episodes per day was also not different between the 2 cohorts, in contrast to the findings in studies of patients not taking glucocorticoids that demonstrate more hypoglycemic episodes in patients on NPH than in patients on glargine (17,22). This might indicate a different glycemic profile in patients on high-dose glucocorticoids, which make either NPH or glargine equally effective.
As mentioned in the preceding text, there is a concept that NPH insulin better matches the pharmacokinetic profile of prednisone, and should be used in patients on this type of glucocorticoid. Our results suggest that although the pharmacokinetic profiles of prednisone and NPH match more closely, a similar glycemic outcome is achieved by both NPH insulin and glargine basal-bolus regimen at the cost of higher insulin doses when using a glargine-based regimen.
Although most patients in our study had type 2 diabetes mellitus and glucocorticoid-induced diabetes mellitus, there was a significant difference in the number of patients in the 2 cohorts with other types of diabetes. This category included patients with cystic fibrosis – and pancreatitis-associated diabetes. In a separate analysis excluding these patients, there was no difference in glycemic outcomes, thus suggesting that this difference does not have a significant influence on the overall outcomes of this study.
Our study population is somewhat unique in that it contained a large percentage of transplant recipients, primarily renal transplant. This may have some impact on the overall glycemic outcomes, as multiple factors besides glucocorticoid use, such as the use of calcineurin inhibitors for immunosuppression, are known to be associated with posttransplant hyperglycemia (8). It is reasonable to question whether our results are broadly translatable to all hospitalized patients on high-dosage glucocorticoid therapy, but the results are likely to be highly relevant to hospitals caring for transplant recipients with diabetes.
CONCLUSION
In conclusion, NPH insulin and insulin glargine appear to be equally effective in the management of inpatient hyperglycemia associated with prednisone use. However, the NPH insulin–based regimen appears to achieve the same goals with a lower dosage of insulin. NPH insulin is also less expensive than insulin glargine; therefore, NPH insulin–based therapy is likely to be more cost-effective in the hospital care of this select patient population. Our study is limited by the fact that it is a retrospective study performed in a relatively unique population, with most patients being transplant recipients, in a tertiary care center with a dedicated inpatient diabetes management team. To fully explore the differences between these 2 regimens, a prospective randomized controlled clinical trial will be necessary in a larger population.
Acknowledgments
The authors would like to thank Zhanhai Li, PhD, for assistance with statistical issues. Dr. Davis has received support from NIDDK (DK083442) and the University of Wisconsin. The contents of this manuscript do not represent the views of the Department of Veterans Affairs or the United States government.
Abbreviation
- NPH
neutral protamine Hagedorn
Footnotes
To purchase reprints of this article, please visit: www.aace.com/reprints.
DISCLOSURE
Drs. Dhital, Shenker, and Davis have no multiplicity of interest to disclose. Dr. Meredith has received research support from Novo-Nordisk and Medtronic for separate projects.
References
- 1.Clore JN, Thurby-Hay L. Glucocorticoid-induced hyperglycemia. Endocr Pract. 2009;15:469–474. doi: 10.4158/EP08331.RAR. [DOI] [PubMed] [Google Scholar]
- 2.Abdelmannan D, Tahboub R, Genuth S, Ismail-Beigi F. Effect of dexamethasone on oral glucose tolerance in normal individuals. Endocr Pract. 2010;16:770–777. doi: 10.4158/EP09373.OR. [DOI] [PubMed] [Google Scholar]
- 3.Gulliford MC, Charlton J, Latinovic R. Risk of diabetes associated with prescribed glucocorticoids in a large population. Diabetes Care. 2006;29:2728–2729. doi: 10.2337/dc06-1499. [DOI] [PubMed] [Google Scholar]
- 4.Donihi AC, Raval D, Saul M, Korytkowski MT, DeVita MA. Prevalence and predictors of corticosteroid-related hyperglycemia in hospitalized patients. Endocr Pract. 2006;12:358–362. doi: 10.4158/EP.12.4.358. [DOI] [PubMed] [Google Scholar]
- 5.Moore R, Ravindran V, Baboolal K. The burden of new-onset diabetes mellitus after transplantation. Clin Transplant. 2006;20:755–761. doi: 10.1111/j.1399-0012.2006.00565.x. [DOI] [PubMed] [Google Scholar]
- 6.Pomposelli JJ, Baxter JK, 3rd, Babineau TJ, et al. Early postoperative glucose control predicts nosocomial infection rate in diabetic patients. JPEN J Parenter Enteral Nutr. 1998;22:77–81. doi: 10.1177/014860719802200277. [DOI] [PubMed] [Google Scholar]
- 7.Malmberg K, Norhammar A, Wedel H, Rydén L. Glycometabolic state at admission: Important risk marker of mortality in conventionally treated patients with diabetes mellitus and acute myocardial infarction: Long-term results from the diabetes and insulin-glucose infusion in acute myocardial infarction (DIGAMI) study. Circulation. 1999;99:2626–2632. doi: 10.1161/01.cir.99.20.2626. [DOI] [PubMed] [Google Scholar]
- 8.Bloom RD, Crutchlow MF. Transplant-associated hyperglycemia. Transplant Rev (Orlando) 2008;22:39–51. doi: 10.1016/j.trre.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 9.Inzucchi SE. Clinical practice. Management of hyperglycemia in the hospital setting. N Engl J Med. 2006;355:1903–1911. doi: 10.1056/NEJMcp060094. [DOI] [PubMed] [Google Scholar]
- 10.Menzin J, Korn JR, Cohen J, et al. Relationship between glycemic control and diabetes-related hospital costs in patients with type 1 or type 2 diabetes mellitus. J Manag Care Pharm. 2010;16:264–275. doi: 10.18553/jmcp.2010.16.4.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: An independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87:978–982. doi: 10.1210/jcem.87.3.8341. [DOI] [PubMed] [Google Scholar]
- 12.Hoogwerf B, Danese RD. Drug selection and the management of corticosteroid-related diabetes mellitus. Rheum Dis Clin North Am. 1999;25:489–505. doi: 10.1016/s0889-857x(05)70083-1. [DOI] [PubMed] [Google Scholar]
- 13.Braithwaite SS, Barr WG, Thomas JD. Diabetes management during glucocorticoid therapy for nonendocrine disease. Endocr Pract. 1996;2:320–325. doi: 10.4158/EP.2.5.320. [DOI] [PubMed] [Google Scholar]
- 14.Lucidi P, Porcellati F, Rossetti P, et al. Pharmacokinetics and pharmacodynamics of therapeutic doses of basal insulins NPH, glargine, and detemir after 1 week of daily administration at bedtime in type 2 diabetic subjects: a randomized cross-over study. Diabetes Care. 2011;34:1312–1314. doi: 10.2337/dc10-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Binder C, Lauritzen T, Faber O, Pramming S. Insulin pharmacokinetics. Diabetes Care. 1984;7:188–199. doi: 10.2337/diacare.7.2.188. [DOI] [PubMed] [Google Scholar]
- 16.Umpierrez GE, Smiley D, Zisman A, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial) Diabetes Care. 2007;30:2181–2186. doi: 10.2337/dc07-0295. [DOI] [PubMed] [Google Scholar]
- 17.Bazzano LA, Lee LJ, Shi L, Reynolds K, Jackson JA, Fonseca V. Safety and efficacy of glargine compared with NPH insulin for the treatment of type 2 diabetes: a meta-analysis of randomized controlled trials. Diabet Med. 2008;25:924–932. doi: 10.1111/j.1464-5491.2008.02517.x. [DOI] [PubMed] [Google Scholar]
- 18.American Diabetes Association. Standards of medical care in diabetes--2012. Diabetes Care. 2012;35 (Suppl 1):S11–S63. doi: 10.2337/dc12-s011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Umpierrez GE, Hellman R, Korytkowski MT, et al. Endocrine Society. Management of hyperglycemia in hospitalized patients in non-critical care setting: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2012;97:16–38. doi: 10.1210/jc.2011-2098. [DOI] [PubMed] [Google Scholar]
- 20.Holman RR, Farmer AJ, Davies MJ, et al. 4-T Study Group. Three-year efficacy of complex insulin regimens in type 2 diabetes. N Engl J Med. 2009;361:1736–1747. doi: 10.1056/NEJMoa0905479. [DOI] [PubMed] [Google Scholar]
- 21.King AB. No higher dose requirements with insulin detemir than glargine in type 2 diabetes: a crossover, double-blind, and randomized study using continuous glucose monitoring. J Diabetes Sci Technol. 2010;4:151–154. doi: 10.1177/193229681000400119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monami M, Marchionni N, Mannucci E. Long-acting insulin analogues vs. NPH human insulin in type 1 diabetes. A meta-analysis. Diabetes Obes Metab. 2009;11:372–378. doi: 10.1111/j.1463-1326.2008.00976.x. [DOI] [PubMed] [Google Scholar]




